Abstract
Gerbera hybrida is a cut-flower crop of global importance, and an understanding of the mechanisms underlying petal development is vital for the continued commercial development of this plant species. Brassinosteroids (BRs), a class of phytohormones, are known to play a major role in cell expansion, but their effect on petal growth in G. hybrida is largely unexplored. In this study, we found that the brassinolide (BL), the most active BR, promotes petal growth by lengthening cells in the middle and basal regions of petals, and that this effect on petal growth was greater than that of gibberellin (GA). The RNA-seq (high-throughput cDNA sequencing) technique was employed to investigate the regulatory mechanisms by which BRs control petal growth. A global transcriptome analysis of the response to BRs in petals was conducted and target genes regulated by BR were identified. These differentially expressed genes (DEGs) include various transcription factors (TFs) that were activated during the early stage (0.5 h) of BL treatment, as well as cell wall proteins whose expression was regulated at a late stage (10 h). BR-responsive DEGs are involved in multiple plant hormone signal pathways, hormone biosynthesis and biotic and abiotic stress responses, showing that the regulation of petal growth by BRs is a complex network of processes. Thus, our study provides new insights at the transcriptional level into the molecular mechanisms of BR regulation of petal growth in G. hybrida.
Keywords: Brassinolide, Regulation, Gerbera hybrida, Petal growth, RNA-seq
Introduction
Brassinosteroids (BRs) are plant-specific steroid hormones involved in many growth and developmental processes, such as cell division and elongation, leaf expansion, vascular differentiation, stress tolerance, senescence and stomatal development (Bajguz & Hayat, 2009; Clouse & Sasse, 1998; Gudesblat et al., 2012; Kim et al., 2012). For example, the role of BR in cell division has been shown in the model plant Arabidopsis thaliana by analysis of mutants defective in BR biosynthesis, such as dwf7-1, which exhibits a slow cell division rate, and det2, in which transcription of the gene encoding cyclin D3 (a protein regulating G1/S transition in the cell cycle) increases after treatment with the 2,4-epibrassinolide (Cheon et al., 2010; Hu, Bao & Li, 2000). Similarly, longitudinal cell expansion is greatly reduced in BR mutants such as cbb, dwf4, cpd and dim, indicating a role for BRs in cell expansion (Azpiroz & Feldmann, 1998; Szekeres et al., 1996; Takahashi et al., 1995).
Coordination of organ growth with cell division, and of cell expansion with cell differentiation, is essential for the determination of organ size in plants (Mizukami, 2001). Large-scale analysis using microarray or proteomic techniques has also demonstrated the importance of BR-regulated genes in cell expansion. These genes are involved in cell wall modification, cellulose biosynthesis, ion and water transport, and cytoskeleton rearrangement (Clouse & Sasse, 1998; Kim & Wang, 2010; Vert et al., 2005). For example, DEVELOPMENTALLY REGULATED PLASMA MEMBRANE POLYPEPTIDE (DREPP), which was first identified as a BR-regulated protein in proteomic studies (Tang et al., 2008), can enhance cell elongation in a BR-deficient mutant, indicating that DREPP is a positive regulator of the BR response (Sun et al., 2010). Further studies in Arabidopsis showed that BRASSINAZOLE-RESISTANT1 (BZR1) and BRI1-EMS-SUPPRESSOR1 (BES1) encode two major transcription factors (TFs) that regulate cell division and elongation through a large number of BR -responsive genes (Guo et al., 2009; He et al., 2005; Sun et al., 2010; Wang et al., 2002; Xie, Yang & Wang, 2011; Yin et al., 2002; Yu et al., 2011).
Gerbera hybrida is a member of the sunflower family (Asteraceae), one of the largest clades of angiosperms. The inflorescence of G. hybrida, which consists of three types of flowers with ray, trans or disc petals, has been used as a model species to study developmental processes during organogenesis in the Asteraceae (Kotilainen et al., 1999; Yu et al., 1999). Consequently, in recent decades, many studies have been conducted on the molecular regulation of flower type differentiation and inflorescence development in G. hybrida. For instance, the CYC2 subclade of CYC/TB1-like TCP domain TFs has been shown to regulate flower-type identity in Asteraceae. Thus, GhCYC2, GhCYC3 and GhCYC4 control the differentiation and petal growth of ray flowers by modulating cell proliferation until the final size and shape of the petals is achieved (Broholm & Elomaa, 2008; Chapman et al., 2012; Fambrini, Salvini & Pugliesi, 2011; Kim et al., 2008; Tähtiharju et al., 2012). Another such TF, GhCYC5, is probably involved in promoting the flower initiation rate during expansion of the capitulum (Juntheikki-Palovaara et al., 2014). Research on orthologs of the flower meristem identity genes LEAFY (LFY) and UNUSUAL FLORAL ORGANS (UFO) in G. hybrida showed that GhUFO is the major regulator of flower meristem identity, while GhLFY has developed a novel, homeotic function during the evolution of head-like inflorescences (Zhao et al., 2016).
In our previous studies, we found that ray petals exhibit substantial cell expansion only after developmental stage 3 (Meng & Wang, 2004). Further work demonstrated that the basal region of ray petals was most sensitive to treatment with gibberellins (GAs) and abscisic acid (ABA) and that these phytohormones regulated cell expansion in an antagonistic manner (Li et al., 2015). Cell elongation is regulated not only by GA, but also by BR in Arabidopsis (Gallego-Bartolomé & Blázquez, 2012; Li et al., 2012). However, little is known about the role and regulatory mechanisms of BR in G. hybrida petal growth. In this study, we used the RNA-seq technique to perform global transcriptomic profiling in petals of G. hybrida and to identify differentially expressed genes (DEGs) responsive to BR treatment. The results showed that many DEGs are involved not only in cell wall organization and transcriptional regulation, but also in multiple plant hormone signaling pathways, hormone biosynthesis, and biotic and abiotic stress responses, indicating that the regulatory effect of BR on petal growth is a complex network of processes.
Materials and Methods
Plant material and growth conditions
G. hybrida “Shenzhen No. 5” seedlings were grown in a greenhouse at Zengcheng Ornamental Center (Guangzhou, China) as described by Zhang et al. (2012) at a temperature of 26/18 °C (day/night) and relative humidity of 65–80%. The developmental stages of inflorescence were defined according to Meng & Wang (2004). Inflorescences at stage 2, with a ray petal length of about 8 mm, were used for the in vivo experiments, while ray petals at stage 3 were used for in vitro studies.
Hormone and inhibitor treatments in vivo and in vitro
Five to six inflorescences at stage 2 were selected for in vivo treatment. The inflorescences were treated by spraying with 3–5 ml 10 µM brassinolide (BL), 10 µM GA3, 10 µM BRZ (brassinazole, a widely used inhibitor of BL biosynthesis), BL + GA3, or BL + BRZ once a day, using 0.1% ethanol as a control. The inflorescences were sampled after nine days. Ten petals of the outermost whorl of ray flowers were detached from the inflorescences at stage 3 for in vitro treatment, following the method described previously (Huang et al., 2008; Zhang et al., 2012). Briefly, the detached petals were placed on two layers of Whatman filter paper soaked in deionized water with or without hormones and/or inhibitor, and treated for nine days. The influence of the duration of BL treatment on petal growth was evaluated by in vitro experiments using the detached petals treated with 10 µM BL for 0.5, 0.75, 1, 2, 4, 10, 12 and 24 h, using deionized water (ddH2O) as a control. Hormones and inhibitors were obtained from Sigma-Aldrich (Shanghai, China). All experiments were replicated at least three times.
Observation and measurement of petal and cell length
To measure petal elongation in vivo, whole petals from more than six inflorescences were collected for each treatment. For each treatment, 60 whole petals from six inflorescences (10 each) were collected. Their images were scanned using an Epson-G850A scanner (Epson, China) and their lengths were measured using Image J software (http://rsb.info.nih.gov/ij/; NIH, MD, USA). Data were expressed as the averages of 60 petals. The lengths of three regions (top, middle and basal) of petals treated in vitro were measured before and after treatment. Data were expressed as the averages of ten petals for each treatment. Elongation rates were calculated according to the equation: elongation rate = (Lf–Li)/Li × 100%, where Lf is the petal length after treatment, and Li is the initial length before treatment.
To measure cell length in petals, a 1 mm2 petal block was dissected from the center of each region and stained by immersion in 0.1 mg mL−1 propidium iodide for 5 min at room temperature. The stained petal block was rinsed thoroughly with deionized water and flattened on a glass slide. Abaxial epidermal cell images were obtained with a laser confocal scanning microscope (LSM710/ConfoCor2; Carl-Zeiss, Jena, Germany) and their cell length was measured using Image J software. More than 100 cells of each of 10 petals from different inflorescences were randomly selected and measured. The cell elongation rate was calculated using the same equation described above. Data were analyzed using SPSS Statistics v. 18.0 (SPSS Inc., Chicago, IL, USA). Duncan’s test was applied to assess the differences between different treatments.
RNA-seq
For each treatment, 200 petals from 20 inflorescences at stage 3 were treated with BL or ddH2O for 0.5 or 10 h, using untreated material as the control. After treatment, petals were frozen in liquid nitrogen and stored at −80 °C. Total RNA was extracted using the TRIzol® reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. DNase I (TaKaRa, Japan) was used to digest genomic DNA. The quality of total RNA was checked with an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Samples with an RNA integrity number >8 were used to prepare cDNA libraries, as previously described (Kuang et al., 2013). Illumina sequencing was performed at Beijing Biomarker Technologies Co. Ltd. (Beijing, China). Five sets of raw reads were obtained, corresponding to Mock (untreated), H 0.5 (0.5 h ddH2O treatment), B 0.5 (0.5 h BL treatment), H 10 (10 h ddH2O treatment), and B 10 (10 h BL treatment).
Data processing and analysis
Raw read processing and primary bioinformatics analysis of the transcript datasets were conducted at Beijing Biomaker Technologies. In brief, clean reads were obtained from the raw data by removing adaptor sequences and reads of low-quality (Q value ≤ 5), fragments less than 20 bp and reads with more than 10% unknown bases. The clean reads were mapped to a G. hybrida transcriptome assembled previously (Kuang et al., 2013) using SOAPaligner/soap2 (Li et al., 2009). Mismatches of no more than two bases were allowed, with separate alignments being performed for each sample independently. Transcripts mapped by at least one read in at least one sample were identified for further analysis. Transcript abundance was expressed as RPKM (reads mapped per 1,000 bp per million sequenced reads) (Mortazavi et al., 2008). RPKM values presenting as “0” were artificially set to “0.001” for subsequent analysis. To analyze DEGs, a modified method described previously (Audic & Claverie, 1997) was used. Comparisons of RPKM between treatments (H 0.5 vs. B 0.5, H 10 vs. B 10) were performed for each transcript. Transcripts with a fold-change of ≥2 and a false discovery rate (FDR) <0.05 in at least one comparison were considered to be significantly differentially expressed and therefore to represent DEGs.
DEGs were used for BLASTX screening of protein databases, including the nr (the non-redundant protein database at NCBI), Swiss-Prot, COG, KEGG, and GO protein databases. GO annotation was performed using the Blast2GO program (Conesa et al., 2005). For GO enrichment analysis, GO annotations for each DEG were retrieved by mapping to GO terms in the database at http://www.geneontology.org. GO terms for Biological Processes (GO-BP) with a FDR ≥ 0.05 were considered significant. For KEGG pathway analysis, KEGG orthology terms for DEGs were retrieved from the KEGG pathway database (http://www.genome.jp/kegg/). The enrichment analysis was performed by comparing the observed DEG count with the expected count based on the transcriptome reported previously (Kuang et al., 2013). Hierarchical clustering analysis was performed using MeV 4.9.0 (http://www.tm4.org/) by considering the RPKM value as the normalized transcript level. Cluster analysis of gene expression patterns was performed using cluster software (Hoon et al., 2004) and Java Treeview software (Saldanha, 2004).
Real-time PCR verification of RNA-seq data
Total RNA was extracted as described above. Each RNA sample was treated with RNase-free DNase I (TaKaRa, Shiga, Japan) following the manufacturer’s protocol to remove any residual genomic DNA. DNase I-treated RNA (2 µg) was subjected to reverse transcriptase reactions using oligo(dT) primer and PrimeScript™ Reverse Transcriptase (TaKaRa, Shiga, Japan) according to the manufacturer’s protocol. For qRT-PCR, transcripts of target genes were amplified in a 20 µl reaction containing 2 µl cDNA (corresponding to 20 ng RNA), 1 µl primers and 5 µl SoFast™ EvaGreen® Supermix (Bio-Rad, Hercules, CA, USA) (Su et al., 2016). The primers are listed in Table S1. Expression levels of the tested genes were normalized to that of the GhACTIN (AJ763915) gene, as previously described (Kuang et al., 2013). Three biological replicates were performed per sample.
Results
The brassinolide modulates petal growth in vivo and in vitro
To examine the role of BRs in the regulation of petal growth, both in vivo and in vitro experiments were carried out. G. hybrida plants treated with BL had an average petal length of 43.55 mm compared to 35.27 mm in the untreated control, whereas treatment with BRZ, a BR biosynthetic inhibitor, resulted in an average petal length of 28.64 mm. The suppression of petal elongation by BRZ could be countered by spraying exogenous BL on the petals (Figs. 1A and 1B). These data clearly show that BL stimulates petal elongation in vivo.
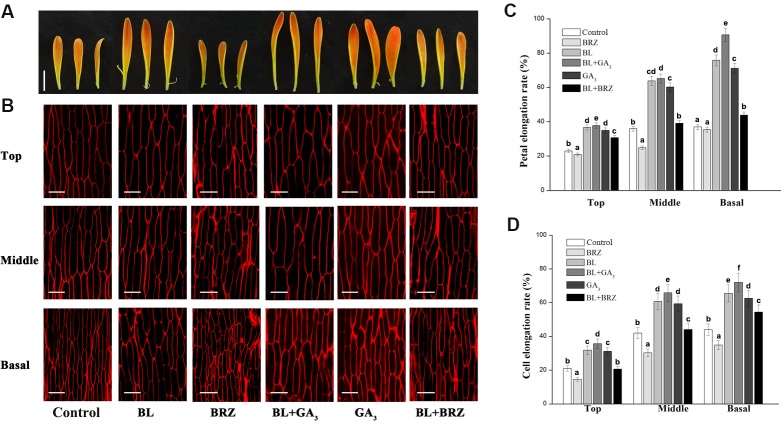
Figure 1. The effects of BRs and GAs on petal growth in G. hybrida.
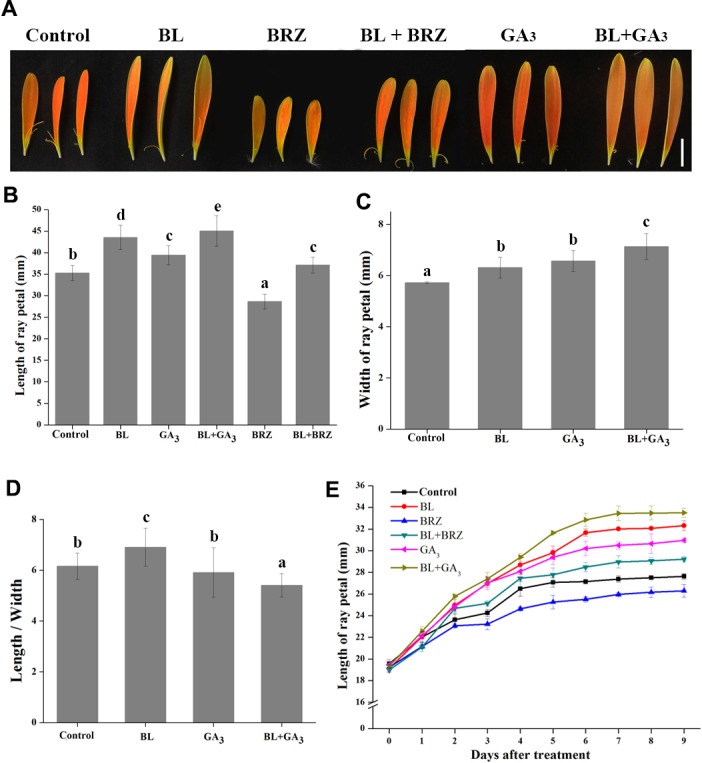
G. hybrida grew in a greenhouse under the conditions described in ‘Materials and Methods’. Plants with inflorescences at stage 2 were sprayed with deionized water (with 0.1% ethanol) (Control), 10 µM BL, 10 µM GA3, 10µM BRZ, a mixture of 10 µM BL and 10 µM GA3 (BL + GA3), or a mixture of 10 µM BL and 10 µM BRZ (BL + BRZ) and were subjected to morphological characterization (A), then the petal length (B), width (C) and length/width (D) were measured and calculated after nine days of treatment. Five to six inflorescences were measured for each treatment (Scale bar represents 1 cm. Value = means ± SE, n > 10, letters above the bars indicate significant differences between the respective values (p < 0.05)). (E) Time-course dynamics of petal length under different treatments. Detached petals were used in this experiment, at least 10 petals for each treatment were cultured for nine days.
Because GAs and BRs coordinately regulate plant growth in Arabidopsis (Li et al., 2012), and GAs promotes petal elongation in G. hybrida (Li et al., 2015), we tested the effect of combined treatment with GA3 and BL on petal growth in G. hybrida. The results showed that the length and width of the petals were both increased after the combined treatment, with a higher elongation rate than that of either BR or GA treatment alone (Figs. 1C and 1D; p < 0.05).
Detached petals were used in the in vitro experiment. After 9 days of hormone and/or inhibitor treatment, petal elongation was greater with BL treatment than with GA treatment, which gave average petal lengths of 32.3 mm and 30.9 mm (p < 0.05), respectively. BRZ-mediated suppression of petal length was reversed by the application of exogenous BL. Moreover, petals treated with both BL and GA3 elongated significantly more than with other treatments (Fig. 1E; p < 0.05), suggesting that BRs and GAs can coordinately regulate petal elongation in G. hybrida. These results are consistent with those obtained from in vivo experiments.
Brassinosteroids preferentially stimulate elongation of the middle and basal regions of petals
We next asked whether different regions of the detached petals experienced different elongation rates when treated with BR (Li et al., 2015). After nine days BL treatment, the elongation rates of the top, middle and basal regions of petals were 36.7, 63.8 and 75.8%, respectively, compared to 23.0, 36.5 and 37.2% in the same regions of the untreated controls. GA treatment also promoted elongation of the three petal regions, giving rates of 35.0, 60.2 and 71.1%, respectively. Elongation rates following BRZ treatment, however, were 21.1, 24.8 and 35.4% for the three regions, respectively; these were significantly lower than those of other treatments and also controls (Figs. 2A and 2C; p < 0.05). Interestingly, elongation rates were extremely high in the middle and basal regions of the petals treated with BL and GA3 combined, indicating a cooperative effect of the two phytohormones mainly concentrated in these regions.
Figure 2. The effects of BRs and GAs on cell elongation of petals in G. hybrida.
Petals of inflorescences at stage 3 were detached and placed on double filter papers soaked with deionized water (with 0.1% ethanol) (Control), 10 µM BL, 10 µM GA3, 10µM BRZ, BL + GA3 or BL + BRZ for nine days (A). The petals were then used for morphological characterization of adaxial epidermal cell in three different regions (top, middle and basal) using a confocal microscope (B) and measurement of elongation rate of each petal region (C), (n = 10) or cell (D), (n > 100). Three biological replicates were performed for each measurement (Scale bar represents 1 cm (A) or 50 µm (B). Value = means ± SE, letters above the bars indicate significant differences between the respective values (p < 0.05)).
We then looked at the petal cells and found that cell elongation rates were greatly increased in the presence of BL, with values of 31.9, 60.7 and 65.6% in the top, middle and basal regions, compared to 21.2, 42.0 and 43.8%, respectively, in the control. In contrast, cell elongation rates were suppressed by BRZ treatment, with values of only 14.6, 30.2 and 34.8% in the three regions, respectively. The cell elongation rates in the top, middle and basal regions were 35.7, 65.8 and 72.2%, respectively, after combined BL and GA3 treatment, compared to 31.2, 59.3 and 62.5%, respectively, with GA3 treatment alone (Figs. 2B and 2D; p < 0.05). These results indicate that petal elongation is associated with cell elongation, and that both BRs and GAs exert their elongation effect predominantly on cells of the middle and basal regions of G. hybrida petals.
Global sequencing analysis
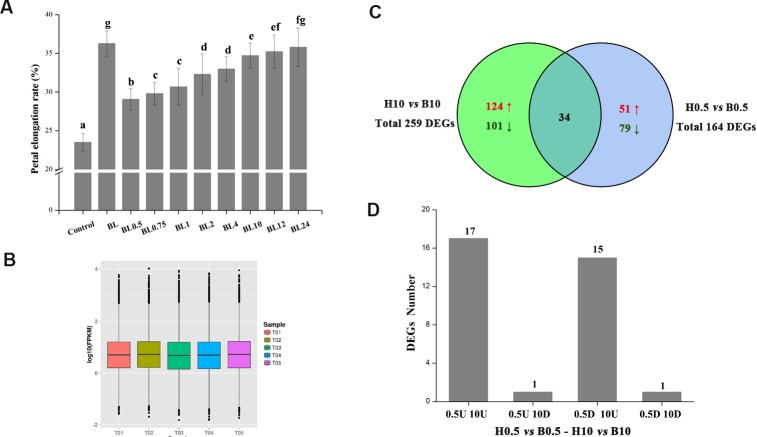
To explore the influence of different BL treatments on the petals at different time points, the growth dynamics of petals were tested in vitro. As shown in Fig. 3A, the petals after 0.5 h BL treatment were longer than those of the control (an increase of 29.8% vs. 23.5%, p < 0.05). The elongation rate increased with time until the 10 h timepoint, at which it was 34.7%. After 10 h, the elongation rate changed little during the remainder of the experiment. Five treatments, including BL treatment for 0.5 or 10 h, H2O treatment for 0.5 or 10 h, and the petals without treatment (Mock), were selected for RNA-seq.
Figure 3. The elongation rates of petals and global analysis of transcript profiles in different treatments.
(A) Detached petals at stage 3 were placed on double filter papers soaked with 10 µM BL for 0.5, 0.75, 1, 2, 4, 10, 12 and 24 h, and then transferred to papers soaked with deionized water. Petals were cultured for a total of 72 h and then the elongation rates were measured. Control: petals kept on double filter papers soaked with deionized water for 72 h. BL: petals kept on double filter papers soaked with10 µM BL for 72 h. (The experiment was repeated at least three times. Value = means ± SE, letters above the bars indicate significant differences between the respective values (p < 0.05)) (B) Global transcript profiles of different treatments. T01: petals without treatment (Mock), T02: H2O treatment for 0.5 h, T03: BL treatment for 0.5 h, T04: H2O treatment for 10 h, T05: BL treatment for 10 h. Each box plot shows the distribution of the relative transcription level (log10 (RPKM)) of genes with at least one read mapped to the transcriptome of G. hybrida in one sample. (C) Venn diagram showing the distribution of DEGs at 0.5 h or 10 h of BL treatment. (D) The number of DEGs in 0.5U 10U, 0.5U 10D, 0.5D 10U and 0.5D 10D. 0.5U 10U refers to DEGs up-regulated at both 0.5 h and 10 h, 0.5U 10D refers to DEGs up-regulated at 0.5 h and down-regulated at 10 h, 0.5D 10U refers to DEGs down-regulated at 0.5 h and up-regulated at 10 h, 0.5D 10D refers to DEGs down-regulated at both 0.5 h and 10 h.
The BL-associated gene regulatory networks that modulate petal growth were explored using RNA-seq data. A total of 16.82 GB clean reads were generated from the five samples, which had phred-like quality scores ranging from 88.71 to 89.09% at the Q30 level, and GC contents between 45.49% and 46.01% (Table 1). All reads were then mapped onto a previously published transcriptome after removing “dirty” data, with the mapped ratios (proportion of reads mapping to the transcriptome) of the five samples ranging from 71.33% to 72.86% (Table 1). The global distribution (using the relative transcription level (log10 (RPKM)) of genes) of each sample is shown in Fig. 3B. Finally, we identified 164 and 259 DEGs after BL treatment for 0.5 and 10 h, respectively, of which 34 transcripts were common to both datasets (Fig. 3C). These 34 transcripts were divided into four groups based on their expression patterns (0.5U 10U: transcripts up-regulated at both 0.5 h and 10 h timepoints; 0.5U 10D: transcripts up-regulated at 0.5 h and down-regulated at 10 h; 0.5D 10U: transcripts down-regulated at 0.5 h and up-regulated at 10 h; 0.5D 10D: transcripts down-regulated at both 0.5 h and 10 h). There were 17 genes with the 0.5U 10U pattern, including genes encoding three zinc finger TFs and five heat shock proteins, plus a TF that regulates heat shock protein genes. Fifteen genes fell into the 0.5D 10U category, many of them involved in secondary metabolite biosynthesis, transport and catabolism. Only one gene each followed the 0.5U 10D and 0.5D 10D expression patterns, encoding a member of the cytochrome P450 family and a homolog of the TF WRKY70, respectively (Table 2).
Table 1. Summary of the mapping reads identified by RNA-seq.
| Samples | Read number | Base number | Mapped reads | Mapped ratio | GC content | % ≥Q30 |
|---|---|---|---|---|---|---|
| Mock | 11,868,506 | 2,990,213,769 | 8,647,966 | 72.86% | 45.49% | 89.09% |
| H 0.5 | 11,042,298 | 2,781,854,022 | 7,876,652 | 71.33% | 45.70% | 89.07% |
| B 0.5 | 14,823,296 | 3,734,419,611 | 10,623,680 | 71.66% | 46.01% | 88.71% |
| H 10 | 14,971,100 | 3,771,890,604 | 10,741,596 | 71.74% | 45.88% | 89.05% |
| B 10 | 14,060,613 | 3,542,095,965 | 10,041,466 | 71.41% | 45.85% | 88.82% |
Table 2. DEGs appeared in both H 0.5 vs. B 0.5 and H 10 vs. B 10.
| Unigene ID | H 0.5 vs. B0.5 | H10 vs. B10 | Annotation |
|---|---|---|---|
| 0.5U 10U | |||
| GACN01031640.1 | 2.14 | 1.93 | Heat stress transcription factor A-7a |
| GACN01005379.1 | 1.74 | 1.95 | RING-H2 zinc finger |
| GACN01029771.1 | 1.74 | 1.47 | Hsp20/alpha crystallin family |
| GACN01043473.1 | 1.95 | 1.28 | Heat shock factor protein HSF30 |
| GACN01001749.1 | 1.52 | 1.41 | Heat shock protein 83 |
| GACN01026124.1 | 1.34 | 1.13 | PREDICTED: uncharacterized protein |
| GACN01046241.1 | 3.30 | 2.99 | Cytokinin dehydrogenase 3 |
| GACN01043054.1 | 1.85 | 1.24 | C3HC4 type RING finger |
| GACN01002323.1 | 1.22 | 1.41 | Hsp70 protein |
| GACN01029465.1 | 1.72 | 1.08 | Hsp20/alpha crystallin family |
| GACN01022828.1 | 1.66 | 3.14 | PREDICTED: uncharacterized protein |
| GACN01042640.1 | 1.94 | 1.76 | Multiprotein-bridging factor 1c |
| GACN01039604.1 | 1.91 | 1.81 | Two-component response regulator ARR4 |
| GACN01006153.1 | 1.41 | 1.37 | C3HC4 type (RING finger) |
| GACN01005141.1 | 1.79 | 1.11 | Histidine kinase 4 |
| GACN01042426.1 | 1.33 | 1.05 | GEM-like protein 5 |
| GACN01015877.1 | 2.65 | 3.65 | Cytokinin dehydrogenase 1 |
| 0.5U 10D | |||
| GACN01030442.1 | 1.51 | −1.46 | Secondary metabolites biosynthesis, transport and catabolism Cytochrome P450 |
| 0.5D 10U | |||
| GACN01011667.1 | −1.30 | 1.19 | Delta fatty acid dehydrogenase |
| GACN01011938.1 | −1.23 | 1.15 | WRKY transcription factor 40 |
| GACN01014026.1 | −1.00 | 1.46 | Secondary metabolites biosynthesis, transport and catabolism Cytochrome P450 |
| GACN01046042.1 | −1.00 | 1.26 | Secondary metabolites biosynthesis, transport and catabolism Multicopper oxidase |
| GACN01001724.1 | −1.13 | 1.72 | AMP-binding enzyme |
| GACN01016941.1 | −1.27 | 1.30 | Secondary metabolites biosynthesis, transport and catabolism NUDIX domain |
| GACN01001199.1 | −1.57 | 3.03 | 2OG-Fe(II) oxygenase superfamily |
| GACN01025109.1 | −1.29 | 1.75 | Anthocyanidin 3-O-glucosyltransferase 4 |
| GACN01017778.1 | −1.01 | 1.74 | Secondary metabolites biosynthesis, transport and catabolism Cytochrome P450 |
| GACN01023881.1 | −1.38 | 5.23 | PREDICTED: uncharacterized protein |
| GACN01046804.1 | −1.27 | 2.30 | AMP-binding enzyme |
| GACN01037971.1 | −1.09 | 1.11 | Taxadien-5-alpha-ol O-acetyltransferase |
| GACN01001030.1 | −1.48 | 1.40 | Secondary metabolites biosynthesis, transport and catabolism Aromatic amino acid lyase |
| GACN01045279.1 | −1.30 | 1.44 | Probable calcium-binding protein CML45 |
| GACN01027915.1 | −1.30 | 1.96 | AMP-binding enzyme C-terminal domain |
| 0.5D 10D | |||
| GACN01040109.1 | −1.32 | −1.45 | Probable WRKY transcription factor 70 |
GO enrichment analyses of DEGs at 0.5 h and 10 h
Using GO enrichment analysis, the DEGs were divided into three major functional categories named “cellular component”, “molecular function” and “biological process”. We focused on the biological process (GO-BP) category and found that, at 0.5 h, most of the enriched subcategories were related to various signal transduction pathways, and the responses to abiotic and biotic stress. At 10 h, most of enriched subcategories were related to cell size and various secondary metabolite biosynthetic processes. These results indicate that BR-responsive DEGs belong to subcategories relating to petal growth (Table 3).
Table 3. DEGs enriched with GO terms.
| GO ID | GO name | DEG counts | p-value |
|---|---|---|---|
| H 0.5vs.B 0.5 | |||
| GO:0023014 | Signal transduction by phosphorylation | 4 | 2.41E−04 |
| GO:0035556 | Intracellular signal transduction | 6 | 3.23E−04 |
| GO:0009736 | Cytokinin-activated signaling pathway | 3 | 3.18E−03 |
| GO:0009873 | Ethylene-activated signaling pathway | 5 | 2.03E−04 |
| GO:0009697 | Salicylic acid biosynthetic process | 8 | 3.41E−06 |
| GO:0009738 | Abscisic acid-activated signaling pathway | 8 | 2.66E−05 |
| GO:0050832 | Defense response to fungus | 12 | 3.61E−08 |
| GO:0006950 | Response to stress | 11 | 2.83E−07 |
| GO:0009408 | Response to heat | 10 | 1.64E−06 |
| H 10vs.B 0.5 | |||
| GO:0008361 | Regulation of cell size | 2 | 1.09E−04 |
| GO:0009932 | Cell tip growth | 3 | 1.20E−04 |
| GO:0009664 | Plant-type cell wall organization | 3 | 1.23E−04 |
| GO:0008152 | Metabolic process | 15 | 2.17E−04 |
| GO:0016099 | Monoterpenoid biosynthetic process | 3 | 1.02E−04 |
| GO:0016114 | Terpenoid biosynthetic process | 3 | 1.86E−03 |
| GO:0009813 | Flavonoid biosynthetic process | 3 | 2.23E−03 |
| GO:0009718 | Anthocyanin biosynthetic process | 5 | 9.08E−06 |
KEGG pathway enrichment analysis of DEGs
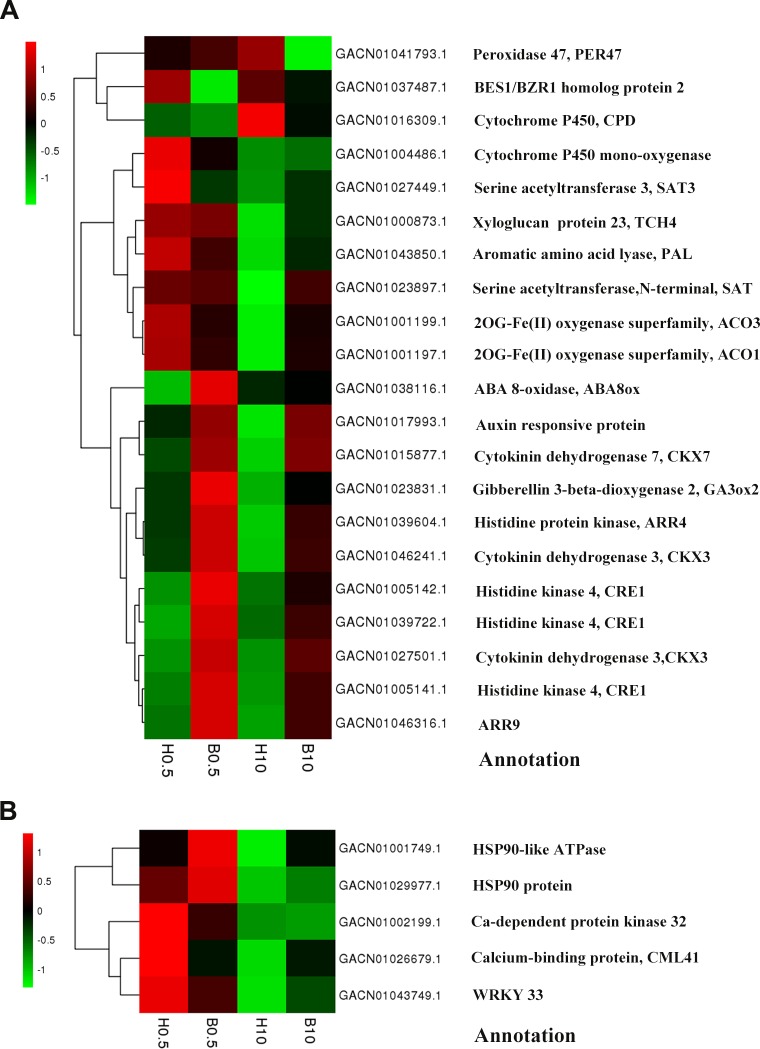
We used the KEGG database to determine which pathways were populated by BR-regulated DEGs in petals. In total, 34 DEGs at 0.5 h and 48 DEGs at 10 h were annotated and mapped to various pathways. DEGs involved in plant hormone signal transduction, including the cytokinin, BR and auxin pathways, were significantly enriched comparing the B 0.5 column with H 0.5, and similarly for the 10 h timepoint (Table 4). For example, in the cytokinin pathway, CRE1 (GACN01005141.1, GACN01005142.1, GACN01039722.1) and two ARRgenes (GACN01039604.1, GACN01046316.1) were up-regulated at both 0.5 h and 10 h. In addition, one of the most important TFs regulating the BR pathway, a BES1/BZR1 homolog (GACN01037487.1), was down-regulated at 0.5 h, while at 10 h, an auxin responsive protein (GACN01017993.1) was up-regulated (Fig. 4A). These data show that BRs regulate the expression of genes involved in multiple hormone signaling pathways. Another representative KEGG process was “biotic and abiotic stresses”, where three DEGs (GACN01002199.1, GACN01026679.1, GACN01043749.1) were down-regulated and two DEGs encoded putative HSP90 proteins (GACN01001749.1, GACN01029977.1) were up-regulated at 0.5 h (Fig. 4B). Moreover, we also observed crosstalk between the biosynthetic pathways of multiple hormones, as follows: zeatin biosynthesis, carotenoid biosynthesis, diterpenoid biosynthesis, BR biosynthesis, cysteine and methionine metabolism and phenylalanine metabolism (Table 4). Most DEGs were down-regulated at 0.5 h in these processes, indicating that BRs might regulate multiple hormone biosynthetic pathways.
Table 4. KEGG annotations of enriched pathways.
| Pathway | ID | H 0.5 vs. B 0.5 | H 10 vs. B 10 | ||
|---|---|---|---|---|---|
| Count percent (%) | P-value | Count percent (%) | P-value | ||
| Plant hormone signal transduction | ko04075 | 17.65% | 1.08E−03 | 8.33% | 2.85E−03 |
| Biotic and abiotic stress | ko04626 | 14.71% | 5.24E−03 | 2.08% | 8.09E−03 |
| Zeatin biosynthesis | ko00908 | 8.82% | 6.96E−04 | 4.17% | 2.58E−03 |
| Carotenoid biosynthesis | ko00906 | 2.94% | 2.15E−03 | ||
| Diterpenoid biosynthesis | ko00904 | 2.94% | 8.28E−03 | ||
| Brassinosteroid biosynthesis | ko00905 | 2.08% | 7.07E−03 | ||
| Cysteine and methionine metabolism | ko00270 | 5.88% | 1.59E−03 | 6.25% | 7.85E−03 |
| Phenylalanine metabolism | ko00360 | 5.88% | 7.66E−03 | 2.08% | 4.81E−03 |
| Phenylpropanoid biosynthesis | ko00940 | 5.88% | 7.49E−03 | 2.08% | 4.77E−03 |
| RNA transport | ko03013 | 2.94% | 8.26E−03 | 8.33% | 2.17E−03 |
| ABC transporters | ko02010 | 2.08% | 1.57E−03 | ||
Figure 4. Heat-maps of DEGs involved in “plant hormone signal transduction” and “biotic and abiotic stress”.
(A) DEGs involved inplant hormone signal transduction”. (B) DEGs involved in “biotic and abiotic stress”. The bar represents the scale of the expression levels for each gene (log10RPKM) in different samples as indicated by green/red rectangles. Green indicates down-regulation of genes, red indicates up-regulation and no change is indicated in black.
Genes modulated by BRs in petal growth
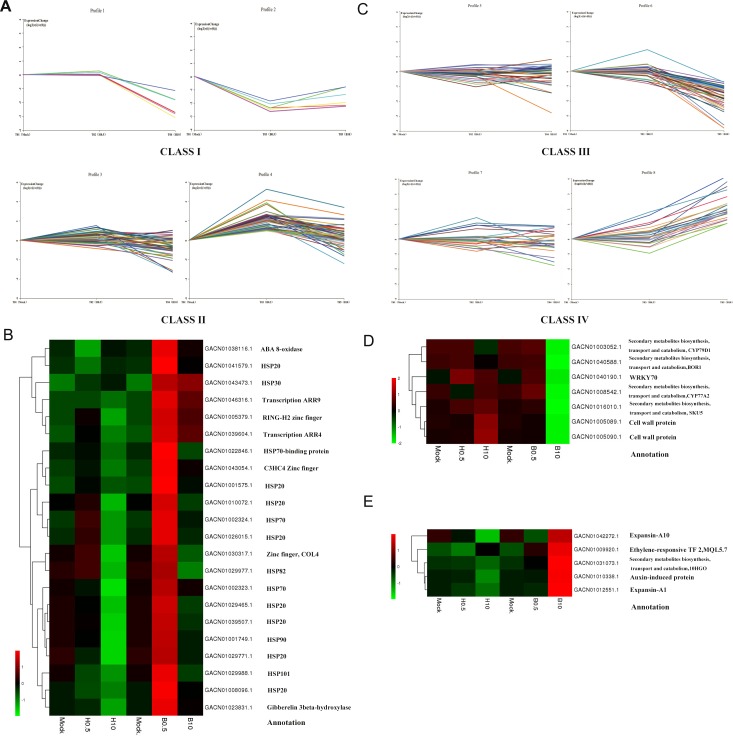
In further analysis, we tried to find DEGs mainly involved in the regulation of petal growth by BRs. The DEGs at 0.5 h and 10 h were divided into four classes according to their expression profiles and a hierarchical clustering analysis based on their RPKM (Fig. 5). Class I DEGs were significantly down-regulated after 0.5 h BL treatment and did not change markedly after 0.5 h H2O treatment (log2 < 1); Class II DEGs were up-regulated after 0.5 h BL treatment and did not change after 0.5 h H2O treatment; Class III DEGs weredown-regulated after 10 h BL treatment and did not change after 10 h H2O treatment; and Class IV DEGs were up-regulated after 10 h BL treatment and did not change after 10 h H2O treatment. As a result, Class I and Class II contained 6 and 48 DEGs, while Class III and Class IV contained 41 and 26 DEGs, respectively. As shown in Fig. 5B, two transcription-related kinases ARR4 (GACN01039604.1) and ARR9 (GACN01046316.1) and three zinc finger TFs were discovered in Class II, indicating that, as might be expected, a number of TFs influence petal growth. Fifteen heat shock proteins (HSPs) were found in this class, suggesting that molecular chaperones are important for the regulation of petal growth by BRs at 0.5 h. Interestingly, two genes that regulated putative cell wall proteins are found in Class III, and two expansin genes were identified in Class IV (Fig. 5D). This implies that one aspect of the effect of BRs on petal growth is regulation the expression of cell wall protein and expansin genes in G. hybrida.
Figure 5. DEGs expression profiles and the heatmaps of some selected DEGs in their profiles.
(A) Class I indicated a down-regulated trend during 0–0.5 h of BL treatment and a gradual trend in H2O treatment. Class II indicated an up-regulated trend during 0–0.5 h of BL treatment and a gradual trend in H2O treatment. (B) Heatmap of DEGs selected from Class II and their annotations. (C) Class III indicated a down-regulated trend during 0.5 h–10 h of BL treatment and a gradual trend in H2O treatment. Class IV indicated an up-regulated trend during 0.5 h to10 h of BL treatment and a gradual trend in H2O treatment. (D) Heatmap of DEGs selected from Class III and their annotations. (E) Heatmap of DEGs selected from Class IV and their annotations.
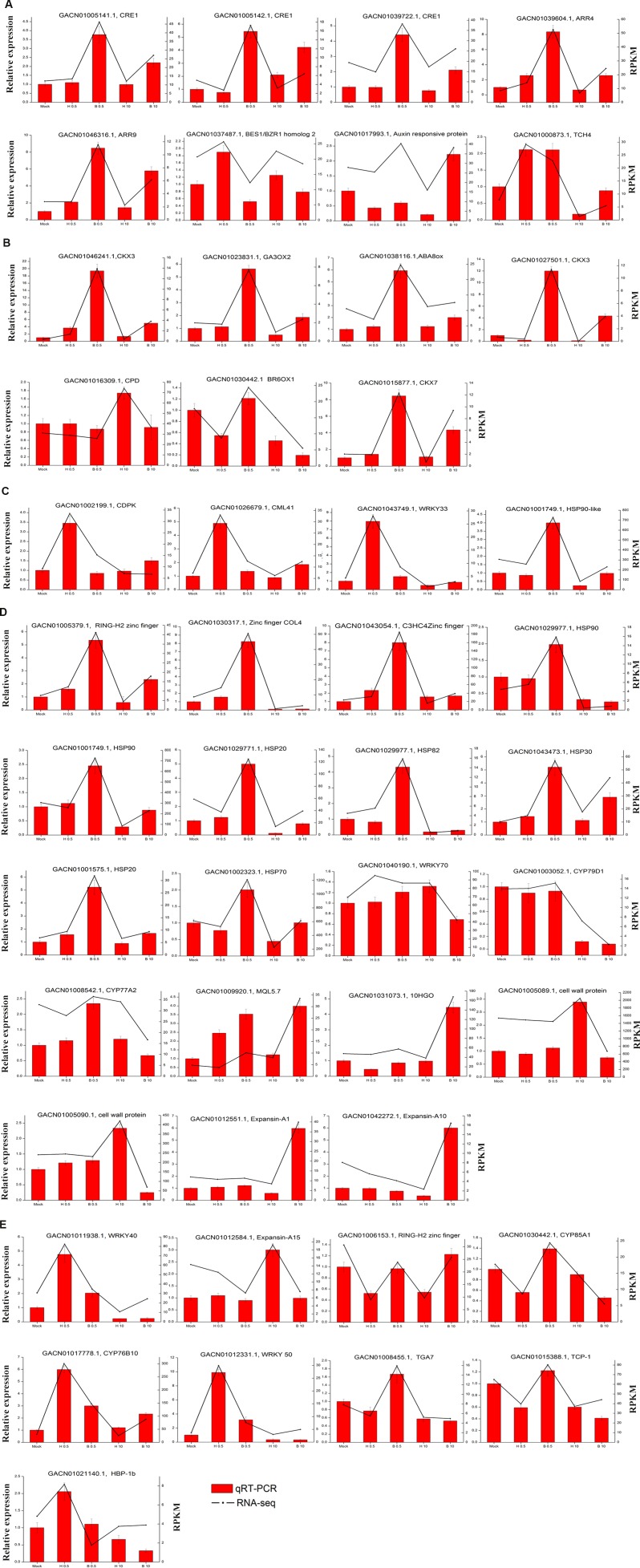
Validation of RNA-seq results by qRT-PCR
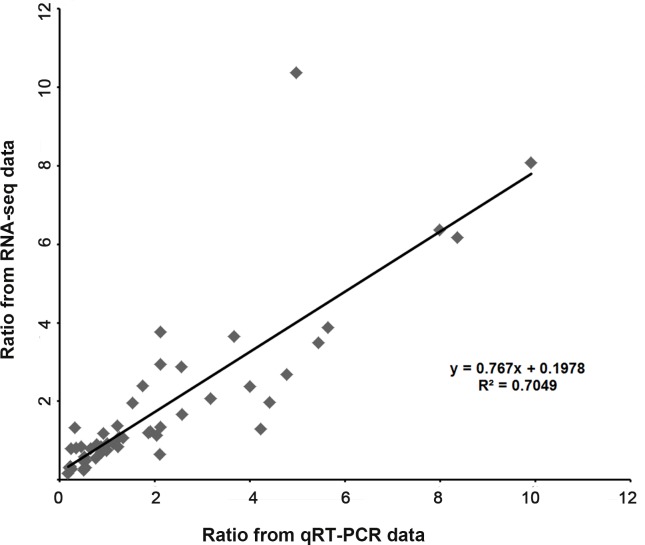
We validated the expression patterns of several DEGs in the five samples by qRT-PCR. The DEGs chosen were those annotated “plant hormone signal transduction” (Fig. 6A) and “hormone biosynthesis” (Fig. 6B), as well as four genes annotated “biotic and abiotic stresses” (Fig. 6C), nineteen DEGs selected from the four Classes defined above (nine in Class II, six in Class III and four in Class IV ) (Fig. 6D) and nine random DEGs (Fig. 6E). The validation results were consistent with the gene expression patterns identified by RNA-seq. Pearson correlation and linear regression analyses of the fold change of the gene expression ratios between RNA-seq and qRT-PCR showed a significantly positive correlation (Fig. 7). These results highlighted the fidelity and reproducibility of the RNA-seq analysis used in the present study.
Figure 6. Comparison of unigene expression levels revealed by qRT-PCR and RNA-seq (RPKM).
(A) DEGs involved in “Plant hormone signal transduction”. (B) DEGs involved in “hormone biosynthesis”. (C) Four DEGs involved in “plant-pathogen interaction”. (D) DEGs selected from the four classes (nine in Class II, six in Class III and four in Class IV) (E) Nine DEGs selected randomly. ACTIN (AJ763915) of G. hybrida was used as the normalization control. Three biological repeats were included for each condition.
Figure 7. Coefficient analysis of fold change data between qRT-PCR and RNA-seq.
Fourteen random unigenes were selected for this analysis. The data of “Mock” was seted as one unit, scatterplots were generated by the ratios of the other four samples from qRT-PCR (x-axis) and RNA-seq (y-axis).
Discussion
BR modulation of petal growth in G. hybrida
Genetic studies suggest that many different pathways act independently to determine flower size. In Arabidopsis, plant hormones, including auxins, cytokinins, BRs and ethylene, play important roles in petal morphogenesis by regulating the expression of key developmental genes (Breuninger & Lenhard, 2010; Weiss, Delgadobenarroch & Egeacortines, 2005). For example, ARL, a gene controlling cell expansion during organogenesis, acts downstream of BRI1 and partially mediates BR-related cell expansion signals during organ growth (Hu, Poh & Chua, 2006). Thus, BRs promote cell expansion to increase floral organ size. Our current research extends these conclusions to G. hybrida, in which BR treatment causes petals to elongate significantly (Fig. 1), largely by promoting lengthening of cells in the middle and basal regions (Fig. 2).
Recent studies in Arabidopsis have shown that crosstalk between the BR and GA pathways is brought about by direct interactions between their target transcriptional regulators (Bai et al., 2012; Gallego-Bartolomé & Blázquez, 2012; Li et al., 2012; Oh et al., 2014). In line with this, our previous research showed that GA and BRs act on the same region of the petal when stimulating petal growth in G. hybrida (Li et al., 2015). In the present work, therefore, we asked whether these two hormones function coordinately in petal growth. Indeed, we found this to be the case, as the elongation rates of both petals and cells was much higher when they were treated with both hormones as opposed to each hormone independently. We also noted that the speed of response to BR is faster than to GA: the petal elongation rate clearly increased after only 0.5 h BR treatment, but required longer treatment times with GA (Li et al., 2015).
DEGs regulated by BR involved in cell wall constitution and transcription regulation in G. hybrida petals
By comparison of transcriptome sets corresponding to various petal treatments, we identified DEGs that were regulated by BL at early (0.5 h) and late (10 h) timepoints. GO analysis for DEGs enriched in the biological process category showed that subcategories involved in cell size were overrepresented after 10 h BR treatment (Table 3). Further analysis found four genes involved in cell wall constitution in the transcriptome set from the late timepoint. It is well documented that plant cell expansion depends on the activity of various proteins, which loosen cell walls through a complex molecular modification network (Xu et al., 2014). The expansins, which are a group of pH-dependent wall-loosening proteins, are considered to be molecular markers of cell elongation (Bai et al., 2012; Ikeda et al., 2012). We found that two putative cell wall proteins were activated by BR treatment, but two expansin genes were repressed. This suggests that BRs may promote cell elongation by the harmonious expression of a network of genes involved in cell wall constitution after 10 h treatment. The regulation pattern of these genes is consistent with that resulting from GA and ABA treatment (Li et al., 2015).
Transcriptional regulation of key genes is essential for petal growth and a number of TFs controlling petal development have been identified recently (Alvarezbuylla et al., 2010; ÓMaoiléidigh, Graciet & Wellmer, 2014). One of these is BES1/BZR1, which plays a central role in BR signaling pathways, as evidenced by the fact that the gain-of-function mutant bzr1-1D shows enhanced BR signaling, resulting in increased flower and silique size (Wang et al., 2002; Yin et al., 2002; Yu et al., 2016). Other TFs characterized in Arabidopsis include the TCP gene family, whose members play an important role in organ growth, and the C2H2 zinc finger TFs, such as JAG and RBE, which regulate the transcription of TCP4 during petal development (Efroni et al., 2008; Jing et al., 2016; Martín-Trillo & Cubas, 2010; Powell & Lenhard, 2012; Schiessl, Muiño & Sablowski, 2014). In G. hybrida, we found that several TFs were regulated after only 0.5 h BR treatment, including a homolog of BES1/BZR1 (GACN01037487.1) and three zinc-finger TFs (GACN01030317.1, GACN01043054.1 and GACN01005379.1), indicating that transcriptional regulation is also important in gerbera petal growth.
BR signaling is mediated by HSP90 activity and via trafficking of BIN2-HSP90 complexes into the cytoplasm. In Arabidopsis, HSP90, as a molecular chaperone, contributes to BR-mediated gene expression through complex formation with two major TFs, BZR1 and BES1 (Samakovli et al., 2014; Shigeta et al., 2015). A recent study reported that HSP90 is pivotal in the transition from vegetative to reproductive phase in Arabidopsis and that depletion of HSP90 mRNA levels causes extreme variation in the expression of flowering genes, and disturbs flower determination and patterning (Margaritopoulou et al., 2016). We found that a large number of HSP genes were regulated by BR treatment, indicating that HSPs also have a central function in BR-regulated petal growth in G. hybrida.
BRs regulate a wide range of physiological and developmental processes in G. hybrida
BRs are involved in a wide range of physiological and developmental processes through their interactions with other phytohormones including auxins, cytokinins, ethylene, GAs, jasmonic acid, ABA, salicylic acid and polyamine in plants (Ahammed et al., 2015; Arteca & Arteca, 2008; Belkhadir & Jaillais, 2015; Wang et al., 2011). Our results in G. hybrida support this view, with GO and KEGG analyses implicating BRs in multiple hormone signaling and biosynthesis pathways (Table 4, Fig. 4). In particular, we found that DEGs induced after both 0.5 h and 10 h BR treatment were highly enriched in the cytokinin pathway, which is an evidence for cooperation between BRs and cytokinins in petal growth. In Arabidopsis, cytokinins are involved in the regulation of cell division and metabolism, and there is crosstalk with BR in the regulation of plant growth and development (Chi et al., 2016; Hwang, Sheen & Müller, 2012; Saini, Sharma & Pati, 2015; Vercruyssen et al., 2011). In our study, two DEGs (GACN01046316.1, GACN01039604.1), corresponding to the ARR9 and ARR4 histidine kinase genes, were clearly up-regulated after 0.5 h BR treatment, indicating that the cytokinin signal pathway might be influenced by BRs in petal growth.
BRs are essential for plant tolerance to environmental factors (drought, flooding, extreme temperatures, salinity) and toxic substances, such as heavy metals, UV-radiation, and ozone (Kim et al., 2012; Kutschera, 2012). BRs may also regulate other phytohormones, such as ABA, SA and ethylene, to induce plant resistance to these abiotic stresses (Ashraf, 2010; Hasan, Hayat & Ahmad, 2011). Our RNA-seq data show that DEGs involved in stress tolerance, including WRKY TFs, were specifically up or down-regulated after 0.5 h or 10 h BL treatment. Together, the above results point to the regulation of petal growth by BRs being a very complex network of processes.
In summary, we report a comprehensive transcriptome dataset relating to petal growth in G. hybrida following BR treatment and have identified a group of DEGs that might regulate petal growth. This is a valuable resource for further Asteraceae genomic studies and will also benefit research in other closely related species with high ornamental and economic value. The DEG annotation we carried out should also provide useful candidate genes, especially TFs, for functional analysis of petal growth and may have great significance for the improvement of the ornamental characteristics of G. hybrida via molecular breeding.
Supplemental Information
Class I DEGs were significantly down-regulated after 0.5 h BL treatment and did not change markedly after 0.5 h H2O treatment (log2 < 1).
Class II DEGs were up-regulated after 0.5 h BL treatment and did not change markedly after 0.5 h H2O treatment.
Class III DEGs were down-regulated after 10 h BL treatment and did not change markedly after 10 h H2O treatment.
Class IV DEGs were up-regulated after 10 h BL treatment and did not change markedly after 10 h H2O treatment.
Acknowledgments
We thank Prof. Xiaojing Wang and Dr. Lingfei Li for guidance. We also thank Cambridge Academic Manuscripts (www.CambridgeAcademicManuscripts.com) for manuscript editing.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31672188, 31601784), and the Science and Technology Plan Project of Guangdong Province (2015A020209156, 2016A020208013, 2015B020231009, 2015B020202007, 2013B020201003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Gan Huang conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Meixiang Han contributed reagents/materials/analysis tools.
Wei Yao performed the experiments.
Yaqin Wang analyzed the data, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplementary File.
References
- Ahammed et al. (2015).Ahammed GJ, Xia XJ, Li X, Shi K, Yu JQ, Zhou YH. Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Current Protein & Peptide Science. 2015;16:462–473. doi: 10.2174/1389203716666150330141427. [DOI] [PubMed] [Google Scholar]
- Alvarezbuylla et al. (2010).Alvarezbuylla ER, Benítez M, Corverapoiré A, Chaos CA, De FS, Gamboa dBA, Garayarroyo A, Garcíaponce B, Jaimesmiranda F, Pérezruiz RV. Flower development. Arabidopsis Book. 2010;8:e0127. doi: 10.1199/tab.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteca & Arteca (2008).Arteca RN, Arteca JM. Effects of brassinosteroid, auxin, and cytokinin on ethylene production in Arabidopsis thaliana plants. Journal of Experimental Botany. 2008;59:3019–3026. doi: 10.1093/jxb/ern159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf (2010).Ashraf M. Inducing drought tolerance in plants: recent advances. Biotechnology Advances. 2010;28:169–183. doi: 10.1016/j.biotechadv.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Audic & Claverie (1997).Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Research. 1997;7:986–995. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- Azpiroz & Feldmann (1998).Azpiroz R, Feldmann KA. An Arabidopsis brassinosteroid-dependent mutant is blocked in cell elongation. The Plant Cell. 1998;10:219–230. doi: 10.1105/tpc.10.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai et al. (2012).Bai MY, Fan M, Oh E, Wang ZY. A triple helix-loop-helix/basic helix-loop-helix cascade controls cell elongation downstream of multiple hormonal and environmental signaling pathways in Arabidopsis. The Plant Cell. 2012;24:4917–4929. doi: 10.1105/tpc.112.105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajguz & Hayat (2009).Bajguz A, Hayat S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiology & Biochemistry. 2009;47:1–8. doi: 10.1016/j.plaphy.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Belkhadir & Jaillais (2015).Belkhadir Y, Jaillais Y. The molecular circuitry of brassinosteroid signaling. New Phytologist. 2015;206:522–540. doi: 10.1111/nph.13269. [DOI] [PubMed] [Google Scholar]
- Breuninger & Lenhard (2010).Breuninger H, Lenhard M. Chapter seven-control of tissue and organ growth in plants. Current Topics in Developmental Biology. 2010;91:185–220. doi: 10.1016/S0070-2153(10)91007-7. [DOI] [PubMed] [Google Scholar]
- Broholm & Elomaa (2008).Broholm SK, Elomaa P. A TCP domain transcription factor controls flower type specification along the radial axis of the Gerbera (Asteraceae) inflorescence. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9117–9122. doi: 10.1073/pnas.0801359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman et al. (2012).Chapman MA, Tang S, Draeger D, Nambeesan S, Shaffer H, Barb JG, Knapp SJ, Burke JM. Genetic analysis of floral symmetry in Van Gogh’s sunflowers reveals independent recruitment of CYCLOIDEA genes in the asteraceae. PLOS Genetics. 2012;8:e1002628. doi: 10.1371/journal.pgen.1002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon et al. (2010).Cheon J, Park SY, Schulz B, Choe S. Arabidopsis brassinosteroid biosynthetic mutant dwarf7-1 exhibits slower rates of cell division and shoot induction. BMC Plant Biology. 2010;10:1–8. doi: 10.1186/1471-2229-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi et al. (2016).Chi W, Li J, He B, Chai X, Xu X, Sun X, Jiang J, Feng P, Zuo J, Lin R. DEG9, a serine protease, modulates cytokinin and light signaling by regulating the level of ARABIDOPSIS RESPONSE REGULATOR 4. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:201601724. doi: 10.1073/pnas.1601724113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse & Sasse (1998).Clouse SD, Sasse JM. BRASSINOSTEROIDS: essential regulators of plant growth and development. Annual Review of Plant Physiology & Plant Molecular Biology. 1998;49:427–451. doi: 10.1146/annurev.arplant.49.1.427. [DOI] [PubMed] [Google Scholar]
- Conesa et al. (2005).Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
- Efroni et al. (2008).Efroni I, Blum E, Goldshmidt A, Eshed Y. A protracted and dynamic maturation schedule underlies arabidopsis leaf development. The Plant Cell. 2008;20:2293–2306. doi: 10.1105/tpc.107.057521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrini, Salvini & Pugliesi (2011).Fambrini M, Salvini M, Pugliesi C. A transposon-mediate inactivation of a CYCLOIDEA-like gene originates polysymmetric and androgynous ray flowers in Helianthus annuus. Genetica. 2011;139:1521–1529. doi: 10.1007/s10709-012-9652-y. [DOI] [PubMed] [Google Scholar]
- Gallego-Bartolomé & Blázquez (2012).Gallego-Bartolomé J, Blázquez MA. Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13446–13451. doi: 10.1073/pnas.1119992109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudesblat et al. (2012).Gudesblat G, Schneider-Pizoń J, Betti C, Mayerhofer J, Vanhoutte I, van Dongen W, Boeren S, Zhiponova M, de Vries S, Jonak C, Russinova E. SPEECHLESS integrates brassinosteroid and stomata signalling pathways. Nature Cell Biology. 2012;14:548–554. doi: 10.1038/ncb2471. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2009).Guo H, Li L, Ye H, Yu X, Algreen A, Yin Y. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7648–7653. doi: 10.1073/pnas.0812346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan, Hayat & Ahmad (2011).Hasan SA, Hayat S, Ahmad A. Brassinosteroids protect photosynthetic machinery against the cadmium induced oxidative stress in two tomato cultivars. Chemosphere. 2011;84:1446–1451. doi: 10.1016/j.chemosphere.2011.04.047. [DOI] [PubMed] [Google Scholar]
- He et al. (2005).He JX, Gendron JM, Sun Y, Gampala SS, Gendron N, Sun CQ, Wang ZY. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science. 2005;307:1634–1638. doi: 10.1126/science.1107580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoon et al. (2004).Hoon MJLD, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20:1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- Hu, Bao & Li (2000).Hu Y, Bao F, Li J. Promotive effect of brassinosteroids on cell division involves a distinct CycD3-induction pathway in Arabidopsis. The Plant Journal. 2000;24:693–701. doi: 10.1046/j.1365-313x.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- Hu, Poh & Chua (2006).Hu Y, Poh HM, Chua NH. The Arabidopsis ARGOS-LIKE gene regulates cell expansion during organ growth. The Plant Journal. 2006;47:1–9. doi: 10.1111/j.1365-313X.2006.02750.x. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2008).Huang Z, Liang M, Peng J, Xing T, Wang X. Exogenous ammonium inhibits petal pigmentation and expansion in Gerbera hybrida. Physiologia Plantarum. 2008;133:254–265. doi: 10.1111/j.1399-3054.2008.01071.x. [DOI] [PubMed] [Google Scholar]
- Hwang, Sheen & Müller (2012).Hwang I, Sheen J, Müller B. Cytokinin signaling networks. Annual Review of Plant Biology. 2012;63:353–380. doi: 10.1146/annurev-arplant-042811-105503. [DOI] [PubMed] [Google Scholar]
- Ikeda et al. (2012).Ikeda M, Fujiwara S, Mitsuda N, Ohme-Takagi M. A triantagonistic basic helix-loop-helix system regulates cell elongation in Arabidopsis. The Plant Cell. 2012;24:4483–4497. doi: 10.1105/tpc.112.105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing et al. (2016).Jing L, Wang Y, Zhang Y, Wang W, Irish VF, Huang T. RABBIT EARS regulates the transcription of TCP4 during petal development in Arabidopsis. Journal of Experimental Botany. 2016;67:6473–6480. doi: 10.1093/jxb/erw419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juntheikki-Palovaara et al. (2014).Juntheikki-Palovaara I, Tähtiharju S, Lan T, Broholm SK, Rijpkema AS, Ruonala R, Kale L, Albert VA, Teeri TH, Elomaa P. Functional diversification of duplicated CYC2 clade genes in regulation of inflorescence development in Gerbera hybrida (Asteraceae) The Plant Journal. 2014;79:783–796. doi: 10.1111/tpj.12583. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2008).Kim M, Cui ML, Cubas P, Gillies A, Lee K, Chapman MA, Abbott RJ, Coen E. Regulatory genes control a key morphological and ecological trait transferred between species. Science. 2008;322:1116–1119. doi: 10.1126/science.1164371. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2012).Kim TW, Michniewicz M, Bergmann DC, Wang ZY. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature. 2012;482:419–422. doi: 10.1038/nature10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim & Wang (2010).Kim TW, Wang ZY. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annual Review of Plant Biology. 2010;61:681–704. doi: 10.1146/annurev.arplant.043008.092057. [DOI] [PubMed] [Google Scholar]
- Kotilainen et al. (1999).Kotilainen M, Helariutta Y, Mehto M, Pöllänen E, Albert VA, Elomaa P, Teeri TH. GEG participates in the regulation of cell and organ shape during corolla and carpel development in gerbera hybrida. The Plant Cell. 1999;11:1093–1104. doi: 10.1105/tpc.11.6.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang et al. (2013).Kuang Q, Li L, Peng J, Sun S, Wang X. Transcriptome analysis of Gerbera hybrida ray florets: putative genes associated with gibberellin metabolism and signal transduction. PLOS ONE. 2013;8:e57715. doi: 10.1371/journal.pone.0057715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera (2012).Kutschera U. Brassinosteroid action in flowering plants: a Darwinian perspective. Journal of Experimental Botany. 2012;63:3511–3522. doi: 10.1093/jxb/ers065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2012).Li QF, Wang C, Jiang L, Li S, Sun SS, He JX. An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Science Signaling. 2012;5:ra72. doi: 10.1126/scisignal.2002908. [DOI] [PubMed] [Google Scholar]
- Li et al. (2009).Li R, Yu C, Li Y, Lam TW, Yiu SM, Kristiansen K, Wang J, Wang J. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics. 2009;25:1966–1967. doi: 10.1093/bioinformatics/btp336. [DOI] [PubMed] [Google Scholar]
- Li et al. (2015).Li L, Zhang W, Zhang L, Li N, Peng J, Wang Y, Zhong C, Yang Y, Sun S, Liang S, Wang X. Transcriptomic insights into antagonistic effects of gibberellin and abscisic acid on petal growth in Gerbera hybrida. Frontiers in Plant Science. 2015;6 doi: 10.3389/fpls.2015.00168. Article 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaritopoulou et al. (2016).Margaritopoulou T, Kryovrysanaki N, Megkoula P, Prassinos C, Samakovli D, Milioni D, Hatzopoulos P. HSP90 canonical content organizes a molecular scaffold mechanism to progress flowering. The Plant Journal. 2016;87:174–187. doi: 10.1111/tpj.13191. [DOI] [PubMed] [Google Scholar]
- Martín-Trillo & Cubas (2010).Martín-Trillo M, Cubas P. TCP genes: a family snapshot ten years later. Trends in Plant Science. 2010;15:31–39. doi: 10.1016/j.tplants.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Meng & Wang (2004).Meng X, Wang X. Regulation of flower development and anthocyanin accumulation in Gerbera hybrida. Journal of Horticultural Science & Biotechnology. 2004;79:131–137. doi: 10.1080/14620316.2004.11511725. [DOI] [Google Scholar]
- Mizukami (2001).Mizukami Y. A matter of size: developmental control of organ size in plants. Current Opinion in Plant Biology. 2001;4:533–539. doi: 10.1016/S1369-5266(00)00212-0. [DOI] [PubMed] [Google Scholar]
- Mortazavi et al. (2008).Mortazavi A, Williams BA, Mccue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nature Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Oh et al. (2014).Oh E, Zhu JY, Bai MY, Arenhart RA, Sun Y, Wang ZY. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. Elife. 2014;3:714–723. doi: 10.7554/eLife.03031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ÓMaoiléidigh, Graciet & Wellmer (2014).ÓMaoiléidigh D, Graciet E, Wellmer F. Gene networks controlling Arabidopsis thaliana flower development. New Phytologist. 2014;201:16–30. doi: 10.1111/nph.12444. [DOI] [PubMed] [Google Scholar]
- Powell & Lenhard (2012).Powell AE, Lenhard M. Control of organ size in plants. Current Biology. 2012;22:R360–R367. doi: 10.1016/j.cub.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Saini, Sharma & Pati (2015).Saini S, Sharma I, Pati PK. Versatile roles of brassinosteroid in plants in the context of its homoeostasis, signaling and crosstalks. Frontiers in Plant Science. 2015;6 doi: 10.3389/fpls.2015.00950. Article 950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha (2004).Saldanha AJ. Java Treeview—extensible visualization of microarray data. Bioinformatics. 2004;20:3246–3248(3243). doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- Samakovli et al. (2014).Samakovli D, Margaritopoulou T, Prassinos C, Milioni D, Hatzopoulos P. Brassinosteroid nuclear signaling recruits HSP90 activity. New Phytologist. 2014;203:743–757. doi: 10.1111/nph.12843. [DOI] [PubMed] [Google Scholar]
- Schiessl, Muiño & Sablowski (2014).Schiessl K, Muiño JM, Sablowski R. Arabidopsis JAGGED links floral organ patterning to tissue growth by repressing Kip-related cell cycle inhibitors. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2830–2835. doi: 10.1073/pnas.1320457111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeta et al. (2015).Shigeta T, Zaizen Y, Sugimoto Y, Nakamura Y, Matsuo T, Okamoto S. Heat shock protein 90 acts in brassinosteroid signaling through interaction with BES1/BZR1 transcription factor. Journal of Plant Physiology. 2015;178:69–73. doi: 10.1016/j.jplph.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Su et al. (2016).Su M, Huang G, Zhang Q, Wang X, Li C, Tao Y, Zhang S, Lai J, Yang C, Wang Y. The LEA protein, ABR, is regulated by ABI5 and involved in dark-induced leaf senescence in Arabidopsis thaliana. Plant Science. 2016;247:93–103. doi: 10.1016/j.plantsci.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Sun et al. (2010).Sun Y, Fan XY, Cao DM, Tang W, He K, Zhu JY, He JX, Bai MY, Zhu S, Oh E. Integration of brassinosteroid signal transduction with the transcription network for plant growth regulation in Arabidopsis. Developmental Cell. 2010;19:765–777. doi: 10.1016/j.devcel.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres et al. (1996).Szekeres M, Nemeth K, Koncz-Kalman Z, Mathur J, Kauschmann A, Altmann T, Redei GP, Nagy F, Schell J, Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996;85:171–182. doi: 10.1016/S0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Tähtiharju et al. (2012).Tähtiharju S, Rijpkema AS, Vetterli A, Albert VA, Teeri TH, Elomaa P. Evolution and diversification of the CYC/TB1 gene family in Asteraceae–a comparative study in Gerbera (Mutisieae) and sunflower (Heliantheae) Molecular Biology & Evolution. 2012;29:1155–1166 (1112). doi: 10.1093/molbev/msr283. [DOI] [PubMed] [Google Scholar]
- Takahashi et al. (1995).Takahashi T, Gasch A, Nishizawa N, Chua NH. The DIMINUTO gene of Arabidopsis is involved in regulating cell elongation. Genes & Development. 1995;9:97–107. doi: 10.1101/gad.9.1.97. [DOI] [PubMed] [Google Scholar]
- Tang et al. (2008).Tang W, Deng Z, Osesprieto JA, Suzuki N, Zhu S, Xin Z, Burlingame AL, Wang ZY. Proteomics studies of brassinosteroid signal transduction using prefractionation and two-dimensional DIGE. Molecular & Cellular Proteomics. 2008;7:151–170. doi: 10.1074/mcp.M700358-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercruyssen et al. (2011).Vercruyssen L, Gonzalez N, Werner T, Schmülling T, Inzé D. Combining enhanced root and shoot growth reveals cross talk between pathways that control plant organ size in Arabidopsis. Plant Physiology. 2011;155:1339–1352. doi: 10.1104/pp.110.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert et al. (2005).Vert G, Nemhauser JL, Geldner N, Hong F, Chory J. Molecular mechanisms of steroid hormone signaling in plants. Annual Review of Cell & Developmental Biology. 2005;21:177–201. doi: 10.1146/annurev.cellbio.21.090704.151241. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2011).Wang ZY, Bai MY, Oh E, Zhu JY. Brassinosteroid signaling network and regulation of photomorphogenesis. Annual Review of Genetics. 2011;46:701–724. doi: 10.1146/annurev-genet-102209-163450. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2002).Wang ZY, Nakano T, Gendron J, He J, Meng C, Vafeados D, Yang Y, Fujioka S, Yoshida S, Asami T. Nuclear-localized BZR1 Mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Developmental Cell. 2002;2:505–513. doi: 10.1016/S1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- Weiss, Delgadobenarroch & Egeacortines (2005).Weiss J, Delgadobenarroch L, Egeacortines M. Genetic control of floral size and proportions. International Journal of Developmental Biology. 2005;49:513–525. doi: 10.1387/ijdb.051998jw. [DOI] [PubMed] [Google Scholar]
- Xie, Yang & Wang (2011).Xie L, Yang C, Wang X. Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. Journal of Experimental Botany. 2011;62:4495–4506. doi: 10.1093/jxb/err164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2014).Xu P, Cai XT, Wang Y, Xing L, Chen Q, Xiang CB. HDG11 upregulates cell-wall-loosening protein genes to promote root elongation in Arabidopsis. Journal of Experimental Botany. 2014;65:4285–4295. doi: 10.1093/jxb/eru202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin et al. (2002).Yin Y, Wang ZY, Mora-Garcia S, Li J, Yoshida S, Asami T, Chory J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell. 2002;109:181–191. doi: 10.1016/S0092-8674(02)00721-3. [DOI] [PubMed] [Google Scholar]
- Yu et al. (1999).Yu D, Kotilainen M, Pöllänen E, Mehto M, Elomaa P, Helariutta Y, Albert VA, Teeri TH. Organ identity genes and modified patterns of flower development in Gerbera hybrida (Asteraceae) The Plant Journal. 1999;17:51–62. doi: 10.1046/j.1365-313X.1999.00351.x. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2011).Yu X, Li L, Zola J, Aluru M, Ye H, Foudree A, Guo H, Anderson S, Aluru S, Liu P. A brassinosteroid transcriptional network revealed by genome-wide identification of BESI target genes in Arabidopsis thaliana. The Plant Journal. 2011;65:634–646. doi: 10.1111/j.1365-313X.2010.04449.x. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2016).Yu Z, Zhang YJ, Yang BJ, Yu XX, Wang D, Zu SH, Xue HW, Lin WH. Functional characterization of GmBZL2(AtBZR1like gene) reveals the conserved BR signaling regulation in Glycine max. Scientific Reports. 2016;6:31134. doi: 10.1038/srep31134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2012).Zhang L, Li L, Wu J, Peng J, Zhang L, Wang X. Cell expansion and microtubule behavior in ray floret petals of Gerbera hybrida: responses to light and gibberellic acid. Photochemical & Photobiological Sciences. 2012;11:279–288. doi: 10.1039/C1PP05218G. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2016).Zhao Y, Zhang T, Broholm SK, Tahtiharju S, Mouhu K, Albert VA, Teeri TH, Elomaa P. Evolutionary co-option of floral meristem identity genes for patterning of the flower-like asteraceae inflorescence. Plant Physiology. 2016;172:284–296. doi: 10.1104/pp.16.00779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Class I DEGs were significantly down-regulated after 0.5 h BL treatment and did not change markedly after 0.5 h H2O treatment (log2 < 1).
Class II DEGs were up-regulated after 0.5 h BL treatment and did not change markedly after 0.5 h H2O treatment.
Class III DEGs were down-regulated after 10 h BL treatment and did not change markedly after 10 h H2O treatment.
Class IV DEGs were up-regulated after 10 h BL treatment and did not change markedly after 10 h H2O treatment.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplementary File.