Abstract
Purpose
Choroidal thinning has been associated with reticular pseudodrusen (RPD) and β-peripapillary atrophy (β-PPA), which have been linked to normal-tension glaucoma (NTG). This analysis sought to determine whether RPD are independently associated with β-PPA in early AMD patients. Secondary outcomes included the association of RPD and preexisting diagnosis of glaucoma, cup-to-disc ratio (CDR), subfoveal choroidal thickness (SFCT), and IOP.
Methods
This prospective cross-sectional study examined 78 age- and sex-matched early AMD patients: 43 RPD patients (63 eyes) and 35 non-RPD patients (64 eyes). Exclusion criteria included advanced AMD, high myopia, and vitreoretinal conditions/surgery. RPD and non-RPD groups were identified by confocal scanning laser ophthalmoscopy. β-PPA as well as CDR were graded on digital, nonstereoscopic fundus photos. SFCT was measured on spectral-domain optical coherence tomography for 69 patients (35 RPD and 34 non-RPD). IOP and glaucoma diagnosis were extracted from charts.
Results
β-PPA had a greater prevalence in RPD than non-RPD (44% vs. 19%, P = 0.002); however, this relationship was not significant when SFCT was added to the model (P = 0.150). A preexisting diagnosis of glaucoma (P = 0.156), CDR (P = 0.176), and IOP (P = 0.98) was not different between groups.
Conclusions
RPD in early AMD are associated with presence of β-PPA, but choroidal thickness is a confounder in this relationship. Because β-PPA is a common finding in NTG, focusing on a potential shared pathway between RPD and NTG could improve the understanding of pathophysiology and expand therapies for each condition.
Keywords: age-related macular degeneration, beta-peripapillary atrophy, normal-tension glaucoma, reticular pseudodrusen, choroidal thinning
Age-related macular degeneration (AMD) is the leading cause of irreparable loss of vision in developing and developed countries.1 It is a disease characterized by the deposition of extracellular material, collectively described as drusen, under the RPE, and in severe cases can lead to the loss of central vision.2 Reticular pseudodrusen (RPD) are an imaging marker in the fundi of some patients with AMD, and have been associated with choroidal thinning3–5 as well as the development of late-stage AMD in the forms of choroidal neovascularization6–8 and geographic atrophy.9,10 In AMD patients, choroidal thinning also has been linked to a diagnosis of glaucoma and the presence of peripapillary atrophy (PPA), or atrophy of the region around the optic nerve.11
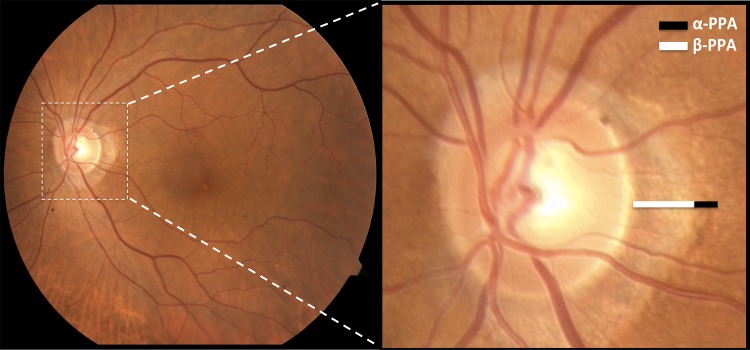
Glaucoma is defined as an optic neuropathy associated with characteristic structural damage to the optic nerve and peripheral visual dysfunction.12 Glaucomatous optic nerve damage13 has been significantly correlated with both the presence and size of the central zone of PPA, which is termed β-PPA.14–16 PPA also has a peripheral α-zone; both are seen in the representative image in Figure 1. Specifically, β-PPA has been associated with normal-tension glaucoma (NTG),13,14,17,18 a type of open angle glaucoma with glaucomatous excavation of the disc with corresponding visual field defects, but with IOP measurements within statistical normal limits.15,19 PPA is hypothesized to originate from age-related changes in the peripapillary RPE, inflammation, or ocular diseases, including glaucoma.20 However, the mechanism of PPA in glaucoma is unknown.
Figure 1.
α- and β-PPA. Color fundus photograph of a patient with PPA with a 55° lens (left) and with magnification of the nerve (right). α-PPA is characterized by an irregular pigmentation and thinning of the chorioretinal tissue layer, with its lateral border abutting the retina and its medial border in contact with β-PPA, which is characterized by marked atrophy of retinal photoreceptors, RPE, and the choriocapillaris, along with the distinct visibility of the large choroidal vessels and the sclera.
Spectral-domain optical coherence tomography (SD-OCT) imaging has greatly increased the resolution of choroidal imaging21 and thus has broadened our understanding of this vascular layer. Recent studies using SD-OCT have demonstrated a significant association between RPD and choroidal thinning.3–5 Park et al.22 likewise found that the peripapillary choroid is significantly thinned in NTG patients relative to both those with primary open angle glaucoma (POAG) and controls. Additionally, Hirooka et al.23 found that the choroid thins nasal to the fovea in NTG patients as compared with controls, and Spaide11 demonstrated an association between choroidal thinning and glaucoma. Choroidal thinning may reflect a common pathway for both RPD in AMD as well as β-PPA and NTG.
The topographic relationship between RPD and the optic nerve also raises interest in the possibility of association between RPD and glaucoma. Although RPD are traditionally considered a macular finding, studies have found that in approximately 50% of RPD eyes, RPD are located nasal to the optic nerve24 and are frequently found in the peripapillary region.10 The association between RPD and PPA has been seen in early work on late, atrophic AMD.25 However, to our knowledge, an association between RPD and β-PPA in patients with early AMD has not been studied.
This study aimed to evaluate the relationship between RPD in early AMD and the following variables: β-PPA, preexisting diagnosis of glaucoma, cup-to-disc ratio (CDR), subfoveal choroidal thickness (SFCT), and IOP. We hypothesized that all of these variables will be associated with the presence of RPD.
Methods
This analysis used images collected by a study that had approval through the Columbia University Medical Center Institutional Review Board/Ethics Board, complied with the Health Insurance Portability and Accountability Act regulations, and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from the subjects after explanation of the nature and possible consequences of the study.
This cross-sectional analysis consisted of an evaluation of 146 consecutive AMD patients who were enrolled in the study and imaged with confocal scanning laser ophthalmoscopy (cSLO) imaging (including infrared [IR] and autofluorescence [AF] imaging) between January 1, 2005, and September 1, 2012.
Exclusion criteria included advanced AMD, which was defined as presence of geographic atrophy or choroidal neovascularization, as well as myopia greater than −6 diopters, central serous chorioretinopathy, other vitreoretinal conditions, and vitreoretinal surgery. Presence of geographic atrophy was defined as ≥500 μm2 area of RPE loss seen on IR or AF imaging, or if atrophy was documented on clinical examination.
All patients' ophthalmic examinations, fundus photographs, and cSLO imaging were performed within 18 months. A comprehensive chart review was performed to document age, sex, race, lens status, best corrected visual acuity (BCVA), a preexisting diagnosis of glaucoma, and IOP.
Image Acquisition and Analysis
High-resolution digital color fundus photographs were taken with an FF 450plus with VISUPAC camera (Carl Zeiss Meditec, Dublin, CA, USA). AF, IR, and SD-OCT imaging were obtained by cSLO imaging (Heidelberg Spectralis HRA+OCT version 1.7.0.0; Heidelberg Engineering, Heidelberg, Germany). For AF images, the instrument uses blue laser light at 488 nm for illumination and a barrier filter at 500 nm. The IR images are obtained at 810 nm. The images were viewed with Heidelberg software (Spectralis Viewing Module 5.4.6.0; Heidelberg Engineering).
Horizontal SD-OCT sections centered on the fovea were used for SFCT calculations. SFCT was defined as the outer portion of the hyperreflective line corresponding to the RPE up to the inner surface of the sclera,11 and was measured using the manual caliper tool in Heidelberg Eye Explorer interactive software. Choroidal thickness measurements were performed in a masked fashion by a grader (AG) and reviewed by a retinal specialist (SB).
Classification of Disease
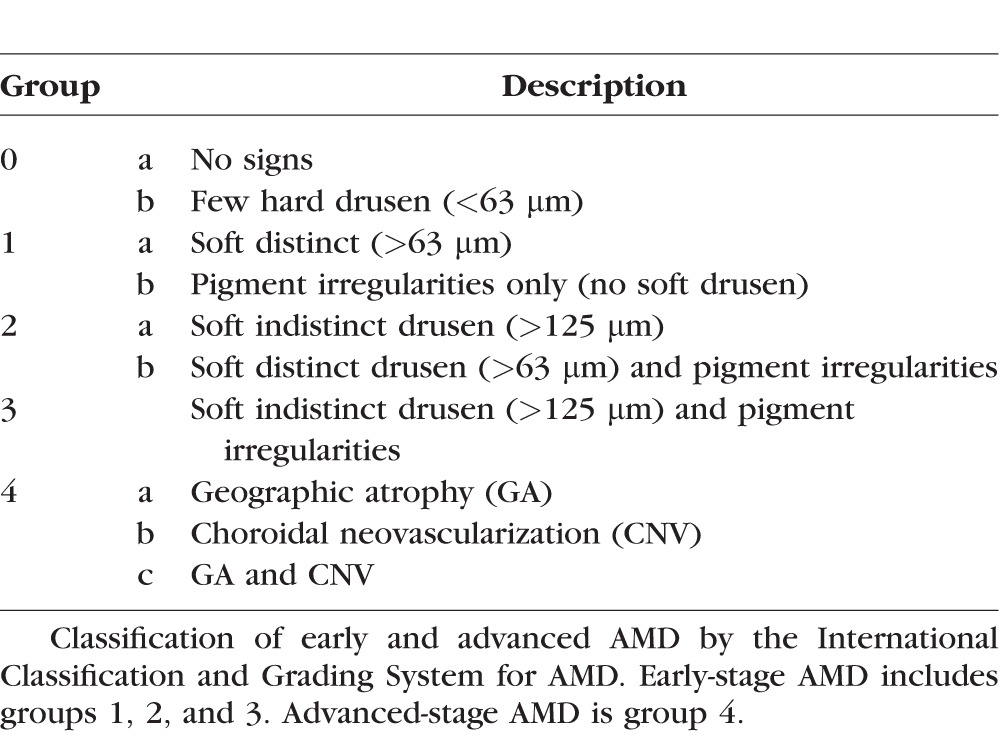
All eyes included in the study were classified as groups 1, 2, or 3 by the International Classification and Grading System for ARM (age-related maculopathy).26 This classification is summarized in Table 1 and denotes early-stage AMD as the presence of few hard, soft distinct, or soft indistinct drusen, and/or RPE hyperpigmentation and/or hypopigmentation. Eyes with geographic atrophy and/or choroidal neovascularization are classified as having advanced AMD.
Table 1.
International Classification and Grading System for AMD
Preexisting glaucoma diagnoses were counted by person, not eye. That is, if a patient's chart listed only one eye as having glaucomatous nerve damage, the patient was considered to have glaucoma. IOPs recorded in charts were considered independently per eye. IOP values were categorized into three categories: low (<12 mm Hg), medium (12–17 mm Hg), and high (>17 mm Hg).
RPD were defined as networks of hypoautofluorescent lesions against a background of mildly elevated autofluorescence on AF imaging, and groupings of hyporeflectant lesions against a background of mild hyperreflectance on IR imaging.27 A patient was defined as having RPD if either eye had evidence of RPD anywhere in the macula.
RPD status was agreed on by three retinal specialists (MO, SY, SB). All graders were masked to the glaucoma specialists' grading of β-PPA and CDR, as well as chart-documented glaucoma and IOP.
Digital, nonstereoscopic color fundus photographs were graded for β-PPA and CDR by two glaucoma specialists (DMB, LA) who were masked to RPD status. The zone of β-PPA was defined by chorioretinal atrophy with large visible choroidal vessels and sclera.14,16,17 Of note, the peripapillary region's scleral ring, which was a thin white band encircling the disc boundary, was not considered to be PPA.28 When differences in CDR values evaluated by the two graders were ≤0.2, they were averaged between the graders. CDR values were stratified into two categories, low (≤0.4) and high (>0.4). Each eye was independently considered for each variable, β-PPA, and CDR; the presence of β-PPA in one eye did not affect the evaluation of the fellow eye, and likewise the value of CDR did not affect evaluation in the fellow eye. The two graders collaboratively arbitrated diagnoses that were discrepant in the evaluation of β-PPA and CDR.
We first examined the association of RPD to these four “glaucoma-related” variables, β-PPA, previous diagnosis of glaucoma, IOP, and CDR. We assessed these variables individually using univariable logistic regression analyses with RPD as the outcome variable. The variables that reached statistical significance by P < 0.20 in the univariable analysis were included in the multivariable analyses.
A multivariable logistic regression was fitted by a generalized estimating equation approach that assumed an exchangeable correlation between outcomes in each eye, with RPD as the outcome variable. For the multivariable regression, person-specific covariates included in the models were age, sex, and race and eye-specific covariates included AMD status, pseudophakic status, and BCVA. SFCT measurements were added to the multivariable regression model for the 69 patients who had this imaging available. Analyses were conducted using Stata 13.1 (StataCorp, College Station, TX, USA).
Results
Patient Demographics and Clinical Characteristics
A total of 78 patients were included in the analysis. Forty-three patients (63 eyes) were diagnosed as having RPD, whereas 35 patients (64 eyes) were classified as non-RPD. Tables 2 and 3 summarize demographics of the studied groups. As mentioned previously, presence of RPD in either or both eyes classified the patient in the RPD group. SD-OCT imaging was available for 69 patients from the analyzed group. Of these, 35 patients (52 eyes) were classified as RPD and 34 patients (59 eyes) were classified as non-RPD.
Table 2.
Demographics of Study Subjects
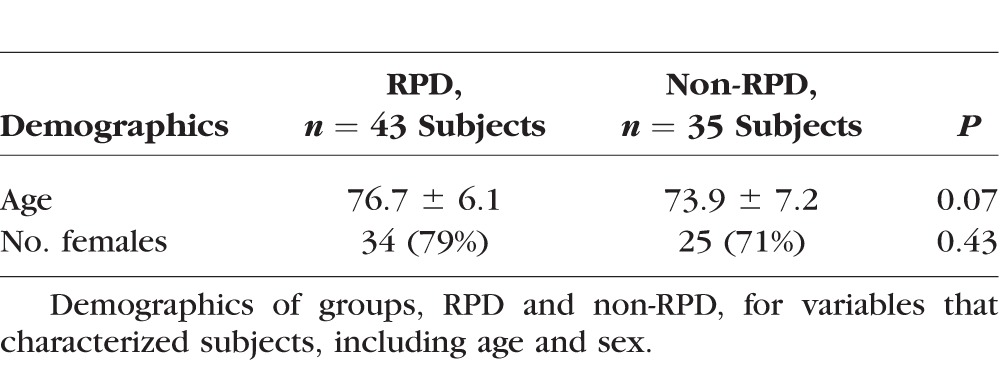
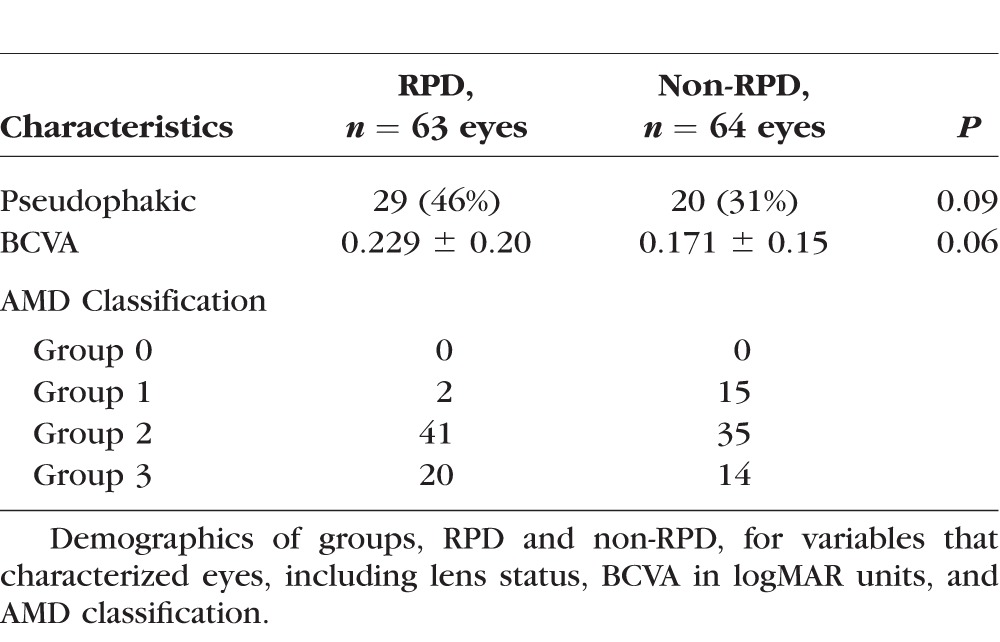
Table 3.
Characteristics of Case and Control Eyes
Of the original group of 146 AMD patients who consecutively enrolled in the study, 68 were excluded for the following reasons: diagnosis of advanced AMD in both eyes (31), incomplete charts and imaging (10), high myopia (1), and poor image quality of both eyes (16). Subjects older than 86 years were excluded (eight RPD, two non-RPD) to age-match the two groups. From the group of 78 included subjects, 23 eyes of 23 subjects were excluded due to advanced AMD, and 6 eyes of 6 subjects were excluded due to poor image quality. As a result, 63 RPD and 64 non-RPD eyes were part of the final analysis.
BCVA was converted to the logMAR scale. The RPD and non-RPD groups had similar distributions with regard to AMD classification by the International Classification and Grading System for ARM.26 No significant differences were found between the RPD and non-RPD groups in age (P = 0.07; Student's t-test for independence), sex (P = 0.43; χ2 test for independence), BCVA (P = 0.06; Student's t-test for independence), and pseudophakia (P = 0.09; χ2 test for independence).
Findings on Image Analysis and Chart Review
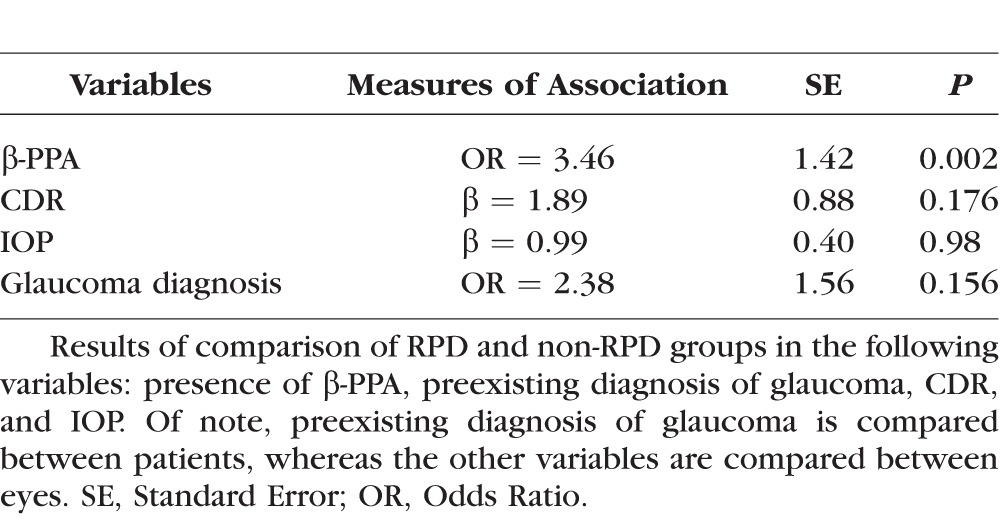
β-PPA had a greater prevalence in RPD than non-RPD (44% vs. 19%, P = 0.002). Table 4 summarizes results of the univariable logistic regression models. After adjusting for confounders described above, the β-PPA group demonstrated a significantly greater prevalence of RPD than the group without β-PPA (adjusted odds ratio [OR] 3.46). However, when choroidal thickness was taken into account, RPD was no longer an independent predictor of β-PPA (P = 0.150), while SFCT was (P = 0.005). RPD was found to be a predictor of SFCT in this study (P = 0.010) as other analyses have demonstrated. There was no difference in the presence of RPD based on IOP (P = 0.98) or CDR (P = 0.176), and no association was found between RPD and preexisting glaucoma diagnosis (P = 0.156).
Table 4.
Univariable Analyses for RPD
Representative images of non-RPD and RPD are demonstrated in Figures 2 and 3.
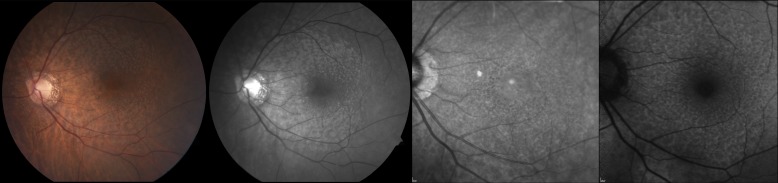
Figure 2.
Images from an 84-year-old female patient with stage 2 AMD and RPD. Color fundus photography (left) and red-free imaging (middle left) demonstrate α- and β-PPA. IR (middle right) and AF (right) imaging show hyporeflectant and hypoautofluorescent networks, respectively, which are consistent with RPD.
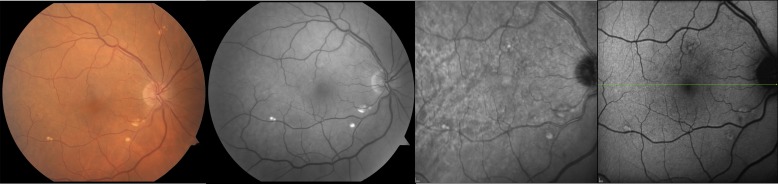
Figure 3.
Images from a 68-year-old male patient with stage 2 AMD without RPD. Color fundus photography (left) and red-free imaging (middle left) demonstrate the lack of α- or β-PPA. IR (middle right) and AF (right) imaging depict the absence of RPD.
Discussion
Our analysis is the first large study that evaluated the relationship between RPD and the presence of β-PPA in an exclusively early AMD population. In this study, the adjusted odds of β-PPA occurring in RPD were 3.46; however, this significant relationship was confounded by SFCT. Thinned choroid appears to be associated with both glaucomatous atrophy and RPD, but we are not able to identify an independent association between the latter two variables. We propose that the connection between RPD and PPA may have a choroidal origin.
Early work has demonstrated an association between β-PPA and choroidal thickness in the setting of late AMD,25 but to our knowledge, no studies, including our own, have shown an independent relationship between β-PPA and RPD. Switzer and colleagues4 examined 90 early AMD eyes with enhanced-depth imaging SD-OCT and found that subfoveal choroidal thinning was significantly associated with β-PPA, RPD, and glaucoma. However, the relationships these variables had with one another were not examined. Our study similarly examined factors associated with glaucoma in an early AMD population; however, our analysis went further. We divided the early AMD population into those with RPD and without RPD and compared the prevalence of various factors associated with glaucoma between the two groups. Although an independent association between β-PPA and RPD was not demonstrated, we found that choroidal thinning may be the common pathway through which these variables are related.
Additionally, a report by Spaide11 on 17 patients with early and advanced AMD found an association between choroidal thinning and a diagnosis of glaucoma. However, this study did not take into consideration the presence or absence of RPD, which was the characteristic that differentiated our case and control groups. Additionally, Spaide's analysis11 was potentially confounded by its mixed population of early and advanced AMD, whereas our study included only early AMD patients. Late dry AMD is characterized by geographic atrophy,26 which in turn is a known risk factor for choroidal29 and retinal thinning30,31 due to degeneration of the RPE and neurosensory retina.32 Thinning of the retinal nerve fiber layer is known to predispose the patient to visual field damage and the development of glaucoma.33,34
Given the confounded relationship among RPD, β-PPA, and SFCT, we hypothesize that the pathogenesis of choroidal thinning may be the same as that of the development of glaucomatous β-PPA.14 Histologic comparison of POAG eyes with normal eyes demonstrated choroidal thinning in glaucomatous eyes, which was attributed to shrinkage of choroidal vessel caliber and decrease in vessel density.35 More recently, thinning of the peripapillary choroid of glaucoma patients has been seen on SD-OCT imaging.23,36 Additionally, peripapillary changes have been demonstrated in angiography of glaucoma patients. Fluorescein angiography of NTG eyes has demonstrated a decrease in blood flow in retinal vessels, peripapillary choroid, and optic disc that correlated with visual field loss.37 Studies using indocyanine green angiography have corroborated this by demonstrating late-phase hypofluorescent areas in the peripapillary region of two-thirds of glaucomatous eyes and only 20% of control eyes.38 One theory for this was the absence of choriocapillaris tissue in the peripapillary region,38 which aligns with our hypothesis that a common pathway of both RPD and β-PPA is choroidal thinning. This is also in agreement with studies using SD-OCT that have found an association between choroidal thinning and the presence of β-PPA,4 as well as choroidal thinning and NTG.22
Given the known association between β-PPA and NTG, we are proposing that future studies investigate the relationship between RPD and NTG. RPD have been associated with vascular abnormalities primarily in the choroid. In a study by Sohrab et al.,39 intravascular choroidal stroma identified on SD-OCT imaging colocalized to RPD seen in red-free, autofluorescence, and infrared images. Additionally, histopathologic examination of an eye with RPD showed loss of choroidal layers and fibrous replacement of stroma.7 More recent SD-OCT studies have found choroidal thinning associated with RPD.3–5 Likewise, NTG has been linked to conditions of vascular instability, such as disc hemorrhage, migraines, and Raynaud's phenomenon.19,40–42 These studies and ours provide supportive evidence that a diagnosis of glaucoma, β-PPA, and RPD may be related by the common pathway of choroidal abnormalities.
There is some conflicting evidence on the association between a thinned choroid and glaucoma. Peripapillary as well as macular choroidal thinning have been associated with glaucoma in several studies,23,36,43 but some analyses refute this theory.44,45 An explanation for these findings may be that choroidal thinning is not linked to all forms of glaucoma, but is specifically found in glaucoma associated with vascular factors, such as NTG. Two studies have found no significant choroidal thinning in NTG patients as compared with healthy controls, but these analyses examined macular as opposed to peripapillary choroidal thickness.46,47
Because of the known association of β-PPA and NTG, we believe our results merit a further investigation of the risk of NTG in the setting of RPD. Although our study did not find an independent association between preexisting glaucoma and RPD, there may be some assessment bias regarding glaucoma detection, as we relied on a preexisting diagnosis and not prospective evaluation. Additionally, the patients in our study were primarily AMD patients, and thus may not have undergone glaucoma surveillance in their visit to the retina clinic. Furthermore, we were not powered to detect differences in glaucoma prevalence between the two groups. Future studies should consider a full glaucoma evaluation rather than relying on a preexisting diagnosis.
This study has some limitations. As mentioned above, our small sample size may have precluded us from reaching significance in the studied variables. Second, nonstereoscopic digital photographs were used to assess CDR and PPA. Last, we do not have axial length measurements on all of our patients. There remains a concern that a myopic disc could be incorrectly read as β-PPA; although some number of myopic discs may exist, we anticipate they would be evenly distributed between the RPD and non-RPD groups.
A link between RPD and NTG deserves further investigation. Focusing on a potential shared pathway could improve the sensitivity of diagnostic screenings and allow for the application of therapies that modulate choroidal structure and function to provide a viable intervention for both AMD and glaucoma.
Acknowledgments
The authors thank Hamed B. Bazargan Lari, MD, for his help in image grading.
Presented at The American Academy of Ophthalmology Annual Meeting, November, 2013: Poster Number PO446.
Supported by a grant from the Doris Duke Charitable Foundation to Columbia University (AG), a National Eye Institute Travel Grant to the 2014 ARVO Annual Meeting (AG), National Eye Institute/National Institutes of Health Grants EY013435 and EY019007 (Core Support for Vision Research), Robert L. Burch III Fund, Columbia University (SB), and the New York Community Trust–Fredrick J. and Theresa Dow Wallace Fund. The funding organizations had no role in the design or conduct of this research.
Disclosure: A. Garg, None; D.M. Blumberg, None; L.A. Al-Aswad, None; M. Oll, None; S. Yzer, None; M. Forbes, None; R.L. Allikmets, None; S. Bearelly, None
References
- 1. Klein R,, Klein BE,, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992; 99: 933– 943. [DOI] [PubMed] [Google Scholar]
- 2. Swaroop A,, Chew EY,, Rickman CB,, Abecasis GR. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annu Rev Genomics Hum Genet. 2009; 10: 19– 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Querques G,, Querques L,, Forte R,, Massamba N,, Coscas F,, Souied EH. Choroidal changes associated with reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2012; 53: 1258– 1263. [DOI] [PubMed] [Google Scholar]
- 4. Switzer DW, Jr,, Mendonca LS,, Saito M,, Zweifel SA,, Spaide RF. Segregation of ophthalmoscopic characteristics according to choroidal thickness in patients with early age-related macular degeneration. Retina. 2012; 32: 1265– 1271. [DOI] [PubMed] [Google Scholar]
- 5. Garg A,, Oll M,, Yzer S,, et al. Reticular pseudodrusen in early age-related macular degeneration is associated with choroidal thinning. Invest Ophthalmol Vis Sci. 2013; 54: 7075– 7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen SY,, Dubois L,, Tadayoni R,, Delahaye-Mazza C,, Debibie C,, Quentel G. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br J Ophthalmol. 2007; 91: 354– 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arnold JJ,, Sarks SH,, Killingsworth MC,, Sarks JP. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina. 1995; 15: 183– 191. [PubMed] [Google Scholar]
- 8. Smith RT,, Chan JK,, Busuoic M,, Sivagnanavel V,, Bird AC,, Chong NV. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006; 47: 5495– 5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prenner JL,, Rosenblatt BJ,, Tolentino MJ,, et al. Risk factors for choroidal neovascularization and vision loss in the fellow eye study of CNVPT. Retina. 2003; 23: 307– 314. [DOI] [PubMed] [Google Scholar]
- 10. Xu L,, Blonska AM,, Pumariega NM,, et al. Reticular macular disease is associated with multilobular geographic atrophy in age-related macular degeneration. Retina. 2013; 33: 1850– 1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009; 147: 801– 810. [DOI] [PubMed] [Google Scholar]
- 12. Foster PJ,, Buhrmann R,, Quigley HA,, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002; 86: 238– 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jonas JB. Clinical implications of peripapillary atrophy in glaucoma. Curr Opin Ophthalmol. 2005; 16: 84– 88. [DOI] [PubMed] [Google Scholar]
- 14. Jonas JB,, Fernandez MC,, Naumann GO. Glaucomatous parapapillary atrophy. Occurrence and correlations. Arch Ophthalmol. 1992; 110: 214– 222. [DOI] [PubMed] [Google Scholar]
- 15. Jonas JB,, Grundler A. Optic disc morphology in “age-related atrophic glaucoma.” Graefes Arch Clin Exp Ophthalmol. 1996; 234: 744– 749. [DOI] [PubMed] [Google Scholar]
- 16. Jonas JB,, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. II. Correlations. Invest Ophthalmol Vis Sci. 1989; 30: 919– 926. [PubMed] [Google Scholar]
- 17. Jonas JB,, Nguyen XN,, Gusek GC,, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Invest Ophthalmol Vis Sci. 1989; 30: 908– 918. [PubMed] [Google Scholar]
- 18. Jonas JB,, Gusek GC,, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci. 1988; 29: 1151– 1158. [PubMed] [Google Scholar]
- 19. Geijssen HC,, Greve EL. Focal ischaemic normal pressure glaucoma versus high pressure glaucoma. Doc Ophthalmol. 1990; 75: 291– 301. [DOI] [PubMed] [Google Scholar]
- 20. Nevarez J,, Rockwood EJ,, Anderson DR. The configuration of peripapillary tissue in unilateral glaucoma. Arch Ophthalmol. 1988; 106: 901– 903. [DOI] [PubMed] [Google Scholar]
- 21. Spaide RF,, Koizumi H,, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146: 496– 500. [DOI] [PubMed] [Google Scholar]
- 22. Park HY,, Lee NY,, Shin HY,, Park CK. Analysis of macular and peripapillary choroidal thickness in glaucoma patients by enhanced depth imaging optical coherence tomography. J Glaucoma. 2014; 23: 225– 231. [DOI] [PubMed] [Google Scholar]
- 23. Hirooka K,, Fujiwara A,, Shiragami C,, Baba T,, Shiraga F. Relationship between progression of visual field damage and choroidal thickness in eyes with normal-tension glaucoma. Clin Exp Ophthalmol. 2012; 40: 576– 582. [DOI] [PubMed] [Google Scholar]
- 24. Schmitz-Valckenberg S,, Alten F,, Steinberg JS,, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011; 52: 5009– 5015. [DOI] [PubMed] [Google Scholar]
- 25. Marsiglia M, Boddu S, Bearelly S, Freund KB, Yannuzzi LA, Smith RT. Correlation of peripapillary atrophy and reticular macular disease in patients with primary geographic atrophy resulting from age-related macular degeneration. Poster presented at: American Academy of Ophthalmology Annual Meeting; November 10– 13, 2012; Chicago, IL, USA. [Google Scholar]
- 26. Bird AC,, Bressler NM,, Bressler SB,, et al. The International ARM Epidemiological Study Group. An international classification and grading system for age-related maculopathy and age-related macular degeneration. Surv Ophthalmol. 1995; 39: 367– 374. [DOI] [PubMed] [Google Scholar]
- 27. Smith RT,, Sohrab MA,, Busuioc M,, Barile G. Reticular macular disease. Am J Ophthalmol. 2009; 148: 733– 743.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park KH,, Park SJ,, Lee YJ,, Kim JY,, Caprioli J. Ability of peripapillary atrophy parameters to differentiate normal-tension glaucoma from glaucomalike disk. J Glaucoma. 2001; 10: 95– 101. [DOI] [PubMed] [Google Scholar]
- 29. Adhi M,, Lau M,, Liang MC,, Waheed NK,, Duker JS. Analysis of the thickness and vascular layers of the choroid in eyes with geographic atrophy using spectral-domain optical coherence tomography. Retina. 2014; 34: 306– 312. [DOI] [PubMed] [Google Scholar]
- 30. Gieser JP,, Mori M,, Blair NP,, Shahidi M. Findings on retinal topography and thickness mapping in age-related macular degeneration. Retina. 2001; 21: 352– 360. [DOI] [PubMed] [Google Scholar]
- 31. Wallsh J,, Gallemore R. Optical coherence tomography difference maps and average macular volume for geographic atrophy. Retin Cases Brief Rep. 2015; 9: 88– 91. [DOI] [PubMed] [Google Scholar]
- 32. Sayegh RG,, Simader C,, Scheschy U,, et al. A systematic comparison of spectral-domain optical coherence tomography and fundus autofluorescence in patients with geographic atrophy. Ophthalmology. 2011; 118: 1844– 1851. [DOI] [PubMed] [Google Scholar]
- 33. Alasil T,, Wang K,, Yu F,, et al. Correlation of retinal nerve fiber layer thickness and visual fields in glaucoma: a broken stick model. Am J Ophthalmol. 2014; 157: 953– 959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miki A,, Medeiros FA,, Weinreb RN,, et al. Rates of retinal nerve fiber layer thinning in glaucoma suspect eyes. Ophthalmology. 2014; 121: 1350– 1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yin ZQ,, Vaegan,, Millar TJ,, Beaumont P,, Sarks S. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma. 1997; 6: 23– 32. [PubMed] [Google Scholar]
- 36. Roberts KF,, Artes PH,, O'Leary N,, et al. Peripapillary choroidal thickness in healthy controls and patients with focal, diffuse, and sclerotic glaucomatous optic disc damage. Arch Ophthalmol. 2012; 130: 980– 986. [DOI] [PubMed] [Google Scholar]
- 37. Sugiyama T,, Schwartz B,, Takamoto T,, Azuma I. Evaluation of the circulation in the retina, peripapillary choroid and optic disk in normal-tension glaucoma. Ophthalmic Res. 2000; 32: 79– 86. [DOI] [PubMed] [Google Scholar]
- 38. O'Brart DP,, de Souza Lima M,, Bartsch DU,, Freeman W,, Weinreb RN. Indocyanine green angiography of the peripapillary region in glaucomatous eyes by confocal scanning laser ophthalmoscopy. Am J Ophthalmol. 1997; 123: 657– 666. [DOI] [PubMed] [Google Scholar]
- 39. Sohrab MA,, Smith RT,, Salehi-Had H,, Sadda SR,, Fawzi AA. Image registration and multimodal imaging of reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2011; 52: 5743– 5748. [DOI] [PubMed] [Google Scholar]
- 40. Kim KE,, Kim DM,, Flammer J,, Kim KN. Central retinal venous pressure in eyes of normal-tension glaucoma patients with optic disc hemorrhage. PLoS One. 2015; 10: e0127920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anderson DR; for the Normal Tension Glaucoma Study Collaborative normal tension glaucoma study. Curr Opin Ophthalmol. 2003; 14: 86– 90. [DOI] [PubMed] [Google Scholar]
- 42. Kim JH,, Lee TY,, Lee JW,, Lee KW. Comparison of the thickness of the lamina cribrosa and vascular factors in early normal-tension glaucoma with low and high intraocular pressures. Korean J Ophthalmol. 2014; 28: 473– 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Usui S,, Ikuno Y,, Miki A,, Matsushita K,, Yasuno Y,, Nishida K. Evaluation of the choroidal thickness using high-penetration optical coherence tomography with long wavelength in highly myopic normal-tension glaucoma. Am J Ophthalmol. 2012; 153: 10– 16.e1. [DOI] [PubMed] [Google Scholar]
- 44. Maul EA,, Friedman DS,, Chang DS,, et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 2011; 118: 1571– 1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ehrlich JR,, Peterson J,, Parlitsis G,, Kay KY,, Kiss S,, Radcliffe NM. Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography. Exp Eye Res. 2011; 92: 189– 194. [DOI] [PubMed] [Google Scholar]
- 46. Mwanza JC,, Hochberg JT,, Banitt MR,, Feuer WJ,, Budenz DL. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011; 52: 3430– 3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rhew JY,, Kim YT,, Choi KR. Measurement of subfoveal choroidal thickness in normal-tension glaucoma in Korean patients. J Glaucoma. 2014; 23: 46– 49. [DOI] [PubMed] [Google Scholar]