Abstract
The introduction of digital pathology to nephrology provides a platform for the development of new methodologies and protocols for visual, morphometric and computer-aided assessment of renal biopsies. Application of digital imaging to pathology made substantial progress over the past decade; it is now in use for education, clinical trials and translational research. Digital pathology evolved as a valuable tool to generate comprehensive structural information in digital form, a key prerequisite for achieving precision pathology for computational biology. The application of this new technology on an international scale is driving novel methods for collaborations, providing unique opportunities but also challenges. Standardization of methods needs to be rigorously evaluated and applied at each step, from specimen processing to scanning, uploading into digital repositories, morphologic, morphometric and computer-aided assessment, data collection and analysis. In this review, we discuss the status and opportunities created by the application of digital imaging to precision nephropathology, and present a vision for the near future.
Keywords: glomerulosclerosis, kidney biopsy, minimal change disease, nephrotic syndrome, proteinuria
Virtual microscopy
The digitization of diagnostic imaging applications began with the development and implementation of digital radiology, replacing X-ray film with digital images. The adaptation of digitization to pathology first occurred when a tissue section on a glass slide, traditionally visualized by bright field or epifluorescent microscopy, was translated into a high-resolution image that could be visualized on a computer screen at a remote site. The remote visualization of these high-resolution images is termed digital pathology or virtual microscopy, encompassing telepathology and whole-slide imaging (WSI). Telepathology is the practice of pathology at a distance, using telecommunications technology to facilitate the transfer of image-rich pathology data between distant computers [1, 2]. WSI involves scanning tissue sections mounted on glass slides at high resolution, typically 20× or 40× resolution. While telepathology involves transmission of images at the time of acquisition, WSI requires an internet-accessible database to upload and store captured images. WSI enables the review of digital slides and metadata by multiple users simultaneously [3]. Although both can be used for education, diagnosis, clinical trials and research, digital pathology for primary clinical diagnosis it is not approved by the US Food and Drug Administration, yet. In Europe, some clinical pathology laboratories fully employ this digital approach [4, 5].
Digital imaging: technical notes
High-throughput, automated microscopes and imaging instruments are sufficiently advanced to produce images suitable for morphologic assessment. Most current instruments capture images with a 20× objective and with a resolution between 0.45 and 0.23 μm/pixel using an optical doubler. The use of a doubler limits the numerical aperture, narrowing the depth of field of the captured image. By this approach, images obtained from 4- to 5-μm thick tissue sections are sharpened sufficiently that they satisfy diagnostic needs without the need to obtain z-stack images. These instruments incorporate additional cameras to document the slide, all tissue on the slide, as well as the information on the label. Batch loading of slides, assortment into appropriate queues based on case ID and linkage to databases facilitates management of slides between the histology laboratory and the pathologist.
In the following paragraphs, we discuss the current application of digital pathology to renal biopsies, the importance of standardization and how digital imaging is evolving to slowly modify the practice of pathology.
Development of new strategies through industry and academia collaboration
As digital imaging technology becomes more sophisticated and financially feasible, medical centers have shown increasing interest in introducing digital pathology in their daily practice. Adoption of this new technology was facilitated by advances in biomedical research, an area that has expanded to encompass biomedical engineering, computer science, image analysis and clinical informatics. For example, the Academia and Industry collaboration for Digital PATHology (AIDPATH, http://aidpath.eu/) is an international effort focused on interoperability of digital pathology applications across multiple projects, institutions and disciplines (Table 1).
Table 1.
INTEGRATE sub-consortia goals and structure overview
| Study focus | Target diseases | Primary aims | Infrastructure | Standardized digital pathology protocol3,a | |
|---|---|---|---|---|---|
|
|
|
|
|
Retrieval from pathology archives of
|
|
Investigation of rare kidney diseases (especially SRNS) by using high-throughput technologies (NGS, urine proteomics, metabolomics) as well as assembling and expanding a number of pre-existent patient registries. | SRNS including:
|
Apply standardized comprehensive morphologic scoring to identify features for correlation with clinical presentation and outcome, genetic and molecular signatures (urine proteomic, metabolomic and miRNAomic profiling). |
|
|
|
|
|
|
|
|
| Ancillary organizations and committees |
|
International effort to facilitate interoperability of the digital pathology applications across multiple projects, institutions and disciplines. (Marie Curie Action by the European Union’s FP7 Framework Programme.) It fosters research and development in image display technologies, novel image analysis solutions for diagnosis and biomarker quantification in pathology. Major activities include networking, workshops, summer schools and conferences. | |||
| Advisory committee | Knowledgeable and prominent expert in their field with the goal of strengthening the programs by reviewing and evaluating mission, programs and studies, and improving relationships with other organizations. | ||||
NGS, next generation sequencing; MCD, minimal change disease; FSGS, focal segmental glomerulosclerosis; MN, membranous nephropathy; DPR, digital pathology repository; NS, nephrotic syndrome; SRNS, steroid-resistant nephrotic syndrome; MPGN, membranoproliferative glomerulonephropathy; IF, immunofluorescence; EM, electron microscopy.
The same protocol to establish and populate the DPR is applied by CureGN.
Pharmaceutical industry-sponsored clinical trials have worked with the digital pathology industry, applying previously underused software to create methodologies that improve reliability and reproducibility of data collection [6]. Compared with conventional light microscopy, digital nephropathology for clinical trials is advantageous in the following ways: (i) it provides a permanent dataset allowing full transparency for regulatory agencies, (ii) scoring protocols can be utilized by multiple users while reducing travel expenses and personnel time, (iii) it allows targeted adjudication and (iv) it improves analytic reproducibility. Overall, digital pathology is transforming clinical trials by introducing new standards of practice.
The importance of standardization
‘Implementing and developing technical standards can help maximize compatibility, interoperability, safety, repeatability, or quality. It can also facilitate commoditization of formerly custom processes’ [7]. Quality management protocols that regulate medical services, and evidence-based medicine practices, bring standardization of measuring and reporting to the forefront. In medicine, standardization is subject to a variety of financial, geographical, political, population-specific and ethical boundaries. Medical informatics has facilitated rapid communication among pathologists and their ability to discuss and share strategies across institutions, regions and countries around the globe. Standardization of every step in pre-analytic, analytic and post-analytic phases is crucial to achieve reliable and reproducible results across multiple groups of users (Table 2).
Table 2.
Summary of pre-analytic, analytic and post-analytic factors that influence reliability and reproducibility of results across multiple groups of users
| Pre-analytic |
| Acquisition (delay in putting samples into fixative) |
| Fixation type and time |
| Tissue processing |
| Slide-drying time and temperature |
| Analytic |
| Histology phase |
| Staining selection |
| Staining optimization |
| Staining validation instrumentation |
| CAP inspection/certification |
| Digital phase |
| Scanner |
| Image resolution |
| Number of colors |
| Monitor resolution |
| Compression ratio |
| Format |
| Post-analytic |
| Analysis or interpretation result |
| Recording of data |
| Pathologist performance |
| Reporting |
| Digital imaging analysis |
CAP, College of American Pathologists.
Technical standardization
Technical standardization in pathology begins with tissue procurement, fixation, processing (pre-analytic phase) and staining prior to visualization at the light microscope, immunofluorescence and electron microscope (analytic—histology phase; Table 2). Multiple factors influence substantial variability across laboratories. The application of digital pathology increases the demand for quality, as the quality of the glass slide is integral to the quality of the image displayed on the computer screen [8]. WSI itself requires substantial standardization to ensure image quality across platforms and institutions (analytic—digital phase). Critical to these issues is (i) defining objective measures of quality to replace subjective or regional/local approaches, and (ii) establishing rigorous protocols.
Standardization of protocols for digital pathology repositories
Beyond the standardized pre-analytic and analytic protocols in specimen and slide preparation, interoperability of digital pathology systems across laboratories can be achieved by implementation of meticulous protocols to standardize pathology material collection, scanning, uploading, display and organization in a digital pathology repository (DPR). Although a single stand-alone system that spans multiple sites and nations is an appealing approach, practicality it requires deploying customized DPR on a protocol-by-protocol basis for both regulatory and technical reasons. As such, international collaborative efforts should focus on developing protocol standards for sharable information, digital images, data elements and scoring/evaluation metrics. Careful planning is required to ensure optimal data sharing because the technical details established initially for a DPR sets boundaries for subsequent studies.
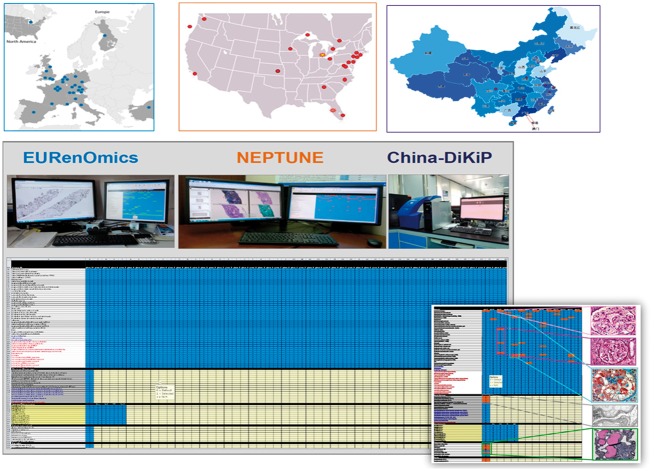
Recognizing the value of a common and standardized digital pathology platform, the NEPhrotic syndrome sTUdy NEtwork (NEPTUNE) established the first multicenter DPR, which subsequently served as a model for other consortia, such as the North American (CureGN), the European (EURenOmics) and Asian (China-DiKiP) consortia. More recently, NEPTUNE, EURenOmics and China-DiKiP, formed the INTErnational diGital nephRopAThology nEtwork (INTEGRATE; Table 1). INTEGRATE sub-consortia not only share a common protocol for pathology material acquisition and storage in the DPR, but they also implement an identical protocol for (i) annotation of glomeruli for multilevel representation, (ii) a descriptor-based scoring system and (iii) morphometry assessment. All data are collected across all INTEGRATE centers using a uniform template for visual and morphometric analysis (Figure 1). This approach allows for unprecedented collection of standardized pathology data.
Fig. 1.
Each INTEGRATE sub-consortia, EURenOmis in Europe, NEPTUNE in North America and China-DiKiP in China, comprises multiple centers. All sub-consortia use similar pathology material acquisition to populate the respective DPR and the same electronic scoring matrix for data collection of morphologic analysis.
Data access and data sharing ethics
Protocols guiding data access ensure governing and coordination of intra- and inter-consortia collaborations. However, data collection across multiple centers and storage in centralized repositories introduce a new set of challenges that include data sharing ethics and integrity. Researchers are expected to maintain the high ethical standards during research and when sharing data. Collection and sharing of research data that relates to people requires obtaining consent, anonymizing data and regulating data access. With the establishment of centralized national and international repositories, data sharing ethics and conduct guidelines are evolving. A data sharing Code of Conduct for international genomic research was proposed in 2011; it includes seven general principles applicable to all fields: scientific quality, accessibility, (including harmonization and transparency of repositories), responsibility of governance, security, transparency, accountability and integrity of scientific and interpersonal conduct [9].
Following this proposal, the Regulatory and Ethics Working Group and the Global Alliance for Genomics and Health established a similar international code to provide a principled and practical framework for the sharing of genomic and health-related data [10]. While a digital pathology-specific code of conduct is not yet established, these principles should be considered universal.
Post-analytic phase: digital morphologic assessment
Reproducibility, accuracy and standardization of language, analysis and reporting are critical elements of the post-analytic phase of the digital pathology assessment. High diagnostic sensitivity and specificity, and pertinent clinical utility are the goals.
Standardization of language
Deriving a standardized and reproducible approach starts with the compilation of detailed and unambiguous descriptions of the individual lesions (observations) believed critical to define diagnoses, followed by training to recognize such lesions, and testing of their reproducibility. The Banff classification for kidney allograft (rejection) provides a successful example of standardization of morphologic assessment [11, 12]. Similarly, the Anti-Neutrophil Circulating Antibodies (ANCA) vasculitis and lupus nephritis classifications provide some quantitative parameters driving the diagnosis, disease class and degree of activity and chronicity [13, 14]. However, classification systems in renal pathology were historically generated without accounting for reproducibility of observations. The developers of the Oxford classification system for IgA nephropathy were the first investigators who considered the concordant performance of pathologists prior to deploying their classification system [15, 16]. A similar approach was implemented by NEPTUNE with the NEPTUNE Digital Pathology Scoring System (NDPSS): a comprehensive and detailed descriptor reference manual encompassing definitions for over 80 histologic, immunofluorescence and ultrastructural descriptors, was first compiled, tested for reproducibility of observations and then refined for clarity of language [17]. The NDPSS was further revised and adopted by INTEGRATE (Table 1). The goal was to establish an international common language and a standardized and robust metric for assessment of the renal parenchyma, from which clinically relevant patient categorization can be derived. Modern telecommunications facilitated the process of international standardization of nomenclature via cost- and time-efficient webinar-based cross-training meeting.
Reproducibility
The issue of reproducibility comprises two elements: (i) reproducibility of analysis by digital imaging compared with conventional light microscopy and (ii) intra- and inter-reader concordance. Studies of glomerular diseases based on conventional microscopy revealed inconsistent concordance for some variables, including poor agreement on the number of glomeruli per section [15, 18–23]. Studies comparing glass slide versus WSI scoring and diagnoses demonstrated better concordance using a digital approach [6, 24–28].
Several elements play a key role in modulating concordance among pathologists:
Clarity of language/definition for the parameters to be evaluated (discussed above).
Overcoming cognitive bias. ‘The human brain’s habit of finding what it wants to find is a key problem for research. Establishing robust methods to avoid such bias will make results more reproducible’ [29].
Qualitative versus quantitative assessment. The overall diagnosis may be comparable between observers although measures of reproducibility (i.e. Kappa Cohen’s coefficient) are low for individual lesions.
Intra- versus inter-institutional tests [18].
Use of jpeg images versus the entire slide to test concordance [17, 31].
Accuracy of denominator (number of glomeruli) and numerator (number of glomeruli with a specific lesion) [32].
Frequency of a lesion as a function of severity (although a lesion may lose specificity as disease progresses).
Diagnostic sensitivity and specificity
Diagnostic sensitivity and specificity. Identification by light microscopy or digital imaging of specific morphologic lesions (descriptors) determining a diagnosis requires substantial training and experience. Traditionally, trainees learn to recognize lesions in isolation first, to some degree using a descriptor-based approach. As the pathologist acquires experience, the process shifts to an integrated qualitative diagnosis derived from pattern recognition and overall case review (e.g. clinical correlation). This process is learned and honed through repetition based on evaluation of individual samples. Conventional observational expertise allows subjective prioritization of heterogeneous lesions from surrounding tissue that creates a context for interpretation. At the level of broad diagnostic categories, this contextual approach may be acceptable for patient categorization; however, the heterogeneity of glomerular diseases has exposed the limitations of such holistic strategy in providing clinically meaningful disease categorization. While descriptor-based scoring systems can capture such complexity and variety of morphologic features [17, 33], intra- and inter-pathologist reproducibility decreases when individual parameters rather than diagnoses are tested [16, 17, 19–22]. For example, when 10 pathologists evaluated the reproducibility of 51 individual glomerular descriptors on 315 glomeruli, good to excellent intra- and inter-pathologist reproducibility was detected for more than 50% of lesions tested, but not all. Additionally, the key descriptors defining three of the five conventional variants for focal segmental glomerulosclerosis, perihilar, Not Otherwise Specified (NOS) and cellular, had poor to moderate concordance, while good concordance was achieved by combining all variants of segmental obliteration. The hopeful prospect is that this study demonstrated the value of training for improving concordance, made possible by the application of digital pathology and webinar meeting [17]. Computer-aided software programs are in process; these, when fully developed, are expected to improve standardization of morphologic data collection and remove subjectivity from this step.
Accuracy
Application of software that allows annotation of specific tissue structures is key in achieving accuracy and reproducibility of digital morphologic analysis. This strategy was first applied in a clinical trial for Fabry’s disease, where annotation of peritubular capillaries increased inter-observer reproducibility of scoring when compared with conventional light microscopy [6]. In glomerular disease studies, accuracy for counting all glomeruli in a biopsy improved using a NEPTUNE/INTEGRATE protocol [3, 15, 16]. Two separate studies, one conducted by NEPTUNE [32] and another by EURenOmics [34], compared glomerular enumeration on annotated WSI; the results indicated that digital annotation significantly increases accuracy and reproducibility of glomerular count.
While accuracy of the denominator can now be addressed by digital annotation, concordance on assessing elements that comprise the numerator remains problematic, asking for new strategies for pathologist and machine training.
Standardization of data recording and reporting
Application of common and transposable electronic scoring matrices should also improve standardization of renal biopsy assessment and data collection (Figure 1). For example, by linking each annotated glomerulus to a specific morphologic profile (glomerular mapping) investigators can test reproducibility not only at the biopsy level, but also at the individual glomerular level, while providing full transparency of analysis for future studies.
Quantitative digital pathology
Current clinical nephropathology practice depends on qualitative assessment of the renal biopsy summarized in a diagnosis line. This diagnostic summary is often combined with a semiquantitative assessment (typically based on four-tiered ordinal scales, 0–4+), which might provide additional prognostic information. Although the reproducibility of some of these parameters is adequate and/or improved by the application of digital imaging [25–28], they often do not accurately quantify the extent of parenchymal damage, since cases within the same semiquantitative category may differ considerably.
Historically, the term ‘quantitative pathology’ was used to imply cytometric, stereologic and morphometric assessment and arose from a need for objective quantitation. The term ‘quantitative’, however, can be paired with the term ‘morphology’ when visual assessment is based on quantitative metrics. Examples of quantitative metrics include: enumeration of glomeruli (annotation), number of events (times a descriptor is selected) per glomerulus or per biopsy, number of observational data (objectives) versus interpretative diagnosis (caring subjectivity) or parameters (descriptors) that are not incorporated in conventional classification systems. Newly proposed scoring systems [17] exploit the digital environment to apply quantitative visual, morphometric and computer-aided analysis.
Digital visual assessment
Visual assessment, whether at the microscope or computer monitor, can be used to quantify conventional parameters in current classifications systems (e.g. global or segmental sclerosis, interstitial fibrosis and tubular atrophy) as well as less commonly used features (halo, synechiae, deflation, etc.; Table 3). DPRs maximize the use of the same image to enhance accuracy of assessment and ultimately of diagnoses. Digitized images allow access by multiple investigators in a blinded fashion to test intra- and inter-observer reproducibility of conventional parameters and classification systems, or to test the performance of different metrics (dichotomous, continuous or ordinal). Additional advantages include: (i) testing for reproducibility of an individual or groups of nonconventional morphologic parameters, (ii) testing for correlation with clinical and molecular parameters and (iii) formulation of new clinically significant morphologic classifications. Best application requires an ongoing Quality Assessment and Improvement Program, the benefits of which have been documented [17].
Table 3.
INTEGRATE digital pathology scoring system descriptor reference manual
| Complete and revised INTEGRATE descriptors reference manual as currently implemented by all international consortia [17]. |
| WSI HISTOLOGY |
| Glomerular damage: Glomerular descriptors listed below are scored as present (1) or absent (0). |
| No (minimal) changes: None of the lesions below are present. |
| Global sclerosis with hyalinosis: Sclerosis involves 100% of the glomerular tuft. Glomerular size is preserved, or, compared with the glomeruli obtained in the same biopsy, increased or decreased by not more than 50%. |
| Global sclerosis without hyalinosis: Sclerosis involves 100% of the glomerular tuft, with no accompanying hyalinosis. Glomerular size is preserved or, compared with the glomeruli obtained in the same biopsy, increased or decreased by not more than 50%. |
| Global deflation: Global wrinkling and folding of the glomerular basement membrane (GBM) (≥80% of the tuft) without epithelial cell (podocyte) hypertrophy and hyperplasia (formerly known an ischemic type of collapse). The urinary space is patent. The wrinkling is generally made by small regular folds of the GBM. |
| Global capillary collapse: Wrinkling and folding of the GBM involving ≥80% of the tuft with occlusion or subocclusion of capillary lumina. Collapse is generally accompanied by hypertrophy and hyperplasia of overlying epithelial cells (pseudo-crescents). Epithelial cell (podocyte) hypertrophy and hyperplasia if present are marked separately as individual descriptors. The wrinkling is generally made by small and/or big irregular folds of the GBM. |
| Obsolescent glomeruli: Glomeruli are small and globally sclerotic without hyalinosis. Bowman’s capsule is completely or partially absent and there is no periglomerular fibrosis. Obsolescent glomeruli are defined when glomerular size is decreased >50% compared with all other glomeruli in the same biopsy. |
| Global mesangial sclerosis: A generalized global increase (100%) of mesangial matrix is present with or without mesangial cell hypercellularity and hypertrophy of overlying epithelial cells. |
| Segmental perihilar sclerosis (vascular pole): Segmental solidification of the glomerular tuft is present with increased extracellular matrix in continuity with the vascular pole. If hyalinosis, foam cells, hypertrophy of overlying epithelial cells (podocytes), halo and adhesion of the tuft to the Bowman’s capsule is present, they should be marked as separate descriptors. |
| Extended segmental perihilar sclerosis (vascular pole): Segmental solidification of the glomerular tuft with increased extracellular matrix in continuity with the vascular pole and extends beyond the middle line of the tuft, with or without involving the tip. If hyalinosis, foam cells, hypertrophy of overlying epithelial cells (podocytes), halo and sinechia/adhesion of the tuft to the Bowman’s capsule is present, they should be marked as separate descriptors. (This lesion includes segmental solidification known as ‘approaching’ global sclerosis.) |
| Segmental sclerosis away from vascular and tubular poles: Segmental solidification of the tuft with increased extracellular matrix. If hyalinosis, foam cells, hypertrophy of overlying epithelial cells (podocytes), halo and adhesion of the tuft to the Bowman’s capsule is present, these features should be marked as separate descriptors. |
| Segmental sclerosis cannot determine location: None of the above. Vascular or tubular pole cannot be seen in section. If hyalinosis, foam cells, hypertrophy of overlying epithelial cells (podocytes), halo and adhesion of the tuft to the Bowman’s capsule is present, they should be marked as separate descriptors. |
| Cellular tip lesion: Foam cells with or without other intracapillary cells within the glomerular tuft at the tubular pole, accompanied by hypertrophy of glomerular epithelial cells (podocytes) exclusive of tubular epithelium, and/or bridging to the Bowman’s capsule/proximal tubule take off area. The presence of foam cells or inflammatory cells needs to be marked separately as individual descriptors when present. |
| Sclerosing tip lesion: Solidification of the tuft at the tubular pole with increased extracellular matrix with or without adhesion to Bowman’s capsule. Glomerular epithelial cells (podocytes) may be hypertrophic and attached to the epithelium at the tubular pole. |
| Extended cellular tip lesion: Foam cells with or without other intracapillary cells within the glomerular tuft at the tubular pole. The process extends through a large portion of the glomerulus (>1/2 of the tuft) but does not involve the vascular pole, accompanied by hypertrophy of epithelial cells (podocytes) and/or bridging to the Bowman’s capsule/proximal tubule take off area. The presence of foam cells or inflammatory cells needs to be marked separately as individual descriptors when present. (This lesion includes segmental solidification not involving the vascular pole but ‘approaching’ global sclerosis.) |
| Extended sclerosing tip lesion: Solidification of the tuft at the tubular pole with increased extracellular matrix and adhesion to Bowman’s capsule that extends through a large portion of the glomerulus but does not involve the vascular pole. No foam cells are present. Glomerular epithelial cells (podocytes) are hypertrophic and attached to epithelial cells at the tubular pole. (This lesion includes segmental solidification not involving the vascular pole but ‘approaching’ global sclerosis.) |
| Mid-tuft/central location of segmental sclerosis: Located neither at the tip, the perihilum or the periphery of the tuft (no adhesion to the Bowman’s capsule). |
| Cellular lesions—non-tip: Endocapillary hypercellularity with epithelial cell hypertrophy (podocyte). Hypercellularity may be due to foam cells and/or endocapillary cells (podocytes) with or without karyhorrexis and is not at the tip of the glomerulus. The presence of foam cells, karyhorrexis or inflammatory cells needs to be marked separately as individual descriptors when present. |
| Segmental capillary collapse: Wrinkling and folding of the GBM involving at least one glomerular lobule and <80% of the tuft, with occlusion or subocclusion of capillary lumina. Collapse is generally accompanied by hypertrophy and hyperplasia of overlying epithelial cells (podocytes); epithelial cell (podocytes) hypertrophy and hyperplasia if present need to be marked separately as individual descriptors. The wrinkling is generally made by small and/or big irregular folds of the GBM. |
| Segmental deflation: Wrinkling and folding of the capillaries without epithelial cell (podocyte) hyperplasia (formerly called ischemic type of collapse) involving <80% of the glomerular tuft. The wrinkling is generally made by small regular folds of the GBM. |
| Periglomerular fibrosis: Circumferential fibrosis in the interstitium surrounding the Bowman’s capsule. |
| Glomerular foam cells: Intracapillary foam cells in the presence or absence of segmental or global sclerosis. |
| Hyaline droplets in epithelial cell (podocyte): Protein droplets are present in glomerular epithelial cells (podocytes). These cells usually are also hypertrophic (if so, both descriptors apply). |
| Hyalinosis at the vascular pole: Hyalinosis is defined as glassy acidophilic, Periodic Acid Schiff (PAS) positive, silver negative material. |
| Hyalinosis at the tubular pole: Hyalinosis is defined as glassy acidophilic, PAS positive, silver negative material. Solidification of the tuft and/or foam cells may be present. |
| Hyalinosis away from the vascular and tubular poles: Hyalinosis is defined as glassy acidophilic, PAS positive, silver negative material. Both the vascular and the tubular pole are present in the glomerular cross section. |
| Hyalinosis cannot determine location: Hyalinosis is defined as glassy acidophilic, PAS positive, silver negative material and can occur with or without adhesion to the Bowman’s capsule in a location that is not the vascular pole of the tip of the glomerulus. The vascular and/or the tubular poles cannot be identified. |
| Synechiae: Continuity of glomerular tuft basement membrane to the Bowman’s capsule with continuity of epithelial cell lining. Note that a sinechia generally includes 1–2 capillaries at the most and it may or may not be associated with segmental sclerosis, hyalinosis or foam cells. Larger adhering section of the glomerular tuft to the Bowman’s capsule in the presence of significant hyalinosis and/or sclerosis is not considered a sinechia but an adhesion part of the segmental sclerosis. |
| Segmental epithelial cell (podocyte) hypertrophy: Hypertrophy is defined as enlarged cytoplasm or enlarged nuclei with prominent nucleoli or both. Segmental hypertrophy is defined when hypertrophic epithelial cells (podocytes) overlying the GBM involve <50% of the glomerular tuft. |
| Global epithelial cell (podocyte) hypertrophy: Hypertrophy is defined as enlarged cytoplasm or enlarged nuclei with prominent nucleoli or both. Global hypertrophy is defined when hypertrophic epithelial cells (podocytes) overlying the GBM involve ≥50% of the glomerular tuft. |
| Segmental epithelial cell (podocyte) hyperplasia: ≥2 layers of epithelial cells (podocytes) overlying the GBM are present, involving <50% of the glomerulus. Hyperplasia may occur with or without hypertrophy. |
| Global epithelial cell (podocyte) hyperplasia: ≥2 layers of epithelial cells (podocytes) overlying the GBM are present, involving ≥50% of the glomerulus. Hyperplasia may occur with or without hypertrophy. |
| Halo (detachment of overlaying podocytes): Detachment of epithelial cells (podocytes) from original underlying GBM is present with intervening new loose basement membrane material (pale on hematoxylin and eosin, PAS, trichrome or silver stain). |
| Segmental mesangial hypercellularity: >3 mesangial cells per mesangial lobule involving <50% of the visible mesangial regions in a glomerulus. |
| Global mesangial hypercellularity: >3 mesangial cells per mesangial lobule involving ≥50% of the visible mesangial regions in a glomerulus. |
| Segmental presence of spikes on silver stain: Spikes are defined as silver positive stain with an irregular profile on the outer side of the GBM and involving <50% of the glomeruli. |
| Global presence of spikes on silver stain: Spikes are defined as silver positive stains with an irregular profile on the outer side of the GBM involving ≥50% of the glomerulus. |
| Infiltrating leukocytes: The presence of leukocytes in glomerular capillaries is recorded when ≥1 inflammatory cell is present in capillary lumina. (Note that the presence of infiltrating leukocytes although initially scored on a semiquantitative scale from 0 to 3 for each glomerulus with 0= none 1 = 1–7, 2 = 8–15, 3 = >15 leukocytes per glomerulus, was revised and scored as a dichotomous value for this study.) |
| Segmental endocapillary hypercellularity: Hypercellularity due to increased number of cells within glomerular capillary lumina causing narrowing of the lumina involving <50% of the glomerulus. |
| Global endocapillary hypercellularity: Hypercellularity due to increased number of cells within glomerular capillary lumina causing narrowing of the lumina’ involving >50% of the glomerulus. |
| Segmental GBM duplication: Defined as a double contour of the GBM involving <50% of the glomerular tuft, with or without endocapillary hypercellularity (endocapillary hypercellularity is independent variable). |
| Global GBM duplication: Defined as a double contour of the GBM involving >50% of the glomerular tuft, with or without endocapillary hypercellularity (endocapillary hypercellularity is independent variable). |
| Segmental increased mesangial matrix: Defined as an increase in the extracellular material in the mesangium such that the width of the interspace exceeds two mesangial cell nuclei in at least one glomerular lobule but <50% of the glomerular tuft. |
| Global increased mesangial matrix: Defined as an increase in the extracellular material in the mesangium such that the width of the interspace exceeds two mesangial cell nuclei in ≥50% of the glomerular tuft. |
| Karyorrhexis: Presence of apoptotic, pyknotic and/or fragmented nuclei. |
| Necrosis: Defined as disruption of the GBM with fibrin exudation and karyorrhexis. |
| Very segmental extracapillary cellular proliferation (cellular crescent): Extracapillary cell proliferation of more than two cell layers with >50% of the lesion occupied by cells, involving <25% of the Bowman’s space. |
| Very segmental extracapillary fibrocellular proliferation (fibrocellular crescent): Defined as part of the circumference of Bowman’s capsule covered by a combination of cells and extracellular matrix, with <50% cells and <90% matrix involving <25% of the Bowman’s space. This lesion is often associated with disruption of Bowman’s capsule. Ischemic, obsolescent glomeruli should be excluded. |
| Very segmental extracapillary fibrosis (fibrous crescent): Defined as >10% of the circumference of Bowman’s capsule covered by a lesion composed of >90% extracellular matrix involving <25% of the Bowman’s space. |
| Extensive extracapillary cellular proliferation (cellular crescent): extracapillary cell proliferation of more than two cell layers with >50% of the lesion occupied by cells, involving >25% of the Bowman’s space. |
| Extensive extracapillary fibrocellular proliferation (fibrocellular crescent): Defined as part of the circumference of Bowman’s capsule covered by a combination of cells and extracellular matrix, with <50% cells and <90% matrix involving >25% of the Bowman’s space. This lesion is often associated with disruption of Bowman’s capsule. Ischemic, obsolescent glomeruli should be excluded. |
| Extensive extracapillary fibrosis (fibrous crescent): Defined as more than 10% of the circumference of Bowman’s capsule covered by a lesion composed of >90% extracellular matrix involving >25% of the Bowman’s space. |
| Tubulo-interstitial damage: The following parameters will be quantitated as present or absent, on a semiquantitative scale (0–3+) or quantitative scale (percentage of cortex involved). |
| (a) present or absent (0 = absent, 1 = present): interstitial edema, microcysts and inflammation with eosinophils or neutrophils >10% |
| (b) semiquantitative scale (0–3+; 0 = absent; 1+ = mild, <25% of cortex; 2+ = moderate, 25–50% of cortex; 3+ = severe, >50% of cortex): acute tubular injury |
| (c) quantitative (percentage of cortex involved): tubular atrophy, interstitial fibrosis, interstitial inflammation and interstitial foam cells |
| Acute tubular damage: Defined by the presence of tubular degenerative changes (such as flattening of the tubular epithelium, loss of proximal cell brush borders, pyknotic cells) and/or tubular regenerative changes (hypertrophic epithelial cells with large nuclei and prominent nucleoli, mitotic activity). |
| Tubular atrophy: Small tubules with thick tubular basement membranes lined by small cuboidal or flat cells. Generally accompanied by fibrosis. Includes ‘thyroidization’ of the parenchyma. |
| Microcysts: Presence of dilated tubules (> twice the diameter of a normal proximal tubule) containing eosinophilic amorphous material, and is generally accompanied by scalloping of the cast profile. The epithelium lining the microcyst is generally flattened and does not reveal brush border. |
| Interstitial fibrosis: The interstitium is expanded by the presence of collagen that stain blue on trichrome. Tubular are not back to back, but rather separated by fibrosis and can be atrophic. |
| Interstitial edema: The interstitium is occupied by pale acellular material. |
| Interstitial inflammation—mononuclear white blood cells: Inflammation involving fibrotic as well as nonfibrotic renal cortex, composed of lymphocytes, monocytes, plasma cells. |
| Interstitial inflammation—eosinophils: If eosinophils are noted in >10% of the cortex and representing >10% of the inflammatory cells. |
| Interstitial inflammation—neutrophils: If neutrophils are noted in >10% of the cortex and representing >10% of the inflammatory cells. |
| Interstitial foam cells: Presence of interstitial foam cells containing optically clear vacuoles. |
| Vascular damage: The following will be scored semiquantitatively on a scale from 0 to 3+ using the vessel with the most severe lesion. If arteries or arterioles are not present in the sections, it will be scored as n/a (999). |
| 0 absent |
| 1 + = mild, thickness of intima of <25% of media width in any number of vessels |
| 2 + = moderate, 25–50% of media width in any number of vessels |
| 3 + = severe, >50% of media width in any number of vessels |
| Arterial sclerosis: Defined as thickening of the intima with fibrosis and/or duplication of the elastic lamina in interlobular and arcuate arteries. |
| Arteriolar hyalinosis: Defined as accumulation of hyaline material in the wall and/or arteriolar sclerosis. |
| ELECTRON MICROSCOPY |
| A minimum of five electron micrographs are reviewed. |
| Podocytes: Foot process effacement, microvillous transformation and condensation of the actin-based cytoskeleton are scored semiquantitatively and loss of primary processes and present or absent. |
| Endothelial cells: Endothelial cells loss of fenestration is recorded on a semiquantitative scale based on percentage of peripheral cytoplasm involved tubuloreticular inclusions and honeycombing-like appearance as present or absent. |
| GBM: Abnormalities in texture are recorded as present or absent. |
| Electron-dense deposits: The percentage of GBM involved by subepithelial deposits in each stage (I–IV) is recorded. The predominance of stages are indicated, e.g. II > III, or II, or II > I > III, etc. Mesangial deposits (including mesangial and paramesangial) and subendothelial deposits are recorded as present or absent. The presence of transmembrane deposits and deposits with nuclear pore appearance is recorded as present or absent. |
| Foot process effacement: Loss of foot processes. Percentage of glomerular capillary surface area affected by effacement will be recorded as semiquantitative value (0 = 0–10%; 1 = 11–25%; 2 = 26–50%; 3 = 51–75%; 4 = 76–100% of the outer GBM surface). |
| Condensation of the actin-based cytoskeleton: Electron-dense cytoskeleton is reorganized and condensed at the GBM aspect of epithelial cell (podocyte) foot processes. Percentage of glomerular capillary surface area affected by effacement will be recorded as semiquantitative value (0 = 0–5%; 1 = ≤50%; 2 = >50% of the outer GBM surface). |
| Microvillous transformation: Cytoplasmic projections into the urinary space that emanate from the luminal side of epithelial cell (podocyte) membrane are present. Percentage of glomerular capillary surface area affected by effacement will be recorded as semiquantitative value (0 = 0–5%; 1 = ≤50%; 2 = >50% of the outer GBM surface). |
| Loss of primary processes: Epithelial cell (podocyte) body sits directly on underlying GBM. This is generally accompanied by complete effacement (loss of foot processes). It will be recorded as present or absent (0 = present, normal; 1 = absent, loss). |
| Epithelial cell (podocyte) detachment: Detachment of epithelial cells from underlying GBM is present with intervening new loose basement membrane material (halo). Recorded as present or absent (0 = absent, 1 = present). |
| Thickening of the GBM: GBM thickness will be assessed on 10 cross sections of capillary loops at foci where there are no capillary wall deposits. |
| − Decreased thickness is scored as such when at least 25% of the GBM appear thinner than normal (0 = absent, 1 = present). |
| − Increased thickness is scored as such when at least 25% of the GBM appear thicker than normal (0 = absent, 1 = present). |
| − Mix pattern when thin and thick areas are present within the same biopsy (0 = absent, 1 = present). |
| GBM abnormal texture: Presence of basket-wave appearance, electron lucent areas alternating with granular or curvilinear electron-dense areas, the presence of microspherule, microparticles different from organized deposits or rests of invaginating cells within the lamina densa of the GBM. Scored as absent or present (0 = absent, 1 = present). |
| Tubuloreticular inclusions: Presence of at least one subcellular organized inclusion in endothelial cell cytoplasm is recorded (0 = absent, 1 = present). |
| Glomerular endothelial cell fenestration: Absence of typical fenestration resulting in a solid rim of endothelial cell cytoplasm away from the perinuclear region (0 = 0–5%; 1 = ≤50%; 2 = >50% of the inner GBM surface). |
| Endothelium honeycombing-like appearance: Presence of cribriform or reticular organization of the endothelial cell cytoplasm, most often, but not exclusively, present at the mesangial side of the capillary lumen. Scored as present or absent, assuming that the absence of it is the pathologic event (0 = present, normal; 1 = absent, loss). |
| Subepithelial deposits Stage I: Electron-dense deposits are present on the outer surface of the GBM with little or no lateral accumulation of basement membrane material. |
| Subepithelial deposits Stage II: Electron-dense deposits are present on the outer surface of the GBM and partially surrounded by extracellular matrix (spikes). |
| Subepithelial deposits Stage III: Electron-dense deposits are embedded in the extracellular matrix (intramembranous). |
| Subepithelial deposits Stage IV: Electron-dense deposits are partially reabsorbed and formed by irregular electron lucent areas and more electron-dense areas. |
| Predominant stage: I = 1; II = 2; III = 3; IV = 4. |
| Transmembranous deposits: Deposits present throughout the entire thickness of the GBM. |
| Nuclear pore configuration: Deposits with a concentric circular (nuclear pore) configuration. |
| Subendothelial deposits: Electron-dense deposits located in between the GBM and the endothelial cell cytoplasm. |
| Mesangial deposits: Electron-dense deposits involving the mesangium and paramesangium. |
Digital morphometric assessment
While morphometry was historically applied to glass slides, recent applications using WSI have proven feasibility [35]. Digital morphometry provides standardized and objective measurements applicable to longitudinal statistical models predicting progression in medicine [36]. Quantitative structural parameters such as morphometric assessment of cortical-interstitial volume, fractional interstitial area, average glomerular tuft volume, cortical density of glomeruli and percentage of globally sclerotic glomeruli correlate with various aspects of renal function [37–41]. Other parameters critical to progression of glomerular diseases are currently explored using new methodologies for estimating podocyte number, size, density and the rate of podocyte detachment from glomeruli into urine (podometrics) [42, 43].
Computer-aided image analysis
Implementation of computer-aided image analysis in research and clinical settings can improve reproducibility and efficiency. Three major current issues need to be addressed when testing and applying software for automatic image analysis: identification of the specific structure to analyze, validation of the methodology compared with visual or morphometric assessment and reproducibility of the analysis. Studies in cancer tissue have shown that image analysis increases workflow, efficiency and throughput, and reduces inter- and intra-observer variability [44, 45]. New image analysis software tools are being tested for their potential to locate specific morphologic features with minimal human intervention. A recent study evaluated automated versus manual segmentation on WSI (tracing the edges of invasive tumor areas) for the purpose of immunohistochemistry scoring for intensity, percentage and combined scores for the estrogen receptor in breast cancer. To enable quantitative assessment, an algorithm was applied using clustering to group segmentation pixels into compact regions, super-pixels, with edges corresponding to the segmentation area edge. When manual segmentation was compared between pathologists and then to computer-aided segmentation of areas of malignancy, pathologists differed in their manual labeling of 9% of pixels, versus 19% when manual was compared with computer-aided image analysis. Estrogen receptor scores computed from automated segmentations were more consistent than those from manual segmentations [46, 47].
Comparable computer-aided analytic methodologies might be applied to renal pathology. Automatic ‘glomeruli finders’ for annotation might result in significant time saving and the implementation of a standardized process that guarantees that the same structures are analyzed. Similarly, automated segmentation of areas of interstitial fibrosis and tubular atrophy are possible if the WSI are qualitatively adequate (Table 2).
Beyond application to glomerular annotation or to tissue segmentation (e.g. evaluation of interstitial fibrosis, inflammation), challenges in applying computer-aided image analysis to renal biopsies rest on the complexity of the parenchymal structure and the variability of the quality and quantity of injury.
Computational nephropathology for precision and prediction medicine
In contrast to the ongoing rapid development of precision pathology in oncology, where immunophenotypic classification of tumors is being overtaken by genomic approaches, renal pathology has evolved slowly over the last 25 years, with diagnostic clinical practice remaining largely dependent on conventional morphologic analysis. The growing awareness of the complexity of clinical, morphologic, genotype and phenotypes of glomerular disorders and advances in ‘-omic’ science stimulated the establishment of consortia to develop a better understanding of the pathogenesis, classification and ultimately treatment of glomerular diseases. Molecular data generated in nephrology and pathology, methodologies utilizing digital technology and powerful new computational approaches for analyzing large sets of data, created an opportunity to establish a new field of computational nephropathology as an approach to renal biopsy analysis. Harmonization of computational nephropathology with computational medicine will provide the opportunity to leverage advanced analytics, algorithms and mathematical approaches from machine learning and biomedical informatics. As computational nephropathology is taking shape to fit within the spectrum of biomedical informatics, new challenges arise, requiring pathologists to learn how to adapt the practice of integrated pathology. This requires that morphologic observations be collected in a manner that allows their incorporation with multiple other sources of data (including clinical electronic medical records, -omics and imaging). Biologically and clinically relevant information can be generated using mathematical models at the individual and population level potentially leading to diagnostic and outcome algorithms. The ultimate goals of these approaches are to: (i) contribute structural information to a hub for data-related research, (ii) provide well-characterized patients according to standardized structural parameters for clinical trials, (iii) advance precision medicine and (iv) increase our ability to predict likelihood of progression (predictive medicine) [48].
The ultimate pathology workstation—immersed in a multidisciplinary cloud
Growing efficiency of pathology services is another aspect of progress that is expected. Digital technologies in pathology present both new challenges and opportunities to shift from traditional pathology workflow, and the ‘physical world of histology glass slides’ managed by conventional laboratory information systems (LIS), to the digital image-based environment integrated with next generation LIS. The emerging field of computational pathology will incorporate robust computational approaches to implement data-driven methods guiding individual and population health care [48]. The complexity of tasks, spanning from data standardization and technical interoperability, to integrated decision support systems and flexible workflow designs, cannot be underestimated [49]. In this new environment, the pathologist’s work will increasingly depend on human–machine interfaces, connecting both to the patient/tissue sample data and to the multidisciplinary team of health practitioners [50]. The new pathology workstation, purely digital or combined with conventional microscopy, is a critical component for this ecosystem of the near future enabling new levels of work efficiency, communication and data management. Proper interpretation of the biopsy findings, based on light, immunofluorescence and electron microscopy methods, requires good understanding of clinical context and good contact with treating physicians. While meetings at the multi-head microscope with clinicians to discuss the biopsy results are historically of great value, they are becoming less feasible with consolidation of specialized pathology services and time pressures in today’s health care. The challenges and advantages of digital solutions for pathology sign-out were recently evaluated in the USA. The authors concluded that such integration of LIS with a digital pathology system was instrumental to streamline workflow, reduce human error and improve the sign-out experience [51]. Accordingly, workstations will evolve and adapt to workflow, enabling effortless viewing of digital images from different techniques. Annotation and targeted morphometry, and smooth online communication systems will be in immediate demand. The ultimate and critical steps would involve integration of structured reporting, image analysis-based quantification and decision support systems [51] for routine native and transplant kidney biopsy standardized diagnoses.
Conclusions
Digital technologies in nephropathology present new opportunities and challenges related to the variety of current techniques, a complex tissue structure and the pathology spectrum. Digital pathology expands the opportunity for broad international standardization of renal biopsy assessment, not previously achievable, and introduces two new concepts: (i) the ‘any time, anywhere’ model (provided available internet connectivity) and (ii) the ‘cloud as the new transparent’. The present circumstances determine the starting point for the next-generation nephropathology: quantitative, robust, agile, interactive with other disciplines and fit for integration into the raising computational medical world. The ultimate goal of next-generation computational nephropathology is to deliver diagnostic information that moves beyond classification of disease, providing prognostic information and contributing to the identification and selection of candidate therapeutic targets. To accomplish these goals, laboratory accreditation agencies, private and public healthcare systems and operators (pathologists and technical staff) will have to capitalize on this technology and embrace new paradigms for clinical workflow and/or international collaboration.
Acknowledgements
The authors acknowledge financial support from (i) the EC Marie Curie Actions, AIDPATH project (no. 612471); (ii) the Nephrotic Syndrome Study Network Consortium (NEPTUNE), part of the National Center for Advancing Translational Sciences (NCATS), the Rare Disease Clinical Research Network (RDCRN), and supported through a collaboration between the Office of Rare Diseases Research (ORDR), NCATS and the National Institute of Diabetes, Digestive, and Kidney Diseases. RDCRN is an initiative of ORDR and NCATS. Additional funding and/or programmatic support for this project has also been provided by the University of Michigan, NephCure Kidney International and the Halperin Foundation. (iii) EURenOmics: this work has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 305608. (iv) China-DiKiP, part of the Clinical Research Program of Jiangsu Province (no. BL2012007), part of the National Key Technology R&D Program (no. 2013BAI09B04), part of NEPTUNE China. (v) The intramural program, Center for Cancer Research, National Cancer Institute, National Institutes of Health. The authors also wish to acknowledge the INTEGRATE group of investigators. NEPTUNE: Laura Barisoni, Serena Bagnasco, Carmen Avila-Casado, Jeffrey Hodgin, Joseph Gaut, Matthew Palmer, Virginie Royal, David Thomas, Avi Rosenberg, Matthias Kretzler, Jun Wu; EURenOmics: Charlotte Gimpel, Renate Kain, Virginie Royale, Ivana Simic, Shane Meehan, Sandrine Florquine, Marion Rabant, Franz Shaefer; China-DiKiP: Zhihong Liu, CaihongZeng Jianghua Chen, Heng Li Zhangsuo Liu, Guolan Xing Shengqiang Yu, Jun Wu, Qiaoling Zhou, Wenbin Tang Li Wang, Guisen Li; AIDPATH: Arvydas Laurinavicious and Gloria Bueno; and INTEGRATE advisory committee: Stephen Hewitt, Helen Liapis, Robert Colvin.
References
- 1. Weinstein RS, Bloom KJ, Rozek LS.. Telepathology and the networking of pathology diagnostic services. Arch Pathol Lab Med 1987; 111: 646–652 [PubMed] [Google Scholar]
- 2. Weinstein RS, Graham AR, Richter LC. et al. Overview of telepathology, virtual microscopy, and whole slide imaging: prospects for the future. Hum Pathol 2009; 40: 1057–1069 [DOI] [PubMed] [Google Scholar]
- 3. Barisoni L, Nast CC, Jennette JC. et al. Digital pathology evaluation in the multicenter Nephrotic Syndrome Study Network (NEPTUNE). Clin J Am Soc Nephrol 2013; 8: 1449–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stathonikos N, Veta M, Huisman A. et al. Going fully digital: perspective of a Dutch academic pathology lab. J Pathol Inform 2013; 4: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thorstenson S, Molin J, Lundstrom C.. Implementation of large-scale routine diagnostics using whole slide imaging in Sweden: digital pathology experiences 2006-2013. J Pathol Inform 2014; 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barisoni L, Jennette JC, Colvin R. et al. Novel quantitative method to evaluate globotriaosylceramide inclusions in renal peritubular capillaries by virtual microscopy in patients with fabry disease. Arch Pathol Lab Med 2012; 136: 816–824 [DOI] [PubMed] [Google Scholar]
- 7.Wikipedia, https://en.wikipedia.org/wiki/Standardization (August 2016, date last accessed)
- 8. Yagi Y, Gilbertson JR.. Digital imaging in pathology: the case for standardization. J Telemed Telecare 2005; 11: 109–116 [DOI] [PubMed] [Google Scholar]
- 9. Knoppers BM, Harris JR, Tasse AM. et al. Towards a data sharing Code of Conduct for international genomic research. Genome Med 2011; 3: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Regulatory, Ethics Working Group GAfG, Health, Sugano S. International code of conduct for genomic and health-related data sharing. Hugo J 2014; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haas M. The revised (2013) Banff classification for antibody-mediated rejection of renal allografts: update, difficulties, and future considerations. Am J Transplant 2016; 16: 1352–1357 [DOI] [PubMed] [Google Scholar]
- 12. Racusen LC, Solez K, Colvin RB. et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 1999; 55: 713–723 [DOI] [PubMed] [Google Scholar]
- 13. Weening JJ, D'Agati VD, Schwartz MM. et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 2004; 15: 241–250 [DOI] [PubMed] [Google Scholar]
- 14. Weening JJ, D'Agati VD, Schwartz MM. et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 2004; 65: 521–530 [DOI] [PubMed] [Google Scholar]
- 15. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Roberts IS, Cook HT. et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int 2009; 76: 546–556 [DOI] [PubMed] [Google Scholar]
- 16. Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R. et al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int 2009; 76: 534–545 [DOI] [PubMed] [Google Scholar]
- 17. Barisoni L, Troost JP, Nast C. et al. Reproducibility of the NEPTUNE descriptor-based scoring system on whole-slide images and histologic and ultrastructural digital images. Mod Pathol 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furness PN, Taub N, Assmann KJ. et al. International variation in histologic grading is large, and persistent feedback does not improve reproducibility. Am J Surg Pathol 2003; 27: 805–810 [DOI] [PubMed] [Google Scholar]
- 19. Lee HS, Lee MS, Lee SM. et al. Histological grading of IgA nephropathy predicting renal outcome: revisiting H. S. Lee’s glomerular grading system. Nephrol Dial Transplant 2005; 20: 342–348 [DOI] [PubMed] [Google Scholar]
- 20. Grootscholten C, Bajema IM, Florquin S. et al. Interobserver agreement of scoring of histopathological characteristics and classification of lupus nephritis. Nephrol Dial Transplant 2008; 23: 223–230 [DOI] [PubMed] [Google Scholar]
- 21. Furness PN, Taub N.. Interobserver reproducibility and application of the ISN/RPS classification of lupus nephritis-a UK-wide study. Am J Surg Pathol 2006; 30: 1030–1035 [DOI] [PubMed] [Google Scholar]
- 22. Furness PN. Histopathology of chronic renal allograft dysfunction. Transplantation 2001; 71: SS31–SS36 [PubMed] [Google Scholar]
- 23. Adam B, Randhawa P, Chan S. et al. Banff initiative for quality assurance in transplantation (BIFQUIT): reproducibility of polyomavirus immunohistochemistry in kidney allografts. Am J Transplant 2014; 14: 2137–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Furness P. A randomized controlled trial of the diagnostic accuracy of internet-based telepathology compared with conventional microscopy. Histopathology 2007; 50: 266–273 [DOI] [PubMed] [Google Scholar]
- 25. Gavrielides MA, Conway C, O'Flaherty N. et al. Observer performance in the use of digital and optical microscopy for the interpretation of tissue-based biomarkers. Anal Cell Pathol (Amst) 2014; 2014: 157308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jen KY, Olson JL, Brodsky S. et al. Reliability of whole slide images as a diagnostic modality for renal allograft biopsies. Hum Pathol 2013; 44: 888–894 [DOI] [PubMed] [Google Scholar]
- 27. Ozluk Y, Blanco PL, Mengel M. et al. Superiority of virtual microscopy versus light microscopy in transplantation pathology. Clin Transplant 2012; 26: 336–344 [DOI] [PubMed] [Google Scholar]
- 28. Reyes C, Ikpatt OF, Nadji M. et al. Intra-observer reproducibility of whole slide imaging for the primary diagnosis of breast needle biopsies. J Pathol Inform 2014; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Let’s think about cognitive bias. Nature 2015; 526: 163. [DOI] [PubMed] [Google Scholar]
- 30. Wang H, Sima CS, Beasley MB. et al. Classification of thymic epithelial neoplasms is still a challenge to thoracic pathologists: a reproducibility study using digital microscopy. Arch Pathol Lab Med 2014; 138: 658–663 [DOI] [PubMed] [Google Scholar]
- 31. Meehan SM, Chang A, Gibson IW. et al. A study of interobserver reproducibility of morphologic lesions of focal segmental glomerulosclerosis. Virchows Arch 2013; 462: 229–237 [DOI] [PubMed] [Google Scholar]
- 32. Rosenberg AZ, Palmer M, Merlino L. et al. The application of digital pathology to improve accuracy in glomerular enumeration in renal biopsies. PLoS One 2016; 11: e0156441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nast CC, Lemley KV, Hodgin JB. et al . Morphology in the digital age: integrating high-resolution description of structural alterations with phenotypes and genotypes. Semin Nephrol 2015; 35: 266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gimpel C, K R, Royal V, Simic I. et al. Nephrol Dial Transplant 2016; 31 (Suppl 1): i373
- 35. Lemley K, Bagnasco SM, Nast CC. et al. Morphometry predicts early GFR change in primary proteinuric glomerulopathies: a longitudinal cohort study using generalized estimating equations. PLoS One 2016; 11: e0157148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tangri N, Kitsios GD, Inker LA. et al. Risk prediction models for patients with chronic kidney disease: a systematic review. Ann Intern Med 2013; 158: 596–603 [DOI] [PubMed] [Google Scholar]
- 37. Bohle A, Mackensen-Haen S, von Gise H. et al. The consequences of tubulo-interstitial changes for renal function in glomerulopathies. A morphometric and cytological analysis. Pathol Res Pract 1990; 186: 135–144 [DOI] [PubMed] [Google Scholar]
- 38. Denic A, Alexander MP, Kaushik V. et al. Detection and clinical patterns of nephron hypertrophy and nephrosclerosis among apparently healthy adults. Am J Kidney Dis 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Denic A, Glassock RJ, Rule AD.. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis 2016; 23: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kremers WK, Glassock RJ, Rule AD. et al. Distinguishing age-related from disease-related glomerulosclerosis on kidney biopsy: the aging kidney anatomy study. Nephrol Dial Transplant 2015; 30: 2034–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lemley KV. Diabetes and chronic kidney disease: lessons from the Pima Indians. Pediatr Nephrol 2008; 23: 1933–1940 [DOI] [PubMed] [Google Scholar]
- 42. Hodgin JB, Bitzer M, Wickman L. et al. Glomerular aging and focal global glomerulosclerosis: a podometric perspective. J Am Soc Nephrol 2015; 26: 3162–3178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kikuchi M, Wickman L, Hodgin JB. et al. Podometrics as a potential clinical tool for glomerular disease management. Semin Nephrol 2015; 35: 245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gurcan MN, Boucheron LE, Can A. et al. Histopathological image analysis: a review. IEEE Rev Biomed Eng 2009; 2: 147–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Veta M, van Diest PJ, Willems SM. et al. Assessment of algorithms for mitosis detection in breast cancer histopathology images. Med Image Anal 2015; 20: 237–248 [DOI] [PubMed] [Google Scholar]
- 46. Akbar S, Jordan LB, Purdie CA. et al. Comparing computer-generated and pathologist-generated tumour segmentations for immunohistochemical scoring of breast tissue microarrays. Br J Cancer 2015; 113: 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gavrielides MA, Gallas BD, Lenz P. et al. Observer variability in the interpretation of HER2/neu immunohistochemical expression with unaided and computer-aided digital microscopy. Arch Pathol Lab Med 2011; 135: 233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Louis DN, Feldman M, Carter AB. et al. Computational pathology: a path ahead. Arch Pathol Lab Med 2016; 140: 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Daniel C, Garcia Rojo M, Bourquard K. et al. Standards to support information systems integration in anatomic pathology. Arch Pathol Lab Med 2009; 133: 1841–1849 [DOI] [PubMed] [Google Scholar]
- 50. Laurinavicius A, Raslavicus P.. Consequences of “going digital” for pathology professionals - entering the cloud. Stud Health Technol Inform 2012; 179: 62–67 [PubMed] [Google Scholar]
- 51. Guo H, Birsa J, Farahani N. et al. Digital pathology and anatomic pathology laboratory information system integration to support digital pathology sign-out. J Pathol Inform 2016; 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]