Abstract
Methamphetamine [METH (“speed”)] is an abused psychostimulant that can cause psychotic, cognitive, and psychomotor impairment in humans. These signs and symptoms are thought to be related to dysfunctions in basal ganglionic structures of the brain. To identify possible molecular bases for these clinical manifestations, we first used cDNA microarray technology to measure METH-induced transcriptional responses in the striatum of rats treated with an apoptosis-inducing dose of the drug. METH injection resulted in increased expression of members of the Jun, Egr, and Nur77 subfamilies of transcription factors (TFs), changes that were confirmed by quantitative PCR. Because pathways linked to these factors are involved in the up-regulation of Fas ligand (FasL), FasL mRNA was quantified and found to be increased. Immunohistochemical studies also revealed METH-induced increased FasL protein expression in striatal GABAergic neurons that express enkephalin. Moreover, there were METH-mediated increases in calcineurin, as well as shuttling of nuclear factor of activated T cells (NFAT)c3 and NFATc4 from the cytosol to the nucleus of METH-treated rats, mechanisms also known to be involved in FasL regulation. Furthermore, METH induced cleavage of caspase-3 in FasL- and Fas-containing neurons. Finally, the METH-induced changes in the FasL-Fas death pathway were attenuated by pretreatment with the dopamine D1 receptor antagonist, SCH23390, which also caused attenuation of METH-induced apoptosis. These observations indicate that METH causes some of its neurodegenerative effects, in part, via stimulation of the Fas-mediated cell death pathway consequent to FasL up-regulation mediated by activation of multiple TFs.
Keywords: calcium, neurodegeneration, gelsolin, Egr
Methamphetamine [METH (”speed”)] is a psychostimulant that is abused throughout the U.S. and the world. Its use can result in euphoria, decreased appetite, and increased alertness, whereas long-term abuse of METH can result in neuropsychiatric complications such as paranoia, coma, stroke, and even death (1, 2). The acute euphoric and intoxicating effects of the drug are thought to be related to the release of dopamine (DA), a neurotransmitter that is very abundant in the mammalian striatum (3). In contrast, the long-term changes that occur in human abusers might be due to neurotoxic or neurodegenerative effects of the drug on monoaminergic terminals (4, 5), abnormalities that have been replicated in animal models of METH toxicity (6). Recent animal studies have also suggested that METH can damage neuronal cell bodies located in the frontal cortex and striatum (6), abnormalities that might also have an impact on the clinical signs and symptoms observed with chronic abuse of the drug. These suggestions are supported by observations that recovery of indices of DA depletion is not associated with significant improvement in neuropsychological parameters in METH abusers (7) and by reports that METH abusers show metabolic abnormalities in other brain regions in addition to the striatum (8, 9). A recent postmortem study has also provided evidence of METH-induced degeneration of nonmonoaminergic cells in the brains of METH abusers (10).
So far, however, only a few studies have tried to unravel the cellular and molecular bases of METH-induced neuronal death in vitro (11, 12) and in vivo (6, 13-15). The in vivo studies have demonstrated that METH can cause neuronal apoptosis via activation of the stress-activated protein kinase/c-Jun N-terminal kinase pathway in the mouse brain (16, 17) and by the concurrent activation of mitochondrial and endoplasmic reticulum death pathways (18).
To identify and characterize additional molecular pathways that might be involved in the deleterious actions of this illicit neurotoxin, we decided to extend our studies of METH-induced apoptosis by further exploiting the METH toxicity model in the rat. Thus, the purpose of this paper is to report that a dose of METH, known to negatively impact on monoaminergic terminals, can also cause apoptosis in the rat striatum. We also provide evidence that METH-induced cell death depends, in part, on calcineurin/nuclear factor of activated T cells (NFAT)-mediated increases in Fas ligand (FasL) expression and activation of the Fas-dependent apoptotic pathway.
Materials and Methods
Animals and Drug Treatment. Male Sprague-Dawley rats (Charles River Breeding Laboratories), weighing 250-300 g, were used. Experiments were carried out in a room with temperature maintained at 22°C. Rats were housed individually and received food and water ad libitum. Rats were given one injection of either METH (40 mg/kg) or saline i.p. This dose was chosen because it has been shown to cause long-term depletion of monoaminergic terminals in the rat brain (19) and to induce apoptosis in the mouse brain (15, 18). This dose of METH was lethal in ≈20% of the rats. To assess the effects of a drug known to protect against METH-induced striatal depletion on some parameters measured in the present study, we pretreated some animals with the DA D1 receptor antagonist, SCH23390 (0.5 mg/kg), 30 min before administering a saline or METH injection. Rectal temperature was measured by using a Yellow Springs Instruments (Yellow Springs, OH) telethermometer; see Supporting Text, which is published as supporting information on the PNAS web site, for more details. All animal use procedures were according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the local animal care committee.
Array and RT-PCR Analyses. For the array experiments, animals were killed 1 h after drug treatment. To confirm some of the array data and to measure the levels of several transcripts, other groups of rats were injected with METH and were killed at various times afterward. Detailed cDNA array preparation and RT-PCR analyses are provided in the Supporting Text. See Table 1, which is published as supporting information on the PNAS web site, for a list of the primers used.
HPLC Measurements. DA and its metabolites were measured by using HPLC with electrochemical detection (see Supporting Text).
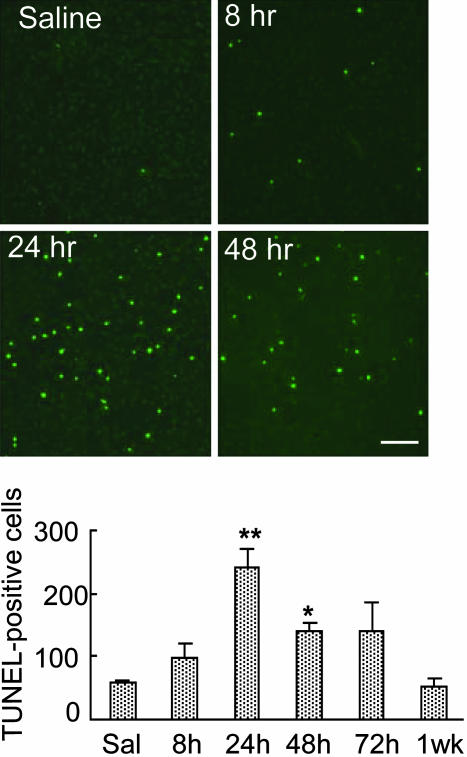
TUNEL Histochemistry. Animal death (time points are in Figs. 1, 2, 3, 4, 5) and section preparations were performed according to previously reported methods (20). The number of TUNEL-positive cells in the striatum (one side only, randomly chosen) was counted by using a Zeiss fluorescent microscope. For statistical analyses, the total number of TUNEL-positive cells in each rat striatum, at Bregma 1.60, 0.48, and -0.92 mm levels, was added to indicate cell death observed in each individual animal.
Fig. 1.
METH causes significant increases in TUNEL-positive cells in the rat brain. (Upper) Representative photomicrographs of TUNEL-stained cells (green) in the rat striatum. The photomicrographs were generated with a Zeiss LSM 410, as described in the text. Lens objective was ×10. (Bar = 200 μm.) (Lower) Quantitative data for these changes. Values represent means ± SEM of five to eight rats per time point. Keys to statistics: *, P < 0.05; **, P < 0.01, in comparison to saline-treated rats.
Fig. 2.
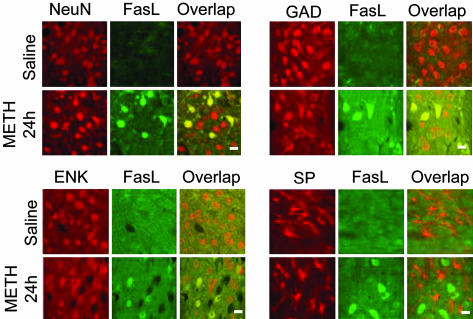
METH causes increases in FasL expression in GABAergic ENK-containing neurons. Double-labeling immunohistochemistry was performed as in Materials and Methods (see Supporting Text). Antibodies against NeuN, GAD, ENK, and SP were selected to costain with the FasL antibody 24 h after METH injection. METH caused colocalization of NeuN and FasL (Upper Left), GAD and FasL (Upper Right), and ENK and FasL (Lower Left) (yellow cells under Overlap). However, METH did not cause any SP-positive cells to express FasL (Lower Right). (Bar = 30 μm.)
Fig. 3.
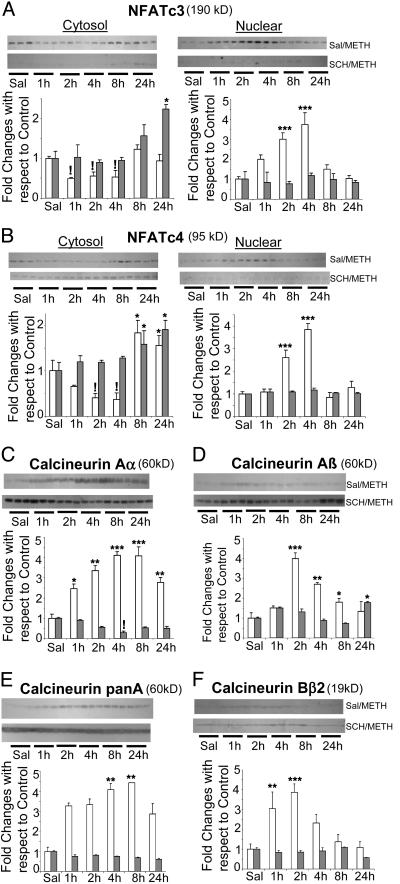
METH administration causes the shuttling of (A) NFATc3 and (B) NFATc4 proteins from the cytosol to the nucleus and increases in the expression of calcineurin (C-F) in the rat striatum: effects of treatment with SCH23390, a DA D1 receptor antagonist. Nuclear and cytosolic fractions were separated as described in the text. The fractions were obtained from individual striatal samples of six animals per time point. Representative photomicrographs show results of three samples per time point. METH caused time-dependent decreases in the cytosolic fraction and parallel increases in the nuclear contents of NFATc3 and NFATc4. Pretreatment with SCH23390 before METH injection completely blocked METH-induced increases in the nuclear fractions. Time-dependent increases were observed by using antibodies against (C) calcineurin Aα, (D) calcineurin Aβ, (E) pan-calcineurin A (which recognizes calcineurin Aα and calcineurin Aβ), and (F) calcineurin Bβ2, the regulatory subunit of calcineurin. These increases were significantly attenuated by pretreatment with SCH23390. The membranes were reprobed with α-tubulin antibody to confirm equal protein loading. The values represent means ± SEM (n = 6) (fold changes with respect to control). Open column, saline-pretreated METH-challenged rats (prior treatment of saline followed by saline or METH); closed column, SCH23390-pretreated METH-challenged rats (prior treatment of SCH23390 followed by saline or METH). *, P < 0.05; **, P < 0.01; ***, P < 0.001, increases in comparison with the respective control group;!, P < 0.05, decreases in comparison with the respective control group.
Fig. 4.
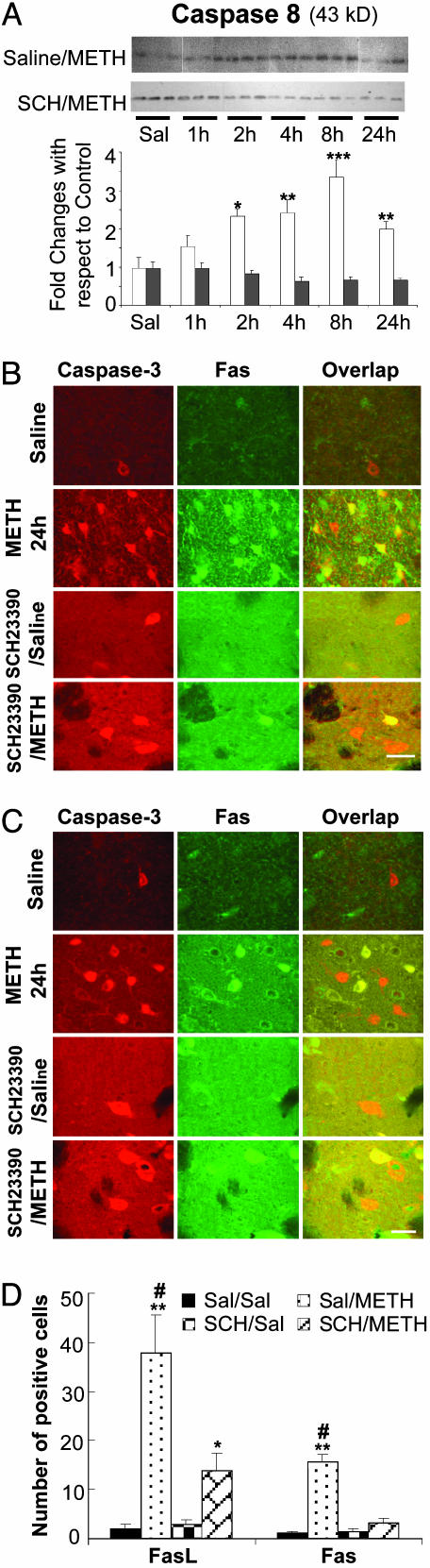
METH injection causes significant increases in the expression of FasL and Fas in caspase-3-expressing striatal cells. (A) Western blot analyses were used to measure the effects of METH and SCH23390 on the cleavage of caspase-8. Cleavage of caspase-8 (43 kDa) was detected 2 h after METH injection. Pretreatment with SCH23390 completely blocked these changes. Statistical analyses were conducted as described in the text. *, P < 0.05; **, P < 0.01; ***, P < 0.001, in comparison to the respective control group. Double-labeled experiments showed that METH induced FasL (B) and Fas (C) expression in cells that express the executioner caspase-3. Pretreatment with SCH23390 significantly reduced the number of cells expressing either FasL or Fas (D). *, P < 0.05; **, P < 0.01, in comparison with the Saline/Saline-treated control group; #, P < 0.01, in comparison with the SCH23390/METH-treated group.
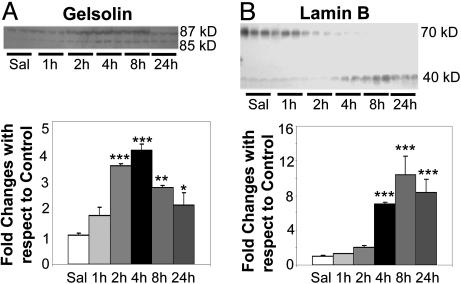
Fig. 5.
METH leads to cleavage of the caspase targets, gelsolin (A) and lamin B(B), in the rat striatum. Analyses were conducted as described in the text. *, P < 0.05; **, P < 0.01; **, P < 0.001, in comparison with the control group.
Double-Label Immunohistochemistry. To identify the neurotransmitter contents of the cells in which METH induced FasL expression, we carried out double-label immunocytochemical experiments as described in the Supporting Text by using antibodies against NeuN (monoclonal antibody, Chemicon), glutamic acid decarboxylase (GAD), enkephalin (ENK), and Substance P (SP) (polyclonal antibodies from Chemicon), all of which were costained with FasL (polyclonal from Oncogene Research Products; monoclonal from BD Biosciences). We also tested the possibility that FasL and Fas proteins might be expressed in cells that exhibited staining with an antibody against active caspase-3. The antibodies used were a polyclonal anticleaved caspase-3 antibody (Cell Signaling Technology, Beverly, MA), a monoclonal antibody against Fas (Santa Cruz Biotechnology), and a monoclonal anti-FasL antibody (BD Biosciences). Animal death and section preparations were performed according to methods published by us (16).
Western Blot Analysis. Nuclear and cytosolic extracts were prepared by using NE-PER nuclear and cytoplasmic extraction reagent (Pierce). The fractions were obtained from individual striatal samples of six animals per time point. The rest of the procedures were essentially as described by us (18). Antibodies used include anticleaved caspase 8 (Cell Signaling Technology), rabbit polyclonal anti-NFATc3; NFATc4; goat polyclonal calcineurin Aα; Aβ; Bβ2; gelsolin; lamin B (Santa Cruz Biotechnology); and rabbit polyclonal anti-calcineurin panA (Chemicon). To confirm equal protein loading, blots were reprobed with α-tubulin antibody (Sigma). Signal intensity was measured by using densitometric analysis and quantitated by using lab-works, Ver. 4.5 (BioImaging System, Ultraviolet Products).
Statistical Analyses. All data are presented as means ± SEM. The data were analyzed by ANOVA followed by Fisher's protected least-significant difference test by using the program statview, Ver. 4.02 (SAS Institute, Cary, NC).
Results
METH Administration Causes Cell Death in the Rat Striatum. METH injections have been reported to cause cell death in the mouse brain via activation of apoptotic mechanisms (13, 18, 20). There is, however, no study that has investigated these processes in the rat striatum. We thus used a dose of METH that has previously been shown (19) to be toxic to monoaminergic systems in the rat to evaluate the possibility that this dose might also cause death of intrinsic striatal neurons. Fig. 1 Upper shows that this dose of the drug did indeed cause the appearance of TUNEL-positive cells in the striatum when examined at various intervals after the injection of the drug. The quantitative data for the appearance of TUNEL-positive cells in the rat brain are also shown in Fig. 1 Lower.
METH Administration Causes Induction of Various Transcription Factors (TFs) in the Rat Striatum. The cDNA array approach was used to identify genes whose transcripts might be regulated in the rat striatum after injection of this toxic METH regimen. We found that several transcripts showed either increases (n = 39) or decreases (n = 22) 1 h after METH injection. Fig. 6, which is published as supporting information on the PNAS web site, shows the cluster analysis of the genes that were affected by METH. These transcripts include genes that fall within multiple functional classes, including genes that code for proteins involved in metabolic pathways, transcriptional regulation, and regulation of apoptosis. The class of TFs includes c-jun, junB, early growth response gene (Egr)-1 and -2, and Nur77, among others (Fig. 6). These results are consistent, in part, with previous observations from experiments in METH-treated rodents (17, 21, 22)
To confirm the array results, we used quantitative PCR to measure transcript levels of the three members of the Jun family of TFs, namely c-Jun, JunB, and JunD. These proteins have been implicated in regulating apoptosis (23). We also extended these studies by using different groups of animals that were killed at various time points after the injection of the drug. Fig. 7, which is published as supporting information on the PNAS web site, shows that these transcripts are affected in a time-dependent fashion. We also confirmed the expression of members of the Fos, Egr, and Nurr77 families of transcriptions factors (see Supporting Text).
METH Administration Induces the Expression of FasL. Egr proteins are widely distributed zinc-finger TFs that are involved in mediating development, differentiation, and death signals via their influence on the expression of various genes (24, 25). One of these genes is FasL (26-28), a member of the TNF superfamily of cytokines (29), which is involved in causing apoptosis in various models of neuronal injury (30). In addition to its regulation by Egr proteins, FasL expression is influenced by an array of TFs that include activator protein 1 (AP-1) and Nur77 factors (28, 31), all of which were also significantly induced by the injection of the toxic dose of METH (see Figs. 6 and 7). Closely related to the theme of this paper, these TFs have also been implicated in the regulation of activity-induced cell death of T cells through cooperative interactions with the FasL promoter, with resulting up-regulation of FasL expression (32-34).
To determine whether the METH-induced changes observed in the various families of immediate early genes might have resulted in up-regulation of FasL, we measured FasL mRNA in the striatum of the rats injected with METH. Fig. 7E shows there were indeed METH-induced increases in the expression of FasL mRNA. These changes reached ≈4-5-fold increases in animals killed 2-4 h after METH administration. We also measured possible METH-induced changes in the expression of Fas, which is a member of the TNF superfamily (29). As can be seen in Fig. 7E, Fas mRNA levels showed significant decreases at 4 h, reverted to normal by 8 h, and showed small increases at 12 h after METH injection (see Fig. 7E).
We also wanted to know whether the increases observed in FasL mRNA translated to increased protein expression. If so, we wanted to determine in which striatal cell types the increases had occurred. As can be seen in Fig. 2, METH caused increases in FasL in cells that stain for NeuN, GAD, and ENK. However, neither SP- nor somatostatin-containing cells were colabeled with FasL (data not shown). In addition, cells that express markers of the cholinergic system were also not labeled with FasL (data not shown). These observations indicate that METH induces FasL protein expression in GABAergic neurons that express ENK but not in those that express SP.
METH, Calcineurin, and NFATs. In addition to Egr, Nur77, and AP-1 TFs, FasL expression is responsive to NFATs (32). The NFAT proteins belong to a family of TFs that are involved in the regulation of cytokine expression (35). At present, five members of the NFAT family have been identified. These include NFAT1 (NFATp or NFATc2), NFAT2 (NFATc1), NFAT3 (NFATc4), NFAT4 (NFATc3 or NFATx), and NFAT5 (35, 36). Although originally described in T cells, the NFATs are now known to participate in the regulation of calcium- and calcineurin-mediated transcriptional activity in the nervous system (37). Because both oxidative stress and calcium dysregulation are also involved in causing METH-induced toxicity (38), we reasoned that METH-induced changes in FasL expression might be due, in part, to METH-induced calcium-mediated calcineurin-dependent NFAT activation (39) followed by increases in FasL mRNA expression (28). These ideas were tested by measuring the levels of NFAT and calcineurin protein levels, because calcineurin-induced NFAT dephosphorylation is essential to NFAT shuttling from the cytoplasm to the nucleus (39, 40), and because calcineurin overexpression has also been shown to predispose neurons to apoptosis (41).
Fig. 3 A and B show that the METH injection did indeed cause time-dependent changes in the cytosolic and nuclear contents of NFATc3 and NFATc4, respectively. Specifically, there were very early decreases in their cytosolic contents with parallel increases in the concentration in their nuclear fraction. Regrettably, NFATc1 and NFATc2 could not be measured in the rat striatum with commercially available antibodies. Figs. 3 C-F show that METH caused changes in calcineurin subunits that reached 4- to 5-fold increases at ≈2-4 h after drug injection.
We further tested the involvement of the calcineurin/NFAT cascade in METH-induced effects on postsynaptic cells by using the DA D1 antagonist, SCH23390, which is known to protect against METH-induced striatal DA depletion (see Fig. 8A, which is published as supporting information on the PNAS web site). Pretreatment with this drug completely blocked the effects of METH on striatal DA levels and also attenuated METH-induced increases in TUNEL-positive cells (see Fig. 8B). Fig. 3 A and B show that prior treatment of SCH23390 significantly blocked the METH-induced increases in NFATc3 and NFATc4 in the nuclear fraction. Moreover, Fig. 3 C-F show that pretreatment with SCH23390 also caused suppression of METH-induced increases in calcineurin expression.
METH Administration Causes Activation of Caspase-3 in FasL- or Fas-Containing Cells. Because FasL/Fas-induced cell death depends on the formation of a death-inducing signaling complex, which causes the autoproteolytic activation of procaspase-8 with subsequent activation of caspase-3 (42), we also assessed the status of caspase-8 by Western blot analysis. As can be seen in Fig. 4A, there was METH-induced cleavage of caspases 8. These changes in caspase-8 were blocked by pretreatment with SCH23390 (Fig. 4A). We also tested the possibility that FasL and Fas might be expressed on cells that express the penultimate executioner, caspase-3. As shown in Fig. 4B, there were time-dependent increases in FasL expression in the striatum of METH-treated rats, with FasL being colocalized with caspase-3 in a large proportion of the cells (Fig. 4B). It is important to note that all sections taken from control rats showed the consistent presence of a few cells that express FasL or active caspase-3 although colocalization of these two proteins was never observed in control animals (Fig. 4B, Saline). Prior treatment with SCH23390 caused significant inhibition of the METH-induced increases in the expression of FasL and caspase-3 in rat striatal cells (Fig. 4B; see Fig. 4D for quantitative data).
Fig. 4C shows METH-induced changes in Fas expression. There was almost no detectable Fas-like immunostaining in the striatum of control rats. However, METH-treated rats showed increased Fas expression in their striatal cells by 24 h after the drug injection. These increases were observed on the cell membrane and in the cytoplasm of some cells but only on the membrane of the majority of the cells. Fas was also colocalized with active caspase-3 in these METH-treated animals. Pretreatment with SCH23390 almost completely eliminated Fas expression in striatal cells of METH-treated rats (Fig. 4C; see Fig. 4D for quantitative data).
METH Administration Causes Proteolysis of Caspase-3 Targets. Because activation of terminal caspases leads to the cleavage of several cytoplasmic and nuclear proteins, we investigated the effects of METH on the status of gelsolin and lamin B, which are known targets of these caspases. When cleaved to an active form by caspase-3, gelsolin severs actin filaments and is thought to participate in some of the structural changes observed in the cytoplasm during apoptosis (43). Proteolysis of lamin B is thought to be involved in the nuclear changes observed during apoptosis (44). Fig. 5A, shows the cleavage product of gelsolin, with the appearance of the cleaved (85-kDa) band occurring 2 h after METH administration. Fig. 5B shows the cleavage product of lamin B.
Discussion
Apoptosis is a suicide program that is involved in the elimination of superfluous or traumatized cells (45). This process is intimately involved in embryonic development, the maintenance of tissue homeostasis, and adaptive responses to injurious events. Apoptosis is mediated by various molecular pathways that cause the activation of several members of the caspase family of proteolytic enzymes (45). Because these pathways are thought to play intimate roles in the causation or progression of neurodegenerative processes (46), knowledge gleaned from the dissection of these molecular events might influence the development of therapeutic approaches to human disorders characterized by accelerated cell death. Because such an argument is relevant to observations of cognitive deficits reported in patients who abuse METH (1), we have been conducting mechanistic studies aimed at elucidating the molecular and cellular bases of METH-induced neuronal apoptosis observed in vitro and in vivo (see ref. 6 for a recent review). So far, our efforts had identified mitochondria- and endoplasmic reticulum-mediated events as important culprits in causing cell death in the rodent brain (15, 18). Thus, the present observations extend our previous work in mouse by showing that METH can regulate the expression of several pro-death transcripts in the rat striatum. Members of the Egr, AP1, and Nur77 subfamilies of TFs were found to sustain very early and profound METH-induced up-regulation. These increases preceded changes in FasL mRNA and protein known to be more intimately involved in degenerative processes. The changes in FasL protein occurred according to a time course that reflects the appearance of cell death in the rat striatum.
Several of the METH-responsive TFs have been shown to participate in various models of apoptosis. These include the Egr, AP1, Nr4a, and NFAT subfamilies of TFs (see above). Of direct relevance to our thesis, these TFs have been shown to be involved in increasing FasL expression in models of activity-induced cell death in T cells (34), observations that are consistent with our demonstration that injection of METH caused early up-regulation of these factors, with subsequent increases in FasL expression. It is also to be noted that NFAT activity can be potentiated by calcium ionophores that cause increases in intracellular calcium (35-37). This occurs through the activation of the calmodulin-dependent phosphatase, calcineurin, which dephosphorylates NFAT and exposes a nuclear localization sequence, with resulting translocation of NFATs from the cytosol to the nucleus (39, 40). Our test of the idea that similar events might occur in the METH toxicity model revealed that METH does cause marked changes in calcineurin levels that were associated with shuttling of NFATc3 and NFATc4 from the cytosol to the nucleus. These results are also consistent with previous reports that METH administration can cause marked increases in glutamate release in the striatum (47), events known to be associated with dysregulation of calcium homeostasis (38). This thesis is also consistent with our previous demonstration (18) that endoplasmic reticulum-mediated events are involved in METH-induced neuronal apoptosis in mice. This suggestion is also supported by our observations that an agent, SCH23390, which causes significant reduction of TUNEL-positive cells in the striatum, can also inhibit METH-induced effects on the calcineurin/NFAT/FasL death cascade. It is of interest to point out that the drug caused complete inhibition of the effects of METH on the dopaminergic system, whereas it caused partial inhibition of the appearance of METH-induced TUNEL-positive cells. These observations suggest there are some differences in the mechanisms involved in METH-induced degeneration of presynaptic terminals and of postsynaptic neurons. These mechanisms might involve METH-induced activation of the mitochondria-dependent death pathway in postsynaptic neurons in mice (20) and in rats (unpublished observations). This discussion is also consistent with the observation that pretreatment with SCH23390 also blocked METH-induced expression of Fas in striatal cells but only partially suppressed activation of caspase-3 (see Fig. 4D). That SCH23390 also blocks METH-induced hyperthermia (see Fig. 8C, which is published as supporting information on the PNAS web site), which is known to play a role in METH-induced striatal DA depletion, might, in part, be relevant to the partial protection that the drug caused against METH-induced neuronal apoptosis.
It is of interest that immunohistochemical studies showed significant increases in the expression of the Fas protein in the striata of METH-treated animals, whereas Fas mRNA levels showed decreases early after METH injection and only small increases later on. Early decreases in Fas mRNA might be related to compensatory down-regulation of Fas mRNA in response to overstimulation of Fas receptor by FasL, which was induced early by METH. In contrast, the somewhat delayed increases in the expression of the Fas protein might be related to METH-induced posttranslational modifications of the Fas receptor. These posttranslational changes might have led to Fas accumulation on cell membranes because of prolongation of its half-life due to decreased degradation and/or increased stabilization. It is also possible that the antibody used in the present study might be better at detecting Fas that has undergone trimerization on the membrane of target cells that are undergoing apoptosis (48). These suppositions notwithstanding, the observation that there does not exist a one-to-one correspondence between Fas mRNA and protein expression is consistent with previous data documenting variable degrees (0.356-0.71) of correlations between mRNA and protein levels in various systems (49, 50).
Conclusion
This paper shows that METH administration can cause calcineurin-mediated shuttling of NFAT from the cytosol to the nucleus, with associated increases in the expression of FasL at both transcript and protein levels. In addition, the observation that METH-induced FasL expression and caspase-3 activation are coincident in a specific striatal neuronal subpopulation suggests that FasL expression is induced in enkephalinergic neurons on their journey toward death.
Supplementary Material
Author contributions: J.L.C. designed research; S.J., X.D., B.L., M.T.M., A.C., and N.-s.C. performed research; S.J., X.D., B.L., A.C., and M.T.M. analyzed data; and S.J., X.D., and J.L.C. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: METH, methamphetamine; DA, dopamine; TF, transcription factor; FasL, Fas ligand; ENK, enkephalin; NFAT, nuclear factor of activated T cells; SP, Substance P; GAD, glutamic acid decarboxylase; Egr, early growth response gene.
References
- 1.Anglin, M. D., Burke, C., Perrochet, B., Stamper, E. & Dawud-Noursi, S. (2000) J. Psychoactive Drugs 32, 137-141. [DOI] [PubMed] [Google Scholar]
- 2.Freese, T. E., Miotto, K. & Reback, C. J. (2002) J. Subst. Abuse Treat. 23, 151-156. [DOI] [PubMed] [Google Scholar]
- 3.Pereira, F. C., Imam, S. Z., Gough, B., Newport, G. D., Ribeiro, C. F., Slikker, W., Jr., Macedo, T. R. & Ali, S. F. (2002) J. Neural Transm. 109, 1151-1158. [DOI] [PubMed] [Google Scholar]
- 4.Volkow, N. D., Chang, L., Wang, G. J., Fowler, J. S., Leonido-Yee, M., Franceschi, D., Sedler, M. J., Gatley, S. J., Hitzemann, R., Ding, Y. S., et al. (2001) Am. J. Psychiatry 158, 377-382. [DOI] [PubMed] [Google Scholar]
- 5.Wilson, J. M., Kalasinsky, K. S., Levey, A. I., Bergeron, C., Reiber, G., Anthony, R. M., Schmunk, G. A., Shannak, K., Haycock, J. W. & Kish, S. J. (1996) Nat. Med. 2, 699-703. [DOI] [PubMed] [Google Scholar]
- 6.Cadet, J. L., Jayanthi, S. & Deng, X. (2003) FASEB J. 17, 1775-1788. [DOI] [PubMed] [Google Scholar]
- 7.Volkow, N. D., Chang, L., Wang, G. J., Fowler, J. S., Franceschi, D., Sedler, M., Gatley, S. J., Miller, E., Hitzemann, R., Ding, Y. S. & Logan, J. (2001) J. Neurosci. 21, 9414-9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volkow, N. D., Chang, L., Wang, G. J., Fowler, J. S., Franceschi, D., Sedler, M. J., Gatley, S. J., Hitzemann, R., Ding, Y. S., Wong, C., et al. (2001) Am. J. Psychiatry 158, 383-389. [DOI] [PubMed] [Google Scholar]
- 9.Chang, L., Ernst, T., Speck, O., Patel, H., DeSilva, M., Leonido-Yee, M. & Miller, E. N. (2002) Psychiatry Res. 114, 65-79. [DOI] [PubMed] [Google Scholar]
- 10.Langford, D., Adame, A., Grigorian, A., Grant, I., McCutchan, J. A., Ellis, R. J., Marcotte, T. D. & Masliah, E. (2003) J. Acquired Immune Defic. Syndr. 34, 467-474. [DOI] [PubMed] [Google Scholar]
- 11.Deng, X., Cai, N. S., McCoy, M. T., Chen, W., Trush, M. A. & Cadet, J. L. (2002) Neuropharmacology 42, 837-845. [DOI] [PubMed] [Google Scholar]
- 12.Stumm, G., Schlegel, J., Schafer, T., Wurz, C., Mennel, H. D., Krieg, J. C. & Vedder, H. (1999) FASEB J. 13, 1065-1072. [DOI] [PubMed] [Google Scholar]
- 13.Deng, X., Ladenheim, B., Tsao, L. I. & Cadet, J. L. (1999) J. Neurosci. 19, 10107-10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisch, A. J., Schmued, L. C. & Marshall, J. F. (1998) Synapse 30, 329-333. [DOI] [PubMed] [Google Scholar]
- 15.Jayanthi, S., Deng, X., Bordelon, M., McCoy, M. T. & Cadet, J. L. (2001) FASEB J. 15, 1745-1752. [DOI] [PubMed] [Google Scholar]
- 16.Deng, X., Jayanthi, S., Ladenheim, B., Krasnova, I. N. & Cadet, J. L. (2002) Mol. Pharmacol. 62, 993-1000. [DOI] [PubMed] [Google Scholar]
- 17.Jayanthi, S., McCoy, M. T., Ladenheim, B. & Cadet, J. L. (2002) Mol. Pharmacol. 61, 1124-1131. [DOI] [PubMed] [Google Scholar]
- 18.Jayanthi, S., Deng, X., Noailles, P. A., Ladenheim, B. & Cadet, J. L. (2004) FASEB J. 18, 238-251. [DOI] [PubMed] [Google Scholar]
- 19.Cappon, G. D., Pu, C. & Vorhees, C. V. (2000) Brain Res. 863, 106-111. [DOI] [PubMed] [Google Scholar]
- 20.Deng, X., Wang, Y., Chou, J. & Cadet, J. L. (2001) Brain Res. Mol. Brain Res. 93, 64-69. [DOI] [PubMed] [Google Scholar]
- 21.Cadet, J. L., McCoy, M. T. & Ladenheim, B. (2002) Synapse 44, 211-226. [DOI] [PubMed] [Google Scholar]
- 22.Hirata, H., Asanuma, M. & Cadet, J. L. (1998) Brain Res. Mol. Brain Res. 58, 209-216. [DOI] [PubMed] [Google Scholar]
- 23.Ham, J., Eilers, A., Whitfield, J., Neame, S. J. & Shah, B. (2000) Biochem. Pharmacol. 60, 1015-1021. [DOI] [PubMed] [Google Scholar]
- 24.Beckmann, A. M. & Wilce, P. A. (1997) Neurochem. Int. 31, 477-510. [DOI] [PubMed] [Google Scholar]
- 25.Thiel, G. & Cibelli, G. (2002) J. Cell Physiol. 193, 287-292. [DOI] [PubMed] [Google Scholar]
- 26.Droin, N. M., Pinkoski, M. J., Dejardin, E. & Green, D. R. (2003) Mol. Cell. Biol. 23, 7638-7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li-Weber, M., Laur, O. & Krammer, P. H. (1999) Eur. J. Immunol. 29, 3017-3027. [DOI] [PubMed] [Google Scholar]
- 28.Li-Weber, M. & Krammer, P. H. (2002) Cell Death Differ. 9, 101-103. [DOI] [PubMed] [Google Scholar]
- 29.Locksley, R. M., Killeen, N. & Lenardo, M. J. (2001) Cell 104, 487-501. [DOI] [PubMed] [Google Scholar]
- 30.Qiu, J., Whalen, M. J., Lowenstein, P., Fiskum, G., Fahy, B., Darwish, R., Aarabi, B., Yuan, J. & Moskowitz, M. A. (2002) J. Neurosci. 22, 3504-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toth, R., Szegezdi, E., Reichert, U., Bernardon, J. M., Michel, S., Ancian, P., Kis-Toth, K., Macsari, Z., Fesus, L. & Szondy, Z. (2001) Eur. J. Immunol. 31, 1382-1391. [DOI] [PubMed] [Google Scholar]
- 32.Latinis, K. M., Norian, L. A., Eliason, S. L. & Koretzky, G. A. (1997) J. Biol. Chem. 272, 31427-31434. [DOI] [PubMed] [Google Scholar]
- 33.Macian, F., Garcia-Rodriguez, C. & Rao, A. (2000) EMBO J. 19, 4783-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mittelstadt, P. R. & Ashwell, J. D. (1999) J. Biol. Chem. 274, 3222-3227. [DOI] [PubMed] [Google Scholar]
- 35.Rao, A., Luo, C. & Hogan, P. G. (1997) Annu. Rev. Immunol. 15, 707-747. [DOI] [PubMed] [Google Scholar]
- 36.Crabtree, G. R. & Olson, E. N. (2002) Cell 109, S67-S79. [DOI] [PubMed] [Google Scholar]
- 37.Hogan, P. G., Chen, L., Nardone, J. & Rao, A. (2003) Genes Dev. 17, 2205-2232. [DOI] [PubMed] [Google Scholar]
- 38.Ermak, G. & Davies, K. J. (2002) Mol. Immunol. 38, 713-721. [DOI] [PubMed] [Google Scholar]
- 39.Jain, J., McCaffrey, P. G., Miner, Z., Kerppola, T. K., Lambert, J. N., Verdine, G. L., Curran, T. & Rao, A. (1993) Nature 365, 352-355. [DOI] [PubMed] [Google Scholar]
- 40.Loh, C., Shaw, K. T., Carew, J., Viola, J. P., Luo, C., Perrino, B. A. & Rao, A. (1996) J. Biol. Chem. 271, 10884-10891. [DOI] [PubMed] [Google Scholar]
- 41.Asai, A., Qiu, J., Narita, Y., Chi, S., Saito, N., Shinoura, N., Hamada, H., Kuchino, Y. & Kirino, T. (1999) J. Biol. Chem. 274, 34450-34458. [DOI] [PubMed] [Google Scholar]
- 42.Nagata, S. (1999) Annu. Rev. Genet. 33, 29-55. [DOI] [PubMed] [Google Scholar]
- 43.Kothakota, S., Azuma, T., Reinhard, C., Klippel, A., Tang, J., Chu, K., McGarry, T. J., Kirschner, M. W., Koths, K., Kwiatkowski, D. J., et al. (1997) Science 278, 294-298. [DOI] [PubMed] [Google Scholar]
- 44.McConkey, D. J. (1996) J. Biol. Chem. 271, 22398-22406. [DOI] [PubMed] [Google Scholar]
- 45.Kerr, J. F., Wyllie, A. H. & Currie, A. R. (1972) Br. J. Cancer 26, 239-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedlander, R. M. (2003) N. Engl. J. Med. 348, 1365-1375. [DOI] [PubMed] [Google Scholar]
- 47.Cadet, J. L. (2003) in Gluamate and Addiction, ed. Herman, B. H. (Humana, Totowa, NJ), pp. 201-210.
- 48.Tschopp, J., Irmler, M. & Thome, M. (1998) Curr. Opin. Immunol. 10, 552-558. [DOI] [PubMed] [Google Scholar]
- 49.Gygi, S. P., Rochon, Y., Franza, B. R. & Aebersold, R. (1999) Mol. Cell. Biol. 19, 1720-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greenbaum, D., Colangelo, C., Williams, K. & Gerstein, M. (2003) Genome Biol. 4, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.