Abstract
Mucus hypersecretion is a prominent manifestation in patients with chronic inflammatory airway diseases. MUC5AC mucin is a major component of airway mucus, and its expression is modulated by a TNF-α-converting enzyme (TACE)–EGF receptor pathway that can be activated by reactive oxygen species (ROS). Dual oxidase 1 (Duox1), a homologue of glycoprotein p91phox, is expressed in airway epithelium and generates ROS. We hypothesize that Duox1 activates TACE, cleaving pro-TGF-α into soluble TGF-α, resulting in mucin expression. To examine this hypothesis, we stimulated both normal human bronchial epithelial cells and NCI-H292 airway epithelial cells with phorbol 12-myristate 13-acetate and with human neutrophil elastase. These stimuli induced TACE activation, TGF-α release, and mucin expression, effects that were inhibited by ROS scavengers, implicating ROS in TACE activation. Inhibition of epithelial NADPH oxidase or knockdown of Duox1 expression with small interfering RNA prevented ROS generation, TGF-α release, and mucin expression by these stimuli, implicating Duox1 in TACE activation and mucin expression. Furthermore, the PKCδ/PKCθ inhibitor rottlerin prevented the effects induced by phorbol 12-myristate 13-acetate and human neutrophil elastase, suggesting that PKCδ and PKCθ are involved in Duox1 activation. From these results, we conclude that Duox1 plays a critical role in mucin expression by airway epithelial cells through PKCδ/PKCθ-Duox1-ROS-TACE-pro-ligand-EGF receptor cascade.
Keywords: EGF receptor, mucus hypersecretion, reactive oxygen species, TNF-α-converting enzyme, type α TGF
Chronic inflammatory airway diseases are associated with mucus hypersecretion (1), which contributes to the morbidity and mortality of these diseases by plugging airways. MUC5AC mucin is a major component of airway mucus, and its expression is modulated by TNF-α-converting enzyme (TACE)-mediated EGF receptor (EGFR) transactivation; activated TACE cleaves EGFR proligand pro-TGF-α into soluble TGF-α, which binds to and activates EGFR, resulting in MUC5AC mucin expression (2–4).
Airway epithelial cells produce reactive oxygen species (ROS) (5). There is increasing evidence showing that ROS are involved in cell signaling (6). Recently, ROS scavengers have been shown to prevent cigarette-smoke-induced TACE activation, EGFR phosphorylation, and mucin production in airway epithelial cells (4), implicating ROS in the activation of the TACE-EGFR pathway.
ROS have long been known to be generated by NADPH oxidase (Nox) of phagocytes (Phox) (7). The core component of Nox is the catalytic subunit glycoprotein p91phox (8). Recently, a homologue of the gp91phox, dual oxidase 1 (Duox1), was identified in human airway epithelial cells and shown to generate ROS (9, 10). We hypothesize that Duox1 activates TACE via ROS generation, cleaving pro-TGF-α into soluble TGF-α, resulting in EGFR activation and mucin expression. To examine this hypothesis, we stimulated normal human bronchial epithelial (NHBE) cells and NCI-H292 airway epithelial cells with phorbol 12-myristate 13-acetate (PMA) and human neutrophil elastase (HNE). PMA is a model inflammatory stimulus known to induce mucin via TACE-EGFR pathway in NCI-H292 airway epithelial cells (2), but the mechanism of activation of this pathway is unknown. HNE is a serine protease secreted by neutrophils and exists in high concentrations (1–20 μM) (11, 12) in the airway surface fluid of patients with chronic inflammatory airway diseases. HNE is known to cause mucin production via TGF-α-dependent EGFR activation (13), but it is unknown how this process is initiated. Here, we show that PMA and HNE activate Duox1 to produce ROS, activating TACE, which cleaves pro-TGF-α into soluble TGF-α, resulting in mucin expression in human airway epithelial cells.
Materials and Methods
Materials. Allopurinol, 1,3-dimethyl-2-thiourea (DMTU), n-propyl gallete (nPG), and PMA were purchased from Sigma. Apocynin, diphenyleneiodonium chloride (DPI), Gö6983, Gö6976, NG-monoethyl-l-arginine, and rottlerin were purchased from Calbiochem. Monoclonal p47phox and rabbit polyclonal p67phox antibodies were purchased from Santa Cruz Biotechnology. HNE was purchased from Elastin Products (St. Louis).
Cell Culture. Proliferating NHBE cells were purchased from Cambrex (Walkersville, MD). When cells reached 80% confluence, they were seeded at density of 2 × 104 per cm2 onto Costar transwell plate inserts (Fisher), growing in bronchial epithelial growth medium (Cambrex) supplemented with defined growth factors and retinoic acid (0.1 μg/liter) contained in the Single-Quot kit (Cambrex) at 37°C in a humidified, 5% CO2/95% air, water-jacketed incubator. After 7 days in an immersed culture condition, cell culture was switched to an air–liquid interface condition for 2–3 weeks.
NCI-H292 airway epithelial cells were cultured as described in ref. 2. After the cells reached confluence, they were serum-starved for 24 h before experiments to maintain low basal levels of MUC5AC expression.
Culture Conditions of Airway Epithelial Cells with Stimuli and Inhibitors. For inhibitory studies, cells were pretreated with inhibitors for 30 min before exposure to stimuli. In studies of PMA or HNE, the cells were treated with PMA (10 ng/ml) or HNE (100 nM) for 1 h, washed three times with medium, and cultured with the same concentrations of inhibitors as in the pretreatment period. After 8 or 24 h, cell-culture supernatants and cell lysates were collected to analyze MUC5AC expression.
Cytotoxicity Detection and Measurement of Total Protein. Lactatedehydrogenase activity in supernatants of cell cultures treated with or without inhibitors was measured by using the Cytotoxicity Detection Kit (Roche Diagnostics). Total protein in cell lysates of cell cultures treated with or without inhibitors was measured by using the BCA Protein Assay kit (Pierce). None of the measurements showed significant cytotoxicity for the inhibitors at the concentrations used in the present studies.
Measurement of H2O2 Production. Cells were treated with PMA (10 ng/ml) or HNE (100 nM) for various times. For inhibitory studies, cells were pretreated with inhibitors for 30 min before exposure to stimuli. H2O2 production in the supernatants was measured by using the Amplex Red Hydrogen Peroxide/Peroxidase Assay kit (Molecular Probes).
RNA Isolation, Reverse Transcription, and TaqMan Real-Time Quantitative PCR. Total RNA was isolated, and reverse transcription was performed as described in ref. 2. TaqMan real-time quantitative PCR analysis of MUC5AC gene expression was achieved by using a sequence detector (Model 7900, Applied Biosystems) according to the manufacturer's instructions. MUC5AC expression was normalized to 18s rRNA. The following TaqMan primers and probes were used: 18s rRNA, GATCCATTGGAGGGCAAGTCT (forward) and GCAGCAACTTTAATATACGCTATTGC (reverse); probe-FAM, TGCCAGCAGCCGCGGTAATTC; MUC5AC, GGAGGTGCCCTTCAGCAA (forward) and CGTGCGGCACTCATCCTT (reverse); probe-FAM, AGTGCGGCACTTGCACCAACGAC; Duox1, GCCCTGTACAACCAGGACTT (forward) and CGCACAAATTGTTCAAGGAC (reverse); and probe-FAM, CCAGGGAGCAGCTCTAGCCA.
MUC5AC Mucin ELISA. Production of MUC5AC mucin protein in cell lysates and cell culture supernatants was measured by ELISA, as described in ref. 3. The amount of MUC5AC mucin in each sample was normalized to total protein in cell lysate and was expressed as μg of mucin per μg of total cellular protein.
Effect of Stimuli on Cleavage and Release of Soluble TGF-α. Cells were stimulated with or without PMA or HNE for 2 h. For inhibitory studies, cells were pretreated with inhibitors for 30 min before adding a stimulus. To prevent soluble TGF-α from binding to EGFR, an EGFR-neutralizing Ab (4 μg/ml) was added 30 min before adding a stimulus. Cell supernatants were collected, and TGF-α was measured with the TGF-α ELISA kit (Oncogene Science).
Small Interfering RNA (siRNA) Preparation and Transfection of Cells Predesigned human Duox1 siRNAs (nos. 24873 and 24969) were purchased from Ambion (Austin, TX). In our preliminary studies, only siRNA no. 24969 (100 nM) had a significant inhibitory effect on MUC5AC mucin production by PMA. Therefore, this siRNA was used for all studies. The 21-nt sequences of Duox1 (siRNA no. 24969) were (sense) GGACUUAUCCUGGCUAGAGtt and (antisense) CUCUAGCCAGGAUAAGUCCtg. Silencer Negative Control no. 1 siRNA (Ambion) was used as a nonspecific siRNA. SiRNA transfection into NCI-H292 cells was carried out by using Lipofectamine 2000 (Invitrogen). Specific silencing of Duox1 was confirmed by using real-time quantitative PCR at 72 h after transfection.
Preparation of Membrane Fraction and Western Blot Analysis. Membrane fraction of cells was prepared according to the method described in ref. 14. Briefly, the cells were harvested and washed twice with PBS. The cell pellet was resuspended in relaxation buffer (100 mM KCl/3 mM NaCl/3.5 mM MgCl2/1 mM EGTA/10 mM Hepes/0.5 mM phenylmethylsulfonyl fluoride) and 1:100 dilution of proteinase inhibitors (Complete Mini, Roche), disrupted by two 10-s cycles of sonication on ice, and then centrifuged at 600 × g for 10 min at 4°C to remove nuclei and unbroken cells. The supernatant was then ultracentrifuged at 100,000 × g for 30 min at 4°C. The pellet was resuspended in relaxation buffer with vigorous mixing and again centrifuged at 100,000 × g for 15 min at 4°C. The final pellet, representing the membrane fraction, was resuspended in relaxation buffer and subjected to Western blot analysis, as described in ref. 2.
Recombinant TACE Cleavage Assay. Purified recombinant TACE without the prodomain was purchased from Calbiochem. TACE inhibitory peptide (TIP) was synthesized from TACE prodomain sequence, PKVCGYLK (15), by BioSource International (Camarillo, CA). TACE activity was determined by continuous kinetic assays using the fluorogenic TACE substrate III, DABCYL-LAQAVRSSSR-EDANS (Calbiochem). The fluorescence intensity was monitored in a CytoFluor 2350 multiwell fluorescent plate reader (Applied Biosystems) by using wavelengths of 340 and 490 nm for excitation and emission, respectively. All reactions were initiated in 50 μl of 50 mM Tricine buffer (pH 7.5) containing 100 mM NaCl, 10 mM CaCl2, and 1 mM ZnCl2. To inhibit TACE, TIP (400 μM) was incubated with TACE (200 nM) for 1 h at 27°C. To test whether HNE can reverse TACE inhibition, HNE was added to the reactions (TACE plus TIP) for final concentrations of 10, 100, and 1,000 nM and incubated for 30 min. H2O2 (4 mM), which has been reported to reverse TACE inhibition by TIP (16), was used as a positive control. Finally, TACE substrate III was added to the reactions for a final concentration of 20 μM, and the fluorescence intensity was monitored every 10 min for 6 h.
Statistical Analysis. Data are presented as mean ± SD (n = 3). ANOVA was used to determine statistically significant differences (P < 0.01).
Results
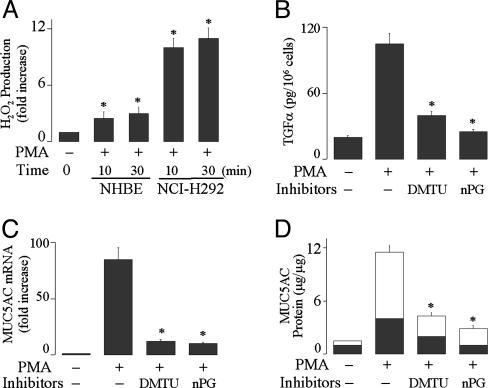
ROS Mediate PMA-Induced TGF-α Release and MUC5AC Expression. PMA (10 ng/ml) induced ROS production in both NHBE and NCI-H292 cells (Fig. 1A). Pretreatment with ROS scavengers DMTU and nPG prevented PMA-induced TGF-α release (Fig. 1B), MUC5AC gene expression (Fig. 1C), and mucin protein production (Fig. 1D), implicating ROS in TACE activation/TGF-α release and mucin expression by PMA in airway epithelial cells.
Fig. 1.
Effect of PMA on H2O2 production and the effects of ROS scavengers on TGF-α release, MUC5AC gene expression, and mucin protein production by PMA. (A) NHBE and NCI-H292 cells were treated with PMA (10 ng/ml) for various times to measure H2O2 production, as described in Materials and Methods. (B) NHBE cells were pretreated with an EGFR-neutralizing Ab and either DMTU (20 mM) or nPG (100 μM) for 30 min. Then, the cells were stimulated with PMA for 2 h to measure soluble TGF-α in the supernatants. (C) After pretreatment with DMTU or nPG for 30 min, NHBE cells were stimulated with PMA for 8 h to analyze MUC5AC gene expression by using real-time quantitative PCR or for 24 h to measure protein production by ELISA (D). Mucins were measured in the cell lysate (dark areas) and in the supernatant (light areas). Similar results were obtained in NCI-H292 cells (data not shown). Data are expressed as mean ± SD (n = 3). *, P < 0.01, compared with PMA alone.
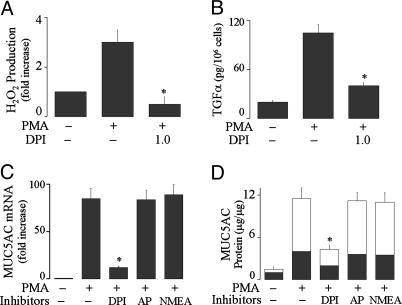
Epithelial NADPH Oxidase Generates ROS, Mediating TACE Activation and Mucin Production by PMA. Pretreatment with a Nox inhibitor, DPI, prevented PMA-induced ROS generation (Fig. 2A), TGF-α release (Fig. 2B), mucin gene expression (Fig. 2C), and protein production (Fig. 2D). To exclude the involvement of other oxidases, we investigated the effect of inhibitors of xanthine oxidase (allopurinol, 100 μM) and nitric oxide synthase (NG-monoethyl-l-arginine, 100 μM). None of the inhibitors had a significant inhibitory effect on MUC5AC gene expression (Fig. 2C) and protein production (Fig. 2D) by PMA. These results implicate Nox in PMA-induced effects in airway epithelial cells.
Fig. 2.
Effect of inhibitors of NADPH oxidase on PMA-induced responses. (A) NHBE cells were pretreated with DPI (1.0 μM) for 30 min and then exposed to PMA (10 ng/ml) for 30 min to measure H2O2 production. (B) NHBE cells were pretreated with an anti-EGFR-neutralizing Ab and with DPI (1.0 μM) for 30 min and then stimulated with PMA for 2 h to measure TGF-α in the supernatants. (C) NHBE cells were pretreated with DPI (1.0 μM), AP (100 μM), or NMEA (100 μM) for 30 min, then treated with PMA for 8 h to analyze MUC5AC gene expression or for 24 h to measure mucin protein production (D). Similar results were obtained in NCI-H292 cells (data not shown). Data are expressed as mean ± SD (n = 3). *, P < 0.01, compared with PMA alone.
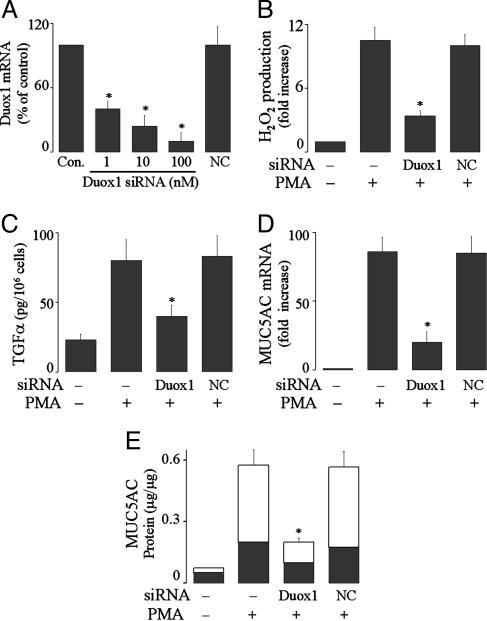
Duox1 Generates ROS, Inducing TACE Activation and Mucin Expression by PMA. Duox1 is a gp91phox homologue expressed in human airway epithelial cells. Knockdown of Duox1 expression in NCI-H292 cells with siRNA (Fig. 3A) prevented ROS generation (Fig. 3B), TGF-α release (Fig. 3C), MUC5AC gene expression (Fig. 3D), and protein production (Fig. 3E) by PMA. Transfection of negative control siRNA was without inhibitory effect (Fig. 3). These results implicate Duox1 in PMA-induced responses.
Fig. 3.
Effect of knockdown of Duox1 expression on PMA-induced responses in NCI-H292 cells. (A) The cells were transfected with Duox1 siRNA at various concentrations or with negative control siRNA (NC, 100 nM), cultured for 72 h, and then analyzed for Duox1 mRNA expression by using real-time PCR. (B)At 72 h after transfection with Duox1 siRNA (100 nM) or NC siRNA (100 nM), the cells were exposed to PMA (10 ng/ml) for 30 min to measure H2O2 production. (C) At 72 h after transfection, the cells were preincubated with an EGFR-neutralizing Ab for 30 min and then stimulated with PMA for 2 h to measure TGF-α in the supernatants. (D and E) At 72 h after transfection, the cells were treated with PMA (10 ng/ml)for 8 h to analyze MUC5AC gene expression (D) or for 24 h to measure mucin protein production (E). Data are expressed as mean ± SD (n = 3). *, P < 0.01, compared with PMA alone and with NC siRNA.
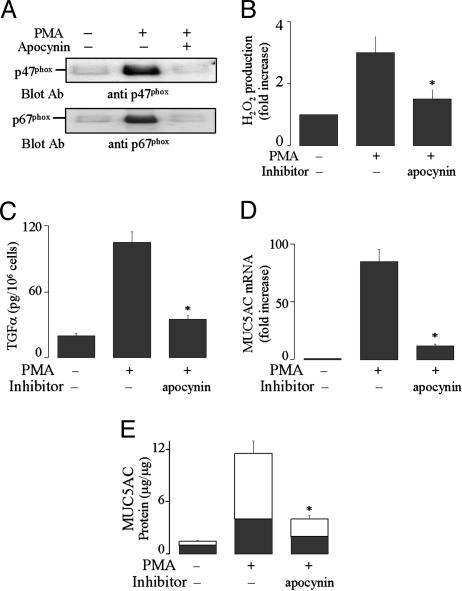
PMA Activates Duox1 by Causing Assembly of the Cytosolic Components of Nox with Duox1. Phagocytic Nox generates ROS when the cytosolic components (e.g., p47phox and p67phox) move to the plasma membrane to form the complete enzyme Nox with gp91phox (17). We examined whether PMA causes Duox1 activation by means of a similar mechanism. PMA caused p47phox and p67phox translocation from cytosol to plasma membrane (Fig. 4A). Pretreatment with apocynin, which blocks Nox assembly (18), prevented translocation of p47phox and p67phox to the plasma membrane (Fig. 4A), ROS generation (Fig. 4B), TGF-α release (Fig. 4C), mucin gene expression (Fig. 4D), and protein production (Fig. 4E). From these results, we conclude that PMA activates Duox1 by causing assembly of the cytosolic components with Duox1.
Fig. 4.
Effect of NADPH oxidase inhibitor apocynin on PMA-induced responses. (A) NHBE cells were pretreated with or without apocynin (1 mM) for 30 min and then stimulated with PMA (10 ng/ml) for 30 min. Plasma membrane fraction was prepared and subjected to Western blot analysis for detection of p47phox with anti-p47phox Ab (Upper) and detection of p67phox with anti-p67phox Ab (Lower) in membranes, as described in Materials and Methods.(B) NHBE cells were pretreated with apocynin (1 mM) for 30 min and then stimulated with PMA for 30 min to measure H2O2 production. (C) NHBE cells were pretreated with an anti-EGFR-neutralizing Ab for 30 min, treated with apocynin for 30 min, and then stimulated with PMA for 2 h to measure TGF-α in the supernatants. (D and E) After pretreatment with apocynin for 30 min, NHBE cells were stimulated with PMA for either 8 h to analyze MUC5AC gene expression (D) or 24 h to measure mucin protein production (E). Similar results were obtained in NCI-H292 cells (data not shown). Data in B–E are expressed as mean ± SD (n = 3). *, P < 0.01, compared with PMA alone.
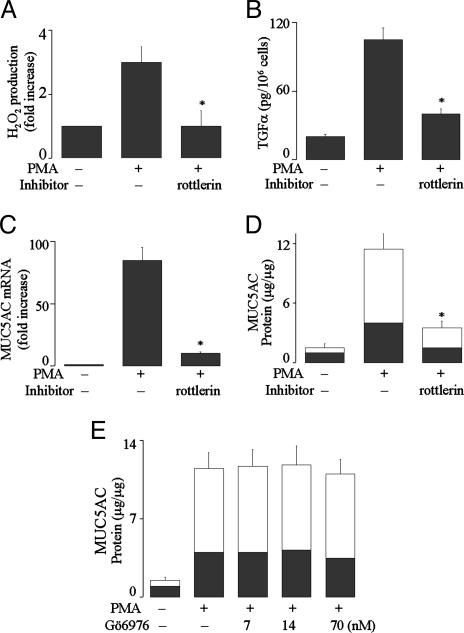
PKC Isoforms PKCδ and PKCθ Mediate Duox1 Activation and Mucin Expression by PMA. PMA exerts functions through activation of PKC. Two major groups of PKC isoforms exist: Ca2+-dependent PKCs including PKCα, PKCβ, and PKCγ; and Ca2+-independent PKCs including PKCδ, PKCθ, and PKCε. Pretreatment with the PKCδ/θ inhibitor rottlerin prevented ROS generation (Fig. 5A), TGF-α release (Fig. 5B), mucin gene expression (Fig. 5C), and protein production (Fig. 5D) by PMA, whereas PKCα/β1 inhibitor Gö 6976 did not inhibit PMA-induced effects (Fig. 5E, data shown for mucin protein production only). These results suggest that PKCδ/θ activates Duox1 to produce ROS, which activate TACE, leading to TGF-α release and mucin expression.
Fig. 5.
Role of PKCδ and PKCθ in PMA-induced responses. (A) NHBE cells were pretreated with PKCδ/θ inhibitor rottlerin (3 μM) for 30 min and then stimulated with PMA (10 ng/ml) for 30 min to measure H2O2 production. (B) NHBE cells were pretreated with an anti-EGFR-neutralizing Ab for 30 min, treated with rottlerin (3 μM) for 30 min, and then stimulated with PMA for 2 h to measure soluble TGF-α.(C–E) After pretreatment with either rottlerin (3 μM) or PKCα/β1 inhibitor Gö-6976 (7–70 nM) for 30 min, NHBE cells were stimulated with PMA for either 8 h to analyze MUC5AC gene expression (C)or 24 h to measure mucin protein production (D and E). Similar results were obtained in NCI-H292 cells (data not shown). Data are expressed as mean ± SD (n = 3). *, P < 0.01, compared with PMA alone.
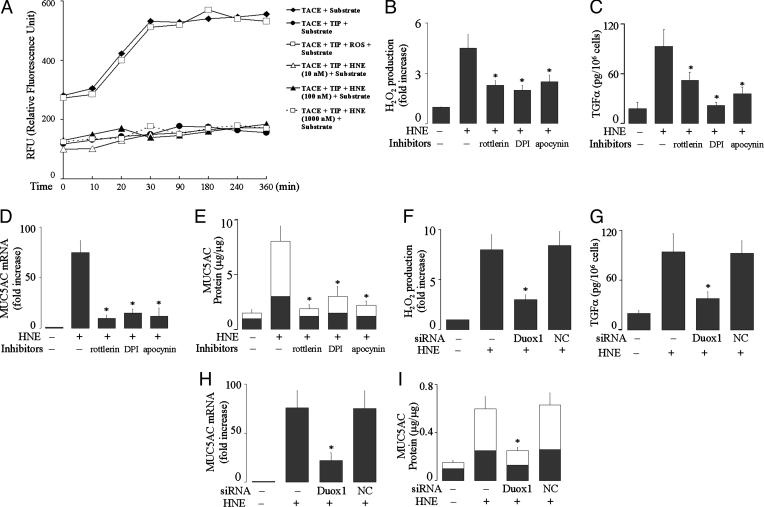
HNE Induces Mucin Expression via a Signaling Pathway Involving PKCδ/θ-Duox1-ROS-TACE. Having established the role of Duox1 in mucin expression by PMA, we examined whether this mechanism could be extended to HNE. First, we examined whether HNE has a direct action on TACE. Preincubation of TACE with TIP prevented cleavage of TACE substrate (Fig. 6A). Exogenous H2O2 (4 mM) reversed the TACE inhibition by TIP, implicating a direct action of ROS on TACE. However, various concentrations of HNE (10, 100, and 1,000 nM) failed to reverse the inhibitory effect of TIP on TACE, suggesting that there is no direct action of HNE on TACE. Second, we examined effects of PKCδ/θ inhibitor rottlerin or Nox inhibitors DPI and apocynin on HNE-induced responses. Pretreatment with these inhibitors prevented HNE-induced ROS generation (Fig. 6B), TGF-α release (Fig. 6C), mucin gene expression (Fig. 6D), and mucin protein production (Fig. 6E) in both NHBE and NCI-H292 cells. Next, we confirmed the role of Duox1 in these responses to HNE by knocking down the expression of Duox1 with siRNA. Knockdown of Duox1 prevented all of these effects of HNE in NCI-H292 cells (Fig. 6 F–I). Together, we conclude that HNE induces MUC5AC gene expression and protein production via a signaling pathway involving PKCδ/θ-Duox1-ROS-TACETGF-α in human airway epithelial cells.
Fig. 6.
Effect of HNE on TACE activation and roles of PKCθ/δ and Duox1 in HNE-induced ROS generation, TACE activation, MUC5AC gene expression, and mucin protein production. (A) First, recombinant TACE (200 nM) was incubated with or without TIP (400 μM)for 1 hat 27°C and then incubated with TACE substrate III (20 μM) to measure fluorescence intensity for 6 h, as described in Materials and Methods. Second, TACE was preincubated with TIP for 1 h to inactivate TACE, and then H2O2 (4 mM) or various concentrations of HNE (10, 100, and 1,000 nM) were added to the reactions for 30 min, followed by TACE substrate, and the fluorescence intensity was monitored for 6 h. (B) NHBE cells were pretreated with rottlerin (3 μM), DPI (1 μM), or apocynin (1 mM) for 30 min and then stimulated with HNE (100 nM) for 30 min to measure H2O2 production. (C) NHBE cells were pretreated with an anti-EGFR neutralizing Ab for 30 min and then treated with rottlerin (3 μM), DPI (1 μM), or apocynin (1 mM) for 30 min and then stimulated with HNE (100 nM) for 2 h to measure TGF-α in the supernatants. (D and E) After pretreatment with or without rottlerin (3 μM) or with DPI (1 μM) or apocynin (1 mM) for 30 min, NHBE cells were stimulated with HNE (100 nM) for 8 h to analyze MUC5AC gene expression (D) or for 24 h to measure mucin protein production (E). (F) NCI-H292 cells were transfected with Duox1 siRNA (100 nM) or NC siRNA (100 nM). After 72 h, the cells were exposed to HNE (100 nM) for 30 min to measure H2O2 production. (G) At 72 h after transfection, NCI-H292 cells were preincubated with an EGFR-neutralizing Ab for 30 min and then stimulated with HNE (100 nM) for 2 h to measure soluble TGF-α. (H and I) At 72 h after transfection, the cells were treated with HNE (100 nM) for 8 h to analyze MUC5AC gene expression (H) or for 24 h to measure protein production (I). Data are expressed as mean ± SD (n = 3). *, P < 0.01, compared with HNE alone and with negative control transfection.
Discussion
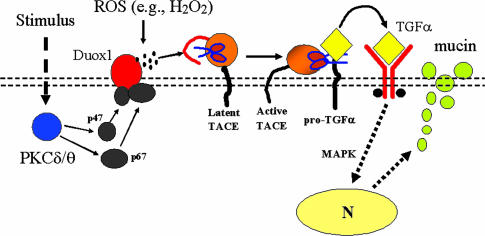
Our previous studies showed that a TACE-EGFR cascade is a primary signaling pathway that regulates MUC5AC mucin expression in response to multiple stimuli in airway epithelial cells (2–4). Here, we show that PMA and HNE activate Duox1 through PKCδ/θ, which causes TACE activation by producing ROS, resulting in TGF-α release and mucin production. Thus, we have identified a signaling pathway mediating mucin production involving PKCδ/θ-Duox1-ROS-TACE-pro-TGF-α-EGFR-mitogen-activated protein kinase in airway epithelial cells (Fig. 7).
Fig. 7.
Diagrammatic scheme of Duox1-dependent mucin production. This model contains our previous work on TACE activation, cleavage of EGFR proligand, and mucin production in airway epithelial cells (2, 4). A stimulus (e.g., PMA or HNE) activates PKC isoforms PKCδ and PKCθ, which recruit cytosolic components (e.g., p47phox and p67phox) to plasma membrane (double-dotted lines) to join Duox1 to form an active enzyme system for generating ROS (e.g., H2O2; represented by dark dots). ROS activate the latent form of TACE, which has an inhibitory prodomain (represented by a red line) covering its catalytic domain (represented by scissors), by removing the prodomain and exposing the catalytic domain to cleave pro-TGF-α (yellow diamond with a dark tail) into soluble TGF-α (yellow diamond without a tail), which binds to and activates EGFR, initiating mitogen-activated protein kinase signaling to the nucleus (arrow to nucleus), leading to mucin gene expression and mucin protein production.
The cellular process of TACE activation has not been defined. TACE is synthesized in a latent form with an inhibitory N-terminal prodomain masking the catalytic domain (19). Schlondorff et al. (20) reported that the prodomain is cleaved at a furin site in a late Golgi compartment. Black et al. (19) reported that the cell-surface TACE is without a prodomain, suggesting that TACE releases its prodomain before reaching the cell-surface membranes. However, conflicting evidence also exists. Doedens et al. (21) showed that PMA increases TACE-specific peptide cleavage without altering TACE expression on cell-surface membrane (7–15 min after PMA stimulation), suggesting that the increased activity of TACE is not due to an increase in TACE abundance on the cell surface but to an unknown mechanism that causes TACE activation. In support of this speculation, Milla et al. (22) showed that the prodomain of TACE is essential for appropriate secretion and processing of TACE catalytic domain. Deletion of the prodomain appears to make the catalytic domain a target for intracellular degradation. Zhang et al. (16) showed that a peptide synthesized from TACE prodomain sequence can inhibit the activity of the recombinant active TACE, a finding that was confirmed in the present study. Moreover, this TACE inhibition can be reversed by treatment with exogenous ROS. Thus, the cellular process controlling the removal of TACE prodomain and TACE activation appears more complicated than previously conceived. We propose a two-step posttranslational processing mechanism for TACE activation.
Cleavage of TACE prodomain at a furin site occurs in a late Golgi compartment. The cleaved prodomain is associatedwith the catalytic domain via an intramolecular bond between the cysteine residue in the prodomain and the zinc atom in the catalytic domain (16). Processed TACE molecules are transported to cell-surface membranes.
Upon cellular stimulation, the cysteine-zinc bond breaks, the prodomain is released, and the catalytic domain is exposed. In PMA- and HNE-stimulated cases, cells generate ROS (e.g., H2O2), which attack the cysteine sulfhydryl moiety and release it from coordination with the catalytic zinc, thus activating TACE.
The identification of Nox homologues in epithelial tissues such as bronchial epithelium (e.g., Duox1) implies that the generation of ROS in these tissues is a fine-tuned biological strategy. Three hypotheses concerning the function of ROS generated by Nox/Duox have been proposed: (i) role of host defense in killing invading microbes and cancer cells, (ii) role in signal transduction, and (iii) role in metabolism. In the present study, we focused on the role of ROS in TACE activation. Here, we found that Duox1 activated by PMA and HNE produces ROS, which activate TACE, resulting in TGF-α release and mucin production in airway epithelial cells. Because TACE cleaves many substrates (e.g., TNF-α, TNF receptors, TGF-α, amphiregulin, and l-selectin), the identification of TACE activation by Duox1 may have broad implications concerning the understanding of both physiological and pathophysiological processes in cells.
Nox has been studied extensively in phagocytes (23, 24). The phagocytic Nox consists of a membrane-bound cytochrome, b558; a complex of gp91phox and p22phox; and four cytosolic components, p47phox, p67phox, p40phox, and Rac1/2 (7). Six-transmembrane gp91phox is the catalytic core of Nox. Nox homologues in noninflammatory cells include Nox1 through Nox5 (25, 26) and Duox1/2 (9). Duox1 is expressed in bronchial epithelium (10). In airways, Duox1 was recently reported to provide H2O2 for lactoperoxidase-catalyzed reactions in antimicrobial activity (10). To explore the role of Duox1 in the signaling pathway mediating TACE activation and mucin production, we knocked down the expression of Duox1 with siRNA: Knockdown of Duox1 prevented PMA- and HNE-induced effects, implicating Duox1 in TACE activation and mucin expression.
HNE is present in the airway secretions of patients with chronic inflammatory airway diseases. HNE plays an important role in mucus hypersecretion. Thus, Kohri et al. (13) showed that HNE induces MUC5AC expression by increasing the release of soluble TGF-α. Fisher et al. (27) reported that ROS are involved in HNE-induced MUC5AC gene expression. Aoshiba et al. (28) showed that HNE induces ROS generation in NHBE cells. However, the mechanisms by which HNE induces ROS and TGF-α shedding were unknown. In the present study, we show that HNE induces H2O2 production, TACE activation/TGF-α shedding, and mucin expression via a signaling pathway involving PKCδ/PKCθ and Duox1 in human airway epithelial cells.
MUC5AC mucin is a large glycoprotein with a number of carbohydrate chains attached to the core protein moiety. Both the protein backbones and the carbohydrates contribute to the viscoelastic properties of airway mucus. Thus, understanding of the signaling pathway regulating mucin expression reveals only one facet in the puzzle of mucus hypersecretion. Further investigation concerning mechanisms such as those regulating mucin glycosylation is needed to uncover the complex picture of mucus hypersecretion.
In summary, we show that PMA and HNE induce mucin expression in human airway epithelial cells via a signaling pathway involving PKCδ/θ-Duox1-ROS-TACE. These findings are especially important because HNE is implicated in mucus hypersecretion associated with chronic inflammatory airway diseases. The discovery that Duox1 is involved in mucin induction suggests new therapies for hypersecretory airway diseases.
Acknowledgments
We thank Iris F. Ueki for technical assistance and useful discussions, and Drs. John Fahy and Silvio Favoreto (both of the University of California, San Francisco) for providing assistance in measuring hydrogen peroxide. This work was supported by private funding.
Author contributions: M.X.G.S. and J.A.N. designed research; M.X.G.S. performed research; M.X.G.S. analyzed data; M.X.G.S. and J.A.N. wrote the paper; and J.A.N. supervised and funded the research by M.X.G.S.
Abbreviations: DMTU, 1,3-dimethyl-2-thiourea; DPI, diphenyleneiodonium chloride; Duox, dual oxidase; EGFR, EGF receptor; NHBE, normal human bronchial epithelial; HNE, human neutrophil elastase; Nox, NADPH oxidase; Phox, phagocyte Nox; nPG, n-propyl gallete; PMA, phorbol 12-myristate 13-acetate; ROS, reactive oxygen species; siRNA, small interfering RNA; TACE, TNF-α-converting enzyme; TIP, TACE inhibitory peptide.
References
- 1.Nadel, J. A. (2002) in Chronic Obstructive Lung Diseases, eds. Voelkel, N. F. & MacNee, W. (BC Decker, Hamilton, ON, Canada), pp. 161-174.
- 2.Shao, M. X. G., Ueki, I. F. & Nadel, J. A. (2003) Proc. Natl. Acad. Sci. USA 100, 11618-11623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeyama, K., Dabbagh, K., Lee, H. M., Agusti, C., Lausier, J. A., Ueki, I. F., Grattan, K. M. & Nadel, J. A. (1999) Proc. Natl. Acad. Sci. USA 96, 3081-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao, M. X. G., Nakanaga, T. & Nadel, J. A. (2004) Am. J. Physiol. 287, L420-L427. [DOI] [PubMed] [Google Scholar]
- 5.Kinnula, V. L., Adler, K. B., Ackley, N. J. & Crapo, J. D. (1992) Am. J. Physiol. 262, L708-L712. [DOI] [PubMed] [Google Scholar]
- 6.Arnold, R. S., Shi, J., Murad, E., Whalen, A. M., Sun, C. Q., Polavarapu, R., Parthasarathy, S., Petros, J. A. & Lambeth, J. D. (2001) Proc. Natl. Acad. Sci. USA 98, 5550-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babior, B. M. (1995) Curr. Opin. Hematol. 2, 55-60. [DOI] [PubMed] [Google Scholar]
- 8.Segal, A. W. & Shatwell, K. P. (1997) Ann. N.Y. Acad. Sci. 832, 215-222. [DOI] [PubMed] [Google Scholar]
- 9.De Deken, X., Wang, D., Many, M. C., Costagliola, S., Libert, F., Vassart, G., Dumont, J. E. & Miot, F. (2000) J. Biol. Chem. 275, 23227-23233. [DOI] [PubMed] [Google Scholar]
- 10.Geiszt, M., Witta, J., Baffi, J., Lekstrom, K. & Leto, T. L. (2003) FASEB J. 17, 1502-1504. [DOI] [PubMed] [Google Scholar]
- 11.Vandivier, R. W., Fadok, V. A., Hoffmann, P. R., Bratton, D. L., Penvari, C., Brown, K. K., Brain, J. D., Accurso, F. J. & Henson, P. M. (2002) J. Clin. Invest. 109, 661-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fahy, J. V., Kim, K. W., Liu, J. & Boushey, H. A. (1995) J. Allergy Clin. Immunol. 95, 843-852. [DOI] [PubMed] [Google Scholar]
- 13.Kohri, K., Ueki, I. F. & Nadel, J. A. (2002) Am. J. Physiol. 283, L531-L540. [DOI] [PubMed] [Google Scholar]
- 14.Clark, R. A., Volpp, B. D., Leidal, K. G. & Nauseef, W. M. (1990) J. Clin. Invest. 85, 714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roghani, M., Becherer, J. D., Moss, M. L., Atherton, R. E., Erdjument-Bromage, H., Arribas, J., Blackburn, R. K., Weskamp, G., Tempst, P. & Blobel, C. P. (1999) J. Biol. Chem. 274, 3531-3540. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, Z., Oliver, P., Lancaster, J. J., Schwarzenberger, P. O., Joshi, M. S., Cork, J. & Kolls, J. K. (2001) FASEB J. 15, 303-305. [DOI] [PubMed] [Google Scholar]
- 17.Vignais, P. V. (2002) Cell. Mol. Life Sci. 59, 1428-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stolk, J., Hiltermann, T. J., Dijkman, J. H. & Verhoeven, A. J. (1994) Am. J. Respir. Cell Mol. Biol. 11, 95-102. [DOI] [PubMed] [Google Scholar]
- 19.Black, R. A., Rauch, C. T., Kozlosky, C. J., Peschon, J. J., Slack, J. L., Wolfson, M. F., Castner, B. J., Stocking, K. L., Reddy, P., Srinivasan, S., et al. (1997) Nature 385, 729-733. [DOI] [PubMed] [Google Scholar]
- 20.Schlondorff, J., Becherer, J. D. & Blobel, C. P. (2000) Biochem. J. 347, 131-138. [PMC free article] [PubMed] [Google Scholar]
- 21.Doedens, J. R. & Black, R. A. (2000) J. Biol. Chem. 275, 14598-14607. [DOI] [PubMed] [Google Scholar]
- 22.Milla, M. E., Leesnitzer, M. A., Moss, M. L., Clay, W. C., Carter, H. L., Miller, A. B., Su, J. L., Lambert, M. H., Willard, D. H., Sheeley, D. M., et al. (1999) J. Biol. Chem. 274, 30563-30570. [DOI] [PubMed] [Google Scholar]
- 23.Royer-Pokora, B., Kunkel, L. M., Monaco, A. P., Goff, S. C., Newburger, P. E., Baehner, R. L., Cole, F. S., Curnutte, J. T. & Orkin, S. H. (1986) Nature 322, 32-38. [DOI] [PubMed] [Google Scholar]
- 24.Babior, B. M. (2002) Isr. Med. Assoc. J. 4, 1023-1024. [PubMed] [Google Scholar]
- 25.Suh, Y. A., Arnold, R. S., Lassegue, B., Shi, J., Xu, X., Sorescu, D., Chung, A. B., Griendling, K. K. & Lambeth, J. D. (1999) Nature 401, 79-82. [DOI] [PubMed] [Google Scholar]
- 26.Cheng, G., Cao, Z., Xu, X., van Meir, E. G. & Lambeth, J. D. (2001) Gene 269, 131-140. [DOI] [PubMed] [Google Scholar]
- 27.Fischer, B. M. & Voynow, J. A. (2002) Am. J. Respir. Cell Mol. Biol. 26, 447-452. [DOI] [PubMed] [Google Scholar]
- 28.Aoshiba, K., Yasuda, K., Yasui, S., Tamaoki, J. & Nagai, A. (2001) Am. J. Physiol. 281, L556-L564. [DOI] [PubMed] [Google Scholar]