Abstract
Background
Weakness of the rotator cuff muscles can lead to imbalances in the strength of shoulder external and internal rotators, change the biomechanics of the glenohumeral joint and predispose an athlete to injury. Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that has demonstrated promising results in a variety of health conditions. However few studies addressed its potential approach in the realm of athletics.
Hypothesis/Purpose
The purpose of this study was to investigate if transcranial direct current stimulation (tDCS) technique increases the isometric muscle strength of shoulder external and internal rotators in handball athletes.
Study Design
Randomized, double-blind, placebo-controlled, crossover study.
Methods
Eight female handball players aged between 17 and 21 years (Mean=19.65; SD=2.55) with 7.1 ± 4.8 years of experience in training, participating in regional and national competitions were recruited. Maximal voluntary isometric contraction (MVIC) of shoulder external and internal rotator muscles was evaluated during and after 30 and 60 minutes post one session of anodal and sham current (2mA; 0.057mA/cm2) with a one-week interval between stimulations.
Results
Compared to baseline, MVIC of shoulder external and internal rotators significantly increased after real but not sham tDCS. Between-group differences were observed for external and internal rotator muscles. Maximal voluntary isometric contraction of external rotation increased significantly during tDCS, and 30 and 60 minutes post-tDCS for real tDCS compared to that for sham tDCS. For internal rotation MVIC increased significantly during and 60 minutes post-tDCS.
Conclusions
The results indicate that transcranial direct current stimulation temporarily increases maximal isometric contractions of the internal and external rotators of the shoulder in handball players.
Level of Evidence
2
Keywords: Isometric contraction, handball, shoulder rotator muscles, Transcranial direct current stimulation (tDCS)
INTRODUCTION
Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique for modulation of brain activity and excitability that has demonstrated promising results in a variety of health conditions, such as chronic pain,1,2 depression3 and chronic stroke.4 For healthy athletes, tDCS is potentially useful due to the possibility of ergogenic or facilitatory effect in muscle performance and sports’ skills.5 Previous authors have shown that an application of anodal tDCS (a-tDCS) over primary motor cortex (M1) of healthy individuals improves muscle endurance6,7; enhances pinch force in the lower leg (grip force of the great toe and the second toe)8 and enhances the consolidation of ballistic thumb movement.9 Other researchers investigated the effect of tDCS over the left premotor cortex and found an improvement performance of a dexterity-demanding task.10
Specifically for muscle performance tasks, the effect of tDCS seems to be related to alterations in motor unit recruitment strategies.7,11,12 Taken together these results suggest that tDCS could be useful as an auxiliary tool for physical and motor performance training. However despite some evidence of the benefits of tDCS in healthy volunteers, few studies have addressed its potential approach in the realm of athletics.
Increase in muscle capabilities even at minimum levels can be useful for athletes, especially for sports demanding excessive and repetitive efforts. For instance, in throwing athletes such as handball players, muscle weakness of external shoulder rotators is associated with shoulder injury.13 Muscle imbalance between the external and internal rotator muscles is also observed in handball players.14 Thus optimal muscle function is highly desired to avoid shoulder injury and impairment in sports performance. The possibility of identifying a safe ergogenic aid to optimize muscle recruitment and muscle strength is of extreme interest to athletes, coaches and researchers, and modulation of the motor cortex by tDCS may be an easy and helpful strategy in this condition. The purpose of this study was to investigate whether tDCS technique increases the isometric muscle strength of shoulder external and internal rotators in handball athletes. Maximal voluntary isometric contraction (MVIC) was evaluated during and after the application of anodal and sham current to test the modulatory and plastic effects of tDCS on isometric muscle strength, respectively.
METHODS
Subjects
This randomized, double-blind, placebo-controlled crossover study included eight female handball athletes participating in regional and national competitions (Table 1). All participants were in the preseason training period and were instructed not to perform any kind of strenuous exercise and not to ingest alcoholic or caffeinated drinks during the two weeks of data collection. Athletes with suspected or confirmed pregnancy, complaints of pain in the upper limbs with intensity ≥ 3, evaluated by numerical pain rating scale (NPRS 0-10), medical history or personal report of epilepsy or convulsive event, or the use of drugs with central action were excluded. The study was approved by the local Research Ethics Committee under the protocol number 269.011 and the participants signed the informed consent.
Table 1.
Participant's characteristics
| Variable | Mean (SD) |
|---|---|
| Age (years) | 19.7 (2.3) |
| Body Mass (Kg) | 64.9 (7.9) |
| Height (m) | 1.66 (0.5) |
| Handball experience (years) | 7.8 (4.5) |
| Week training (hours) | (10.0) |
Experimental paradigm
The study was conducted in two weeks. In the first week, the participants were randomly distributed based on an online generating random numbers software (www.randomization.com) into two groups: (1) real anodal tDCS or (2) sham anodal tDCS. The randomization and allocation concealment were carried out by an external collaborator, not involved in the study, through individual opaque envelopes containing the identification number of the participants and their type of stimulation (real / sham). In the second week the type of stimulus was inverted between the participants. Muscle performance evaluation was carried out (1) immediately pre-tDCS, (2) during tDCS (after 13 min); (3) 30 minutes post-tDCS, and (4) 60 minutes post-tDCS. Evaluations during and after the application of anodal current were designed to test the modulatory and plastic effects of tDCS, respectively. The same researcher performed all the evaluations. He did not know the characteristics of the applied stimulations (real or simulated), nor did the participants.
Muscle Strength Evaluation
Initially, the participants were instructed to perform warm-up exercise for five minutes on an upper body ergometer. Afterwards, they made two attempts of submaximal isometric voluntary contraction of the external and internal rotator muscles, in the dominant limb, to familiarize themselves with the methods of muscle strength evaluation. One minute of rest was allowed between contractions. The maximum voluntary isometric contractions of the internal and external rotator muscles in the dominant limb were collected. To minimize the risks of muscle fatigue during MVIC tests, all participants were allowed a rest interval of one minute between contractions.15 Counter-balancing of the rotator muscles was performed to minimize the participant's learning factor. To perform the tests, the hand held dynamometer (MIcroFet2, Hoggan Health industries, USA) was positioned at the athlete's wrist area (2cm below the radial styloid process) on the dorsal aspect of the wrist to test the lateral rotators, and ventral aspect to test the internal rotators.16
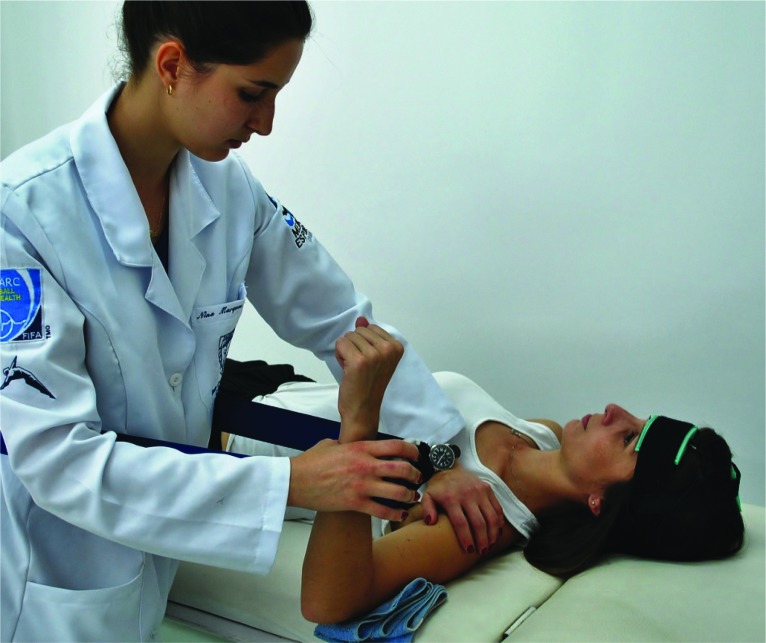
A rigid band was used to stabilize the dynamometer. The participants remained in the supine position, with shoulder at 90 ° of abduction and the elbow was flexed to 90 °.17 During the test the assessor stabilized the participant's shoulder to avoid accessory movements (Figure 1).
Figure 1.
Manual muscle testing with a hand-held dynamometer during tDCS application. Anodal pole positioned over M1 contralateral to the evaluated upper limb. Cathodal pole positioned over ipsilateral supraorbital contralateral to anode pole.
Anodal tDCS
Anodal tDCS was applied through a battery powered DC generator (Activadose II, USA) using two electrodes measuring 5 x 7cm (35cm2) (Ibramed, Brazil) covered with an electrode sponge, saturated with physiological saline solution, and fixed onto the head by means of velcro straps. The electrodes were mounted in accordance with the International 10-20 EEG System18 for optimal focalization of the primary motor cortex.
The electrode with the positive charge (anode – excitatory pole) was positioned at C3 or C4 (contralateral to the dominant limb), and the electrode with the negative charge (cathode – inhibitory pole) in the ipsilateral supraorbital region of the dominant limb (Figure 1). Real tDCS was applied with electric current amplitude of 2 mA, electric current density of 0.057 mA/cm2, for 20 minutes. Sham tDCS was applied with the same parameters, maintaining the electrodes in place for 20 minutes over the head, but stimulation was on for only the first 30 seconds.19
During the application of tDCS the participants remained seated. After 13 minutes of stimulation, they laid down on the stretcher for the MVIC evaluation, corresponding to time interval 2 (during tDCS). The decision was made to wait until 13 minutes of stimulation had passed, because previous studies have demonstrated that this was the minimum time required to obtain an increase in cortical excitability for up to 1.5 hours.20 The adverse effects were evaluated after each application through spontaneous reports of any unpleasant sensations such as burning, tingling, itching, headache, or nausea.
Statistical Analysis
The averages of three maximum contractions in each time interval were analyzed separately with regards to the external and internal rotator muscles. Normalization of the data was performed by using the body mass (kg) of each participant.21 Differences in the MVIC of the rotator muscles within evaluation time points and between real and sham stimulation were calculated using linear mixed models by using group, time and group-versus-time interaction terms. Post hoc tests with Bonferroni corrections were used when necessary. Mean differences of MVIC at baseline between the first and second week were analyzed by paired t-tests for external and internal rotator muscles. Level of significance was established at p < 0.05. The analyses were performed using the software program IBM SPSS v.20 for Windows.
RESULTS
No adverse events were reported during and after application of the intervention protocols. Paired t-tests showed no differences between the means of MVIC in the baseline interval between the first and second week, for external (t(7) = 0.03, p = 0.98) and internal (t(7) = 10.50, p = 0.18) rotator muscles. Intraclass correlation coefficient (ICC) showed good to excellent intra-rater reliability between week 1 and week 2 for baseline measures of external rotation (ICC = 0.94; 95%CI = 0.72 to 0.99). For internal rotation ICC showed poor to excellent intra-rater reliability (ICC = 0.72; 95%CI = 0.37 to 0.94).
Significant differences within time intervals were observed for external and internal rotator muscles. Compared to baseline, MVIC increased significantly during, after 30 and 60 minutes for real but not for sham tDCS for external rotation. Internal rotation increased significantly after 30 and 60 minutes for real but not for sham tDCS (Table 2). Between-group differences were observed for external and internal rotator muscles. Maximal voluntary isometric contraction increased significantly during tDCS, and 30 and 60 minutes post-tDCS for real tDCS than during sham tDCS (Table 2).
Table 2.
Mean (SD) for external and internal muscle strength (N/Kg) across intervals and mean difference (95% CI) within and between groups.
| tDCS intervals | Within-group differences | Between-group differencesa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| External Rotation | T0 | T20 | T50 | T80 | T20-T0 | T50-T0 | T80-T0 | T20 | T50 | T80 |
| Real tDCS | 0.9 (0.1) | 1.0 (0.1) | 1.1 (0.2) | 1.1 (0.2) | 0.1* (0.0 to 0.2) | 0.2* (0.1 to 0.3) | 0.2* (0.1 to 0.3) | 0.1* (0.0 to 0.2) | 0.2* (0.1 to 0.3) | 0.2* (0.1 to 0.3) |
| Sham tDCS | 0.9 (0.2) | 0.9 (0.2) | 0.9 (0.1) | 0.9 (0.1) | 0.0 (-0.1 to 0.1) | 0.0 (-0.1 to 0.1) | 0.0 (-0.2 to 0.1) | |||
| Internal Rotation | ||||||||||
| Real tDCS | 0.9 (0.1) | 1.0 (0.1) | 1.1 (0.1) | 1.1 (0.1) | 0.1 (0.0 to 0.2) | 0.1* (0.0 to 0.2) | 0.1* (0.0 to 0.2) | 0.1* (0.0 to 0.2) | 0.1* (0.0 to 0.2) | 0.1* (0.0 to 0.2) |
| Sham tDCS | 0.9 (0.1) | 0.9 (0.1) | 0.9 (0.2) | 0.9 (0.2) | 0.0 (-0.1 to 0.1) | 0.0 (-0.1 to 0.1) | 0.0 (-0.1 to 0.1) | |||
tDCS: Transcranial direct current stimulation. T0: Baseline; T20: 20min of stimulation; T50: 30min post-stimulation; T80: 60min post-stimulation.
Between-group differences are adjusted.
Significant difference (p<0.05).
DISCUSSION
The aim of this study was to investigate whether anodal tDCS applied to the motor cortex has ergogenic effects on isometric strength of shoulder muscles in handball players. The results showed that anodal tDCS can induce a temporary and progressive increase in MVIC of shoulder rotator muscles, which did not happen in sham tDCS intervention.
Compared to baseline muscle strength, real tDCS improved 10.2, 18.6 and 19.3% for external rotation during and after 30 and 60 minutes post-stimulation, respectively. Internal rotation muscles showed improvement of 5.6%, 11.1% and 15.1%, respectively. For sham tDCS no improvements were observed for external and internal rotation in all time frames analyzed. When compared to sham tDCS, stimulation results also showed increases in MVIC during and after tDCS for external and internal rotator muscles. These results indicate that tDCS can temporarily induce an incremental increase in maximal isometric strength of shoulder rotators in previously physically conditioned subjects.
There is some evidence in the literature that tDCS influences fatigue and muscle strength in normal volunteers. Cogiamanian et al. found increased isometric force endurance in a submaximal isometric contraction of elbow flexors after 10 minutes of anodal but not cathodal tDCS applied over the motor areas of the cerebral cortex (1.5mA, 0.043mA/cm2).6 In a similar protocol of submaximal contraction of elbow flexors, Williams et al. demonstrated that anodal, but not sham tDCS (1.5mA, 0.043mA/cm2) enhanced time to task failure during but not at the end of task execution. For lower limbs, Tanaka and collaborators demonstrated that 10 minutes of real but not sham or cathodal tDCS applied to the motor cortex (2mA, 0.057mA/cm2) could temporarily enhance maximal leg pinch.
A reasonable explanation for these improvements in muscle strength is that the enhancement of corticospinal excitability via tDCS may alter motor unit recruitment strategies.7,11,12 Hence, as more motor units are recruited, more muscle strength is generated. Although the authors’ did not measure muscle activation, the current results are likely to be more related to optimization of neuromuscular function than other effects (such as motivational effects), as the same participants were assessed in both conditions in the crossover design.
In the current study, changes in MVIC during real stimulation indicated that tDCS technique could positively modulate the isometric strength of external and internal shoulder rotators in handball players. Muscle imbalance, as represented by the ratio of external to internal rotators, is often present in handball players14 and may lead to injury.22 tDCS could be applied as a complementary tool in muscle strengthening programs to help counteract these imbalances in combination with specific exercises, as this transitory increment in muscle strength per se does not necessarily result in improved sports skills if considered as an unique intervention.5
In this context, tDCS could act to increase sports performance through facilitating the activation of corticospinal tract and potentiating the entire motor pathway. Favorable results have been demonstrated when tDCS is associated with active exercises. Anodal tDCS (2mA, 0.08mA/cm2) combined simultaneously with voluntary exercise produces a two-fold increase in the amplitude of motor excitability compared with tDCS or exercise alone.23 Nonetheless, the effect of tDCS combined with exercise seems to be dependent on the characteristic of exercises, such as type of muscle contractions.24 It is possible that tDCS has a relevant effect in specific physical fitness and in specific conditions. The “ideal” protocols for motor cortex modulation that fit the needs of athletes and coaches still need further investigation.
The present study has some limitations and factors that may have influenced the results. (1) Small sample size: Given the high variability between subjects during and after tDCS application these results must be replicated with large samples; (2) Variability in muscle representation in the primary motor cortex: transcranial magnetic stimulation was not utilized to determine the hot spot for external and internal muscle rotators in the cortex, which would have helped to potentiate the effects of the intervention; (3) The authors did not include cathodal stimulation. Although many studies have demonstrated that cortical excitability is polarity-dependent,20,25 other studies have verified that anodal polarity may exert an inhibitory effect during motor tasks26 just as cathodal polarity may exert an excitatory effect according to the intensity of stimulation.27 Future studies should include cathodal stimulation to investigate if the effects of tDCS on muscle strength are dependent on the polarity.
CONCLUSION
The results of this randomized, crossover trial indicate that transcranial direct current stimulation temporarily increases the isometric strength of the shoulder rotator muscles in handball players.
REFERENCES
- 1.O’Connell NE Wand BM Marston L Spencer S, et al. Non-invasive brain stimulation techniques for chronic pain. Cochrane Database Syst Rev. 2014;4:CD008208. [DOI] [PubMed] [Google Scholar]
- 2.Vaseghi B Zoghi M Jaberzadeh S. Does anodal transcranial direct current stimulation modulate sensory perception and painϿ. A meta-analysis study. Clin Neurophysiol. 2014;125(9):1847-1858. [DOI] [PubMed] [Google Scholar]
- 3.Meron D Hedger N Garner M Baldwin DS. Transcranial direct current stimulation (tDCS) in the treatment of depression: Systematic review and meta-analysis of efficacy and tolerability. Neurosci Biobehav Rev. 2015;57:46-62. [DOI] [PubMed] [Google Scholar]
- 4.Kang N Summers JJ Cauraugh JH. Transcranial direct current stimulation facilitates motor learning post-stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87(4):345-355. [DOI] [PubMed] [Google Scholar]
- 5.Banissy MJ Muggleton NG. Transcranial direct current stimulation in sports training: potential approaches. Front Hum Neurosci. 2013;7:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cogiamanian F Marceglia S Ardolino G Barbieri S, et al. Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur J Neurosci. 2007;26(1):242-249. [DOI] [PubMed] [Google Scholar]
- 7.Williams PS Hoffman RL Clark BC. Preliminary evidence that anodal transcranial direct current stimulation enhances time to task failure of a sustained submaximal contraction. PLoS One. 2013;8(12):e81418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka S Hanakawa T Honda M Watanabe K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp Brain Res. 2009;196(3):459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyama S Tanaka S Tanabe S Sadato N. Dual-hemisphere transcranial direct current stimulation over primary motor cortex enhances consolidation of a ballistic thumb movement. Neurosci Lett. 2015;588:49-53. [DOI] [PubMed] [Google Scholar]
- 10.Pavlova E Kuo MF Nitsche MA Borg J. Transcranial direct current stimulation of the premotor cortex: effects on hand dexterity. Brain Res. 2014;1576:52-62. [DOI] [PubMed] [Google Scholar]
- 11.Krishnan C Ranganathan R Kantak SS Dhaher YY, et al. Anodal transcranial direct current stimulation alters elbow flexor muscle recruitment strategies. Brain Stimul. 2014;7(3):443-450. [DOI] [PubMed] [Google Scholar]
- 12.Dutta A Krishnan C Kantak SS Ranganathan R, et al. Recurrence quantification analysis of surface electromyogram supports alterations in motor unit recruitment strategies by anodal transcranial direct current stimulation. Restor Neurol Neurosci. 2015;33(5):663-669. [DOI] [PubMed] [Google Scholar]
- 13.Clarsen B Bahr R Andersson SH Munk R, et al. Reduced glenohumeral rotation, external rotation weakness and scapular dyskinesis are risk factors for shoulder injuries among elite male handball players: a prospective cohort study. Br J Sports Med. 2014;48(17):1327-1333. [DOI] [PubMed] [Google Scholar]
- 14.Andrade MS Vancini RL de Lira CA Mascarin NC, et al. Shoulder isokinetic profile of male handball players of the Brazilian National Team. Braz J Phys Ther. 2013;17(6):572-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong OM Cheung RT Li RC. Isokinetic knee function in healthy subjects with and without Kinesio taping. Phys Ther Sport. 2012;13(4):255-258. [DOI] [PubMed] [Google Scholar]
- 16.Marcondes FB de Jesus JF Bryk FF de Vasconcelos RA, et al. Posterior shoulder tightness and rotator cuff strength assessments in painful shoulders of amateur tennis players. Rev Bras Fisioter. 2013;17(2):185-194. [DOI] [PubMed] [Google Scholar]
- 17.Donatelli R Ellenbecker TS Ekedahl SR Wilkes JS, et al. Assessment of shoulder strength in professional baseball pitchers. J Orthop Sports Phys Ther. 2000;30(9):544-551. [DOI] [PubMed] [Google Scholar]
- 18.Klem GH Luders HO Jasper HH Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 1999;52:3-6. [PubMed] [Google Scholar]
- 19.Gandiga PC Hummel FC Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845-850. [DOI] [PubMed] [Google Scholar]
- 20.Nitsche MA Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899-1901. [DOI] [PubMed] [Google Scholar]
- 21.Hurd WJ Morrey BF Kaufman KR. The effects of anthropometric scaling parameters on normalized muscle strength in uninjured baseball pitchers. J Sport Rehabil. 2011;20(3):311-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page P. Shoulder muscle imbalance and subacromial impingement syndrome in overhead athletes. Int J Sports Phys Ther. 2011;6(1):51-58. [PMC free article] [PubMed] [Google Scholar]
- 23.Kim GW Ko MH. Facilitation of corticospinal tract excitability by transcranial direct current stimulation combined with voluntary grip exercise. Neurosci Lett. 2013;548:181-184. [DOI] [PubMed] [Google Scholar]
- 24.Thirugnanasambandam N Sparing R Dafotakis M Meister IG, et al. Isometric contraction interferes with transcranial direct current stimulation (tDCS) induced plasticity: evidence of state-dependent neuromodulation in human motor cortex. Restor Neurol Neurosci. 2011;29(5):311-320. [DOI] [PubMed] [Google Scholar]
- 25.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527Pt 3:633-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antal A Terney D Poreisz C Paulus W. Towards unravelling task-related modulations of neuroplastic changes induced in the human motor cortex. Eur J Neurosci. 2007;26(9):2687-2691. [DOI] [PubMed] [Google Scholar]
- 27.Batsikadze G Moliadze V Paulus W Kuo MF, et al. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591(Pt 7):1987-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]