Abstract
Context:
Individuals with anterior cruciate ligament reconstruction (ACLR) have quadriceps dysfunction that contributes to physical disability and posttraumatic knee osteoarthritis. Quadriceps function in the ACLR limb is commonly evaluated relative to the contralateral uninjured limb. Bilateral quadriceps dysfunction is common in individuals with ACLR, potentially biasing these evaluations.
Objective:
To compare quadriceps function between individuals with ACLR and uninjured control participants.
Design:
Cross-sectional study.
Setting:
Research laboratory.
Patients or Other Participants:
Twenty individuals with unilateral ACLR (age = 21.1 ± 1.7 years, mass = 68.3 ± 14.9 kg, time since ACLR = 50.7 ± 21.3 months; females = 14; Tegner Score = 7.1 ± 0.3; 16 patellar tendon autografts, 3 hamstrings autografts, 1 allograft) matched to 20 control participants (age = 21.2 ± 1.2 years, mass = 67.9 ± 11.3 kg; females = 14; Tegner Score = 7.1 ± 0.4) on age, sex, body mass index, and Tegner Activity Scale.
Main Outcome Measure(s):
Maximal voluntary isometric knee extension was performed on an isokinetic dynamometer. Peak torque (PT), rate of torque development (RTD), electromyographic (EMG) amplitude, central activation ratio (CAR), and hamstrings EMG amplitude were assessed during maximal voluntary isometric knee extension and compared between groups using independent-samples t tests. Relationships between hamstrings co-activation and quadriceps function were assessed using Pearson correlations.
Results:
Participants with anterior cruciate ligament reconstruction displayed lesser quadriceps PT (1.86 ± 0.74 versus 2.56 ± 0.37 Nm/kg, P = .001), RTD (39.4 ± 18.7 versus 52.9 ± 16.4 Nm/s/kg, P = .03), EMG amplitude (0.25 ± 0.12 versus 0.37 ± 0.26 mV, P = .04), and CAR (83.3% ± 11.1% versus 93.7% ± 3.2%, P = .002) and greater hamstrings co-activation (27.2% ± 12.8% versus 14.3% ± 3.7%, P < .001) compared with control participants. Correlations were found between hamstrings co-activation and PT (r = −0.39, P = .007), RTD (r = −0.30, P = .03), and EMG amplitude (r = −0.30, P = .03).
Conclusions:
Individuals with ACLR possessed deficits in PT, RTD, and CAR compared with control participants. Peak torque is the net result of all agonist and antagonist activity, and lesser PT in individuals with ACLR is partially attributable to greater hamstrings co-activation.
Key Words: muscles, strength, arthrogenic muscle inhibition, electromyography
Key Points
Individuals with anterior cruciate ligament reconstruction had deficits in quadriceps function that may contribute to posttraumatic knee osteoarthritis.
Deficits in knee-extensor strength were partially attributable to elevated hamstrings co-activation after anterior cruciate ligament reconstruction.
Approximately 250 000 anterior cruciate ligament (ACL) injuries occur annually in the United States,1 which result in a substantial financial cost of up to $17 billion and a significant amount of lost time due to rehabilitation each year.2 Surgical procedures and rehabilitative efforts improve physical function after ACL reconstruction (ACLR), but many individuals report long-term disability.3 Importantly, individuals with ACLR are up to 5 times more likely to develop knee osteoarthritis, with 50% of patients developing knee osteoarthritis within 2 decades of their injury.3−6
Quadriceps dysfunction is common after ACLR7,8 due, in part, to arthrogenic muscle inhibition (AMI),9 which is caused by altered afferent input from pain, inflammation, swelling, and damaged mechanoreceptors. Additionally, AMI hinders voluntary quadriceps activation,10 which is commonly assessed using the central activation ratio (CAR). Deficits in quadriceps CAR persist after reconstruction and impair the efficacy of quadriceps-strengthening protocols during rehabilitation.11,12 Quadriceps muscle function is strongly related to athletic tasks such as vertical jump and predicts self-reported disability after ACLR.13 As such, proper quadriceps rehabilitation is essential to patients with ACL injury who wish to return to sport, avoid long-term disability, or both. Also, the quadriceps are important to attenuating impact forces during the early stance phase of gait,14 and quadriceps dysfunction may contribute to a high rate/impulsive loading15 that is linked to cartilage breakdown in animal models.16 Therefore, the quadriceps may play an important role in preserving joint health after ACLR.
A common criterion for successful rehabilitation is achieving 85% to 90% of the maximal strength of the contralateral limb.17 However, maximal quadriceps strength may not be a suitable indicator of physical performance in tasks that require rapid movements and neuromuscular control.18 First, the time required to generate the strength necessary for functional tasks is shorter (<200 milliseconds) than the time it takes to generate maximal force (>300 milliseconds).19 The ability to produce quick or explosive movements is often more important than muscle strength19 in sporting activities, and speed rather than magnitude is associated with self-reported physical function in individuals with ACLR.20 Therefore, the rate of torque development (RTD) is an important indicator of quadriceps function in individuals with ACLR21 and is associated with greater ground reaction forces and loading rates during walking gait.14 Importantly, RTD remains depressed after successful rehabilitation, despite a return to preinjury strength.21 Overall, few data are available on RTD in individuals with ACLR.
A common limitation among prior studies22,23 that may confound the interpretation of strength data is the use of the uninjured limb for comparison because quadriceps dysfunction is often observed in the uninjured limb.10 Therefore, the uninjured limb may not provide a suitable control, and an uninjured limb from a healthy individual may be more appropriate. Second, experimental factors such as joint position during strength assessments influence the severity of observed quadriceps dysfunction in individuals with ACLR. Quadriceps strength has frequently been assessed during maximal isometric knee extension in 90° of knee flexion.13,24,25 Recent evidence26 indicates that isometric strength at 90° of knee flexion may not reflect physical function in individuals with ACLR. Krishnan and Theuerkauf26 found a stronger association between quadriceps strength and physical function at 45° than at 90° of knee flexion. Similarly, Hall et al24 observed sagittal-plane knee alterations in gait at 90° of knee flexion during stair ascent in the absence of quadriceps strength deficits. Alternatively, Pietrosimone et al13 noted that isometric strength assessed at 90° of knee flexion predicted self-reported physical disability (International Knee Documentation Committee score27) in individuals with ACLR. Finally, the discrepancy in findings among studies could also be attributed to hamstrings co-activation, which was not assessed in the aforementioned studies.4,24,26 Heightened hamstrings co-activation is common in individuals with knee injuries28−30 and influences knee-extensor torque production. Greater hamstrings co-activation reduces the net knee-extensor torque as measured using dynamometry and may confound the interpretation of quadriceps dysfunction in individuals with ACLR.
The purpose of our study was to compare quadriceps function (isometric strength, RTD, electromyographic [EMG] amplitude, CAR) and hamstrings co-activation between individuals with unilateral ACLR and uninjured control participants matched for sex, age, body mass index, and Tegner31 Activity Scale score. We hypothesized that individuals with ACLR would have deficits in outcomes related to quadriceps function and greater hamstrings co-activation compared with uninjured control participants. A secondary aim was to examine the association between hamstrings co-activation and quadriceps function in individuals with ACLR. We hypothesized that hamstrings co-activation and quadriceps function would have a negative relationship.
METHODS
Experimental Design
The data reported here are from a larger investigation that examined the effects of vibratory stimuli on neuromuscular function in uninjured participants and individuals with ACLR. Several indices of neuromuscular function (quadriceps function, spinal motoneuron excitability, and corticomotor excitability) and self-report surveys (eg, Tegner Activity Scale) were evaluated in 3 testing sessions separated by 7 days in a random order. Only quadriceps function data (isometric strength, EMG amplitude, RTD, and CAR) are reported here, and these data were obtained during a single session.
Participants
Twenty individuals with unilateral ACLR and 20 control participants were recruited for this study (Table 1). Participants were required to be recreationally active and between the ages of 18 and 30 years old but were excluded if they had a history of musculoskeletal injury within 6 months of testing, lower extremity surgery (other than unilateral ACLR; those with ACL reinjury or revision surgery were also excluded), neurologic disorder, cardiovascular disease, hypertension, diabetes mellitus, concussion or head injury, stroke, epilepsy, peripheral neuropathy, migraine headaches, cranial nerve surgery, cancer in the brain or thigh musculature, cardiac pacemaker, implanted foreign metal object, or diagnosed psychiatric disorder. Injured individuals had undergone unilateral ACLR and were cleared by a physician for participation in physical activity. Uninjured participants were matched to individuals with ACLR on sex, age (±1 year), body mass index (±1 kg/m2), and Tegner Activity Scale score21 (±1). The university's institutional review board approved the study, and all participants provided written informed consent before data collection.
Table 1. .
Demographic Values
| Group |
||
| Demographic |
Injured (n = 20) |
Uninjured (n = 20) |
| Men/women | 6/14 | 6/14 |
| Graft type | 16 Patellar tendon autografts, 3 hamstrings autografts, 1 allograft |
Not applicable |
| Mean ± SD |
||
| Age, y | 21.1 ± 1.7 | 21.2 ± 1.1 |
| Height, cm | 168.4 ± 9.5 | 168.0 ± 9.1 |
| Mass, kg | 68.3 ± 14.9 | 67.9 ± 11.3 |
| Body mass index, kg/m2 | 23.8 ± 3.0 | 23.3 ± 2.3 |
| Tegner Activity Scale score | 7.1 ± 0.3 | 7.2 ± 0.4 |
| Time since anterior cruciate ligament reconstruction, mo | 50.7 ± 21.3 | Not applicable |
Quadriceps Function Assessments
Maximal voluntary isometric contraction (MVIC) knee extension was used to assess quadriceps strength, RTD, EMG amplitude, and CAR. Participants completed a 5-minute warmup on a cycle ergometer at a self-selected pace, followed by a series of submaximal quadriceps contractions to reduce the chance of injury. Participants were seated in an isokinetic dynamometer (model HUMAC NORM; Computer Sports Medicine Inc, Stoughton, MA) with straps secured over the torso, thigh, and leg to isolate knee extension. The knee was positioned in 60° of flexion, and the lever arm was adjusted so that the ankle strap was 2 fingerwidths above the medial malleolus (Figure 1). Injured limbs were assessed in individuals with ACLR, and the dominant limb (defined as the limb used to kick a ball) was assessed in uninjured individuals.32 A prior investigation33 has shown that CAR does not differ between limbs in healthy individuals.
Figure 1. .
Participant position for maximal voluntary isometric contraction.
Participants performed knee-flexion and -extension MVICs during which they were instructed to flex or extend the knee maximally “as fast and as hard” as possible against the dynamometer. Three trials were conducted for each muscle group (quadriceps and hamstrings) with 60 seconds' rest between trials, and the highest peak torque (PT) value was selected for analysis. Verbal encouragement was provided during all trials. Central activation ratio was assessed during the knee-extension MVICs described earlier. After plateau of the torque signal, a brief electrical stimulus (10-pulse train, 600-μs duration, 100 Hz, 125 V) was delivered via 2 adhesive electrodes (model Dura-Stick; Chattanooga Group, Hixon, TN) placed on the proximal and distal anterior thigh over the quadriceps via an isolated stimulator (model SK48; Grass Telefactor, Warwick, RI; Figure 2). The electrical stimulus was used to activate motor units that the participants were not able to voluntarily activate. Central activation ratio was calculated as the ratio of peak voluntary torque to the PT resulting from the electrical stimulus (Figure 3), and the maximal value of 3 trials was used for analysis.
Figure 2. .
Electrode placement for measurement of central activation ratio.
Figure 3. .
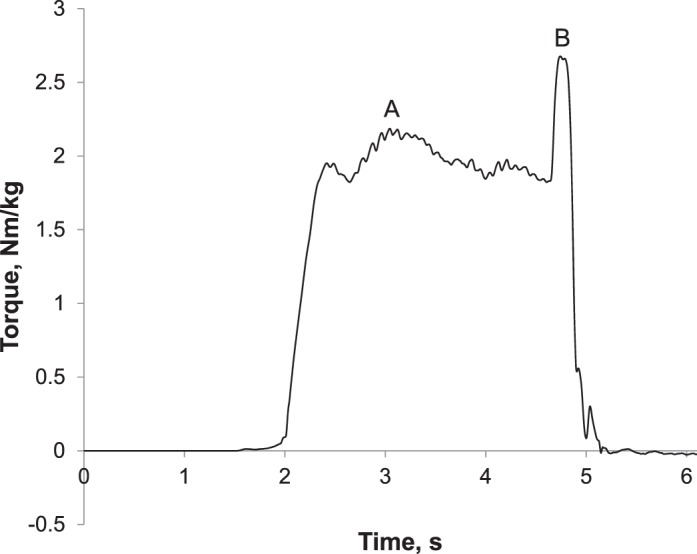
Central activation ratio calculation. A represents voluntary peak torque, and B represents additional torque produced by the electrical stimulus (central activation ratio = A/B × 100).
Quadriceps and hamstrings EMG amplitudes were assessed during MVICs using surface EMG sampled at 2 KHz (model MP150WSW and model EMG100C amplifiers; BIOPAC Systems, Inc, Goleta, CA; input impedance = 1.0 MΩ; common mode rejection ratio = 110-dB minimum, gain = 10 000) with electrodes (model EL503, Ag/AgCl contact 11-mm diameter; BIOPAC Systems, Inc) placed over the vastus medialis, vastus lateralis, rectus femoris, biceps femoris, and semitendinosus according to Surface Electromyography for the Noninvasive Assessment of Muscles guidelines (www.seniam.org). The raw EMG signal was corrected for direct current bias, and band-pass (20−350 Hz) and notch (59.5−60.5 Hz) filtered (fourth–order, zero-phase–lag Butterworth filter). The filtered data were smoothed using a 20-millisecond root mean square sliding-window function. Maximal EMG amplitude (millivolts) was calculated as the largest 1-second moving average of the processed signal and averaged across the muscles to create composite values for the quadriceps and hamstrings. The largest 1-second moving average of hamstrings EMG during the knee-extension MVIC was normalized to the maximal EMG value during knee-flexion MVICs to assess hamstrings co-activation (%MVIC).
Torque data were collected at 2 KHz and low-pass filtered at 50 Hz (fourth order Butterworth), and PT and RTD were calculated from the torque-time curve of the same trial. Peak torque was defined as the maximal voluntary torque value and was normalized to body mass for analysis (Nm/kg). Rate of torque development was calculated as the first derivative of the torque-time curve, and the peak value was identified and normalized to body mass for statistical analyses (Nm/s × kg−1).
Statistical Analyses
We inspected data for normality by evaluating the skewness and kurtosis statistics (ratio of statistic to standard error) and the Shapiro-Wilk test. Boxplots were used to identify outliers, defined as values that exceed 1.5 times the interquartile range away from the median. The association between hamstrings co-activation and quadriceps function (PT, RTD, CAR, and quadriceps EMG amplitude) was assessed using Pearson product moment correlations in the injured participants. Dependent variables (PT, RTD, CAR, quadriceps EMG amplitude, hamstrings co-activation) were compared between groups using independent-samples t tests. Additionally, 1-way analysis of covariance was used to compare quadriceps function between groups with hamstrings co-activation as a covariate. All hypotheses were developed a priori via SPSS software (version 23.0; IBM Corp, Armonk, NY), and the analyses were directional 1-tailed tests (α = .05).
RESULTS
No outliers were identified, all cases were included for further analyses, and all data were confirmed as being normally distributed. Individuals with ACLR had greater hamstrings co-activation compared with the control group (P < .001; Table 2). Greater hamstrings co-activation was associated with lesser quadriceps PT (r = −0.39, P = .007), RTD (r = −0.30, P = .03), CAR (r = −0.40, P = .005), and EMG amplitude (r = −0.30, P = .03) in individuals with ACLR.
Table 2. .
Quadriceps Function and Hamstrings Coactivation After Anterior Cruciate Ligament Reconstruction: Independent-Samples t Tests (Mean ± SD)
| Group |
||
| Dependent Variable |
Injured |
Uninjured |
| Peak torque, Nm/kga | 1.86 ± 0.74 | 2.56 ± 0.37 |
| Peak rate of torque development, Nm/s/kga | 39.4 ± 18.7 | 52.9 ± 16.4 |
| Central activation ratio, %a | 83.3 ± 11.1 | 93.7 ± 3.2 |
| Quadriceps electromyographic amplitude, mVa | 0.25 ± 0.12 | 0.37 ± 0.26 |
| Hamstrings co-activation, % maximal voluntary isometric contractiona | 27.2 ± 12.8 | 14.3 ± 3.7 |
P < .05 indicates the injured-group mean was different than the uninjured-group mean.
Individuals with ACLR had lesser quadriceps PT (P < .001), RTD (P = .03), EMG amplitude (P = .049), and CAR (P = .001) than the control group when assessed using independent-samples t tests (Table 2). However, only PT (P = .001) and CAR (P = .003) were greater in the control group compared with the ACLR group after covarying for hamstrings co-activation (Table 3). No difference was observed in quadriceps EMG amplitude (P = .21) or RTD (P = .06) between groups after covarying for hamstrings co-activation.
Table 3. .
Analysis of Covariance (Least Squares Mean, [95% Confidence Interval])
| Dependent Variable |
Injured |
Uninjured |
| Peak torque, Nm/kga | 1.91 (1.61, 2.20) | 2.50 (2.22, 2.80) |
| Peak rate of torque development, Nm/s/kg | 40.9 (32.1, 49.7) | 51.4 (42.6, 60.2) |
| Central activation ratio, %a | 84.1 (80.0, 88.1) | 93.0 (88.9, 97.0) |
| Quadriceps electromyographic amplitude, mV | 0.28 (0.18, 0.38) | 0.34 (0.24, 0.44) |
P < .05.
DISCUSSION
Our main findings were that quadriceps PT, RTD, EMG amplitude, and CAR were lesser in individuals with ACLR than in control participants, whereas hamstrings co-activation during maximal knee extension was greater in those with ACLR compared with controls. However, only PT and CAR differed between groups after controlling for hamstrings co-activation. Therefore, reductions in knee-extensor function in individuals with ACLR are likely due to a combination of quadriceps dysfunction and additional hamstrings co-activation. These results provide further evidence of persistent quadriceps dysfunction34 in individuals with ACLR who have returned to unrestricted physical activity (at a mean of 51 months since ACLR). As such, current rehabilitation strategies appear to be ineffective for improving quadriceps function.11,12
The findings support our first hypothesis that individuals with ACLR would have deficits associated with quadriceps function compared with control participants. These values are in agreement with those of previous researchers10,13,22,23 who identified quadriceps dysfunction in individuals with ACLR. However, prior authors18,19 have commonly compared quadriceps function between the injured and uninjured limbs of individuals with ACLR. A bilateral comparison ignores the likelihood that individuals with ACLR have bilateral deficits in quadriceps function.22 Furthermore, a strength of our study was that the control group was matched on age, sex, body mass index, and Tegner Activity Scale score.31 In a previous study35 that reported quadriceps dysfunction (ie, RTD) in individuals with ACLR compared with control participants, sample size and sex were imbalanced. Given the disparity in ACL injury prevalence between males and females,36 it is important that study samples exhibit similar demographic characteristics.
Quadriceps dysfunction has been implicated as a contributor to self-reported physical disability after ACLR.13 Furthermore, RTD is especially important to athletic tasks and physical function19 and is associated with gait biomechanics that are linked to cartilage degradation in individuals with ACLR.14 Although RTD did not differ between ACLR and control participants after covarying for hamstrings co-activation, we treat this result with caution because the effect size was moderate (d = 0.61). It is likely that individuals with ACLR have deficits in RTD, yet our research may have been underpowered (observed power = 0.48) to identify a statistical difference. The ability to rapidly develop submaximal force is essential for attenuating loading immediately after heel contact during gait. In a recent investigation,14 quadriceps RTD obtained from an MVIC was associated with vertical ground reaction force magnitude and loading rate during walking gait. Overall, RTD should be considered an important target for rehabilitation programs.
We did not observe a group difference in quadriceps EMG amplitude despite lesser PT and CAR in the injured group after covarying for hamstrings co-activation. Although the results were not statistically different, we found a moderate effect size (d = 0.63, observed power = 0.49) between means, suggesting that further study with a larger sample may identify differences in quadriceps EMG amplitude between groups. An additional 11 participants would be required to achieve a power of 0.8. This was further evidenced by an exploratory evaluation of the 95% confidence interval (CI) of the mean difference (Δ = 0.11, 95% CI = −0.02, 0.24) between injured and uninjured participants. Deficits in quadriceps activation are further indicated by lesser CAR, which provides an estimation of overall motor-unit recruitment and firing frequency.37 Healthy individuals typically have a quadriceps CAR of 95% or greater38; our injured cohort's value (83%) was substantially lower than this threshold. We do take note that our healthy cohort's value was slightly below 95%. However, this is within the range of previously reported values (86%−99%) for healthy individuals39–41 and also could have been influenced by our methods. We conducted the MVIC in 60° of knee flexion, and the previously reported normative value of 95% was obtained during an MVIC in 90° of knee flexion. Finally, it should be noted that the injured group had a relatively large standard deviation for CAR (11.1%). Therefore, not all injured individuals exhibited a lower CAR than control participants, and other factors such as time since reconstruction may influence the magnitude of quadriceps dysfunction.
Quadriceps EMG amplitude is a contributor to knee-extensor torque, whereas PT is the net result of all agonist and antagonist activity. We observed greater hamstrings co-activation during knee-extension MVIC in individuals with ACLR compared with matched control participants. Therefore, we conclude that a combination of lesser quadriceps activity and greater hamstrings co-activation contributed to lower knee-extension torque produced by individuals with ACLR, as evidenced by the relationship between hamstrings co-activation and PT. Greater hamstrings co-activation is common among individuals with ACLR and is a protective response to limit anterior tibial translation and increase joint stability.28,42 Our findings suggest that deficits in quadriceps activation commonly observed in individuals with ACLR could be due in part to reciprocal inhibition from heightened hamstrings co-activation. The negative associations between hamstrings co-activation and quadriceps function and lack of group differences between quadriceps RTD and EMG amplitude after covarying for hamstrings co-activation suggest reciprocal inhibition. Our findings support our hypothesis and agree with those of previous researchers who examined hamstrings activity during muscle contractions28 and functional tasks such as gait30 in individuals with ACLR. However, our results differed from those of Krishnan and Williams,43 who found no difference in hamstrings co-activation between injured and uninjured individuals. Yet Krishnan and Williams43 evaluated hamstrings co-activation at 90° of knee flexion and noted that their results were specific to joint angle. Furthermore, they did not report the graft types in their sample, which may have influenced their findings. Future investigators and clinicians should consider the role of hamstrings co-activation when interpreting knee-extensor torque in individuals with knee injuries.
There are limitations to consider when interpreting the results of this study. First, the cross-sectional study design precludes us from determining the influence of quadriceps function and hamstrings co-activation on long-term joint health. Second, we included injured participants with all graft types, and graft type may influence the location and magnitude of muscle dysfunction. However, this factor is unlikely to have influenced our results, as 80% of our sample (16/20) received the same type of graft (patellar tendon). Future studies are needed to evaluate the physiological mechanisms that may cause persistent knee-extensor weakness and compare quadriceps function and hamstrings co-activation in individuals with different graft types. Third, injured participants in this study were treated by multiple surgeons and may have had varying rehabilitation programs. It is unclear if deficits in quadriceps function are attributable to their rehabilitation programs or chronic AMI. The injured cohort also demonstrated wide variability in time since ACLR (50.3 ± 21.3 months), which may have influenced the level of quadriceps dysfunction. Finally, we assessed isometric muscle function in only a single testing position, which may not specifically mimic the modes of contraction performed during functional and athletic tasks.
CONCLUSIONS
Our results indicate that compared with uninjured participants, individuals with ACLR demonstrated deficits in quadriceps function (PT, RTD, and CAR) more than 4 years after ACLR. Although quadriceps EMG amplitude is a contributor to knee-extensor torque, PT is the net result of all agonist and antagonist activity. Therefore, lesser knee-extensor torque in individuals with ACLR can also be attributed to greater hamstrings co-activation, which should be considered when researchers and clinicians interpret deficits in knee-extensor torque.
REFERENCES
- 1. Griffin LY, Albohm MJ, Arendt EA, et al. . Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006; 34 9: 1512– 1532. [DOI] [PubMed] [Google Scholar]
- 2. Mather RC 3rd, Koenig L, Kocher MS, et al. . Societal and economic impact of anterior cruciate ligament tears. J Bone Joint Surg Am. 2013; 95 19: 1751– 1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oiestad BE, Holm I, Aune AK, et al. . Knee function and prevalence of knee osteoarthritis after anterior cruciate ligament reconstruction: a prospective study with 10 to 15 years of follow-up. Am J Sports Med. 2010; 38 11: 2201– 2210. [DOI] [PubMed] [Google Scholar]
- 4. Luc B, Gribble PA, Pietrosimone BG. . Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014; 49 6: 806– 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harris KP, Driban JB, Sitler MR, Cattano NM, Balasubramanian E. . Tibiofemoral osteoarthritis after surgical or nonsurgical treatment of anterior cruciate ligament rupture: a systematic review. J Athl Train. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lohmander LS, Ostenberg A, Englund M, Roos H. . High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004; 50 10: 3145– 3152. [DOI] [PubMed] [Google Scholar]
- 7. Palmieri-Smith RM, Thomas AC, Wojtys EM. . Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008; 27 3: 405– 424, vii–ix. [DOI] [PubMed] [Google Scholar]
- 8. Urbach D, Nebelung W, Becker R, Awiszus F. . Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris: a prospective twitch interpolation study. J Bone Joint Surg Br. 2001; 83 8: 1104– 1110. [DOI] [PubMed] [Google Scholar]
- 9. Palmieri-Smith RM, Thomas AC. . A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009; 37 3: 147– 153. [DOI] [PubMed] [Google Scholar]
- 10. Hart JM, Turman KA, Diduch DR, Hart JA, Miller MD. . Quadriceps muscle activation and radiographic osteoarthritis following ACL revision. Knee Surg Sports Traumatol Arthrosc. 2011; 19 4: 634– 640. [DOI] [PubMed] [Google Scholar]
- 11. Hopkins J, Ingersoll C. . Arthrogenic muscle inhibition: a limiting factor in joint rehabilitation. J Sport Rehabil. 2000; 9 2: 135– 159. [Google Scholar]
- 12. Hurley MV, Jones DW, Newham DJ. . Arthrogenic quadriceps inhibition and rehabilitation of patients with extensive traumatic knee injuries. Clin Sci (Lond). 1994; 86 3: 305– 310. [DOI] [PubMed] [Google Scholar]
- 13. Pietrosimone BG, Lepley AS, Ericksen HM, Gribble PA, Levine J. . Quadriceps strength and corticospinal excitability as predictors of disability after anterior cruciate ligament reconstruction. J Sport Rehabil. 2013; 22 1: 1– 6. [DOI] [PubMed] [Google Scholar]
- 14. Blackburn JT, Pietrosimone B, Harkey MS, Luc BA, Pamukoff DN. . Quadriceps function and gait kinetics after anterior cruciate ligament reconstruction. Med Sci Sports Exerc. 2016; 48 9: 1664– 1670. [DOI] [PubMed] [Google Scholar]
- 15. Mikesky AE, Meyer A, Thompson KL. . Relationship between quadriceps strength and rate of loading during gait in women. J Orthop Res. 2000; 18 2: 171– 175. [DOI] [PubMed] [Google Scholar]
- 16. Ewers BJ, Jayaraman VM, Banglmaier RF, Haut RC. . Rate of blunt impact loading affects changes in retropatellar cartilage and underlying bone in the rabbit patella. J Biomech. 2002; 35 6: 747– 755. [DOI] [PubMed] [Google Scholar]
- 17. Ardern CL, Webster KE, Taylor NF, Feller JA. . Return to sport following anterior cruciate ligament reconstruction surgery: a systematic review and meta-analysis of the state of play. Br J Sports Med. 2011; 45 7: 596– 606. [DOI] [PubMed] [Google Scholar]
- 18. Myer GD, Schmitt LC, Brent JL, et al. . Utilization of modified NFL combine testing to identify functional deficits in athletes following ACL reconstruction. J Orthop Sports Phys Ther. 2011; 41 6: 377– 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. . Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol (1985). 2002; 93 4: 1318– 1326. [DOI] [PubMed] [Google Scholar]
- 20. Hsieh CJ, Indelicato PA, Moser MW, Vandenborne K, Chmielewski TL. . Speed, not magnitude, of knee extensor torque production is associated with self-reported knee function early after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015; 23 11: 3214– 3220. [DOI] [PubMed] [Google Scholar]
- 21. Angelozzi M, Madama M, Corsica C, et al. . Rate of force development as an adjunctive outcome measure for return-to-sport decisions after anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther. 2012; 42 9: 772– 780. [DOI] [PubMed] [Google Scholar]
- 22. Kline PW, Morgan KD, Johnson DL, Ireland ML, Noehren B. . Impaired quadriceps rate of torque development and knee mechanics after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2015; 43 10: 2553– 2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knezevic OM, Mirkov DM, Kadija M, Nedeljkovic A, Jaric S. . Asymmetries in explosive strength following anterior cruciate ligament reconstruction. Knee. 2014; 21 6: 1039– 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hall M, Stevermer CA, Gillette JC. . Gait analysis post anterior cruciate ligament reconstruction: knee osteoarthritis perspective. Gait Posture. 2012; 36 1: 56– 60. [DOI] [PubMed] [Google Scholar]
- 25. Lewek M, Rudolph K, Axe M, Snyder-Mackler L. . The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech (Bristol, Avon). 2002; 17 1: 56– 63. [DOI] [PubMed] [Google Scholar]
- 26. Krishnan C, Theuerkauf P. . Effect of knee angle on quadriceps strength and activation after anterior cruciate ligament reconstruction. J Appl Physiol (1985). 2015; 119 3: 223– 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hefti F, Müller W, Jakob RP, Stäubli HU. . Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993; 1 3–4: 226– 234. [DOI] [PubMed] [Google Scholar]
- 28. Grabiner MD, Weiker GG. . Anterior cruciate ligament injury and hamstrings coactivation. Clin Biomech (Bristol, Avon). 1993; 8 4: 215– 219. [DOI] [PubMed] [Google Scholar]
- 29. Rudolph KS, Axe MJ, Buchanan TS, Scholz JP, Snyder-Mackler L. . Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg Sports Traumatol Arthrosc. 2001; 9 2: 62– 71. [DOI] [PubMed] [Google Scholar]
- 30. Hurd WJ, Snyder-Mackler L. . Knee instability after acute ACL rupture affects movement patterns during the mid-stance phase of gait. J Orthop Res. 2007; 25 10: 1369– 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tegner Y, Lysholm J. . Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985; 198: 43– 49. [PubMed] [Google Scholar]
- 32. Coren S, Porac C, Duncan P. . A behaviorally validated self-report inventory to assess four types of lateral preference. J Clin Neuropsychol. 1979; 1 1: 55– 64. [Google Scholar]
- 33. Pietrosimone BG, Park CM, Gribble PA, Pfile KR, Tevald MA. . Inter-limb differences in quadriceps strength and volitional activation. J Sports Sci. 2012; 30 5: 471– 477. [DOI] [PubMed] [Google Scholar]
- 34. Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. . Quadriceps activation following knee injuries: a systematic review. J Athl Train. 2010; 45 1: 87– 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jordan MJ, Aagaard P, Herzog W. . Rapid hamstrings/quadriceps strength in ACL-reconstructed elite Alpine ski racers. Med Sci Sports Exerc. 2015; 47 1: 109– 119. [DOI] [PubMed] [Google Scholar]
- 36. Gwinn DE, Wilckens JH, McDevitt ER, Ross G, Kao TC. . The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med. 2000; 28 1: 98– 102. [DOI] [PubMed] [Google Scholar]
- 37. Kent-Braun JA, Le Blanc R. . Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve. 1996; 19 7: 861– 869. [DOI] [PubMed] [Google Scholar]
- 38. Park J, Hopkins JT. . Quadriceps activation normative values and the affect of subcutaneous tissue thickness. J Electromyogr Kinesiol. 2011; 21 1: 136– 140. [DOI] [PubMed] [Google Scholar]
- 39. Luc BA, Harkey MH, Arguelles GD, Blackburn JT, Ryan ED, Pietrosimone B. . Measuring voluntary quadriceps activation: effect of visual feedback and stimulus delivery. J Electromyogr Kinesiol. 2016; 26: 73– 81. [DOI] [PubMed] [Google Scholar]
- 40. Lewek MD, Rudolph KS, Snyder-Mackler L. . Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res. 2004; 22 1: 110– 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuenze C, Hertel J, Saliba S, Diduch DR, Weltman A, Hart JM. . Clinical thresholds for quadriceps assessment after anterior cruciate ligament reconstruction. J Sport Rehabil. 2015; 24 1: 36– 46. [DOI] [PubMed] [Google Scholar]
- 42. Friemert B, Franke S, Gollhofer A, Claes L, Faist M. . Group I afferent pathway contributes to functional knee stability. J Neurophysiol. 2010; 103 2: 616– 622. [DOI] [PubMed] [Google Scholar]
- 43. Krishnan C, Williams GN. . Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res. 2011; 29 5: 633– 640. [DOI] [PMC free article] [PubMed] [Google Scholar]