Abstract
Purpose:
Research in palliative care demonstrates improvements in overall survival, quality of life, symptom management, and reductions in the cost of care. Despite the American Society of Clinical Oncology recommendation for early concurrent palliative care in patients with advanced cancer and high symptom burden, integrating palliative services is challenging. Our aims were to quantitatively describe the palliative referral rates and symptom burden in a South Texas cancer center and establish a palliative referral system by implementing the Edmonton Symptom Assessment Scale (ESAS).
Methods:
As part of our Plan-Do-Study-Act process, all staff received an educational overview of the ESAS tool and consultation ordering process. The ESAS form was then implemented across five ambulatory oncology clinics to assess symptom burden and changes therein longitudinally. Referral rates and symptom assessment scores were tracked as metrics for quality improvement.
Results:
On average, one patient per month was referred before implementation of the intervention compared with 10 patients per month after implementation across all clinics. In five sample clinics, 607 patients completed the initial assessment, and 430 follow-up forms were collected over 5 months, resulting in a total of 1,037 scores collected in REDCap. The mean ESAS score for initial patient visits was 20.0 (standard deviation, 18.1), and referred patients had an initial mean score of 39.0 (standard deviation, 19.0).
Conclusion:
This project highlights the low palliative care consultation rate, high symptom burden of oncology patients, and underuse of services by oncologists despite improvements with the introduction of a symptom assessment form and referral system.
INTRODUCTION
Increasingly, research has demonstrated that early palliative care improves quality of life, treatment decision making, care satisfaction, and health care use.1-5 Thus, the American Society of Clinical Oncology has recommended that “combined standard oncology care and palliative care should be considered early in the course of illness for any patient with metastatic cancer and/or high symptom burden.”1(p880) In addition, to improve the overall management of distress in patients with cancer, the National Comprehensive Cancer Network has recommended that all patients be screened throughout the trajectory of their disease course.6 However, in a national survey of oncologists, these guidelines did not seem to be widely disseminated.7 Studies have identified barriers to integration of palliative care, which include a lack of consensus regarding a symptom assessment tool and the level of distress that is appropriate for referral, an inadequately trained workforce, and cultural stigma.8 The strategies to overcome these barriers are a key aspect of ongoing research and include methods for identification, assessment, and management of palliative care needs.
A recent systemic review discussed the heterogeneity in tools used for distress screening, timing, and the referral process.9 One such tool is the Edmonton Symptom Assessment Scale (ESAS), which has been validated in multiple languages (including English and Spanish) as a symptom assessment tool in patients with cancer that can be completed in less than 2 minutes.10-20 The ESAS tool consists of 10 items that capture general symptom burden (eg, fatigue, nausea) scored on a scale from 0 to 10; scores of ≥ 7 indicate severe symptom burden.21-24 As one of four National Cancer Institute–designated cancer centers in Texas, our center plays an active role in reducing distress and morbidity of cancer and related therapies for the large and diverse South Texas population. Our main objective for this quality improvement (QI) project was to quantitatively define palliative referral rates and symptom burden in ambulatory oncology patients at our cancer center using the ESAS tool. Furthermore, we aim to implement a screening and referral process to better integrate palliative services in the oncology clinic. Using the Model for Improvement, we report the results of our first Plan-Do-Study-Act (PDSA) cycle and examine potential triggers for providers to initiate palliative care referral, which we are currently studying in the second cycle of the model.25
METHODS
Initially, our cancer center had no established workflow for symptom assessment or palliative referral. Off-site palliative referral was available; however, in data pulled from the electronic medical record, less than 0.1% of patient encounters resulted in referrals across all oncology clinics. For this QI project, we established relationships with ambulatory palliative consultation services and developed a referral pathway for our center, with the primary aim of increasing referrals for patients with high symptom burden. Second, the ESAS tool was implemented in oncology clinics to improve symptom assessment and serve as a metric for symptom management.
An educational session was held with providers to introduce the ESAS tool and explain the palliative consultation process. All ambulatory clinics had access to the use of the ESAS form and consultation process; however, the tool was piloted specifically in five clinics treating patients with breast, GI, lung, and head and neck cancers. The questionnaire was administered by trained medical assistants at each clinic visit, regardless of disease status or prior responses or referrals. The completed ESAS form was reviewed by the provider during each visit to decide if a palliative referral was appropriate based on patient-reported symptom burden. From October 2015 through February 2016, in selected clinics, 1,099 ESAS forms were collected, with 1,037 completed forms.
Study data, including patient demographics, cancer type, ESAS score, and palliative care referral, were collected and managed using REDCap (Research Electronic Data Capture).26 Data were collected to measure referrals and quantify symptom burden to facilitate the development of an institutional trigger-based referral system to be studied in the second iteration of the PDSA model. Here, we report the impact of a standardized symptom assessment and referral process on trends in symptom scores and palliative care referral rates.
RESULTS
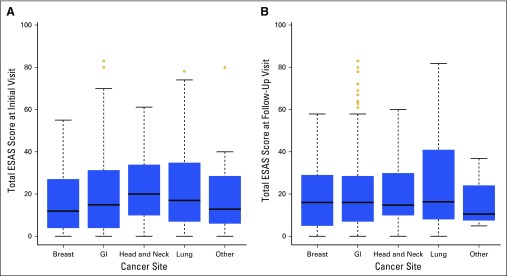
A total of 669 initial forms were collected in the five selected clinics, with 607 patients (90.7%) completing an initial ESAS questionnaire, and 430 follow-up forms were collected, resulting in a total of 1,037 completed scores. The mean ESAS score for the initial visit was 20.0 (standard deviation, 18.1; range, 0 to 83; maximum range possible, 0 to 100); it was 20.6 (standard deviation, 18.2; range, 0 to 83; maximum range possible, 0 to 100) on first follow-up. Severe symptoms (≥ 7) for any category were reported in 41.4% (n = 251) and 40.8% (n = 98) of patients on initial and follow-up visits, respectively. The most common malignancy was GI cancer (53.9%), followed by breast cancer (21.9%). To assess trends in symptom burden, we examined the distribution of total ESAS score by cancer type, which revealed similar symptom burden at initial and follow-up visits by cancer type (Fig 1). Within the five clinics sampled, only 3.5% (n = 21) of initial encounters and 2.5% (n = 11) of follow-up encounters resulted in palliative referrals.
Fig 1.
Box and whisker plots showing distribution of total Edmonton Symptom Assessment Scale (ESAS) score by cancer type at (A) initial and (B) follow-up visits.
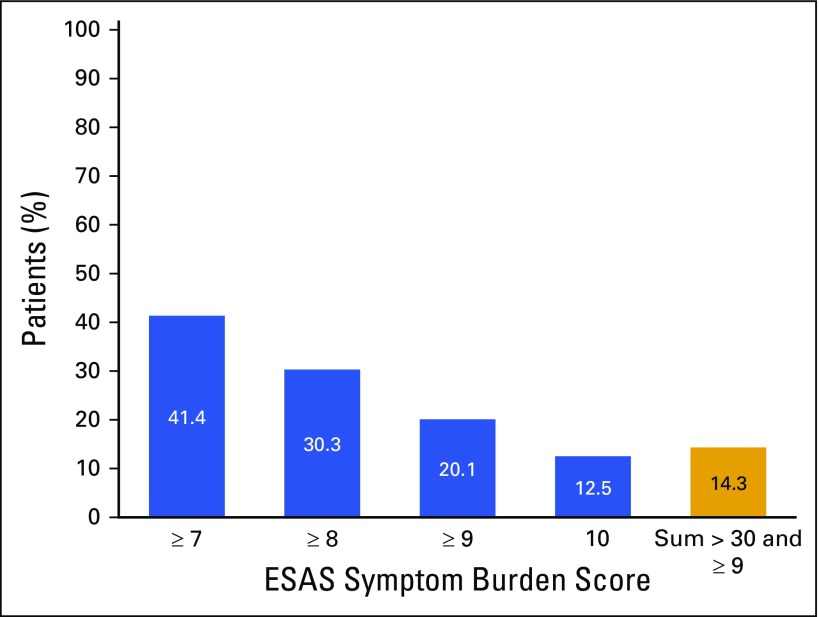
To determine if we could improve the referral system, we examined the distribution of total ESAS scores (data not shown) and severe symptom burden (Appendix Fig A1, online only). Approximately 30% and 20% of patients reported a score of ≥ 8 and ≥ 9, respectively, on any symptom. Additionally, 12.1% (n = 125) of patients marked the worst possible score in at least one of the assessment areas. We found that 25% of patients had a total score greater than 30 (data not shown).
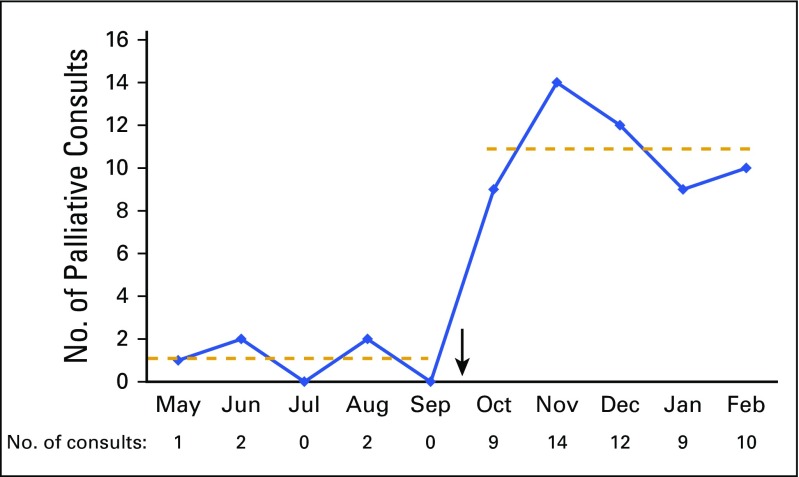
Across all oncology clinics at our center before the QI initiative, five encounters (0.07%) resulted in referral from May 2015 through September 2015, with 6,547 total patient encounters. In contrast, after the educational session and initiation of the ESAS forms, 54 encounters (0.8%) resulted in referral, with 6,421 unique encounters, across all clinics during the 5-month period. A run chart of this consecutive 10-month period shows an average monthly referral rate of one referral versus 10.8 referrals after the implementation of the ESAS tool and referral system (Fig 2).
Fig 2.
No. of palliative consults per month. Arrow indicates implementation of Edmonton Symptom Assessment Scale and referral process; dashed lines indicate average No. of consults per month.
DISCUSSION
With provider education and initiation of a symptom screening tool and palliative referral process, we improved referral rates 10-fold. Despite this improvement, the overall percentage of referrals remained less than 1% across the cancer center. Additionally, assessment of the ESAS scores suggests that symptom burden remains similarly high from initial to follow-up encounters. Therefore, we explored potential criteria to develop and implement a trigger based on patient-rated symptom scores.
To our knowledge, this is the first QI project to characterize the symptom burden of ambulatory oncology patients at all spectrums of the disease course by using the ESAS tool to determine appropriate palliative care referral triggers and help patients and providers more actively manage cancer symptoms. A previous study of palliative care referrals among patients with cancer at different stages in the disease trajectory indicated that although clinical symptoms did not differ, patients who were referred earlier tended to have fewer medical visits per month despite the involvement of more medical services compared with patients referred late.27 We found that more than 40% of ambulatory oncology patients indicated having at least one severe symptom (score ≥ 7); however, we only saw an overall palliative care referral rate of 0.8% across the cancer center. The low palliative care consultation rate and the underuse of services by oncologists at the cancer center despite use of a symptom assessment tool demonstrate the need to better integrate symptom assessment and palliative care referrals into ambulatory settings to achieve the benefits of early integration.
Barriers to integration included preconceived notions on the role of palliative care in oncology, system efficiency concerns, and patient payer source. Some physicians opted to only give ESAS forms to individuals who had metastatic disease, because they felt patients receiving curative-intent treatment would not benefit from palliative care. Misunderstanding of the benefits of palliative care services seems to be our greatest barrier to implementation.
In the era of electronic medical records and clinical practice guidelines, the development of clinical decision support systems is increasingly important but should be approached carefully to avoid provider alert fatigue.28,29 On the basis of the principles of developing clinical decision support systems and our pilot data, future projects will evaluate using an electronic medical record trigger to improve use of palliative services, patient outcomes, and symptom management.28 Our data suggest that if we captured all patients who self-identify with a severe score of ≥ 7, approximately 40% of our patients would be included. This speaks to both the high percentage of patients with cancer who perceive a significant symptom burden and the need for increasing the cancer care focus on symptom management.
As an extension of this initiative, the second PDSA cycle aims to study the use of the ESAS tool with an electronic medical record–based provider alert (ie, trigger), which captures a predefined percentage of patients in all medical oncology clinics at our cancer center. The trigger thereby facilitates the identification of patients with high symptom burden, prompting providers to initiate palliative care referral. Future studies will determine if this trigger-based approach improves referral to palliative services as well as symptom control and outcomes for patients at our institution.
ACKNOWLEDGMENT
Supported by Grant No. KL2 TR001118 from the National Center for Advancing Translational Sciences, National Institutes of Health (NIH; S.S.). Presented at the National Hospice and Palliative Care Organization Virtual Conference, August 9-11, 2016; University of Texas Health Science Center at San Antonio (UTHSCSA) Medicine Research Day, San Antonio, TX, May 24, 2016; and UTHSCSA Passport Poster Day, April 6, 2016. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Appendix
Definition of Terms
GI cancers: include esophageal, gastric, pancreatic, hepatobiliary, colorectal, and anal cancers; unique encounter: single patient visit with provider; first follow-up visit: visit at which a second Edmonton Symptom Assessment Scale form was completed.
Fig A1.
Percentage of patients reporting severe symptom burden (n = 607). ESAS, Edmonton Symptom Assessment Scale.
AUTHOR CONTRIBUTIONS
Conception and design: Sherri L. Rauenzahn, Laura L. Tenner
Collection and assembly of data: Sherri L. Rauenzahn, Ifeoma O. Aduba, Jessica T. Jones, Nazneen Ali, Laura L. Tenner
Data analysis and interpretation: Sherri L. Rauenzahn, Susanne Schmidt, Laura L. Tenner
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Integrating Palliative Care Services in Ambulatory Oncology: An Application of the Edmonton Symptom Assessment System
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jop/site/misc/ifc.xhtml.
Sherri L. Rauenzahn
No relationship to disclose
Susanne Schmidt
No relationship to disclose
Ifeoma O. Aduba
No relationship to disclose
Jessica T. Jones
No relationship to disclose
Nazneen Ali
No relationship to disclose
Laura L. Tenner
Consulting or Advisory Role: Community First Health Plan, Bayer HealthCare Pharmaceuticals
REFERENCES
- 1.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol. 2012;30:880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 2.Bruera E, Michaud M, Vigano A, et al. Multidisciplinary symptom control clinic in a cancer center: A retrospective study. Support Care Cancer. 2001;9:162–168. doi: 10.1007/s005200000172. [DOI] [PubMed] [Google Scholar]
- 3.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 4.Smith TJ, Coyne P, Cassel B, et al. A high-volume specialist palliative care unit and team may reduce in-hospital end-of-life care costs. J Palliat Med. 2003;6:699–705. doi: 10.1089/109662103322515202. [DOI] [PubMed] [Google Scholar]
- 5.Salins N, Ramanjulu R, Patra L, et al. Integration of early specialist palliative care in cancer care and patient related outcomes: A critical review of Evidence. Indian J Palliat Care. 2016;22:252–257. doi: 10.4103/0973-1075.185028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network: NCCN clinical practice guidelines in oncology: Distress management (version 2.2014). https://www.nccn.org/professionals/physician_gls/pdf/distress.pdf [DOI] [PMC free article] [PubMed]
- 7.Pirl WF, Muriel A, Hwang V, et al. Screening for psychosocial distress: A national survey of oncologists. J Support Oncol. 2007;5:499–504. [PubMed] [Google Scholar]
- 8.Ramchandran K, Tribett E, Dietrich B, et al. Integrating palliative care into oncology: A way forward. Cancer Contr. 2015;22:386–395. doi: 10.1177/107327481502200404. [DOI] [PubMed] [Google Scholar]
- 9.Hui D, Meng Y-C, Bruera S, et al. Referral criteria for outpatient palliative cancer care: A systematic review. Oncologist. 2016;21:895–901. doi: 10.1634/theoncologist.2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvajal A, Centeno C, Watson R, et al: A comprehensive study of psychometric properties of the Edmonton Symptom Assessment System (ESAS) in Spanish advanced cancer patients. Eur J Cancer 47:1863-1872, 2011 [DOI] [PubMed]
- 11.Chinda M, Jaturapatporn D, Kirshen AJ, et al. Reliability and validity of a Thai version of the Edmonton Symptom Assessment scale (ESAS-Thai) J Pain Symptom Manage. 2011;42:954–960. doi: 10.1016/j.jpainsymman.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe SM, Nekolaichuk CL, Beaumont C. The Edmonton Symptom Assessment System, a proposed tool for distress screening in cancer patients: Development and refinement. Psychooncology. 2012;21:977–985. doi: 10.1002/pon.1996. [DOI] [PubMed] [Google Scholar]
- 13.Dong Y, Chen H, Zheng Y, et al. Psychometric validation of the Edmonton Symptom Assessment System in Chinese patients. J Pain Symptom Manage. 2015;50:712–717.e2. doi: 10.1016/j.jpainsymman.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 14.Yokomichi N, Morita T, Nitto A, et al. Validation of the Japanese version of the Edmonton Symptom Assessment System-Revised. J Pain Symptom Manage. 2015;50:718–723. doi: 10.1016/j.jpainsymman.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Kwon JH, Nam S-H, Koh S, et al. Validation of the Edmonton Symptom Assessment System in Korean patients with cancer. J Pain Symptom Manage. 2013;46:947–956. doi: 10.1016/j.jpainsymman.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claessens P, Menten J, Schotsmans P, et al. Development and validation of a modified version of the Edmonton Symptom Assessment Scale in a Flemish palliative care population. Am J Hosp Palliat Care. 2011;28:475–482. doi: 10.1177/1049909111400724. [DOI] [PubMed] [Google Scholar]
- 17.Moro C, Brunelli C, Miccinesi G, et al. Edmonton symptom assessment scale: Italian validation in two palliative care settings. Support Care Cancer. 2006;14:30–37. doi: 10.1007/s00520-005-0834-3. [DOI] [PubMed] [Google Scholar]
- 18.Paiva CE, Manfredini LL, Paiva BSR, et al. The Brazilian version of the Edmonton Symptom Assessment System (ESAS) is a feasible, valid and reliable instrument for the measurement of symptoms in advanced cancer patients. PLoS One. 2015;10:e0132073. doi: 10.1371/journal.pone.0132073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 20.Wong A, Rodriguez-Nunez A, Tayjasanant S, et al: Edmonton Symptom Assessment Scale (ESAS): Time duration of self-completion versus assisted-completion in palliative care patients—A randomized controlled trial. J Clin Oncol 34, 2016 (suppl 26S; abstr 67)
- 21.Selby D, Cascella A, Gardiner K, et al. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton Symptom Assessment System. J Pain Symptom Manage. 2010;39:241–249. doi: 10.1016/j.jpainsymman.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Kang JH, Kwon JH, Hui D, et al. Changes in symptom intensity among cancer patients receiving outpatient palliative care. J Pain Symptom Manage. 2013;46:652–660. doi: 10.1016/j.jpainsymman.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Swarm R, Abernethy AP, Anghelescu DL, et al. Adult cancer pain. J Natl Compr Canc Netw. 2010;8:1046–1086. doi: 10.6004/jnccn.2010.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedard G, Zeng L, Zhang L, et al. Minimal clinically important differences in the Edmonton Symptom Assessment System in patients with advanced cancer. J Pain Symptom Manage. 2013;46:192–200. doi: 10.1016/j.jpainsymman.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Langley G, Moen R, Nolan K, et al. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. (ed 2) San Francisco, CA: Jossey-Bass Publishers; 2009. [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon JH, Hui D, Chisholm G, et al. Clinical characteristics of cancer patients referred early to supportive and palliative care. J Palliat Med. 2013;16:148–155. doi: 10.1089/jpm.2012.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beeler PE, Bates DW, Hug BL. Clinical decision support systems. Swiss Med Wkly. 2014;144:w14073. doi: 10.4414/smw.2014.14073. [DOI] [PubMed] [Google Scholar]
- 29.Footracer KG. Alert fatigue in electronic health records. JAAPA. 2015;28:41–42. doi: 10.1097/01.JAA.0000465221.04234.ca. [DOI] [PubMed] [Google Scholar]