Abstract
The present study examined the association of dopamine-related genes with short- and long-term neurobehavioral recovery, as well as neurobehavioral recovery trajectories over time, in children who had sustained early childhood traumatic brain injuries (TBI) relative to children who had sustained orthopedic injuries (OI). Participants were recruited from a prospective, longitudinal study evaluating outcomes of children who sustained a TBI (n = 68) or OI (n = 72) between the ages of 3 and 7 years. Parents completed ratings of child executive function and behavior at the immediate post-acute period (0–3 months after injury); 6, 12, and 18 months after injury; and an average of 3.5 and 7 years after injury. Thirty-two single nucleotide polymorphisms (SNPs) in dopamine-related genes (dopamine receptor D2 [DRD2], solute carrier family 6 member 3 [SLC6A3], solute carrier family 18 member A2 [SLC18A2], catechol-o-methyltransferase [COMT], and ankyrin repeat and kinase domain containing 1 [ANKK1]) were examined in association with short- and long-term executive function and behavioral adjustment, as well as their trajectories over time. After controlling for premorbid child functioning, genetic variation within the SLC6A3 (rs464049 and rs460000) gene was differentially associated with neurobehavioral recovery trajectories over time following TBI relative to OI, with rs464049 surviving multiple testing corrections. In addition, genetic variation within the ANKK1 (rs1800497 and rs2734849) and SLC6A3 (rs464049, rs460000, and rs1042098) genes was differentially associated with short- and long-term neurobehavioral recovery following TBI, with rs460000 and rs464049 surviving multiple testing corrections. The findings provide preliminary evidence that genetic variation in genes involved in DRD2 expression and density (ANKK1) and dopamine transport (SLC6A3) plays a role in neurobehavioral recovery following pediatric TBI.
Keywords: : behavior, genetic factors, neurotransmitters, pediatric brain injury, TBI
Introduction
Traumatic brain injury (TBI) is one of the leading causes of morbidity and mortality in childhood.1 Currently, the meager amount of scientific evidence available to guide management of pediatric TBI is disproportionate to its large societal and medical impact. The majority of children sustaining moderate to severe TBI demonstrate neurobehavioral impairments in the first few months following injury, spanning domains of language, nonverbal skills, memory, attention, and executive functioning, as well as behavioral regulation and social skills.2 Considerable recovery of function can occur during the first 1–2 years post-injury; however, many children display long-term deficits that may extend into adulthood.3,4 Among the most persistent and disruptive sequelae are executive dysfunction and related child behavior problems.5,6 Executive and behavioral deficits have been shown to negatively impact long-term academic, occupational, social, and overall adaptive functioning.7,8 Increased understanding of the various factors that influence recovery of these functions is imperative to informing clinical management of TBI.6
The unexplained heterogeneity in recovery and outcomes following TBI is one of the most significant barriers to the development of effective prognostic tools and therapeutic interventions.9,10 The most well-established predictor of outcome following TBI is injury severity, most often measured using the Glasgow Coma Scale (GCS).11 Injury factors, in combination with demographic factors (e.g., age), however, typically account for only ∼35% of the variance in outcomes following moderate to severe TBI.12 Family environmental factors appear to account for additional variance in outcomes.13,14 Despite the known contributions of injury and environmental factors to recovery, substantial unexplained heterogeneity exists in neurobehavioral outcomes following pediatric TBI. Injuries of similar severity in similar environmental contexts can result in significantly disparate outcomes. Individual factors, such as genetic variability, may influence the pathophysiological response to injury, recovery, and repair processes, and response to intervention.15 Therefore, investigation of the influence of genetic factors on recovery is a critical step for advancing our understanding of variability in neurobehavioral outcomes following pediatric TBI.
Genes modulating the dopamine systems are of particular interest among those likely to influence neurobehavioral recovery following early TBI. Dopamine is a neurotransmitter that plays a key role in cognition and behavior, especially in the executive functions, including working memory, cognitive control, cognitive flexibility, and planning,16,17 as well as in related behaviors, including hyperactivity, impulse control, and reward sensitivity.18–20 Executive functions are mediated by the mesocortical and mesolimbic dopamine pathways, which originate in the ventral tegmental area of the midbrain and project to the prefrontal cortex, anterior cingulate cortex, anterior temporal structures, and the basal forebrain.17,21 In the developing brain, dopamine plays a role in myelination, synaptogenesis, and pruning processes.22–25 Throughout childhood, the development of executive functions and related behaviors is associated with maturation of the dopamine system.26 Dopamine, dopamine transporter, and D1 and D2 receptor levels increase in the striatum until middle childhood, with more protracted development of dopaminergic modulation of the prefrontal cortex occurring into adolescence.27–29
Modulation of various components of the dopamine system by dopamine-related genes can result in disrupted neurobehavioral functioning. The “inverted-U” hypothesis suggests that optimal functioning of frontostriatal circuits underlying executive functions and related behaviors relies on optimal levels of dopamine, whereby too much or too little results in impaired function.17,30 Differences in executive functions and related behaviors are associated with variation in dopamine-related genes in typically developing children,31–33 as well as in conditions such as attention-deficit/hyperactivity disorder (ADHD),34,35 oppositional defiant disorder, and conduct disorder.19 Evidence from in vivo TBI models suggests that functional reductions in dopamine can occur after TBI.36 Moreover, pharmacotherapies targeting dopamine system pathways are beneficial for post-injury neurobehavioral function in both adults and children; however, the specific mechanisms of TBI pathology targeted by these therapies, the most effective time course, and which individuals will reap the most benefit remain unclear.36 Recent studies of TBI in adults have hypothesized that the effects of low dopamine on neurobehavioral functioning may be related to genetic variants that modulate dopamine levels.37–39
Several sources of variation within genes that modulate dopamine signaling may influence neurobehavioral recovery following pediatric TBI. Dopamine receptors are distributed at various densities throughout the frontostriatal dopamine system and receive the transmission of dopamine across the synapse. Genetic variability associated with dopamine receptor D2 (DRD2) expression and density has been examined by assessing variation within the DRD2 gene as well as the ankyrin repeat and kinase domain containing 1 (ANKK1) gene immediately upstream of DRD2.39 Studies have shown that genetic variation within polymorphisms rs1800497, rs11604671, and rs4938016 in ANKK1 and rs6279 in DRD2 is associated with neuropsychological performance within the 1st year post-injury in adults.38–41 Less is known regarding the influence of other dopamine receptor genes on neurobehavioral function following TBI.
In addition to dopamine receptors, dopamine's effects are regulated through complex processes of release, reuptake, and metabolism. The vesicular monoamine transporter (VMAT) facilitates dopamine transport into synaptic vesicles for release. A recent study found that the rs363226 genotype within the vesicular monoamine transporter 2 (VMAT2) gene, also known as solute carrier family 18 member A2 (SLC18A2), was associated with cognitive impairment at 6 months following severe TBI in adults.42 In most brain regions, dopamine is removed from the synapse primarily by the dopamine transporter (DAT). Several studies have implicated a variable number tandem repeat (VNTR rs28363170) within the dopamine transporter gene, solute carrier family 6 member 3 (SLC6A3), in influencing both short-term43–45 and long-term37 dopamine-relevant TBI pathology; however, a study in adults with severe TBI failed to find a significant association with composite cognitive scores at 6 or 12 months post-injury.38
In the prefrontal cortex, dopamine transporter expression is lower and dopamine clearance is mediated by catechol-o-methyltransferase (COMT), an enzyme that metabolizes dopamine.46,47 The COMT gene has received the most research attention in TBI, but has produced mixed results. Variation within the Val158Met (rs4680) single nucleotide polymorphism (SNP) has been associated with poorer neuropsychological performance in a cohort of adults with mild-severe TBI assessed within 1 year post-injury,48 and in a cohort of adults with mild TBI examined 6 months post-injury;49 however, the COMT gene showed no significant associations with post-injury performance in a cohort of adults with moderate-severe TBI relative to healthy controls assessed within 3 months post-injury,50 or in the largest investigated cohort of adults with moderate-severe TBI assessed 1 month post-injury.51 In the only prior study examining a dopamine-related gene in association with neurobehavioral function following TBI in childhood, variation within rs4680 was associated with poorer neuropsychological function across groups at 18 months following mild-severe TBI or orthopedic injury (OI).52
Although initial investigations of the influence of dopamine-related genes on neurobehavioral function following TBI are promising, the present study addresses three important limitations of the existing literature. First, few studies have included a non-brain-injured comparison group for examining the differential association of dopamine-related genes with neurobehavioral recovery following injury to the brain. Without examination of the moderating effect of brain injury, previous studies have been limited in their ability to differentiate effects of dopamine genes on neurobehavioral recovery after TBI from differences that may reflect premorbid genetic influences on neurobehavioral function, as have been found in individuals without brain injuries.49 Second, no studies have examined the association of dopamine-related genes with neurobehavioral outcomes beyond 18 months post-injury. The effects of dopamine on neurobehavioral function may vary over time post-injury, with different pathophysiological processes occurring during the acute and chronic phases of recovery and influencing recovery trajectories over time. Third, the relation of dopamine-related genes to neurobehavioral function following TBI has almost exclusively been studied in adults. Given the important role that dopamine systems play in brain development and development of executive functions and related behaviors throughout childhood and into adolescence, dopamine-related genes may play an even more prominent role in neurobehavioral recovery following TBI sustained during childhood.
Therefore, the primary aim of the present study was to examine the differential effects of dopamine-related genes on neurobehavioral recovery trajectories over time in children who sustained early childhood TBI relative to children who sustained OI. In addition, because we might expect different genetic effects during the short- and long-term phases of recovery, we also had an exploratory aim of examining the differential effects of dopamine-related genes on short-term (∼ 6 months post-injury) and long-term (∼7 years post-injury) neurobehavioral recovery. We hypothesized that variation within dopamine-related genes would be associated with changes in executive function and behavioral adjustment over time post-injury, as well as in the short and long term, and that these associations would be moderated by injury type (e.g., TBI vs. OI).
Materials and Methods
Participants
Participants were part of a prospective, longitudinal study evaluating outcomes of children who sustained a TBI or OI between the ages of 3 and 7 years.5,6,53 They were recruited from three tertiary care children's hospitals and one tertiary care, general hospital in Ohio. Participants completed assessments at multiple visits, including the immediate post-acute period (0–3 months after injury); 6 (referred to as “short-term” throughout), 12, and 18 months after injury; and an average of 3.5 and 7 years after injury (referred to as “long-term” throughout).
Inclusion criteria included hospitalization overnight for traumatic injury (TBI or OI), no evidence of child abuse as the cause of the injury, no history of documented neurological problems or developmental delays pre-injury, and English as the primary language spoken in the home. Severity of TBI was characterized using the lowest post-resuscitation GCS score.11 Severe TBI was defined as a GCS score ≤8. Moderate TBI was defined as a GCS score of 9–12 with or without abnormal neuroimaging (moderate TBI) or a higher GCS score with abnormal neuroimaging as defined by an intracranial or parenchymal injury or depressed skull fracture (complicated mild TBI). Mild TBI was defined as a GCS score ≥13 without abnormal neuroimaging. The OI group included children who had sustained a bone fracture (not including skull fractures), had an overnight stay in the hospital, and did not exhibit alterations in consciousness or other signs or symptoms of head trauma or brain injury. The study was approved by the institutional review boards at each of the participating medical centers and informed consent was obtained from participating caregivers.
DNA collection and genotyping
DNA was collected from saliva samples and purified using the United States Food and Drug Administration (FDA)-cleared Oragene OG-500 self-collection tubes (DNA Genotek, Ottawa, Canada). Oragene allows the collection, stabilization, and long-term storage of DNA from saliva at ambient temperature. Salivary DNA is a valid and reliable alternative to blood DNA for high-throughput genotyping.54 The HumanExome v1.1 Bead Chip (Illumina, San Diego, CA) was used to perform genotyping using the Illumina iScan system to identify SNPs associated with the following dopamine-related genes: DRD2, SLC6A3, SLC18A2, COMT, and ANKK1. SNPs were selected within genes' boundaries plus 5 kilo bases upstream and downstream from the genes. These candidate genes were chosen based on their associations with outcomes following TBI in adults and their biological relevance in dopamine pathways in adults and children as described. Prior to analysis, quality of SNP calls from the chip was evaluated. SNPs that failed Hardy–Weinberg equilibrium (p < 0.0001) or had minor allele frequencies <10% were excluded. Thresholds for quality control for call rates at individual and SNP levels were 99% and 90%, respectively. Cryptic relatedness was checked using Graphical Representation of Relationships (GRR)55 (http://csg.sph.umich.edu/abecasis/GRR). Principal component analysis was employed to confirm European and African continental ancestry, which aligns with self-reported white and black race, using 200 validated ancestry informative markers and HapMap genotypic data from individuals of known ancestry as referent groups. Concordance with self-reported race was >95%. Results of primary and exploratory analyses were similar when run with only participants of European ancestry; therefore, both ancestral groups were retained in analyses to preserve power. JMP Genomics 8.0 (SAS, Cary, NC) was used to determine the degree of linkage disequilibrium (LD) between SNPs within this sample in genes exhibiting nominal association (p < 0.05) with study outcomes.
Outcome measures
Parents completed the age-appropriate form of the Behavior Rating Inventory of Executive Function (BRIEF) at all visits, the first of which was based on the child's behavior prior to injury, used in analysis to control for premorbid differences in child functioning. The BRIEF is a parent-report measure of child executive function as evident in everyday behavior.56–58 We analyzed the age-standardized global executive composite T score (BRIEF GEC) to assess global executive function behaviors. Higher scores reflect poorer executive functioning.
Parents completed the age-appropriate form of the Child Behavior Checklist (CBCL)59 at all visits, the first of which was based on retrospective recall of the child's behavior prior to injury to control for premorbid differences in child functioning. The CBCL is a parent-report measure of child behavioral adjustment and possesses high test–retest reliability and criterion-related validity. We analyzed the age- and sex-standardized Total Problems T score to assess child behavioral adjustment. Higher scores reflect poorer behavioral adjustment.
Both outcome measures are National Institute of Neurological Disorders and Stroke (NINDS) recommended common data elements for pediatric TBI and are well validated for the pediatric TBI population.60
Statistical analysis
Statistical analyses were conducted using SAS 9.4 (SAS, Cary, NC). Prior to analyses, child outcome data were reviewed for plausibility according to the following rules to reduce the potential influence of outliers: 1) participants with changes in outcome scores between the short-term and long-term time point that exceeded three standard deviations of change were excluded from respective analyses (n = 2 from BRIEF analysis; n = 0 from CBCL analysis); and 2) outcome scores of T > 90 (4 SDs above the normative mean) were winsorized to 90 (n = 5 from BRIEF analysis; n = 1 from CBCL analysis).
Our primary analysis examined genetic associations with outcome trajectories over time, using a random coefficients longitudinal mixed model including all time points. Using longitudinal analyses, we examined the association of each SNP with executive function and behavioral adjustment recovery trajectories over time and the moderating effect of injury group (TBI vs. OI) on these associations (e.g., SNP × injury group × time interaction). Mixed models included random intercepts, slopes, and subjects. Random slopes were modeled over time. Our exploratory analysis used multiple linear models to examine genetic associations with cross-sectional outcomes in the short-term (6 months post-injury; 0.62 ± 0.08 years) and long-term (6.82 ± 1.12 years post-injury) phases of recovery, corresponding to the second and sixth study visits, respectively. We employed multiple linear models to examine the association of each SNP with short-term and long-term executive functioning and behavioral adjustment, and the moderating effect of injury group (TBI vs. OI) on these associations (e.g., SNP × injury group interaction). In all models, higher level interactions were examined first, followed by each lower level interaction if nonsignificant at a nominal (p < 0.05) level. In all association tests, we used an additive genetic model in which major homozygotes were coded as 0, heterozygotes were coded as 1, and minor homozygotes were coded as 2.
Covariates initially included and then trimmed if nonsignificant were the child's pre-injury functioning on the measure of interest and socioeconomic status (SES) (defined by averaging sample z scores for maternal education and median income).53 Continental ancestry was covaried in all models, dichotomized as European versus African, to account for potential confounding related to race. Results were evaluated after correcting for multiple testing using a Bonferroni adjustment taking into account average pairwise LD correlation between all SNPs considered in the analyses (r = 0.16) as a way to correct for the number of independent tests.55 The results of the SNP times injury group interaction for each SNP are provided in Manhattan plots with both nominal (p < 0.05) and multiple testing-adjusted (p = 0.0027) significance thresholds indicated. For each interaction that was at least nominally significant, we plotted regression lines for each group by allele status to examine the direction of the interaction and identify the risk allele. When plotting the data, SNPs with <10 participants for minor allele homozygotes were combined with heterozygotes to ensure more robust estimates.
Results
Sample description
Of the 221 participants enrolled in the original study, 141 provided DNA samples. Participants with genetic data did not differ significantly from those without genetic data in demographic characteristics or on various study measures (see Table 1). Of those with genetic data, 140 of 141 participants had covariate and outcome data for at least one outcome visit to be included in the present analyses. Of those included in the present analyses, 10 had mild TBI, 42 had complicated mild to moderate TBI, 16 had severe TBI, and 72 had OI. The TBI and OI groups did not differ significantly in race, sex, age at injury, age at assessment, or SES (Table 2). As shown in Table 2, children with TBI had poorer executive functioning and poorer behavioral adjustment relative to children with OI during both the short-term and long-term phases of recovery. These results are consistent with our prior reports based on the larger sample from this cohort.61,62
Table 1.
Comparison of Participants with and without Genetic Data
| Demographics and outcome measures | Participants with genetic data (n = 141) | Participants without genetic data (n = 80) | p |
|---|---|---|---|
| Gender, n (%) | 0.108 | ||
| Male | 76 (53.9) | 52 (65.0) | |
| Female | 65 (46.1) | 28 (35.0) | |
| Race, n (%) | 0.548 | ||
| White | 104 (73.8) | 56 (70.0) | |
| Black | 37 (26.2) | 24 (30.0) | |
| Age at injury in years, mean (SD) | 5.10 (1.11) | 4.95 (1.09) | 0.345 |
| Median family income, mean (SD) | $59,606 (22,780) | $61,998 (26,978) | 0.489 |
| Highest maternal education, n (%) | 0.579 | ||
| < high school | 15 (10.6) | 10 (13.2) | |
| ≥ high school | 126 (89.4) | 66 (86.8) | |
| BRIEF: Global Executive Composite (T-Score), mean (SD) | 49.60 (13.90) | 50.19 (11.87) | 0.762 |
| CBCL Total Problems, (T-Score), mean (SD) | 47.92 (12.82) | 48.68 (12.38) | 0.687 |
| GCS, mean (SD) | 11.23 (4.45) | 11.45 (4.44) | 0.814 |
BRIEF, Behavior Rating Inventory of Executive Function; CBCL, Child Behavior Checklist; GCS, Glasgow Coma Scale.
Table 2.
Participant Characteristics by Injury Group
| OI (n = 72) | TBI (n = 68) | p | |
|---|---|---|---|
| Gender, n (%) | 0.479 | ||
| Male | 37 (51.4) | 39 (57.4) | |
| Female | 35 (48.6) | 29 (42.6) | |
| Race, n (%) | 0.231 | ||
| White | 59 (81.9) | 50 (73.5) | |
| Non-white | 13 (18.1) | 18 (26.5) | |
| Age at injury in years, mean (SD) | 5.10 (1.08) | 5.08 (1.15) | 0.912 |
| Age at short-term follow-up | 5.74 (1.08) | 5.70 (1.14) | 0.827 |
| Age at long-term follow-up | 11.92 (1.09) | 11.94 (1.17) | 0.906 |
| zSES, mean (SD) | 0.12 (0.96) | -0.12 (0.98) | 0.134 |
| GCS, mean (SD) | 11.25 (4.48) | ||
| BRIEF GEC short-term, LSM (SE) | 50.71 (1.12) | 54.32 (1.19) | 0.032a |
| BRIEF GEC long-term, LSM (SE) | 50.18 (1.23) | 58.14 (1.26) | <0.001a |
| CBCL, Total Problems short-term, LSM (SE) | 46.71 (0.83) | 49.73 (0.88) | 0.016a |
| CBCL Total Problems long-term, LSM (SE) | 47.08 (1.18) | 54.19 (1.20) | <0.001a |
Analysis of covariance (ANCOVA) controlling for premorbid child functioning on each measure and zSES.
BRIEF GEC, Behavior Rating Inventory of Executive Function; CBCL, Child Behavior Checklist; GCS, Glasgow Coma Scale; LSM, least squares mean; OI, orthopedic injury; TBI, traumatic brain injury; zSES, socioeconomic status z score.
SNPs investigated
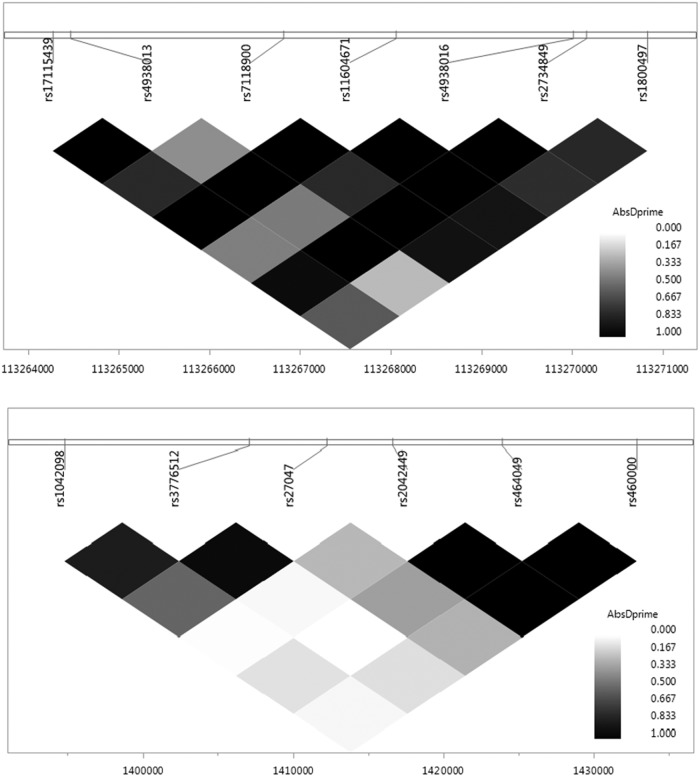
Thirty-two SNPs across the five dopamine-related genes met quality control and inclusion criteria. The majority of SNPs (30 out of 32) were located within boundaries of selected genes, and two SNPs were located within the 5 kilo bases interval downstream from DRD2 and COMT genes. Table 3 contains descriptive information for each SNP. LD plots were generated for the ANKK1 and SLC6A3 genes based on their associations with study outcomes (see Fig. 1).
Table 3.
Descriptive Information for SNPs Investigated
| Gene | SNP | Chrom | Position | Major allele | Minorallele | Minor allele frequency |
|---|---|---|---|---|---|---|
| SLC6A3 | rs1042098 | 5 | 1394815 | A | G | 0.22 |
| SLC6A3 | rs3776512 | 5 | 1407116 | G | A | 0.13 |
| SLC6A3 | rs27047 | 5 | 1412251 | A | G | 0.28 |
| SLC6A3 | rs2042449 | 5 | 1416646 | G | A | 0.18 |
| SLC6A3 | rs464049 | 5 | 1423905 | G | A | 0.47 |
| SLC6A3 | rs460000 | 5 | 1432825 | C | A | 0.27 |
| SLC18A2 | rs363341 | 10 | 119010465 | G | A | 0.33 |
| SLC18A2 | rs363343 | 10 | 119014948 | A | C | 0.25 |
| ANKK1 | rs17115439 | 11 | 113264272 | G | A | 0.38 |
| ANKK1 | rs4938013 | 11 | 113264470 | C | A | 0.30 |
| ANKK1 | rs7118900 | 11 | 113266821 | G | A | 0.20 |
| ANKK1 | rs11604671 | 11 | 113268059 | G | A | 0.44 |
| ANKK1 | rs4938016 | 11 | 113270015 | C | G | 0.35 |
| ANKK1 | rs2734849 | 11 | 113270160 | A | G | 0.44 |
| ANKK1 | rs1800497 | 11 | 113270828 | G | A | 0.23 |
| DRD2a | rs10891549 | 11 | 113278447 | A | G | 0.46 |
| DRD2 | rs2002453 | 11 | 113289298 | G | A | 0.31 |
| DRD2 | rs2734833 | 11 | 113292920 | A | G | 0.47 |
| DRD2 | rs1079597 | 11 | 113296286 | G | A | 0.15 |
| DRD2 | rs4620755 | 11 | 113309619 | G | A | 0.15 |
| DRD2 | rs4245148 | 11 | 113320419 | G | A | 0.22 |
| COMT | rs737866 | 22 | 19930109 | A | G | 0.26 |
| COMT | rs174674 | 22 | 19934025 | G | A | 0.32 |
| COMT | rs5993883 | 22 | 19937638 | C | A | 0.49 |
| COMT | rs740603 | 22 | 19945177 | A | G | 0.50 |
| COMT | rs165656 | 22 | 19948863 | G | C | 0.48 |
| COMT | rs2239393 | 22 | 19950428 | A | G | 0.45 |
| COMT | rs4818 | 22 | 19951207 | G | C | 0.39 |
| COMT | rs4680 | 22 | 19951271 | G | A | 0.41 |
| COMT | rs9332381 | 22 | 19956553 | C | G | 0.12 |
| COMT | rs165599 | 22 | 19956781 | A | G | 0.39 |
| COMTa | rs165815 | 22 | 19959473 | A | G | 0.26 |
Single nucleotide polymorphism (SNP) is outside of gene region, within 5 kilo bases of extension downstream.
SLC6A3, solute carrier family 6 member 3; SLC18A2, solute carrier family 18 member A2; ANKK1, ankyrin repeat and kinase domain containing 1; DRD2, dopamine receptor D2; COMT, catechol-o-methyltransferase.
FIG. 1.
Linkage disequilibrium (LD) plots for single nucleotide polymorphisms (SNPs) examined in the ankyrin repeat and kinase domain containing 1 (ANKK1) (top) and solute carrier family 6 member 3 (SLC6A3) (bottom) genes.
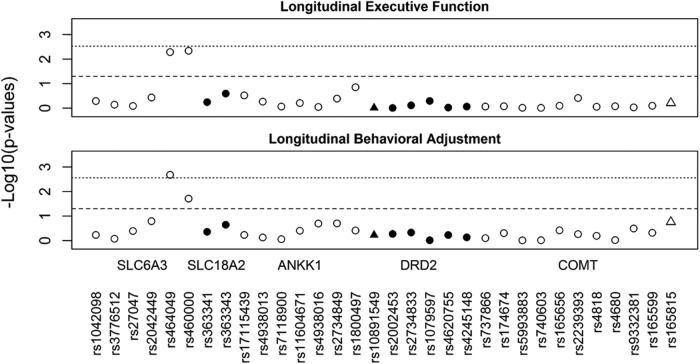
Associations of dopamine genes with longitudinal recovery trajectories
Figure 2 illustrates the SNP by injury group interactions that met nominal and multiple testing-adjusted significance thresholds for their effect on longitudinal recovery trajectories of executive functioning and behavioral adjustment. The three-way interaction of SNP by injury group by time was not significant for either child outcome, suggesting that differences in recovery trajectories among groups remained consistent across time since injury. Lower level analyses revealed significant interactions of SNP by injury group across time for rs464049 and rs460000 SNPs in SLC6A3 on both executive functioning (p = 0.005 and p = 0.005) and behavioral adjustment (p = 0.002 and p = 0.019) recovery trajectories. The interactions of SNP by injury group across time for rs464049 SNP in SLC6A3 on behavioral adjustment remained significant after multiple testing corrections.
FIG. 2.
Manhattan plots for all single nucleotide polymorphisms (SNPs) examined in association with longitudinal recovery trajectories of executive function and behavioral adjustment. Triangles represent SNPs located in 5 Kb extensions from dopamine receptor D2 (DRD2) and catechol-o-methyltransferase (COMT). The dashed line indicates nominal significance at p < 0.05. The dotted line indicates multiple testing-adjusted significance (p = 0.0027), taking into account linkage disequilibrium (LD) correlation between SNPs.
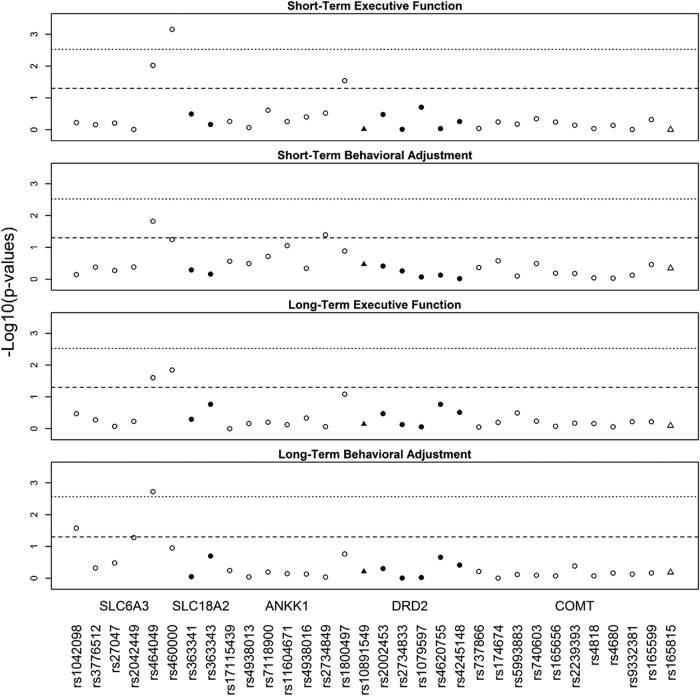
Associations of dopamine genes with short- and long-term recovery
Figure 3 illustrates the SNP by injury group interactions that met nominal and multiple testing significance thresholds for their effect on short- and long-term executive functioning and behavioral adjustment. The rs464049 and rs460000 SNPs in SLC6A3 exhibited interactions with injury group on both short- (p = 0.010 and p < 0.001) and long-term (p = 0.025 and p = 0.014) executive functioning. The rs1800497 SNP (p = 0.029) in ANKK1 also exhibited interactions with injury group on short-term executive functioning. The rs464049 SNP in SLC6A3 also exhibited interactions with injury group for both short- (p = 0.015) and long-term (p = 0.002) behavioral adjustment. In addition, the rs2734849 SNP (p = 0.040) in ANKK1 exhibited an interaction with injury group on short-term behavioral adjustment. Finally, the rs1042098 SNP (p = 0.027) in SLC6A3 exhibited an interaction with injury group on long-term behavioral adjustment. The interactions of SNP by injury group for rs460000 in SLC6A3 on short-term executive functioning and for rs464049 SNP in SLC6A3 on long-term behavioral adjustment remained significant after multiple testing corrections.
FIG. 3.
Manhattan plots for all single nucleotide polymorphisms (SNPs) examined in association with short- and long-term executive function and behavioral adjustment. Triangles represent SNPs located in 5 Kb extensions from dopamine receptor D2 (DRD2) and catechol-o-methyltransferase (COMT). The dashed line indicates nominal significance at p < 0.05. The dotted line indicates multiple testing-adjusted significance (p = 0.0027), taking into account linkage disequilibrium (LD) correlation between SNPs.
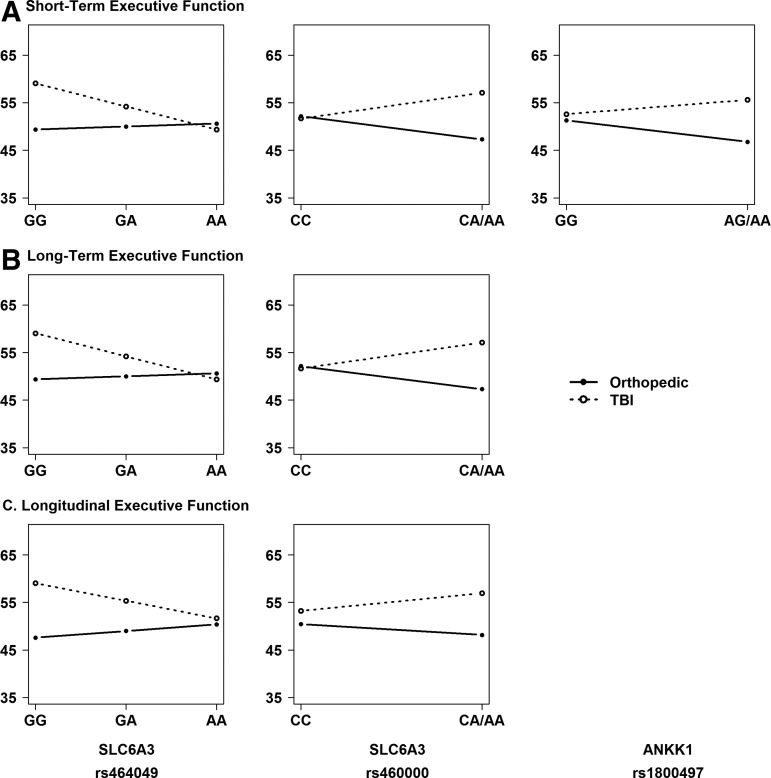
Identification of risk alleles
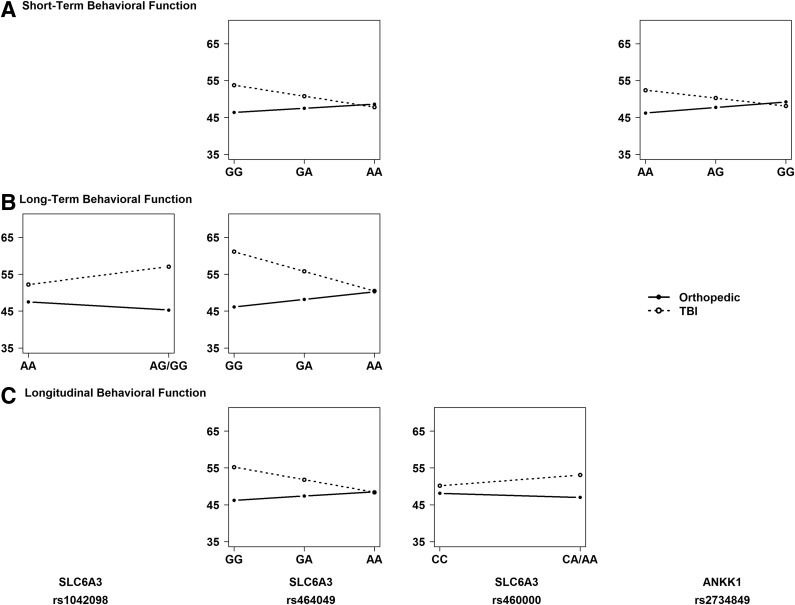
Given the presence of significant SNP by injury group interactions, we performed stratified, descriptive analyses to understand the direction of the association of the variants with outcomes within each injury group and to identify the risk allele. Figures 4 and 5 illustrate the direction of associations of genetic variation in SLC6A3 and ANKK1 with executive function (Fig. 4) and behavioral adjustment (Fig. 5) outcomes within each injury group at the short- and long-term phases of recovery, as well as on longitudinal recovery trajectories. Homozygotes for the minor allele were combined with heterozygotes because of small cell sizes for rs1042098, rs460000, and rs1800497. Results revealed the following risk alleles: G for rs1042098, G for rs464049, A for rs460000, A for rs2734849, and A for rs1800497.
FIG. 4.
Regression lines for each injury group by allele status plotted to examine the direction of the interaction and identify the risk allele. An additive model was used to plot regression lines for all single nucleotide polymorphisms (SNPs). SNPs with <10 participants for minor allele homozygotes were combined with heterozygotes.
FIG. 5.
Regression lines for each injury group by allele status plotted to examine the direction of the interaction and identify the risk allele. An additive model was used to plot regression lines for all single nucleotide polymorphisms (SNPs). SNPs with <10 participants for minor allele homozygotes were combined with heterozygotes.
Discussion
The present study sought to examine the association of dopamine-related genes with neurobehavioral recovery trajectories over time, as well as short- and long-term neurobehavioral recovery, in children who had sustained early childhood TBI relative to children with OI. Genetic variation within the SLC6A3 (rs464049 and rs460000) gene was differentially associated with neurobehavioral recovery trajectories over time following TBI relative to OI. In addition, genetic variation within ANKK1 (rs1800497 and rs2734849) and SLC6A3 (rs464049, rs460000, and rs1042098) was differentially associated with short- and long-term neurobehavioral recovery following TBI. The findings provide preliminary evidence that genetic variation in genes involved in DRD2 expression and density (ANKK1) and dopamine transport (SLC6A3) plays a role in neurobehavioral recovery following pediatric TBI.
Our most robust and consistent findings were for SNPs within the SLC6A3 gene that encodes expression of the dopamine transporter. The rs1042098 SNP exhibited an interaction with injury group on long-term behavioral adjustment, and the rs464049 and rs460000 SNPs exhibited interactions with injury group across time on both executive functioning and behavioral adjustment recovery trajectories. In the cross-sectional analyses, the rs464049 and rs460000 SNPs exhibited interactions with injury group on both short- and long-term executive functioning, and the rs464049 SNP exhibited interactions with injury group for both short- and long-term behavioral adjustment. Risk alleles were A for rs460000, G for rs464049, and G for rs1042098. These results provide the first evidence for an association of variation within the SLC6A3 gene with clinical outcomes following TBI.
Although little is known about the functional impact of the significant SNPs associated with recovery in the present findings, other polymorphisms within this gene have shown associations with dopamine transporter expression and with pathology in adult TBI. Experimental TBI studies have demonstrated reduced maximal evoked dopamine overflow, altered dopamine clearance kinetics, and dopamine transporter protein expression changes post-injury.63 Prior studies in adult TBI have shown reduced striatal dopamine transporter binding in association with the VNTR rs28363170 in SLC6A3,37 although examination of clinical outcomes failed to find a significant association of this polymorphism with composite cognitive scores at 6 or 12 months following severe TBI.38,39,64 As we did not directly assess this VNTR variant, it is not possible to determine if our variants are contributing independently to TBI recovery. Nevertheless, the significance of SLC6A3 for both trajectories of neurobehavioral recovery over time and long-term recovery in the present study might suggest that genetic variation in dopamine transporter expression may underlie the chronic pathology associated with persistent neurobehavioral impairment years after early childhood TBI.
Our exploratory cross-sectional analyses revealed additional differential associations with outcomes following TBI involving the ANKK1 gene. The rs1800497 SNP in ANKK1 exhibited interactions with injury group on short-term executive functioning, with post-hoc analyses showing poorest executive functioning in children with TBI who were carriers of the A allele, referred to as A1 in prior literature. Although this association failed to survive multiple comparison corrections, confidence in the findings is bolstered by its consistency with prior research in adult TBI. Several studies have shown associations of rs1800497 with cognitive outcomes following TBI in adulthood,38–41 although findings have been mixed with regard to the risk allele or genotype most closely associated with these outcomes.39,64 Because our study included a non-brain-injured control group, our findings not only generalize the involvement of the ANKK1 gene to outcomes following TBI in childhood, but also lend evidence for a specific role of this gene in recovery from injury to the brain. The results for ANKK1 rs1800497 suggest a role for DRD2 expression and density in neurobehavioral recovery following TBI in childhood. Studies of healthy individuals suggest that A1 carriers show lower receptor densities, reduced receptor binding, and increased dopamine synthesis rate, especially in the striatum.65–67 In view of the mixed findings on the role of the rs1800497 in neurobehavioral outcomes of adult TBI, further study is needed to clarify the effects of genetic variation within ANKK1 on dopamine neurotransmission following brain injury.
Also within ANKK1, our results showed an interaction between SNP rs2734849 and injury group on short-term behavioral adjustment, with post-hoc analyses indicating poorest behavioral adjustment in children with TBI who were homozygous for the A allele. In contrast to the previous evidence for a role of rs1800497 in outcome following TBI, no previous studies have implicated rs2734849 in recovery from TBI. Moreover, little is known regarding the role of this SNP aside from possible involvement in dyskinesia in Parkinson's disease68 and in nicotine dependence.69 This SNP may serve as a new target of investigation in future studies of pediatric TBI.
Given that our exploratory cross-sectional analyses identified an additional gene (ANKK1) that was not significantly associated with outcomes in our longitudinal models, these findings might suggest that genetic variation related to DRD2 expression and density is more salient in determining neurobehavioral outcomes in the first several months post-injury, whereas genetic variation related to dopamine transport (SLC6A3) has more lasting influences on neurobehavioral recovery over time. Our longitudinal mixed models may have been underpowered to detect a three-way interaction among our ANKK1 SNPs, injury group, and time. Viewed from a more conservative perspective, however, it is possible that our associations of ANKK1 with short-term outcomes reflect type I error, as these findings were not identified in the more conservative longitudinal mixed models. Studies with larger samples are needed to replicate the present results.
The findings of significant associations of dopamine-related genes with neurobehavioral recovery following pediatric TBI may add to a conceptual model that incorporates the various risk and protective factors contributing to neurobehavioral phenotypes following pediatric TBI. In addition to being implicated in recovery from TBI, the SLC6A3 gene and other dopamine genes are also implicated in risk for ADHD70,71 and related endophenotypes, including poor inhibitory control,72 mood instability,73 and reduced working memory.74 The substantial overlap of such traits in children who have sustained TBI and in those with ADHD, combined with potential commonalities in genetic risk factors, suggests that shared pathological mechanisms may contribute to shared clinical presentations.71 This hypothesis is supported by the frequent diagnosis of secondary ADHD in children with TBI,75,76 as well as evidence for poorer recovery and exacerbation of symptoms following TBI in children with premorbid neurobehavioral difficulties.77–80 Conceptualizing traumatic injury to the brain as an environmental event that interacts with an individual's genetic predisposition, outcome, and recovery following TBI in childhood would be consistent with a diathesis-stress model in which children with genetic vulnerabilities for dopamine-related neurobehavioral difficulties experience poorer outcomes because of an emergence or exacerbation of these problems following traumatic disruption to the dopamine system.
The other side of the risk perspective discussed is the potential protective effects that certain genotypes may confer following TBI. Interestingly, cognitive neuroscience research into learning and cognitive training has also implicated roles for both striatal dopamine D2 receptor density/binding and dopamine transporter expression, as well as their genetic determinants. Dopamine may be important for plasticity by enhancing neural sprouting and synaptogenesis.81 Positron emission tomography (PET) studies have shown changes in striatal D2 binding following working memory training,82 and lower D2 densities have been associated with better motor sequence learning83 in healthy adults. Recently, children and adolescents who were T/A1 carriers of ANKK1 rs1800497 demonstrated greater improvements during working memory training.84 Similarly, variation in SLC6A3 influences improvements in working memory following cognitive training in preschool and school-age children.85,86 Taken together, these studies suggest that the ANKK1 and SLC6A3 genes may confer higher cognitive or neural plasticity84,87 that may be especially relevant to recovery from injury to the brain. Clinically, individuals with certain genotypes may be more responsive to cognitive or medical interventions following TBI. Identifying those who are more or less likely to respond to specific interventions is critical in treatment selection and optimizing recovery.
The present findings should be considered in light of several study limitations. Although the present study is one of the largest investigations of the association of genetic factors after TBI in childhood, larger sample sizes are needed to replicate the findings. Because the study population consisted primarily of children with moderate TBI and all injuries were sustained during early childhood, sample size limitations precluded investigation of potential differences in genetic effects in relation to injury severity or age at injury. Another significant limitation is that measurement of child outcomes relied entirely on parent report, which may be subject to bias. Nevertheless, parent reports, especially of executive function behaviors, may be more ecologically valid than paper-and-pencil tests administered in a controlled environment.57 Future studies that also include objective, performance-based measures of neuropsychological functioning, however, would be informative. Related, our reliance on retrospective recall of premorbid child functioning is another limitation. Given our focus on dopamine-related genes, it would have also been informative to know how many children were taking dopaminergic agents during their recovery and whether these medications influenced the results. Unfortunately, however, sufficiently reliable and valid data on medication use was unavailable for analysis. Finally, the exome genotyping chip that we used did not capture all common variation in our genes of interest, hampering the identification of the most promising variants for future follow- up. Because our candidate gene approach evaluated only preselected dopamine genes, we did not explore the potential role of other polymorphisms or gene–gene interactions. Given the known moderating role of the family environment on recovery following TBI,53,88 examination of gene–environment interactions in relation to dopamine genes is underway.
This work aimed to advance understanding of unexplained heterogeneity in neurobehavioral outcomes following pediatric TBI through the identification of genetically influenced biological processes affecting recovery. The present study is the first to provide preliminary evidence for the influence of variation in dopamine-related genes on neurobehavioral recovery following TBI in children. Our inclusion of the OI group was a significant strength, allowing us to identify genetic effects related specifically to recovery from brain injury. The fact that genetic associations were specific to the TBI group and were evident even when controlling for premorbid child functioning further increases our confidence that these associations are related to recovery processes rather than reflecting premorbid expression of genetic differences. The longitudinal study design also permitted examination of genetic effects on recovery trajectories over time as well as on both short- and long-term recovery. Elucidation of biological processes underlying neurobehavioral recovery following pediatric TBI has the potential to substantially advance the field by facilitating more individualized prognosis, the development of novel targets for intervention, and a reduction in public health burden.
Acknowledgments
Funding for this study was supported in part by the Rehabilitation Medicine Scientist Training Program (RMSTP) K-12 HD001097-16, National Institute for Child Health and Human Development K23HD074683-01A1, R01 HD42729, and Grant 8 UL1 TR000077 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other supporting agencies.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Faul M., Wald M.M., and Coronado V.G. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta [Google Scholar]
- 2.Beauchamp M.H., and Anderson V. (2013). Cognitive and psychopathological sequelae of pediatric traumatic brain injury. Handb. Clin. Neurol. 112, 913–920 [DOI] [PubMed] [Google Scholar]
- 3.Muscara F., Catroppa C., and Anderson V. (2008). The impact of injury severity on executive function 7–10 years following pediatric traumatic brain injury. Dev. Neuropsychol. 33, 623–636 [DOI] [PubMed] [Google Scholar]
- 4.Catroppa C., Anderson V.A., Morse S.A., Haritou F., and Rosenfeld J.V. (2007). Children's attentional skills 5 years post-TBI. J. Pediatr. Psychol. 32, 354–369 [DOI] [PubMed] [Google Scholar]
- 5.Wade S.L., Zhang N., Yeates K.O., Stancin T., and Taylor H.G. (2016). Social environmental moderators of long-term functional outcomes of early childhood brain injury. JAMA Pediatr. 170, 343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treble-Barna A., Zang H., Zhang N., Taylor G., Yeates K.O., and Wade S. (2017). Long-term neuropsychological profiles and their role as mediators of adaptive functioning after traumatic brain injury in early childhood. J. Neurotrauma 34, 353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson V., Brown S., Newitt H., and Hoile H. (2009). Educational, vocational, psychosocial, and quality-of-life outcomes for adult survivors of childhood traumatic brain injury. J. Head Trauma Rehabil. 24, 303–312 [DOI] [PubMed] [Google Scholar]
- 8.Rosema S., Muscara F., Anderson V., Godfrey C., Hearps S., and Catroppa C. (2015). The trajectory of long-term psychosocial development 16 years following childhood traumatic brain injury. J. Neurotrauma 32, 976–983 [DOI] [PubMed] [Google Scholar]
- 9.Hawryluk G.W., and Manley G.T. (2015). Classification of traumatic brain injury: past, present, and future. Handb. Clin. Neurol. 127, 15–21 [DOI] [PubMed] [Google Scholar]
- 10.Saatman K.E., Duhaime A.C., Bullock R., Maas A.I., Valadka A., Manley G.T., and Workskhop Scientific Team and Advisory Panel Members (2008). Classification of traumatic brain injury for targeted therapies. J. Neurotrauma 25, 719–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teasdale G., and Jennett B. (1974). Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 12.Maas A.I., Lingsma H.F., and Roozenbeek B. (2015). Predicting outcome after traumatic brain injury. Handb. Clin. Neurol. 128, 455–474 [DOI] [PubMed] [Google Scholar]
- 13.Yeates K.O., Taylor H.G., Walz N.C., Stancin T., and Wade S. (2010). The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology 24, 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wade S.L., Cassedy A., Walz N.C., Taylor H.G., Stancin T., and Yeates K.O. (2011). The relationship of parental warm responsiveness and negativity to emerging behavior problems following traumatic brain injury in young children. Dev. Psychol. 47, 119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conley Y.P., and Alexander S. (2011). Genomic, transcriptomic, and epigenomic approaches to recovery after acquired brain injury. PM R 3, S52–58 [DOI] [PubMed] [Google Scholar]
- 16.Diamond A. (2011). Biological and social influences on cognitive control processes dependent on prefrontal cortex. Prog. Brain Res. 189, 319–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cools R., and D'Esposito M. (2011). Inverted-U–shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 69, e113–e125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berridge K.C. (2007). The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology 191, 391–431 [DOI] [PubMed] [Google Scholar]
- 19.Matthys W., Vanderschuren L., and Schutter D. (2013). The neurobiology of oppositional defiant disorder and conduct disorder: altered functioning in three mental domains. Dev. Psychopathol. 25, 193–207 [DOI] [PubMed] [Google Scholar]
- 20.Pattij T., and Vanderschuren L.J.M.J. (2008). The neuropharmacology of impulsive behavior. Trends Pharmacol. Sci. 29, 192–199 [DOI] [PubMed] [Google Scholar]
- 21.Bannon M.J., and Roth R.H. (1983). Pharmacology of mesocortical dopamine neurons. Pharmacol. Rev. 35, 53–68 [PubMed] [Google Scholar]
- 22.Feng Y. (2008). Convergence and divergence in the etiology of myelin impairment in psychiatric disorders and drug addiction. Neurochem. Res. 33, 1940–1949 [DOI] [PubMed] [Google Scholar]
- 23.Shen W., Tian X., Day M., Ulrich S., Tkatch T., Nathanson N.M., and Surmeier D.J. (2007). Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat. Neurosci. 10, 1458–1466 [DOI] [PubMed] [Google Scholar]
- 24.Brocki K., Clerkin S.M., Guise K.G., Fan J., and Fossella J.A. (2009). Assessing the molecular genetics of the development of executive attention in children: focus on genetic pathways related to the anterior cingulate cortex and dopamine. Neuroscience 164, 241–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fasano C., Poirier A., DesGroseillers L., and Trudeau L.E. (2008). Chronic activation of the D2 dopamine autoreceptor inhibits synaptogenesis in mesencephalic dopaminergic neurons in vitro. Eur. J. Neurosci. 28, 1480–1490 [DOI] [PubMed] [Google Scholar]
- 26.Luciana M., Wahlstrom D., Porter J.N., and Collins P.F. (2012). Dopaminergic modulation of incentive motivation in adolescence: age-related changes in signaling, individual differences, and implications for the development of self-regulation. Dev. Psychol. 48, 844–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benes F.M. (2001). The development of prefrontal cortex: the maturation of neurotransmitter systems and thier interactions, in: Handbook of Developmental Cognitive Neuroscience. Alexander C., and Nelson M.L. (eds.). MIT Press: Boston, pps. 79–92 [Google Scholar]
- 28.Haycock J.W., Becker O., Ang L., Furukawa Y., Hornkiewicz O., and Kish S.J. (2003). Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J. Neurochem. 87, 574–585 [DOI] [PubMed] [Google Scholar]
- 29.Seeman P., Bzowej N.H., Guan H.C., Bergeron C., Becker L.E., Reynolds G.P., Bird E.D., Riederer P., Jellinger K., and Watanabe S. (1987). Human brain dopamine receptors in children and aging adults. Synapse 1, 399–404 [DOI] [PubMed] [Google Scholar]
- 30.Robbins T.W., and Arnsten A.F.T. (2009). The neuropsychopharmacology of fronto-executive function: Monoaminergic modulation. Annu. Rev. Neurosci. 32, 267–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnett J.H., Heron J., Goldman D., Jones P.B., and Xu K. (2009). Effects of catechol-O-methyltransferase on normal variation in the cognitive function of children. Am. J. Psychiatry 166, 909–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith H.J., Kryski K.R., Sheikh H.I., Singh S.M., and Hayden E.P. (2013). The role of parenting and dopamine D4 receptor gene polymorphisms in children's inhibitory control. Dev. Sci. 16, 515–530 [DOI] [PubMed] [Google Scholar]
- 33.Smith H.J., Sheikh H.I., Dyson M.W., Olino T.M., Laptook R.S., Durbin C.E., Hayden E.P., Singh S.M., and Klein D.N. (2012). Parenting and child DRD4 genotype interact to predict children's early emerging effortful control. Child Dev. 83, 1932–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faraone S.V., Perlis R.H., Doyle A.E., Smoller J.W., Goralnick J.J., Holmgren M.A., and Skylar P. (2005). Molecular genetics of attention-deficit/hyperactivity disorder. Biol. Psychiatry 57, 1313–1323 [DOI] [PubMed] [Google Scholar]
- 35.Li D., Sham P.C., Owen M.J., and He L. (2006). Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum. Mol. Genet. 15, 2276–2284 [DOI] [PubMed] [Google Scholar]
- 36.Bales J.W., Wagner A.K., Kline A.E., and Dixon C.E. (2009). Persistent cognitive dysfunction after traumatic brain injury: a dopamine hypothesis. Neurosci. Biobehav. Rev. 33, 981–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner A.K., Scanlon J.M., Becker C.R., Ritter A.C., Niyonjuru C., Dixon C.E., Conley Y.P., and Price J.C. (2014). The influence of genetic variants on striatal dopamine transporter and D2 receptor binding after TBI. J. Cereb. Blood Flow Metab. 34, 1328–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myrga J.M., Failla M.D., Ricker J.H., Dixon E., Conley Y.P., Arenth P.M., and Wagner A.K. (2015). A dopamine pathway gene risk score for cognitive recovery following traumatic brain injury: methodological considerations, preliminary findings, and interactions with sex. J. Head Trauma Rehabil. 31, E15–E29 [DOI] [PubMed] [Google Scholar]
- 39.Failla M.D., Myrga J.M., Ricker J.H., Dixon C.E., Conley Y.P., and Wagner A.K. (2015). Posttraumatic brain injury cognitive performance is moderated by variation within ANKK1 and DRD2 genes. J Head Trauma Rehabil. 30, E54–E66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McAllister T.W., Flashman L.A., Rhodes C.H., Tyler A.L., Moore J.H., Saykin A.J., McDonald B.C., Tosteson T.D., and Tsongalis G.J. (2008). Single nucleotide polymorphisms in ANKK1 and the dopamine D2 receptor gene affect cognitive outcome shortly after traumatic brain injury: a replication and extension study. Brain Inj. 22, 705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue J.K., Pronger A.M., Ferguson A.R., Temkin N.R., Sharma S., Rosand J., Sorani M.D., McAllister T.W., Cooper S.R., Puccio A.M., Okonkwo D.O., Diaz-Arrastia R., and Manley G.T. (2015). Association of a common genetic variant within ANKK1 with six-month cognitive performance after traumatic brain injury. Neurogenetics 16, 169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markos S.M., Failla M.D., Ritter A.C., Dixon C.E., Conley Y.P., Ricker J.H., Arenth P.M., Juengst S.B., and Wagner A.K. (2016). Genetic variation in the vesicular monoamine transporter: preliminary associations with cognitive outcomes after severe traumatic brain injury. J. Head Trauma Rehabil. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 43.Yan H.Q., Kline A.E., Ma X., Li Y., and Dixon C.E. (2002). Traumatic brain injury reduces dopamine transporter protein expression in the rat frontal cortex. NeuroReport 13, 1899–1901 [DOI] [PubMed] [Google Scholar]
- 44.Shimada R., Abe K., Furutani R., and Kibayashi K. (2014). Changes in dopamine transporter expression in the midbrain following traumatic brain injury: an immunohistochemical and in situ hybridization study in a mouse model. Neurol. Res. 36, 239–246 [DOI] [PubMed] [Google Scholar]
- 45.Wagner A.K., Ren D., Conley Y.P., Ma X., Zafonte R.D., Puccio A.M., Marion D.W., and Dixon C.E. (2007). Sex and genetic associations with cerebrospinal fluid dopamine and metabolite production after severe traumatic brain injury. J. Neurosurg. 106, 538–547 [DOI] [PubMed] [Google Scholar]
- 46.Chen J., Lipska B.K., Halim N., Ma Q.D., Matsumoto M., Melhem S., Kolachana B.S., Hyde T.M., Herman M.M., Apud J., Egan M.F., Kleinman J.E., and Weinberger D.R. (2004). Functional analysis of genetic variation in Catechol-O-Methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 75, 807–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sesack S.R., Hawrylak V.A., Matus C., Guido M.A., and Levey A.I. (1998). Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J. Neurosci. 18, 2697–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipsky R.H., Sparling M.B., Ryan L.M., Xu K., Salazar A.M., Goldman D., and Warden D.L. (2005). Association of COMT Val158Met genotype with executive functioning following traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 17, 465–471 [DOI] [PubMed] [Google Scholar]
- 49.Winkler E.A., Yue J.K., McAllister T.W., Temkin N.R., Oh S.S., Burchard E.G., Hu D., Ferguson A.R., Lingsma H.F., Burke J.F., Sorani M.D., Rosand J., Yuh E.L., Barber J., Tarapore P.E., Gardner R.C., Sharma S., Satris G.G., Eng C., Puccio A.M., Wang K.K.W., Mukherjee P., Valadka A.B., Okonkwo D.O., Diaz-Arrastia R., Manley G.T., and Investigators T.T.-T. (2016). COMT Val158Met polymorphism is associated with nonverbal cognition following mild traumatic brain injury. Neurogenetics 17, 31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willmott C., Ponsford J., McAllister T.W., and Burke R. (2013). Effect of COMT Val158Met genotype on attention and response to methylphenidate following traumatic brain injury. Brain Inj. 27, 1281–1286 [DOI] [PubMed] [Google Scholar]
- 51.Willmott C., Withiel T., Ponsford J., and Burke R. (2014). COMT Val158Met and Cognitive and functional outcomes after traumatic brain injury. J. Neurotrauma 31, 1507–1514 [DOI] [PubMed] [Google Scholar]
- 52.Kurowski B.G., Backeljauw B., Zang H., Zhang N., Martin L.J., Pilipenko V., Yeates K., Taylor H.G., and Wade S. (2015). Influence of Catechol-O-methyltransferase on executive functioning longitudinally after early childhood traumatic brain injury: preliminary findings. J. Head Trauma Rehabil. 31, E1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeates K.O., Taylor H.G., Walz N.C., Stancin T., and Wade S.L. (2010). The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children. Neuropsychology 24, 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abraham J.E., Maranian M.J., Spiteri I., Russell R., Ingle S., Luccarini C., Earl H.M., Pharoah P.P., Dunning A.M., and Caldas C. (2012). Saliva samples are a viable alternative to blood samples as a source of DNA for high throughput genotyping. BMC Med. Genomics 5, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abecasis G.R., Cherny S.S., Cookson W.O.C., and Cardon L.R. (2001). GRR: graphical representation of relationship errors. Bioinformatics 17, 742–743 [DOI] [PubMed] [Google Scholar]
- 56.Gioia G.A., Isquith P.K., Guy S.C., and Kenworthy L. (2000). Behavior Rating Inventory of Executive Function. Psychological Assessment Resources: Odessa, FL [Google Scholar]
- 57.Gioia G.A., and Isquith P.K. (2004). Ecological assessment of executive function in traumatic brain injury. Dev. Neuropsychol. 25, 135–158 [DOI] [PubMed] [Google Scholar]
- 58.Derogatis L., and Spencer P. (1982). The Brief Symptom Inventory: Administration, Scoring and Procedures Manual I. Clinical Psychology Research: Baltimore [Google Scholar]
- 59.Achenbach T.M., and Rescorla L.A. ( 2001). Manual for ASEBA School-Age Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families: Burlington, VT [Google Scholar]
- 60.McCauley S.R., Wilde E.A., Anderson V.A., Bedell G., Beers S.R., Campbell T.F., Chapman S.B., Ewings-Cobbs L., Gerring J.P., Gioia G.A., Levin H.S., Michaud L.J., Prasad M.R., Swaine B.R., Turkstra L.S., Wade S.L., Yeates K.O., and Workgroup P.T.O. (2012). Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J. Neurotrauma 29, 678–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narad M.E., Treble-Barna A., Peugh J., Yeates K.O., Taylor H.G., Stancin T. and Wade S.L. (2016). Recovery trajectories of executive functioning after pediatric TBI: a latent class growth modeling analysis. J. Head Trauma Rehabil. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 62.Treble-Barna A., Schultz H., Minich N., Taylor H.G., Yeates K.O., Stancin T., and Wade S.L. (in press). Long-term classroom functioning and its association with neuropsychological and academic performance following traumatic brain injury during early childhood. Neuropsychology [DOI] [PMC free article] [PubMed]
- 63.Wagner A.K., Sokoloski J.E., Ren D., Chen X., Khan A.S., Zafonte R.D., Michael A.C., and Dixon C.E. (2005). Controlled cortical impact injury affects dopaminergic transmission in the rat striatum. J. Neurochem. 95, 457–465 [DOI] [PubMed] [Google Scholar]
- 64.Myrga J.M., Juengst S.B., Failla M.D., Conley Y.P., Arenth P.M., Grace A.A., and Wagner A.K. (2016). COMT and ANKK1 genetics interact with depression to influence behavior following severe TBI: an initial assessment. Neurorehabil. Neuronal Repair 30, 920–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laakso A., Pohjalainen T., Bergman J., Kajander J., Haaparanta M., Solin O., Syvälahti E., and Hietala J. (2005). The A1 allele of the human D2 dopamine receptor gene is associated with increased activity of striatal L–amino acid decarboxylase in healthy subjects. Pharmacogenet. Genomics 15, 387–391 [DOI] [PubMed] [Google Scholar]
- 66.Thompson J., Thomas N., Singleton A., Piggott M., Lloyd S., Perry E.K., Morris C.M., Perry R.H., Ferrier I.N., and Court J.A. (1997). D2 dopamine receptor gene (DRD2) Taql A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics 7, 479–484 [DOI] [PubMed] [Google Scholar]
- 67.Jönsson E.G., Nöthen M.M., Grünhage F., Farde L., Nakashima Y., Propping P., and Sedvall G.C. (1999). Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol. Psychiatry 4, 290–296 [DOI] [PubMed] [Google Scholar]
- 68.Rieck M., Schumacher–Schuh A.F., Altmann V., Francisconi C.L., Faqundes P.T., Monte T.L., Callegari–Jacques S.M., Rieder C.R., and Hutz M.H. (2012). DRD2 haplotype is associated with dyskinesia induced by levodopa therapy in Parkinson's disease patients. Pharmacogenetics 13, 1701–1710 [DOI] [PubMed] [Google Scholar]
- 69.Huang W., Payne T.J., Ma J.Z., Beuten J., Dupont R.T., Inohara N., and Li M.D. (2009). Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology 34, 319–330 [DOI] [PubMed] [Google Scholar]
- 70.Gizer I.R., Ficks C., and Waldman I.D. (2009). Candidate gene studies of ADHD: a meta-analytic review. Hum. Genet. 126, 51–90 [DOI] [PubMed] [Google Scholar]
- 71.Hawi Z., Cummins T.D.R., Tong J., Johnson B., Lau R., Samarrai W., and Bellgrove M.A. (2015). The molecular genetic architecture of attention deficit hyperactivity disorder. Mol. Psychiatry 20, 289–297 [DOI] [PubMed] [Google Scholar]
- 72.Albrecht B., Brandeis D., Uebel-von Sandersleben H., Valko L., Heinrich H., Xu X., Drechsler R., Heise A., Kuntsj J., Müller U.C., Asherson P., Steinhausen H.C., Rothenberger A., and Banaschewski T. (2014). Genetics of preparation and response control in ADHD: the role of DRD4 and DAT1. J. Child Psychol. Psychiatry 55, 914–923 [DOI] [PubMed] [Google Scholar]
- 73.Jeong S.H., Choi K.S., Lee K.Y., Kim E.J., Kim Y.S., and Joo E.J. (2015). Association between the dopamine transporter gene (DAT1) and attention deficit hyperactivity disorder-related traits in healthy adults. Psychiatr. Genet. 25, 119–126 [DOI] [PubMed] [Google Scholar]
- 74.Shang C.Y., and Gau S.S. (2014). Association between the DAT1 gene and spatial working memory in attention deficit hyperactivity disorder. Int. J. Neuropsychopharmacol. 17, 9–21 [DOI] [PubMed] [Google Scholar]
- 75.Max J.E., Lansing A.E., Koele S.L., Castillo C.S., Bokura H., Schachar R., Collings N., and Williams K.E. (2004). Attention deficit hyperactivity disorder in children and adolescents following traumatic brain injury. Dev. Neuropsychol. 25, 159–177 [DOI] [PubMed] [Google Scholar]
- 76.Yang L.Y., Huang C.C., Chiu W.T., Huang L.T., Lo W.C., and Wang J.Y. (2016). Association of traumatic brain injury in childhood and attention-deficit/hyperactivity disorder: a population-based study. Pediatr. Res. 80, 356–362 [DOI] [PubMed] [Google Scholar]
- 77.Schachar R., Levin H.S., Max J.E., Purvis K., and Chen S. (2004). Attention deficit hyperactivity disorder symptoms and response inhibition after closed head injury in children: do preinjury behavior and injury severity predict outcome? Dev. Neuropsychol. 25, 179–198 [DOI] [PubMed] [Google Scholar]
- 78.Catroppa C., Anderson V.A., Morse S.A., Haritou F., and Rosenfeld J.V. (2008). Outcome and predictors of functional recovery 5 years following pediatric traumatic brain injury (TBI). J. Pediatr. Psychol. 33, 707–718 [DOI] [PubMed] [Google Scholar]
- 79.Levin H., Hanten G., Max J., Li X., Swank P., Ewings–Cobbs L., Dennis M., Menefee D.S., and Schachar R. (2007). Symptoms of attention-deficit/hyperactivity disorder following traumatic brain injury in children. J. Dev. Behav. Pediatr. 28, 108–118 [DOI] [PubMed] [Google Scholar]
- 80.Anderson V., Le Brocque R., Iselin G., Eren S., Dob R., Davern T.J., McKinlay L., and Kenardy J. (2012). Adaptive ability, behavior and quality of life pre and posttraumatic brain injury in childhood. Disabil. Rehabil. 34, 1639–1647 [DOI] [PubMed] [Google Scholar]
- 81.Stroemer R.P., Kent T.A., and Hulsebosch C.E. (1998). Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke 29, 2381–2393 [DOI] [PubMed] [Google Scholar]
- 82.Bäckman L., Nyberg L., Soveri A., Johansson J., Anderson M., Dahlin E., Neely A.S., Virta J., Laine M., and Rinne J.O. (2011). Effects of working-memory training on striatal dopamine release. Science 333, 718. [DOI] [PubMed] [Google Scholar]
- 83.Karabanov A., Cervenka S., de Manzano O., Forssberg H., Farde L., and Ullén F. (2010). Dopamine D2 receptor density in the limbic striatum is related to implicit but not explicit movement sequence learning. Proc. Natl. Acad. Sci. U.S.A. 107, 7574–7579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Söderqvist S., Matsson H., Peyrard–Janvid M., Kere J., and Klingberg T. (2013). Polymorphisms in the dopamine receptor 2 gene region influence improvements during working memory training in children and adolescents. J. Cogn. Neurosci. 26, 54–62 [DOI] [PubMed] [Google Scholar]
- 85.Brehmer Y., Westerberg H., Bellander M., Fürth D., Karlsson S., and Bäckman L. (2009). Working memory plasticity modulated by dopamine transporter genotype. Neurosci. Lett. 467, 117–120 [DOI] [PubMed] [Google Scholar]
- 86.Söderqvist S., Bergman Nutley S., Peyrard–Janvid M., Matsson H., Humphreys K., Kere J., and Klingberg T. (2012). Dopamine, working memory, and training induced plasticity: implications for developmental research. Dev. Psychol. 48, 836–843 [DOI] [PubMed] [Google Scholar]
- 87.Klingberg T. (2014). Childhood cognitive development as a skill. Trends Cogn. Sci. 18, 573–579 [DOI] [PubMed] [Google Scholar]
- 88.Treble-Barna A., Zang H., Zhang N., Taylor H.G., Stancin T., Yeates K.O., and Wade S.L. (in press). Observed parenting behaviors as time-varying moderators of early traumatic brain injury on child behavior problems. Dev. Psychol. 52, 1777–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]