Abstract
Growth arrest–specific gene (Gas)6 is a secreted vitamin K–dependent protein with pleiotropic effects via activation of receptor tyrosine kinase Tyro3, Axl, and Mertk receptors, but little is known about its role in allergic airway disease. We investigated the role of Gas6 in the development of fungal allergic airway disease in mice. The immune response was evaluated in Gas6-deficient (Gas6−/−) and wild-type (WT) mice and in recombinant Gas6-treated WT mice during Aspergillus fumigatus–induced allergic airway disease. Gas6 plasma levels were significantly elevated in adult clinical asthma of all severities compared with subjects without asthma. In a murine model of fungal allergic airway disease, increased protein expression of Axl and Mertk were observed in the lung. Airway hyperresponsiveness (AHR), whole lung Th2 cytokine levels, goblet cell metaplasia, and peribronchial fibrosis were ameliorated in Gas6−/− mice compared with WT mice with fungal allergic airway disease. Intranasal Gas6 administration into WT mice had a divergent effect on airway inflammation and AHR. Specifically, a total dose of 2 μg of exogenous Gas6 (i.e., low dose) significantly increased whole lung Th2 cytokine levels and subsequent AHR, whereas a total dose of 7 μg of exogenous Gas6 (i.e., high dose) significantly suppressed Th1 and Th2 cytokines and AHR compared with appropriate control groups. Mechanistically, Gas6 promoted Th2 activation via its highest affinity receptor Axl expressed by myeloid DCs. Intranasal administration of Gas6 consistently exacerbated airway remodeling compared with control WT groups. These results demonstrate that Gas6 enhances several features of fungal allergic airway disease.
Keywords: Gas6, Axl, allergic inflammation, Aspergillus fumigatus
Clinical Relevance
Growth-arrest specific gene (Gas)6 is elevated in clinical asthma but its role in this disease was unknown. The present study addressed the role of Gas6 in an experimental model of fungal allergic airway disease.
Allergic asthma is an airway-specific inflammatory process that promotes persistent physiological and structural remodeling events in the lung (1). Several environmental allergens and/or viruses are known to trigger and exacerbate asthma via their effects on innate and adaptive immune cells (2, 3). For example, dendritic cells (DCs) in the airways induce sensitization to allergens, leading to the development of IL-4–, IL-5–, and IL-13–induced Th2-dependent airway inflammation (4, 5). Th2-type cytokines, such as IL-4 and IL-13, are involved in the differentiation of alternatively activated (M2) macrophages (4), which is a source of airway remodeling factors such as cytokines and chitinase-like molecules (6) and is found in inflammatory zone-1 (FIZZ1) (7, 8). Recent attention has also been directed at characterizing factors that modulate immune cell responses in response to environmental allergens.
We hypothesized that the vitamin K–dependent soluble protein known as growth arrest-specific protein (Gas)6 exerted a key role in the development and exacerbation of asthma. Several cell types express and release Gas6, including structural and immune cells (9). This protein has an N-terminal γ-carboxylated glutamic acid domain (9), which is the ligand that binds and activates TAM (Tyro3, Axl, Mertk) receptor (10). More is known about Gas6, which binds to Axl and Tyro3 with equal affinity but binds Mertk with approximately 10-fold lower affinity (11, 12). TAM receptors belong to a distinct receptor protein–tyrosine kinase subfamily and are expressed in various cells and tissues. First described as critical receptors for the clearance of apoptotic cells (13–15) and as proproliferative mediators on smooth muscle and fibroblasts (16, 17), it is now known that TAM receptors have immunosuppressive functions related to their induction of suppressor of cytokine signaling (SOC)1, SOCS3, and Twist, which inhibit TLR- and cytokine-driven immune responses (18, 19). Although Gas6 and TAM receptors are active in a variety of diseases (13, 20–22), studies addressing the role of Gas6 and TAM receptors in asthma have not been reported despite evidence of Gas6 expression in this disease (23).
In the present study, we addressed the expression of Gas6 in clinical allergic asthma and examined the role of this protein in a well-described model of fungal allergic airway disease. Our data demonstrate that Gas6 modulates Th2-mediated airway inflammation and promotes airway remodeling.
Materials and Methods
Gas6-Deficient Mice
Gas6-deficient (Gas6−/−) mice were generated as previously described in detail (13) and were subsequently bred and maintained in a specific pathogen-free colony at the University of Michigan.
Fungal Allergic Airway Disease in Mice
Female C57BL/6 wild-type (WT) mice, 6 to 8 weeks of age (Taconic Farms, Germantown, NY) or Gas6−/− mice (same age) were sensitized with Aspergillus fumigatus antigens as previously described in detail (24). Prior approval for mouse use was obtained from the University Committee on Use and Care of Animals at the University of Michigan. After sensitization, mice were challenged via intratracheal instillation with live, swollen A. fumigatus conidia. Beginning at Day 14 after conidia challenge, mice received PBS alone or recombinant mouse Gas6 (either 300 ng/dose or 1 μg/dose) (R&D Systems, Minneapolis, MN) every other day until Day 28 after conidia challenge for a total of seven doses of Gas6. At Day 28 after intratracheal administration of A. fumigatus conidia, airway hyperresponsiveness (AHR) was assessed in all groups of mice using a plethysmograph (Buxco, Troy, NY). Briefly, sodium pentobarbital (0.04 mg/g of mouse body weight) (Butler, Columbus, OH) was used to anesthetize mice before their intubation and ventilation. Once baseline airway resistance was established, increasing doses (i.e., 210 or 420 μg/kg) of methacholine were administered via a tail vein, and AHR was monitored for approximately 2 minutes. The peak increase in airway resistance after methacholine challenge was then recorded. In these studies, we observed that the 420 μg/kg dose of methacholine evoked maximal changes in airway resistance, and these values are reported herein.
Bone Marrow–Derived DCs and Macrophage Isolation
Bone marrow–derived DCs or macrophages were prepared from control or allergic mice at various times before and after conidia challenge. To generate DCs, bone marrow cells were cultured for 6 days with granulocyte-macrophage colony-stimulating factor (20 ng/ml) (R&D Systems), and DCs were sorted for CD11c+ expression using magnetic-activated cell sorting (Miltenyi Biotech, Bergisch Gladbach, Germany). To generate macrophages, bone marrow cells were cultured for 6 days with L-cell supernatant containing macrophage colony-stimulating factor and collected cells. The resultant cells were comprised of approximately 97.5% macrophages as determined by flow cytometry.
Naive T Cell Isolation, Co-culture with DCs, and Proliferation Assays
Splenic CD4+CD62L+ T cells were isolated using a magnetic bead column (Miltenyi Biotech). More than 96.2% of the T cells were CD4 positive after isolation, and these cells (2 × 105 cells/well) were exposed to cultured DCs prestimulated with Aspergillus antigen and/or rGas6 (1 μg/ml) for 24 hours at a DC/CD4+ cell ratio of 1:5 for 6 days. After resting the co-cultured T cells for another 48 hours, these cells (2 × 105 cells/well) were exposed to 0.5 μg/ml of anti-CD3 and 2.0 μg/ml of soluble anti-CD28 mAbs (BD, Franklin Lakes, NJ) for an additional 48 hours before analysis for intracellular cytokines.
Statistical Analysis
All results are expressed as the mean ± SEM. A Student's t test or an ANOVA and a Student-Newman-Keuls multiple comparison test were used to determine statistical significance between groups. P < 0.05 was considered statistically significant.
Results
Gas6 Levels Are Elevated in Clinical and Experimental Allergic Airway Disease
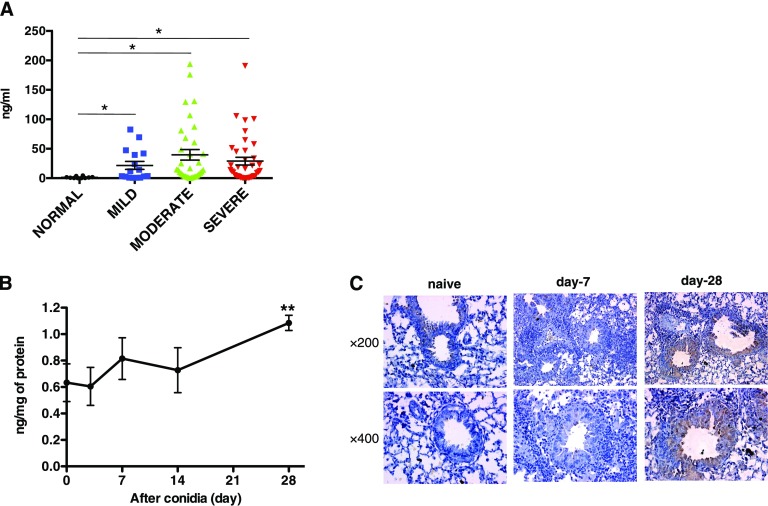
Plasma levels of Gas6 were significantly increased in patients with asthma with mild, moderate, and severe forms of this disease compared with plasma levels detected in nonasthmatic control plasma (Figure 1A). Likewise, Gas6 was significantly increased in whole lung only at Day 28 after conidia when compared with sensitized mice before conidia challenge (Figure 1B). In addition, Gas6-positive macrophages and epithelial cells were detected by immunohistochemistry at Day 28 after conidia challenge (Figure 1C and see Figure E1A in the online supplement). Macrophages isolated before and at Days 7 and 28 after conidia expressed FIZZ1 and significantly higher amounts of Gas6 compared with naive macrophages at Day 28 after conidia (Figure E1B). M2 macrophages expressed significantly greater Gas6 compared with M1 macrophages (Figure E1C). Together, these data demonstrate that M2 macrophages exhibit enhanced Gas6 generation during the course of fungal allergic airway disease model.
Figure 1.
Temporal changes in growth-arrest specific gene (Gas)6 transcript and protein levels in clinical asthma and experimental allergic airway disease. (A) Protein level of Gas6 in plasma from patients with asthma categorized according to disease severity (normal, n = 8; mild, n = 15; moderate, n = 25; severe, n = 26). *P < 0.05 versus the normal control group. (B) Protein level of Gas6 in whole lung samples before and at 3, 7, 14, and 28 days after conidia challenge. Results are expressed as the mean ± SEM for n = 5 per group. Data are representative of two separate experiments. **P < 0.01 versus the naive group. (C) Lung tissue sections from naive and allergic mice were subjected to immunohistochemical analysis for Gas6. Original magnification: ×400.
Gas6 Contributes to Fungal Allergic Airway Disease
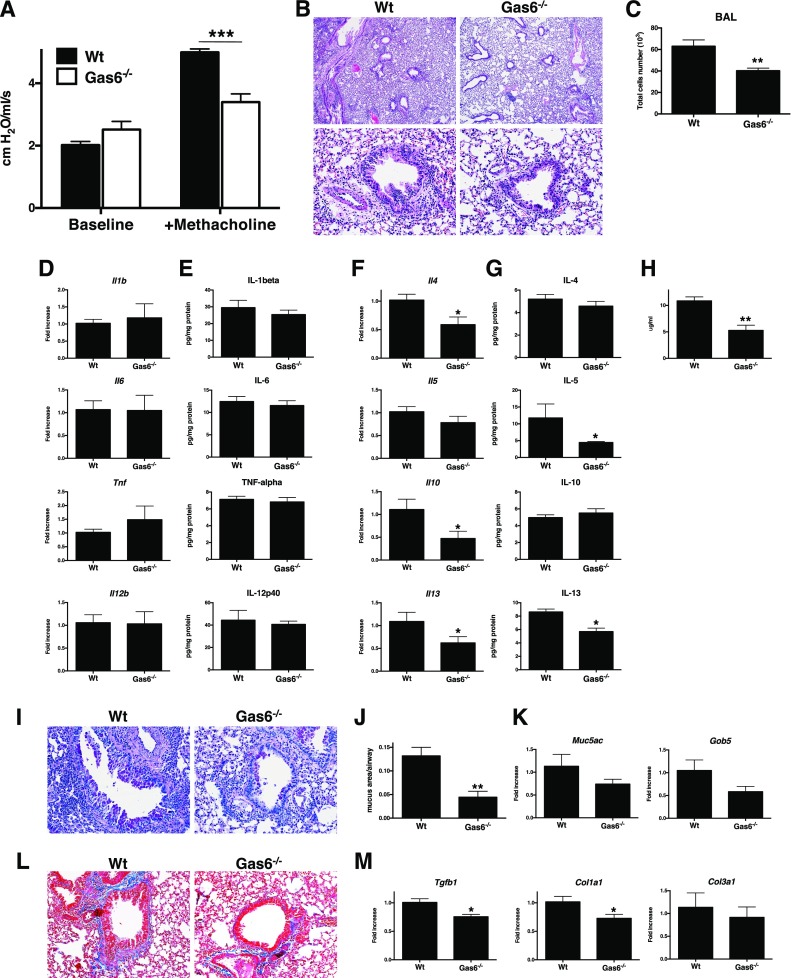
We next compared A. fumigatus–sensitized Gas6 sufficient (i.e., WT) with similarly sensitized Gas6−/− mice at Day 28 after A. fumigatus conidia challenge. AHR in Gas6−/− mice was significantly suppressed compared with the WT group (Figure 2A). Accordingly, peribronchial accumulation of inflammatory cells was significantly higher in the WT groups compared with the Gas6−/− group (Figure 2B), and the number of cells in bronchoalveolar lavage (BAL) was significantly decreased in the Gas6−/− mice compared with the WT group (Figure 2C). Whole lung levels of IL-1β, IL-6, IL-12p40, and TNF-α were not different at either the transcript or protein level (Figures 2D and 2E), but IL-13 transcript and protein levels were significantly reduced in Gas6−/− mice compared with the WT group (Figures 2F and 2G). Statistically significant changes in IL-4, IL-10, and IL-13 transcripts were also observed between these two groups (Figure 2F). IgE level in the serum from Gas6−/− group were significantly decreased compared with the WT group (Figure 2H). Histological analysis revealed a reduction in Periodic acid-Schiff (PAS)-positive cells (Figure 2I), which was confirmed quantitatively (Figure 2J), and Muc5ac and Gob5 transcript expression (Figure 2K) were inhibited in Gas6−/− versus WT mice. Similarly, the collagen production was lower (Figure 2L), and Tgfb1 and Col1a1 transcript expression (Figure 2M) were also significantly lower in Gas6−/− versus WT mice. Together, these results indicated that Gas6 modulates airway inflammatory and remodeling events in allergic airway disease.
Figure 2.
Endogenous Gas6 facilitates methacholine-induced bronchoconstriction in allergic airway disease. (A) Airway hyperresponsiveness (AHR) to a methacholine challenge (420 μg/kg) was determined in Aspergillus fumigatus–sensitized wild-type (Wt) mice or Gas6-deficient (Gas6−/−) mice at Day 28 after conidia challenge. (B) Representative hematoxylin and eosin–stained lung tissue sections from Wt and Gas6−/− mice at Day 28 after conidia challenge. Original magnification: ×40 (upper) and ×200 (lower). (C) Bronchoalveolar lavage (BAL) cell counts from both groups of mice. (D and E) Transcript (D) and protein (E) levels of IL-1β, IL-6, IL-12p40, and TNF-α in Wt and Gas6−/− mice at Day 28 after conidia challenge. (F and G) Whole lung transcript (F) and protein (G) levels of IL-4, IL-5, IL-10, and IL-13 in Wt and Gas6−/− mice at Day 28 after conidia challenge. (H) Serum IgE levels in Wt and Gas6−/− mice at Day 28 after conidia challenge. (I) Representative Periodic acid-Schiff (PAS)-stained lung tissue sections from Wt and Gas6−/− mice at Day 28 after conidia challenge. (J) Quantitative pathological analysis of mucus area in G. (K) Quantitative PCR analysis of Muc5ac and Gob5 in Wt and Gas6−/− mice at Day 28 after conidia challenge. (L) Representative Masson trichrome–stained lung tissue sections from Wt and Gas6−/− mice at Day 28 after conidia challenge. (M) TGF-β, Col1a1, and Col3a1 transcript levels in Wt and Gas6−/− mice at Day 28 after conidia challenge. Results are expressed as the mean ± SEM for n = 5 per group. *P < 0.05, **P < 0.01, and ***P < 0.001 versus Wt.
Exogenous Gas6 Exacerbates Experimental Allergic Airway Disease
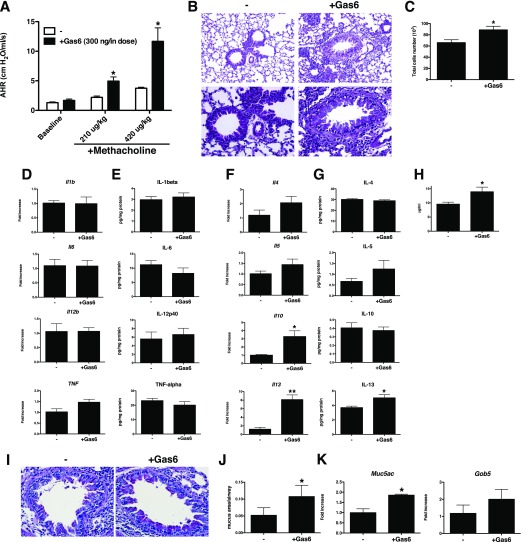
We next examined the effect of exogenously administered Gas6 by intranasal administration in A. fumigatus–sensitized WT mice from Days 14 to 28 after conidia challenge. This time frame was selected on the basis of our finding that TAM receptors were increased in expression during this time (data not shown). When seven Gas6 treatments (300 ng/dose) were administered over this time via intranasal challenge, methacholine-induced AHR was significantly increased compared with controls (Figure 3A). In the Gas6-treated group, peribronchial inflammation was increased compared with the control group (Figure 3B), and the number of cells in the BAL was significantly increased in the Gas6-treated group compared with the control group (Figure 3C). In the Gas6-treated group, levels of inflammatory cytokines such as IL-12p40, IL-1β, IL-6, and TNF-α were unchanged at the transcript (Figure 3D) and protein (Figure 3E) levels, but IL-13 (Figures 3F and 3G) and serum IgE levels (Figure 3H) were significantly increased compared with the control group. Likewise, whole lung histological sections from both Gas6-treated groups exhibited increased PAS-positive cells in airway (Figures 3I and 3J), and the transcript levels of Muc5ac and Gob5 were significantly increased in Gas6-treated lungs (Figure 3K). Together, these results demonstrate that exogenous Gas6 exacerbates fungal-induced allergic airway disease.
Figure 3.
Exogenous Gas6 increased Th2 inflammation followed by AHR and mucus cell metaplasia in fungal allergic airway disease. (A) AHR to a methacholine challenge was determined in A. fumigatus–sensitized mice with PBS (-) as a control or with (+Gas6) intranasal challenges with recombinant Gas6 (300 ng/dose × 7 doses = 2.1 μg total) at Day 28 after conidia challenge. (B) Representative hematoxylin and eosin–stained lung tissue sections from control and Gas6-treated groups at Day 28 after conidia challenge. Original magnification: ×100 (upper) and ×400 (lower). (C) BAL cell counts from both groups of mice. (D and E) Whole lung transcript (D) and protein (E) levels of IL-1β, IL-6, IL-12p40, and TNF-α in control and Gas6-treated groups at Day 28 after conidia challenge. (F and G) Whole lung transcript (F) and protein (G) levels of IL-4, IL-5, IL-10, and IL-13 in control and Gas6-treated groups at Day 28 after conidia challenge. (H) Serum IgE levels in wild-type (WT) and Gas6−/− mice at Day 28 after conidia challenge. (I) Representative PAS-stained lung tissue sections from control and Gas6-treated groups at Day 28 after conidia challenge. Original magnification: ×400. (J) Quantitative pathological analysis of mucus area in G. (K) Quantitative PCR analysis of Muc5ac and Gob5 at Day 28 after conidia. Results are expressed as the mean ± SEM for n = 5 per group. *P < 0.05 and **P < 0.01 versus control (indicated as -).
Higher Exogenous Gas6 Suppresses AHR but Augments Airway Remodeling during Fungal Allergic Airway Disease
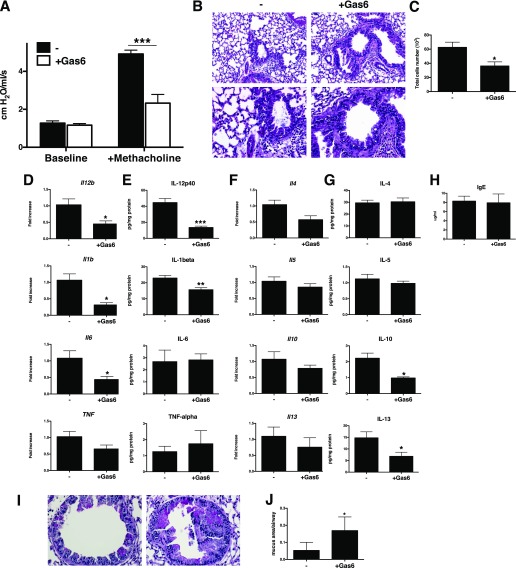
We hypothesized that the previously described inhibitory effects of Gas6 on cytokine production by immune cells (19) might provide a beneficial effect if higher amounts were administered. To address this, exogenous Gas6 was given at 1 μg/dose (referred to as “high dose”) to sensitized WT mice from Days 14 to 28 after conidia challenge. The high-dose Gas6 treatment protocol significantly decreased methacholine-induced AHR (Figure 4A), the peribronchial accumulation of inflammatory cells (Figure 4B), and the accumulation of cells in the BAL compared with the control group (Figure 4C). Whole lung levels of IL-12p40, IL-1β, IL-6, and TNF-α were lower in the high-dose group compared with the control group at the transcriptional (Figure 4D) and protein (Figure 4E) levels. IL-4, IL-5, IL-10, and IL-13 levels were also suppressed by the high-dose Gas6 treatment (Figures 4F and 4G). Similarly, many other transcripts were suppressed by the high-dose Gas6 treatment protocol (exceptions included CD19, CD28, CD34, and IL-15; Figure E2). Exogenous Gas6 treatment at this dose did not change the serum levels of IgE compared with control group (Figure 4H). However, airway remodeling was markedly enhanced in the Gas6-treated group, as evidenced by thickened smooth muscle cells around airway. Accordingly, whole lung histological sections from the high-dose Gas6 group showed significantly increased numbers of PAS-positive cells in the airways compared with the control group (Figures 4I and 4J). Together, these data demonstrate that higher exogenous Gas6 modulated AHR and certain proinflammatory cytokines, but this mediator consistently promoted and exacerbated airway remodeling in this model.
Figure 4.
High-dose Gas6 suppresses inflammatory response and subsequent AHR and induces severe mucus production in airway. (A) AHR to a methacholine challenge was determined in A. fumigatus–sensitized mice with PBS (-) as a control or with (+Gas6) intranasal challenges with recombinant Gas6 (1 μg/dose × 7 doses = 7.0 μg total) at Day 28 after conidia challenge. (B) Representative hematoxylin and eosin–stained lung tissue sections from control and Gas6-treated groups at Day 28 after conidia challenge. Original magnification: ×100 (upper) and ×400 (lower). (C) BAL cell counts from both groups of mice. (D and E) Whole lung transcript (D) and protein (E) levels of IL-1β, IL-6, IL-12p40, and TNF-α in control and Gas6-treated groups at Day 28 after conidia challenge. (F and G) Whole lung transcript (F) and protein (G) levels of IL-4, IL-5, IL-10, and IL-13 in control and Gas6-treated groups at Day 28 after conidia challenge. (I) Representative PAS-stained lung tissue sections from control and Gas6-treated groups at Day 28 after conidia challenge. Original magnification: ×400. (J) Quantitative pathological analysis of mucus area in I. Results are expressed as the mean ± SEM for n = 5 per group. *P < 0.05, **P < 0.01, and ***P < 0.001 versus control (indicated as -).
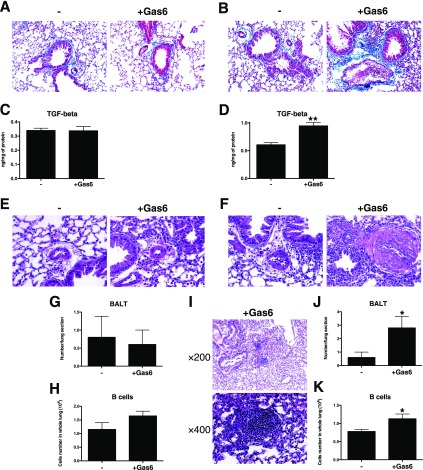
Exogenous Gas6 Treatments Promoted Airway and Blood Vessel Remodeling and Bronchial-Associated Lymphoid Tissue Hypertrophy during Fungal Allergic Airway Disease
Low- and high-dose Gas6 treatments consistently altered the degree of airway remodeling during fungal allergic airway disease, including collagen deposition, around airways in the low-dose (Figure 5A) and high-dose (Figure 5B) Gas6-treated mice. Although not changed in the low-dose group (Figure 5C), whole lung TGF-β protein was significantly increased in the high-dose group compared with the control group (Figure 5D). Although the low-dose Gas6 group did not show the same alterations (Figures 5E, 5G, and 5H), the high-dose group exhibited significant vascular alterations (Figure 5F) and a significant increase in the number of lymphoid follicles or BALT (Figures 5I and 5J) containing significantly increased numbers of B cells (Figure 5K) compared with the control group. Thus, these results suggest that Gas6 contributes uniformly to airway and vascular remodeling and to BALT hypertrophy during fungal allergic airway disease.
Figure 5.
Exogenous Gas6 uniformly induced fibrosis, vascular smooth muscle hypertrophy, and B cell accumulation. Representative Masson trichrome–stained lung tissue sections from the low-dose (2 μg) Gas6 (A) and high-dose (7 μg) Gas6 groups (B) at Day 28 after conidia challenge. TGF-β transcript and protein levels in the low-dose (C) and high-dose (D) Gas6-treated groups at Day 28 after conidia challenge. Vascular wall hypertrophy was detected in the low-dose Gas6 (E) and high-dose Gas6 (F) groups at Day 28 after conidia challenge. The number of bronchus-associated lymphoid tissue (BALT) follicles was not changed in the low-dose Gas6-treated lung (G), but the number of B cells in the BALT from this group was increased (H). Expanded BALT was observed in histological sections from the high-dose Gas6-treated group (I), and the number of follicles (J) and the number of B cells in these follicles (K) were significantly increased compared with the control group. Results are expressed as the mean ± SEM for n = 5 per group. *P < 0.05 and **P < 0.01 versus control (indicated as -).
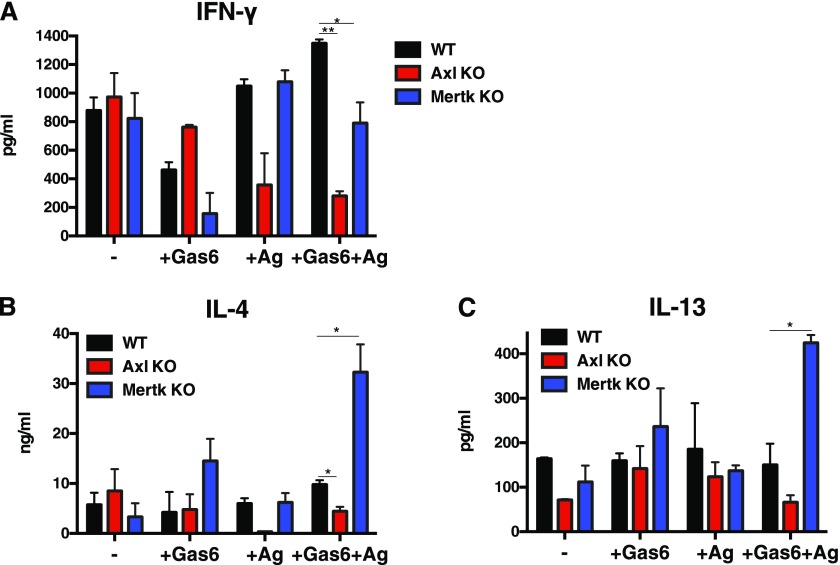
Gas6/Axl Signaling Promotes a Th2 Response via DC Activation
The divergent effects of Gas6 on fungus-induced airway disease led us to focus on the manner in which Gas6 exerted its immune effects via specific TAM receptors. Gas6 has several-fold higher affinity for Axl compared with Mertk or Tyro3, but Tyro3 is not present on mouse myeloid DCs (10). In a co-culture system, we observed that the transcript levels of IFN-γ were significantly increased, whereas IL-4 and IL-13 were significantly reduced in anti-CD3– and anti-CD28–stimulated T cells when Axl−/− DCs were stimulated with Gas6 and Aspergillus antigen and co-cultured with naive WT T cells compared with WT only co-cultures (Figure 6). In contrast, co-culture of naive WT T cells and Mertk−/− DCs led to the generation of significantly higher levels of IL-13 in T cells compared with WT-only co-cultures (Figure 6). Together, these results indicate that Gas6/Axl signaling in DCs led to the activation of Th2 cells and that the absence of this ligand/receptor interaction enhanced the activation of Th1 cells. Gas6/Mertk signaling in DCs appeared to be necessary for Th1 activation, and, in the absence of this ligand/receptor interaction, we observed enhanced IL-13 in this co-culture system. Thus, these findings suggest that specific TAM receptor activation could uniquely affect DC-driven T cell activation.
Figure 6.
Gas6 modulated the Th2-inducing properties of isolated dendritic cells via Axl. Bone marrow–derived DCs generated from naive WT, Axl−/−, and Mertk−/− mice were treated with Aspergillus antigen and Gas6 and co-cultured with naive CD4 T cells. After co-culture, CD4 cells were activated with anti-CD3 and anti-CD28 before cytokine analysis. Transcript expression of IFN-γ, IL-4, and IL-13 was determined using quantitative PCR. Results are expressed as the mean ± SEM for n = 3 independent experiments in which a minimum of three mice per group were used to isolate DCs and T cells. *P < 0.05 and **P < 0.01.
Discussion
Gas6 was found to be significantly elevated in a pediatric asthma population, particularly in children who experienced an infectious exacerbation of this disease, but its role remains unclear (24). We observed that the plasma level of Gas6 was elevated in adult patients with asthma regardless of disease severity. In further experimental studies, Gas6 was constitutively expressed in the lung of A. fumigatus–sensitized WT mice, but it was markedly induced after conidia challenge in these mice. A major source of Gas6 protein appeared to be M2 macrophages, but airway epithelial cells also expressed this mediator. In this model of fungal allergic airway disease, AHR, airway inflammation, goblet cell metaplasia, and airway remodeling were markedly reduced in Gas6−/− versus WT mice. Gas6 activated DCs via Axl receptor, leading to Th2-type cytokine polarization, and exogenous Gas6 uniformly enhanced airway remodeling during fungal allergic airway disease. Together, these data highlight that Gas6 has a key role in inflammatory and remodeling processes during fungal allergic airway disease.
The increased expression of Gas6 in human asthma and by immune and nonimmune cells in lung samples during the course of fungal allergic airway disease model was a key finding from the present study. Increased M2 macrophage activation appears to be a cellular biomarker of asthma (25), and we observed that M2 macrophages generated large amounts of Gas6 in response to Th2-type cytokines. However, our immunohistochemical analysis indicated that other cells types, including epithelial cells, expressed this ligand, which is consistent with previous studies (26). Gas6 is known to inhibit apoptosis and is a strong survival factor for epithelial cells, fibroblasts, and vascular endothelial cells (27, 28), and Gas6/Axl (but not Gas6/Mertk) signaling prevents apoptosis and stimulates proliferation of fibroblasts and vascular smooth muscle cells via the activation of ERK kinase (17, 28). Exogenous Gas6 uniformly led to increased airway remodeling, as evidenced by goblet cell hyperplasia, peribronchial fibrosis, and vascular remodeling. Our in vivo studies also showed that exogenous Gas6 increased the expansion of BALT (particularly B cells) in this model. The effects of exogenous Gas6 were not observed in the absence of conidia challenge, presumably because constitutive expression of TAM receptors is below the limits of detection (i.e., immunohistochemistry) in naive and A. fumigatus–sensitized mouse lung. Gas6 was expressed by and exerted effects on a number of lung-resident immune and nonimmune cells during experimental fungal allergic airway disease.
Methacholine-evoked AHR is an important physiological parameter in experimental allergic airway disease models. Various factors contribute to the development of AHR, but it is widely accepted that the Th2-type mediators, such as IL-4 and IL-13, play a major role in driving this response (30, 31). In the present study, Gas6−/− mice exhibited attenuated fungal allergic airway disease, including AHR, compared with their WT counterparts, and this finding coincided with significantly less Th2-type inflammation. These findings were also consistent with the experiment in which a total of 2 μg of exogenous Gas6 in WT mice significantly increased AHR and IL-13 transcript and protein levels compared with a WT control group. In this model, IL-13 has been previously shown to have a very prominent role in the expression of AHR, particularly at Day 28 after conidia challenge (32). However, the high dose of exogenous Gas6 significantly suppressed AHR, and this coincided with decreased IL-13 levels. Also, the high-dose Gas6 treatment inhibited proinflammatory IL-1β and IL-6 at the transcript and/or protein levels and many other transcripts. We hypothesized that the suppressive effect by high-dose Gas6 on the inflammatory response depended on the induction of SOCS1, SOCS3, and/or Twist that inhibit TLR- and cytokine-driven immune responses (18, 19), but we did not detect changes in these suppressive proteins in the high-dose Gas6-treated group (data not shown).
The differential responses of exogenous Gas6 on AHR and the airway inflammatory response led us to examine immunoregulatory mechanisms, and we observed that the role of Gas6 in DC-mediated T cell activation differed markedly depending on the expression of Axl or Mertk on DCs. Gas6 expresses a carboxyterminal domain, with which it binds to TAM receptors according to the following affinities: Axl > Tyro3 > >> Mertk (11, 12), suggesting that Gas6 preferentially bind to Axl. We therefore speculate that Axl signaling is predominant under endogenous or low-dose exogenous Gas6 conditions, whereas Gas6/Mertk and Gas6/Axl activation occurs under conditions in which a high dose of exogenous Gas6 was applied to the fungal allergic airway disease model. Axl and Mertk were expressed in the lungs of allergic mice, and the expression of both increased with time, in contrast to Tyro3, which showed decreased expression with time. From our in vitro T cell–DC co-culture experiments, Gas6/Axl signaling in DCs was necessary for the expression of IL-13, and the absence of this ligand/receptor interaction enhanced the activation of Th1 cells. In contrast, Gas6/Mertk signaling in DCs appeared to be necessary for IFN-γ transcript expression (under all culture conditions), and in the absence of this ligand/receptor interaction we observed enhanced IL-13 in this co-culture system. Additional studies are required to more fully elucidate the cellular mechanisms by which Gas6/Mertk interactions modulate that allergic inflammatory response.
Aside from direct effects of Gas6 on the structural cells, the increase in IL-13 and the changes in TGF-β might account for the airway remodeling changes observed (32–35). Observations in a carbon tetrachloride liver fibrosis model coincide with our observations, specifically that TGF-β was found to be markedly reduced in Gas6−/− mice compared with WT controls (35), strongly suggesting that Gas6 regulates TGF-β expression in vivo. Thus, Gas6 is a potent modulator of the lung remodeling responses apparently due to its direct effects on the various cellular components of airway and blood vessels.
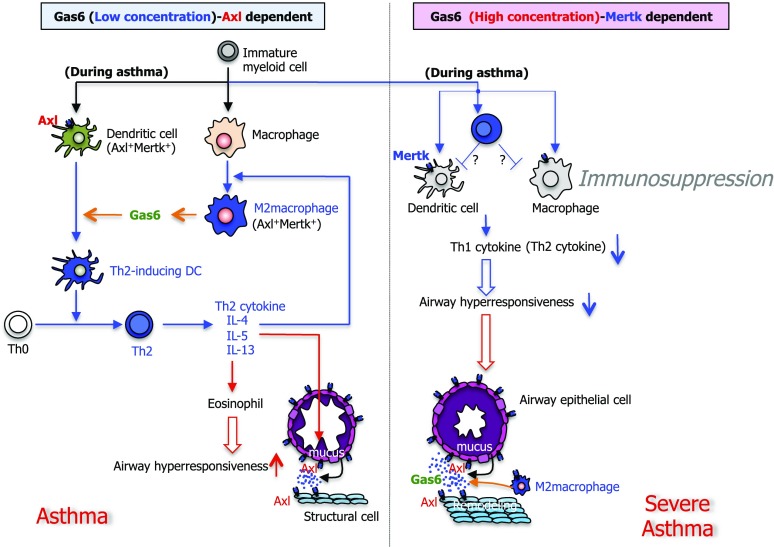
In summary, we used gene targeting and recombinant protein approaches to reveal that Gas6 modulates Th2-type inflammation, AHR, and airway and blood vessel remodeling during fungal allergic airway disease. Its mechanism of action appears to involve the generation of Gas6 by M2 macrophages leading to DC activation via Axl, which in turn polarized antigen-specific Th2 cells. However, Gas6 also exhibited immunoregulatory effects via Mertk activation of DCs. Regardless of the lung levels of Gas6, it was apparent that this mediator exerted prominent airway remodeling in fungal allergic airway disease. On the basis of our in vivo and in vitro findings, we have developed a working hypothesis, which is outlined in Figure 7, highlighting the dose-dependent role of Gas/TAM receptor interactions leading to allergic inflammation and remodeling during fungal allergic airway disease. Thus, targeting Gas6 and specifically Axl might provide clear therapeutic effects in clinical asthma.
Figure 7.
Summary scheme outlining the manner in which Gas6 dose-dependently regulates lung immune and remodeling responses during primary allergic airway disease. In a dose-dependent manner, Gas6 modulated Th2-type inflammation, AHR, and airway and blood vessel remodeling during fungal allergic airway disease. Its mechanism of action appears to involve the generation of Gas6 by M2 macrophages leading to DC activation via Axl, which in turn polarized Th2 cells (left). However, Gas6 also exhibited immunoregulatory effects via Mertk activation in DCs and presumably other myeloid cell populations (right). Regardless of the lung levels of Gas6, it was apparent that this mediator exerted prominent airway remodeling characterized by goblet cell metaplasia and peribronchial fibrosis.
Footnotes
Author Contributions: T.S. and C.M.H. designed the experiments. T.S. performed the experiments. U.B.I., N.A.K., A.P.M., and A.L.C. provided technical advice and assistance. G.L.C. provided human samples. S.L.K. and N.W.L. provided expertise and reagents. T.S. and C.M.H. analyzed data and wrote the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0049OC on May 8, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011;128:1165–1174. doi: 10.1016/j.jaci.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Byrne PM. Therapeutic strategies to reduce asthma exacerbations. J Allergy Clin Immunol. 2011;128:257–263, quiz 264–265. doi: 10.1016/j.jaci.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 4.Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106:551–559. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304:1678–1682. doi: 10.1126/science.1095336. [DOI] [PubMed] [Google Scholar]
- 7.Mikita T, Campbell D, Wu P, Williamson K, Schindler U. Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol Cell Biol. 1996;16:5811–5820. doi: 10.1128/mcb.16.10.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett. 2003;85:173–180. doi: 10.1016/s0165-2478(02)00225-0. [DOI] [PubMed] [Google Scholar]
- 9.Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol. 2010;22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem. 1996;271:30022–30027. doi: 10.1074/jbc.271.47.30022. [DOI] [PubMed] [Google Scholar]
- 12.Fisher PW, Brigham-Burke M, Wu SJ, Luo J, Carton J, Staquet K, Gao W, Jackson S, Bethea D, Chen C, et al. A novel site contributing to growth-arrest-specific gene 6 binding to its receptors as revealed by a human monoclonal antibody. Biochem J. 2005;387:727–735. doi: 10.1042/BJ20040859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angelillo-Scherrer A, de Frutos P, Aparicio C, Melis E, Savi P, Lupu F, Arnout J, Dewerchin M, Hoylaerts M, Herbert J, et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 14.Chen JCK, Carey K, Godowski PJ. Identification of Gas6 as a ligand for Mer, a neural cell adhesion molecule related receptor tyrosine kinase implicated in cellular transformation. Oncogene. 1997;14:2033–2039. doi: 10.1038/sj.onc.1201039. [DOI] [PubMed] [Google Scholar]
- 15.Scott RSME, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 16.Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB, Ivaska J. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30:1436–1448. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- 17.Melaragno MG, Cavet ME, Yan C, Tai L-K, Jin Z-G, Haendeler J, Berk BC. Gas6 inhibits apoptosis in vascular smooth muscle: role of Axl kinase and Akt. J Mol Cell Cardiol. 2004;37:881–887. doi: 10.1016/j.yjmcc.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MBA, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Sharif MNSD, Sosic D, Rothlin CV, Kelly E, Lemke G, Olson EN, Ivashkiv LB. Twist mediates suppression of inflammation by type I IFNs and Axl. J Exp Med. 2006;203:1891–1901. doi: 10.1084/jem.20051725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang L, Liu CY, Yang QF, Wang P, Zhang W. Plasma level of growth arrest-specific 6 (GAS6) protein and genetic variations in the GAS6 gene in patients with acute coronary syndrome. Am J Clin Pathol. 2009;131:738–743. doi: 10.1309/AJCP3CX3AUVRBHCF. [DOI] [PubMed] [Google Scholar]
- 21.Mc Cormack O, Chung WY, Fitzpatrick P, Cooke F, Flynn B, Harrison M, Fox E, Gallagher E, Goldrick AM, Dervan PA, et al. Growth arrest-specific gene 6 expression in human breast cancer. Br J Cancer. 2008;98:1141–1146. doi: 10.1038/sj.bjc.6604260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borgel D, Clauser S, Bornstain C, Bièche I, Bissery A, Remones V, Fagon JY, Aiach M, Diehl JL. Elevated growth-arrest-specific protein 6 plasma levels in patients with severe sepsis. Crit Care Med. 2006;34:219–222. doi: 10.1097/01.ccm.0000195014.56254.8a. [DOI] [PubMed] [Google Scholar]
- 23.Aoki T, Matsumoto Y, Hirata K, Ochiai K, Okada M, Ichikawa K, Shibasaki M, Arinami T, Sumazaki R, Noguchi E. Expression profiling of genes related to asthma exacerbations. Clin Exp Allergy. 2009;39:213–221. doi: 10.1111/j.1365-2222.2008.03186.x. [DOI] [PubMed] [Google Scholar]
- 24.Hogaboam CM, Blease K, Mehrad B, Steinhauser ML, Standiford TJ, Kunkel SL, Lukacs NW. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am J Pathol. 2000;156:723–732. doi: 10.1016/S0002-9440(10)64775-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Byers DE, Holtzman MJ. Alternatively activated macrophages and airway disease. Chest. 2011;140:768–774. doi: 10.1378/chest.10-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tjwa M, Moons L, Lutgens E. Pleiotropic role of growth arrest-specific gene 6 in atherosclerosis. Curr Opin Lipidol. 2009;20:386–392. doi: 10.1097/MOL.0b013e328330982e. [DOI] [PubMed] [Google Scholar]
- 27.Korshunov VA, Mohan AM, Georger MA, Berk BC. Axl, a receptor tyrosine kinase, mediates flow-induced vascular remodeling. Circ Res. 2006;98:1446–1452. doi: 10.1161/01.RES.0000223322.16149.9a. [DOI] [PubMed] [Google Scholar]
- 28.Valverde P, Obin MS, Taylor A. Role of Gas6/Axl signaling in lens epithelial cell proliferation and survival. Exp Eye Res. 2004;78:27–37. doi: 10.1016/j.exer.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Stenhoff J, Dahlbäck B, Hafizi S. Vitamin K-dependent Gas6 activates ERK kinase and stimulates growth of cardiac fibroblasts. Biochem Biophys Res Commun. 2004;319:871–878. doi: 10.1016/j.bbrc.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 30.Finkelman FD, Hogan SP, Hershey GKK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol. 2010;184:1663–1674. doi: 10.4049/jimmunol.0902185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie ANJ.Innate IL-13–producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity J Allergy Clin Immunol 2012;129:191–198, e1–4. [DOI] [PubMed] [Google Scholar]
- 32.Blease K, Jakubzick C, Westwick J, Lukacs N, Kunkel SL, Hogaboam CM. Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J Immunol. 2001;2001:5219–5224. doi: 10.4049/jimmunol.166.8.5219. [DOI] [PubMed] [Google Scholar]
- 33.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, Hogaboam C, Taraseviciene-Stewart L, Voelkel NF, Rabinovitch M, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med. 2008;205:361–372. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitamura H, Cambier S, Somanath S, Barker T, Minagawa S, Markovics J, Goodsell A, Publicover J, Reichardt L, Jablons D, et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J Clin Invest. 2011;121:2863–2875. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choy DF, Modrek B, Abbas AR, Kummerfeld S, Clark HF, Wu LC, Fedorowicz G, Modrusan Z, Fahy JV, Woodruff PG, et al. Gene expression patterns of Th2 inflammation and intercellular communication in asthmatic airways. J Immunol. 2011;186:1861–1869. doi: 10.4049/jimmunol.1002568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fourcot ACD, Couchie D, Chobert MN, Zafrani ES, Mavier P, Laperche Y, Brouillet A. Gas6 deficiency prevents liver inflammation, steatohepatitis, and fibrosis in mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1043–G1053. doi: 10.1152/ajpgi.00311.2010. [DOI] [PubMed] [Google Scholar]