Abstract
Heterocellular communication between different cell types of the vasculature, both within the blood vessel wall and cells interacting with the blood vessel wall, is absolutely vital and must be tightly regulated. In this Forum, the role of four different gaseous transmitters [nitric oxide [NO], carbon monoxide (CO), hydrogen sulfide (H2S), and superoxide (O2•−)] is examined by four different research groups in detail, with two original articles and two reviews of the literature. In this editorial, we discuss how each of them may contribute their own component to heterocellular signaling in the vasculature. Antioxid. Redox Signal. 26, 881–885.
Keywords: : heterocellular, gaseous transmitters, endothelium, red blood cells, macrophage
As the primary transport system of the body, the vasculature is responsible for directing the flow of blood and its contents among a multitude of tissues. Intricate heterocellular communication pathways regulate the interactions between vascular cells, whether within the vascular wall, between blood cells and the vascular wall, or among blood cells. This communication facilitates the diverse and complex processes that take place in the vasculature.
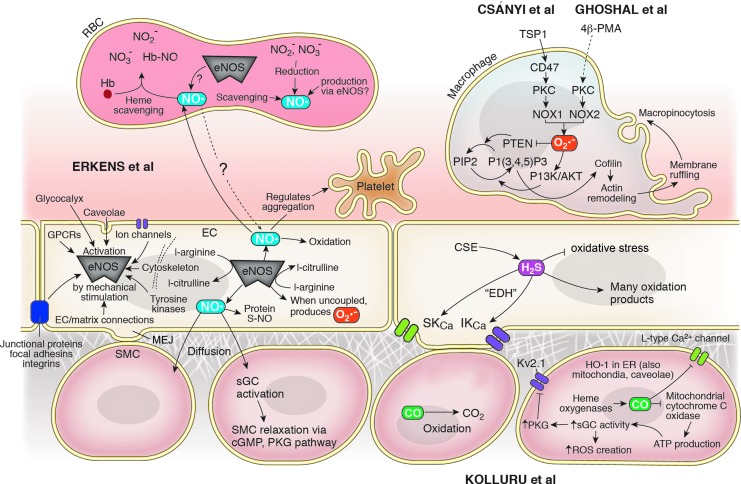
Four articles in this Forum explore the role of gasotransmitter molecules in our evolving understanding of heterocellular signaling: two reviews examine the roles of the gasotransmitters nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S) in a variety of signaling contexts, while two original research articles examine the role of superoxide (O2•−) in the initiation of macrophage macropinocytosis. Figure 1 shows some of the heterocellular signaling pathways in which these gasotransmitters participate.
FIG. 1.
Gasotransmitters as messengers in vascular heterocellular signaling pathways. Endogenous H2S, CO, and NO are all produced enzymatically in the vascular wall. H2S and CO modulate the function of various ion channels and regulate ROS availability, whereas eNOS participates in a vasodilatory signaling cascade, regulates platelet aggregation, and facilitates the post-translational modification of a wide variety of proteins. Not only a byproduct of uncoupled eNOS, O2•− is also produced by NOX enzymes in macrophages as part of the signaling pathway by which actin remodeling initiates macropinocytosis. eNOS, endothelial nitric oxide synthase; ROS, reactive oxygen species.
In the new review “Gasotransmitter Heterocellular Signaling,” Kolluru et al. from Louisiana State University broadly review heterocellular signaling mechanisms involving NO, CO, and H2S, which they collectively term “gasotransmitters” (4). Among the smallest of any signaling molecules, these share characteristics of both gases and dissolved solutes, facilitating their roles in heterocellular communication.
Perhaps the most obvious function of gasotransmitters, being members of the reactive nitrogen species (RNS) and reactive sulfur species families, is modulation of cellular redox balances that drive the abundance of reactive oxygen species (ROS) and various free radical species. H2S serves as a reducing agent with weak antioxidant effects, protecting cells from redox stress both directly and via indirect routes such as increased glutathione. Conversely, endothelial nitric oxide synthase (eNOS), the enzyme that catalyzes production of NO in the endothelium, produces superoxide ions when uncoupled from nicotinamide adenine dinucleotide phosphate (NADPH) oxidation (4).
However, gasotransmitters also serve important roles in heterocellular communication throughout the vasculature. H2S recently gained attention when Tang et al. identified the gasotransmitter as an endothelium-derived hyperpolarization (EDH) factor. Tang et al. demonstrated that genetic deletion of cystathionine γ-lyase (CSE), an enzyme that catalyzes production of H2S, significantly decreased endothelium-dependent relaxation of phenylephrine-preconstricted resistance mesenteric arteries (MAs), but not aortas, in response to treatment with methacholine, a nonselective muscarinic receptor agonist (6). Furthermore, application of the H2S donor NaHS produced a similarly dose-dependent endothelium-dependent relaxation of wild-type MAs.
NaHS application also produced dose-dependent hyperpolarization of wild-type MA smooth muscle cells, as did application of l-cysteine, the substrate of CSE-catalyzed H2S production. Tang et al. further clarified endogenous CSE as the relevant source of H2S by showing that dl-propargylglycine, a CSE inhibitor, caused smooth muscle cell (SMC) depolarization in wild-type MAs but not in CSE-knockout MAs. Finally, inhibition of methacholine-induced endothelial-dependent wild-type MA SMC hyperpolarization by charybdotoxin and apamin, which block IKCa and SKCa confirmed the involvement of these potassium channels downstream of H2S in the EDH pathway.
Interestingly, charybdotoxin and apamin-sensitive hyperpolarization in response to both of these H2S-inducing stimulations was found to be stronger in female wild-type MA SMCs versus SMCs from equivalent male vessels. Beyond its direct effects on these channels, H2S was also found to enhance the expression of SK2.3 but not IK3.1 channels, via an unknown mechanism (6).
The vascular role of H2S as an EDH is an important addition to our knowledge about the gas as a signaling molecule, reframing the dual identity of this chemical. Although endogenous production of H2S is well established, until recently it has been known primarily as a highly poisonous gas and pollutant in the environment. Kolluru et al. elaborate on the current and rapidly expanding knowledge of H2S, from the enzymatic pathways controlling its production in different tissue beds to the chemical interactions governing its nonenzymatic production and the balance between different related species. The overall picture is one of exquisite chemical complexity, with 10 oxidation products of H2S alone, in addition to its many chemical interactions with biomolecules such as protein sulfhydration (4).
CO carries a dual identity similar to H2S, known most predominantly as a poisonous component of smoke, yet it too is an important and endogenously produced signaling molecule, as Kolluru et al. review in detail. Produced by three heme oxygenase enzymes and oxidized to the ubiquitous CO2, CO interacts with iron-carrying molecules including hemoglobin and myoglobin and disrupts their normal interactions with O2. However, Kolluru et al. note additional and potentially important functions of CO. Beyond the poisonous mechanisms for which it is chiefly known, CO is also capable of inhibiting mitochondrial cytochrome c oxidase to reduce adenosine triphosphate (ATP) production and inducing soluble guanylate cyclase activity, blunting ROS creation by NADPH oxidases. Given increasing interest in the vascular roles of CO and H2S, the yearly article counts for both have increased steadily over the past two decades (4).
The third gasotransmitter discussed by Kolluru et al. is no newcomer to vascular biology discussions. Although NO was indeed also first known as an endogenous pollutant, it is now well established as an endogenous gasotransmitter with important canonical functions in the vasculature. The best-understood sources of NO are three nitric oxide synthase (NOS) enzymes, although it can also be produced via nonenzymatic redox processes. Redox pathways also facilitate the conversion of NO into NO2− and NO3−, among other oxidation products. In vascular physiology, the creation of NO in endothelial cells (ECs) via the eNOS-catalyzed conversion of l-arginine to l-citrulline and the subsequent diffusion of this NO into adjacent SMCs facilitate a key vasodilatory pathway.
The binding of NO to heme on soluble guanylyl cyclase (sGC) in SMCs activates sGC production of cyclic guanosine monophosphate and promotes smooth muscle relaxation. Protein S-nitrosylation further allows NO to exert a broad array of effects on the myriad signaling pathways controlled by post-translational modification of participating proteins. A prominent example noted by Kolluru et al. is S-nitrosylation of cysteine 271 on connixin 43, which activates the opening of connexin 43 gap junctions expressed at myoendethelial junctions. Thus, NO also facilitates the diffusive exchange of signaling molecules between, and electrical connectivity of, adjacent ECs and SMCs. The full range of proteins modified by S-nitrosylation and these subsequent indirect impacts of NO bioavailability are still being discovered (4).
Beyond the individual functions of each gasotransmitter, Kolluru et al. stress the interconnectivity of H2S, CO, and NO with each other. NO and H2S in particular are believed to synergistically facilitate vasodilation, as the two can react to form additional chemical species with vasodilatory activity. In addition, NO is believed to be a necessary component of the cardioprotective effects of H2S therapy, and the two appear to share further cooperative homeostatic functions. Conversely, CO is a regulator of H2S production, and H2S produced in carotid body glomus cells contributes to ventilation control mechanisms. The complicated interactions between gasotransmitters further underlie the extent to which they exert control over diverse and important cardiovascular functions.
In another review in this Forum, “Modulation of local and systemic heterocellular communication by mechanical forces: a role of eNOS,” Erkens et al. from the Heinrich Heine University of Dusseldorf in Germany discuss the role of eNOS in heterocellular communication in response to mechanical forces. NO is not only an important gasotransmitter in vasodilatory pathways but also regulates platelet aggregation and a variety of other heterocellular communication processes. Erkens et al. draw particular attention to mechanical regulation of NO production in the endothelium, where the dynamics of blood flow in the lumen is known to influence physiological responses. Intracellular signal transduction pathways activated by mechanosensors in the membrane and cytoskeleton of ECs lead to broader heterocellular communication upon activation of eNOS and production of NO. Not only can NO diffuse across membranes to signal in paracrine manner, but it can also interconvert between NO and longer-lived metabolites such as nitrite (NO2−) and nitrate (NO3−).
The mechanisms of mechanosensation in ECs are diverse, encompassing the glycocalyx surrounding ECs; the caveolae, ion channels, and G protein-coupled receptors on the surface of ECs; the junctional complex proteins, focal adhesions, and integrins involved in EC–EC junctions and EC–extracellular matrix connections; and the cytoskeleton and cytoplasmic tyrosine kinases intracellularly. Erkens et al. stress the importance of this complexity, as it facilitates precise homeostatic regulation to maintain appropriate vascular function, and highlight the need to identify the “common control nodes” that regulate different regulatory mechanisms (2).
Heterocellular communication via NO is one such node in EC mechanotransduction pathways, resulting in communication between ECs and the lumen as well as between ECs and SMCs. EC-derived NO interacts with both blood cells and plasma. By activating sGC and thus protein kinase G, NO inhibits platelet activation and helps maintain hemostasis. Conversely, red blood cells (RBCs) scavenge EC-derived NO, resulting in a reduction in the half-life of NO in the lumen by five orders of magnitude in whole blood compared with plasma alone.
Hemoglobin (Hb) oxidation of NO in RBCs produces NO3−, which, although once viewed only as a sink for RNS, is gaining new consideration as a reservoir for long-distance storage and potential rebioactivation (2). Conversely, in hypoxic conditions, deoxygenated Hb is now believed to catalyze the conversion of NO2− to NO, complicating the effects of Hb on RNS redox state based on O2 saturation in different locations in the vasculature. RBCs also appear to produce NO in normoxic conditions via eNOS, although the ultimate impact of RBC-derived NO in heterocellular communication requires more study given the complex details of NO production and scavenging in the lumen. Proposed as a regulator of RBC deformability, it is possible that NO could also play a role in mechanical pathways in these blood cells as well as in the vessel wall.
Two original research articles from Pagano and Csányi at the University of Pittsburgh and Medical College of Georgia demonstrate a novel role for superoxide (O2•−) in macropinocytosis, the process by which the plasma membrane ruffles and folds to internalize large vesicles filled with fluid from the extracellular environment.
Macropinocytosis is a clathrin-independent endocytic pathway distinguished both by its route of vesicle construction—actin cytoskeletal reorganization that folds lamellipodia into themselves to create large, heterogeneous vesicles—and by the size of these vesicles, which range from 0.2 to 5 μM in diameter (5). Macropinosomes undergo endocytic vesicular maturation once internalized, although this subsequent process appears to be diverse based on cell type. Many contexts for macropinocytosis exist, as it facilitates a variety of cellular functions including chemitactic response and motility, antigen presentation by cells such as macrophages and dendritic cells, entry of pathogens into host cells, and a cellular uptake mechanism for drug delivery (5).
Although many details of this process remain poorly understood, Ghoshal et al. and Csányi et al. demonstrate the role of redox signaling in the regulation of cytoskeletal remodeling that initiates macropinocytosis via two original research articles in this Forum. “Nox2-Mediated PI3K and Cofilin Activation Confers Alternate Redox Control of Macrophage Pinocytosis” (3) identifies NADPH oxidase 2 (Nox2)-derived ROS as a regulator of macropinocytosis via activation of the phosphoinositide-3-kinase (PI3K)/Akt pathway and inhibition of phosphatase and tensin homolog (PTEN); simultaneously, “CD47 and Nox1 Mediate Dynamic Fluid-Phase Macropinocytosis of Native LDL” (1) details a novel signaling pathway in macrophages by which matrix protein thrombospondin-1 (TSP1) and CD47 stimulate oxidation-independent macropinocytic uptake of native low-density lipoprotein (LDL) in macrophages.
Reduced Nox2, the primary inducible Nox isoform in phagocytic cells, is compartmentalized to the intracellular and plasma membranes. Nox2 production of O2•− is substantial, to the point that phagocytes increase their oxygen consumption to obtain sufficient O2 for O2•− production. Given existing reports of macropinocytosis dependence upon signaling mechanisms known also to activate Nox2, Ghoshal et al. investigated whether Nox2 plays a role in the initiation of macropinocytosis in macrophages. Stimulation with the 4β stereoisomer of phorbol 12-myristate 13-acetate (4β-PMA), which activates Nox2 catalysis of O2•− generation, in combination with native LDL (nLDL) treatment, caused lipid accumulation.
Ghoshal et al. clarified macropinocytosis as the uptake route inhibiting this lipid uptake with a PI3K inhibitor, an actin perturbant, and an Na+/H+ blocker, all of which inhibit macropinocytosis, but finding no effect from inhibitors of clathrin- and caveolin-mediated endocytosis (3). They further clarified the role of Nox2 by observing decreased uptake of FITC-dextran in macrophages transfected with Nox2 siRNA versus scrambled siRNA. Noting a significant decrease in 4β-PMA-induced lipid internalization after polyethylene glycol-conjugated superoxide dismutase, a cell-permeable intracellular O2•−scavenger, but not in macrophages lacking CD36, a major extracellular O2•− scavenger receptor, Ghoshal et al. identified the intracellular pool O2•− as a likely signaling participant. 1,2-Dioctanoyl-sn-glycerol, a cell-permeable analog of diacylglycerol and activator of protein kinase C (PKC), was sufficient to stimulate FITC-dextran by macrophages, and could be blocked by the PKC inhibitor calphostin c (3).
Observing membrane ruffle formation via scanning electron microscopy (SEM), Ghoshal et al. demonstrated that the previously described pathway regulates this step of macrophage macropinocytosis initiation. They further showed that 4β-PMA caused phosphorylation of PTEN and Akt, suppressing the phosphatase activity of PTEN and stimulating the PI3K/Akt signaling pathway to activate cofilin, which facilitates actin network reconstruction to induce membrane ruffle formation. Cofilin was subsequently confirmed as a participant in the pathway via cofilin siRNA-driven attenuation of 4β-PMA-induced FITC-dextran uptake.
To demonstrate the importance of O2•− production to initiation of macropinocytosis in a physiologically relevant stimulation condition, stimulation of macropinocytosis by macrophage colony stimulating factor was inhibited by an Na+/H+ antiporter blocker, a cell-permeant antioxidant, and an oxidase inhibitor. Finally, adoptive transfer of Nox2y/− macrophages into an ApoE−/− mouse model demonstrated an attenuation of lipid uptake after 20 h in comparison with wild-type (WT) macrophages (3). These findings demonstrate the importance of Nox2 signaling via O2•− to activate the PI3K/Akt pathway, and in turn cofilin, to facilitate membrane ruffle formation and initialize macropinocytosis.
Csányi et al. build upon the findings detailed in Ghoshal et al. by examining the role of LDL oxidation in its macropinocytic uptake. Although oxidative modification of LDL in the subendothelial matrix is a popular explanation for its uptake by macrophages, this theory is not universally agreed upon. Csányi et al. proposed that TSP1 stimulates uptake of nLDL independently of scavenger receptor-mediated pathways, achieving lipid accumulation instead via macropinocytosis.
Treatment of macrophages with physiological concentrations of TSP1 resulted in the initiation of membrane ruffling within 30 min, as identified via SEM and differential interference contrast microscopy. TSP1 treatment furthermore resulted in uptake of fluorescent beads into the cytosol of macrophages, identified by confocal microscopy. SiRNA silencing of the clathrin heavy chain and caveolin 1 genes, as well as pharmacological inhibition of either pathway, both failed to disrupt TSP1-induced uptake of extracellular fluorophores, ruling out these endocytic pathways, nor did macrophages lacking the LDL receptor exhibit decreased uptake. However, a PI3K inhibitor, an Na+/H+ antiporter blocker, and an actin polymerization inhibitor all prevented TSP1-induced fluorescent bead uptake, implicating macropinocytosis as the internalization mechanism (1).
To distinguish between oxidized LDL uptake and nLDL uptake mechanisms, Csányi et al. demonstrated that TSP1 treatment does not change the expression of the enzymes responsible for cholesterol esterification and hydrolysis. Furthermore, TSP1 treatment had no effect on LDL electrophoretic mobility, showing that nLDL is not oxidized by the pathway in question. As in Ghoshal et al., Csányi et al. were able to inhibit TSP1-induced lipid uptake with calphostin c, the PKC inhibitor, suggesting a role for oxidase-mediated signaling. Nox1y/− macrophages showed no lipid uptake in response to TSP1 treatment, highlighting a specific role for this isoform.
In addition, Csányi et al. identified intracellular O2•− as the downstream signaling mechanism, as extracellular O2•− production was unchanged after TSP1 treatment of WT macrophages. By inhibiting CD47, a known receptor of TSP1 and activator of Nox1, with a blocking antibody, Csányi et al. prevented O2•− production, showing CD47 to be a key intermediate between TSP1 treatment and Nox1 activation. Accordingly, CD47−/− macrophages did not exhibit membrane ruffling or LDL accumulation in response to TSP1 treatment.6 These experiments thus highlight macropinocytic uptake of nLDL, independent of LDL oxidation, in response to TSP1 via a pathway, including CD47, PKC, and Nox1.
Ghoshal et al. and Csányi et al. offer two perspectives into the induction of macropinocytosis in macrophages via ROS-dependent mechanisms, providing an argument for the O2•− radical ion as a second messenger essential to the physiological response that enables solute uptake by macrophages in multiple contexts such as atherosclerosis and infectious diseases.
Taken in the context of the two reviews in this Forum that examine gasotransmitters in a broader context, it is clear that there is much still to learn about gasotransmitter-dependent signaling pathways in the vasculature. Canonical and novel roles for NO signaling, as well as an emerging understanding of the functions of other gasotransmitters, are poised to fill in many of the gaps in our current understanding of vascular heterocellular signaling pathways (Fig. 1). Future studies will need to explore both the novel functions of different gasotransmitters and their numerous metabolites, and the interactions between this interconnected family of signaling molecules.
Abbreviations Used
- CSE
cystathionine γ-lyase
- eNOS
endothelial nitric oxide synthase
- EC
endothelial cells
- EDH
endothelium-derived hyperpolarization
- LDL
low-density lipoprotein
- MAs
mesenteric arteries
- NADPH
nicotinamide adenine dinucleotide phosphate
- nLDL
native LDL
- NOS
nitric oxide synthase
- 4β-PMA
4β stereoisomer of phorbol 12-myristate 13-acetate
- PTEN
phosphatase and tensin homolog
- PI3K
phosphoinositide-3-kinase
- PKC
protein kinase C
- RBC
red blood cell
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SEM
scanning electron microscopy
- sGC
soluble guanylyl cyclase
- SMC
smooth muscle cell
- TSP1
thrombospondin-1
- WT
wild-type
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Csányi G, Feck DM, Ghoshal P, Singla B, Lin H, Nagarajan S, Meijles DN, Al Ghouleh I, Cantu-Medellin N, Kelley EE, Mateuszuk L, Isenberg JS, Watkins S, and Pagano PJ. CD47 and Nox1 mediate dynamic fluid-phase macropinocytosis of native LDL. Antioxid Redox Signal 26: 886–901, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erkens R, Suvorava T, Kramer CM, Diederich L, Kelm M, and Cortese-Krott MM. Modulation of local and systemic heterocellular communication by mechanical forces: a role of eNOS. Antioxid Redox Signal 26: 917–935, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghoshal P, Singla B, Lin H, Feck DM, Cantu-Medellin N, Kelley EE, Haigh S, Fulton D, and Csányi G. Nox2-mediated PI3K and cofilin activation confers alternate redox control of macrophage pinocytosis. Antioxid Redox Signal 26: 902–916, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolluru G, Yuan S, Shen X, and Kevil CG. Gasotransmitter heterocellular signaling. Antioxid Redox Signal 26: 936–960, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim JP, and Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol 89: 836–843, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Tang G, Yang G, Jiang B, Ju Y, Wu L, and Wang R. H(2)s is an endothelium-derived hyperpolarizing factor. Antioxid Redox Signal 19: 1634–1646, 2013 [DOI] [PubMed] [Google Scholar]