Abstract
The receptor for advanced glycation end-products (RAGE), a multiligand member of the Ig family, may play a crucial role in the regulation of lung fluid balance. We quantified soluble RAGE (sRAGE), a decoy isoform, and advanced glycation end-products (AGEs) from the bronchoalveolar lavage fluid of smokers and nonsmokers, and tested the hypothesis that AGEs regulate lung fluid balance through protein kinase C (PKC)–gp91phox signaling to the epithelial sodium channel (ENaC). Human bronchoalveolar lavage samples from smokers showed increased AGEs (9.02 ± 3.03 μg versus 2.48 ± 0.53 μg), lower sRAGE (1,205 ± 292 pg/ml versus 1,910 ± 263 pg/ml), and lower volume(s) of epithelial lining fluid (97 ± 14 ml versus 133 ± 17 ml). sRAGE levels did not predict ELF volumes in nonsmokers; however, in smokers, higher volumes of ELF were predicted with higher levels of sRAGE. Single-channel patch clamp analysis of rat alveolar epithelial type 1 cells showed that AGEs increased ENaC activity measured as the product of the number of channels (N) and the open probability (Po) (NPo) from 0.19 ± 0.08 to 0.83 ± 0.22 (P = 0.017) and the subsequent addition of 4-hydroxy-2, 2, 6, 6-tetramethylpiperidine-N-oxyl decreased ENaC NPo to 0.15 ± 0.07 (P = 0.01). In type 2 cells, human AGEs increased ENaC NPo from 0.12 ± 0.05 to 0.53 ± 0.16 (P = 0.025) and the addition of 4-hydroxy-2, 2, 6, 6-tetramethylpiperidine-N-oxyl decreased ENaC NPo to 0.10 ± 0.03 (P = 0.013). Using molecular and biochemical techniques, we observed that inhibition of RAGE and PKC activity attenuated AGE-induced activation of ENaC. AGEs induced phosphorylation of p47phox and increased gp91phox-dependent reactive oxygen species production, a response that was abrogated with RAGE or PKC inhibition. Finally, tracheal instillation of AGEs promoted clearance of lung fluid, whereas concomitant inhibition of RAGE, PKC, and gp91phox abrogated the response.
Keywords: acute respiratory distress syndrome, chronic obstructive pulmonary disease, pulmonary edema, alveolar microenvironment, lung injury
Clinical Relevance
Receptor for advanced glycation end-products (RAGE) plays a critical role in regulating inflammation in the lung and may be a therapeutic target in the treatment of acute and chronic lung diseases. Herein we describe the signal transduction pathway through which RAGE regulates the epithelial sodium channel.
The receptor for advanced glycation end-products (RAGE) is a multiligand transmembrane protein of the Ig superfamily that functions to amplify and perpetuate the inflammatory response (1–3). RAGE is most abundantly expressed on the alveolar epithelium, and is highly expressed in alveolar epithelial type (T) 1 cells, which make up more than 95% of the alveolar surface area (4, 5). In addition to its full-length transmembrane form, RAGE exists in an endogenously secreted and cleaved form collectively termed soluble RAGE (sRAGE). sRAGE acts as a decoy signal—that is, sRAGE binds to ligands, thereby reducing the bioavailability of agonists that could activate RAGE signaling. There are many RAGE ligands, including advanced glycation end-products (AGEs), amyloid fibrils, amphoterins, S100/calgranulins, and macrophage-1 (1, 6–9).
Studies show that smoking and aging increase AGE formation. This is significant to chronic lung disease, because smoking is the primary etiology, and accelerated aging is recognized as a contributor to the pathogenesis of chronic obstructive pulmonary disease (COPD) (10). The role of RAGE, RAGE ligands, and sRAGE in the pathogenesis and progression of pulmonary disease is gaining attention in the biomedical community. Indeed, studies show increased expression of RAGE in airway biopsies of individuals with COPD, and deficient levels of sRAGE were observed in the plasma of individuals with COPD (11, 12). Comprehending the role of RAGE, RAGE ligands, and sRAGE in disease development and progression could lead to improved therapies for acute and chronic lung diseases.
Appropriate lung fluid balance is critical for effective gas exchange. Lung fluid balance is controlled through regulated activity of the amiloride-sensitive epithelial sodium channel (ENaC). ENaC is a member of the degenerin family of ion channels, and is composed of α, β, and γ subunits arranged in a trimeric stoichiometry (13). The α subunit is abundantly expressed at the cell membrane, and is capable of forming amiloride-sensitive current alone, or alongside β-and γ-ENaC subunits (14, 15). A δ subunit has recently been characterized in the human lung, albeit the functional role of this subunit remains unclear (16, 17). Together, these subunits play an important role in reabsorbing sodium from the epithelial lining fluid (ELF), which is then extruded transcellularly via the basolaterally located Na+-K+ATPases. The movement of sodium from the apical to the basolateral membrane creates an osmotic gradient that drives water absorption. As such, normal regulation of ENaC activity plays an important role in the resolution of pulmonary edema (18).
It is also clear that inappropriate ENaC activity plays a critical role in a variety of acute and chronic lung diseases, such as acute respiratory distress syndrome (ARDS), cystic fibrosis, and chronic bronchitis. Recently, we have shown that cigarette smoke extract increases ENaC activity in T1 and T2 cells, which resulted in altered lung fluid volumes (19). This implicates ENaC dysfunction in smoking-related lung diseases, such as COPD.
ENaC can be regulated through a variety of cell signaling events. In particular, protein kinase C (PKC) and oxidants produced by reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (Nox) are of primary interest, because the former is an upstream regulator of Nox activation (20). There are multiple PKC isoforms expressed in the human lung, and general activation of PKC has been shown to increase β- and γ-ENaC subunits, but not α-ENaC (21–23). Although PKC has been shown to directly phosphorylate ENaC when it is overexpressed, there is still uncertainty as to whether endogenous ENaC is phosphoregulated (24–26). There is evidence in vivo that PKC acts as a second messenger that regulates ENaC via a complex signal transduction pathway. Recently, PKC isoforms have been shown to phosphorylate the p47phox subunit of Nox leading to assembly and activation. We, and others have recently shown that the seven Nox isoforms (Nox1–5, dual oxidase 1, and dual oxidase 2) are expressed in the human lung, albeit the cellular distribution of each Nox isoform in the human lung remains unclear (27). Importantly, we have shown that Nox2-derived reactive oxygen species (ROS) are critical regulators of lung ENaC activity (28, 29). Evidence suggests that PKC/Nox signaling regulates oxidant production in a variety of pathological conditions, such as atherosclerosis, hypertension, diabetes, and cancer (24). Herein, we propose that PKC/Nox signaling plays an important role in RAGE regulation of lung fluid balance. We show that RAGE signaling plays an important role in the regulation of lung fluid balance by measuring ELF, AGEs, and sRAGE from the bronchoalveolar lavage (BAL) fluid of smokers and nonsmokers residing in a major metropolitan area, and obtained mechanistic insight by performing single-channel patch clamp analysis and complementary biochemical assays.
Materials and Methods
Materials used and detailed methods are provided in the online supplement.
Results
Differences in BAL Characteristics between Smokers and Nonsmokers
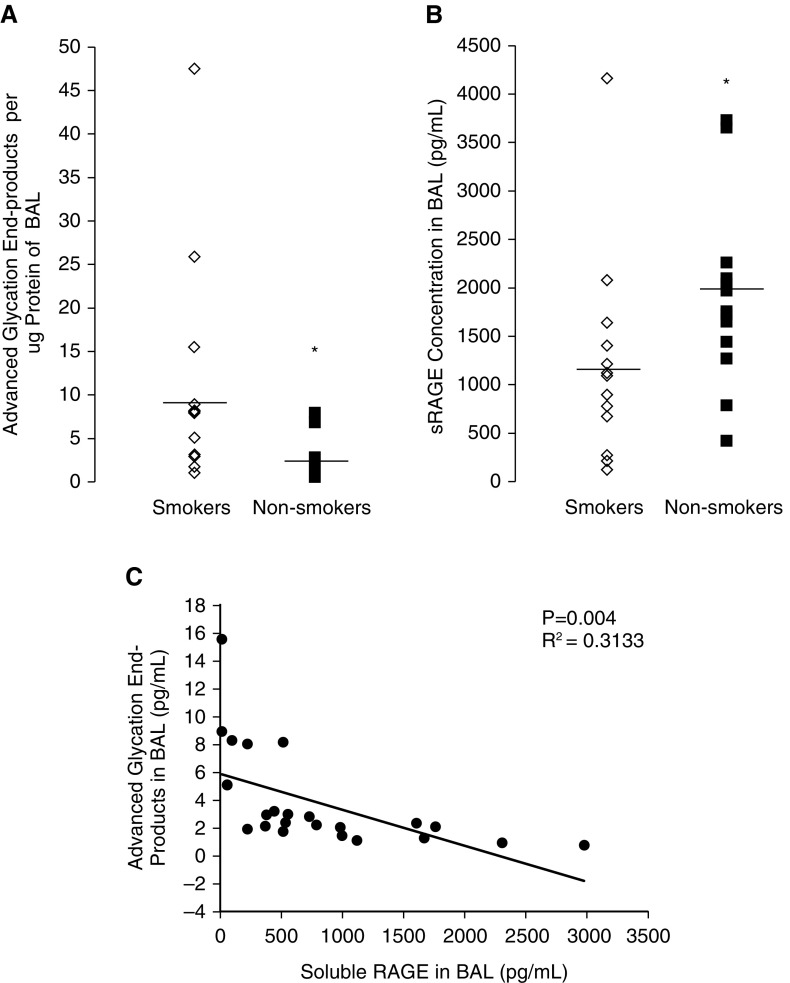
BAL fluid obtained from 16 smokers (eight female and eight male) and 16 nonsmokers (eight female and eight male) was assayed for concentration of AGEs and sRAGE. AGE concentration in BAL was higher in smokers compared with nonsmokers (9.0 ± 3.03 pg/ml versus 2.5 ± 0.52 pg/ml; P < 0.05; Figure 1A). Smokers had lower levels of sRAGE in the BAL compared with nonsmokers (1,205 ± 292 pg/ml versus 1,910.4 ± 262 pg/ml; P < 0.05; Figure 1B). Simple linear regression was performed to ascertain the relationship between AGEs and sRAGE in the BAL fluid. In Figure 1C, we show that lower levels of AGEs correspond to higher levels of sRAGE in the BAL fluid.
Figure 1.
Advanced glycation end products (AGEs) and soluble receptor for advanced glycation end-products (sRAGE) from bronchoalveolar lavage (BAL) fluid of smokers and nonsmokers. BAL fluid from smokers exhibited (A) higher concentrations of AGEs and (B) lower concentrations of sRAGE. (C) Simple linear regression showing that higher levels of sRAGE correlated with lower levels of AGEs. n = 16/group; *P < 0.05.
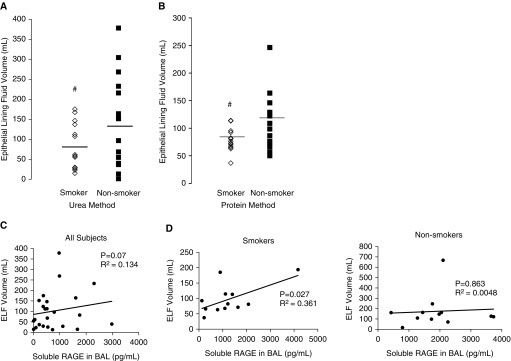
In Figure 2, we show that smokers have lower volumes of ELF compared with nonsmokers using the urea (74 ± 14 ml versus 138 ± 27 ml; Figure 2A, #P < 0.05) and protein methods (81 ± 6 ml versus 121 ± 14 ml; Figure 2B, #P < 0.05). In Figure 2C, we used linear regression to evaluate the relationship between sRAGE levels in the BAL and ELF volume in all subjects. Data were then dichotomized into smoking versus non-smoking, and a linear correlation between ELF and sRAGE level was observed (Figure 2D). Interestingly, greater volume of ELF obtained from the BAL of smokers correlated with higher levels of sRAGE, suggesting that sRAGE may play a critical role in regulating ELF volume. There was not a significant relationship between sRAGE and ELF volume in nonsmokers (Figure 2E).
Figure 2.
Predictors of epithelial lining fluid (ELF) volume in smokers. ELF volume from smokers and nonsmokers calculated using (A) urea and (B) protein methods. #P < 0.05. (C) Linear regression model depicting a characteristic of BAL fluid correlated with the level of sRAGE on the x axis and ELF volume on the y axis. Elevated levels of sRAGE correlate with greater volume of ELF regardless of smoking status (P = 0.07). (D) Linear regression model depicting characteristics of BAL fluid from smokers with sRAGE concentration on the x axis and ELF volume on the y axis. Greater concentrations of sRAGE correlated with greater ELF volume in smokers (P = 0.027). There was not a significant correlation between sRAGE and ELF volume in nonsmokers (P = 0.863).
Human AGEs Increase ENaC Activity in T1 and T2 Cells via Pro-Oxidant Signaling
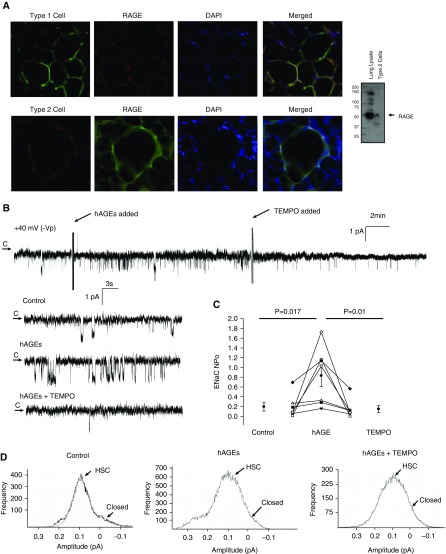
Because AGE levels in BAL fluid obtained from smokers were nearly three times the quantity of AGEs measured in nonsmokers after normalization to protein concentration, we sought mechanistic insight into the signaling events through which RAGE regulates ELF fluid levels. First, we assessed RAGE expression in the distal rat lung (Figure 3A) to document the presence of the protein in alveolar epithelial T1 and T2 cells. RAGE expression was observed in both cell types. Next, we assessed the effect of human AGEs (hAGEs; obtained from My Biosource, San Diego, CA) on ENaC activity in primary rat T1 (Figure 3) and T2 (Figure 4) cells. We used single-channel patch clamp analysis in the cell-attached configuration to determine the effects of hAGEs on the biophysical properties of ENaC. In Figure 3B, we provide a representative trace of a continual patch clamp recording (∼ 30-min duration) obtained from a T1 cell accessed from a rat lung tissue slice. The arrow in Figure 3B denotes the closed state, and downward deflections are indicative of inward current (e.g., sodium transport). After a 5-minute control recording period, cells were treated with hAGEs added to the bath (as indicated). After approximately 15 minutes, 4-hydroxy-2, 2, 6, 6-tetramethylpiperidine-N-oxyl (TEMPO; a superoxide dismutase [SOD] mimetic) was subsequently added to the cell bath. Enlarged portions of the control, hAGE challenge, and TEMPO treatment time points are provided in Figure 3B to highlight the hAGE-induced increase in ENaC activity, followed by TEMPO attenuation of Na+ transport. ENaC activity was measured and averaged as the product of the number of channels (N) and the open probability (Po). Figure 3C reports that ENaC NPo increased from 0.19 ± 0.08 to 0.83 ± 0.22 (P = 0.017) with the addition of hAGE. ENaC NPo decreased to 0.15 ± 0.07 (P = 0.01) with the addition of TEMPO. Point amplitude histograms taken from the control and hAGE- and TEMPO-treated conditions reveal that hAGEs increase the frequency of both types of ENaC channels, the highly selective cation (HSC; with average conductances near 6.1 pS) channels. Nonselective cation (NSC; with larger conductances near 12.9 pS) channels were also observed, albeit at lower frequency.
Figure 3.
Human AGEs (hAGEs) regulate epithelial sodium channel (ENaC) activity via oxidant signaling in rat primary alveolar type 1 cells accessed in lung tissue slices. (A) Immunohistochemistry of rat lung slices probed for RAGE and cell-specific markers (erythrina crista galli lectin for alveolar type 1 cells, lysotracker red, which binds to surfactant-producing lysosomes in alveolar type 2 cells) demonstrate that RAGE is expressed in alveolar type 1 and type 2 cells. Merged fluorescent signals indicate colocalization of RAGE- and cell-type markers; 4′,6-diamidino-2-phenylindole (DAPI)–labeled nuclei. Western blot from alveolar type 2 cell lysate immunoblotted for RAGE confirms that RAGE protein is expressed in type 2 cells. (B) Continuous cell-attached single-channel recording of a primary alveolar type 1 cell accessed from a lung slice preparation. Arrow represents the closed (c) state, with downward deflections from the arrow representing inward Na+ channel openings (−40 mV holding potential [−Vp]). Enlarged portions of the representative recording represent control, hAGE treatment, and 4-hydroxy-2, 2, 6, 6-tetramethylpiperidine-N-oxyl (TEMPO), a superoxide dismutase (SOD) mimetic, conditions. (C) Results from seven independent observations shown on dot plot with ENaC activity on the y axis. Challenge with hAGEs increased ENaC number of channels (N) and the open probability (Po) (NPo) from 0.19 ± 0.08 to 0.83 ± 0.22 (P = 0.017), and the addition of TEMPO decreased ENaC NPo to 0.15 ± 0.07 (P = 0.01). (D) Point amplitude histograms demonstrate a challenge with hAGEs increases highly selective cation (HSC) and nonselective cation (NSC) activity in primary alveolar type 1 cells.
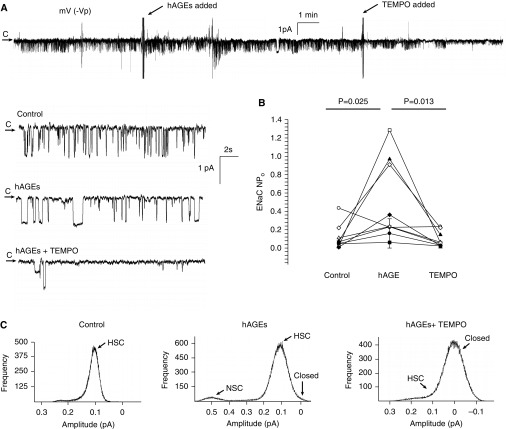
Figure 4.
hAGEs regulate ENaC activity via oxidant signaling in rat primary alveolar type 2 cells. (A) Continuous cell-attached single-channel recording of a primary alveolar type 2 cell accessed from a lung slice preparation. Arrow represents the closed (c) state, with downward deflections from the arrow representing inward Na+ channel openings (−40 mV [−Vp] holding potential). Enlarged portions of the representative recording represent control, hAGE treatment, and TEMPO, a SOD mimetic, conditions. (B) Results from eight independent observations shown on dot plot, with ENaC activity on the y axis. Challenge with hAGEs increased ENaC NPo from 0.12 ± 0.05 to 0.53 ± 0.16 (P = 0.025), and the addition of TEMPO decreased ENaC NPo to 0.10 ± 0.03 (P = 0.013). (C) Point amplitude histograms demonstrate that a challenge with hAGEs increase HSC channel and NSC channel activity in isolated alveolar type 2 cells.
Next, we examined the effects of hAGEs in isolated primary rat T2 cells, which make up roughly 5% of the alveolar surface area (Figure 4). A representative trace in the cell-attached configuration from an isolated T2 cell is shown in Figure 4A. The arrow denotes the closed state, with downward deflections indicative of inward Na+ current. Enlarged portions from the control and hAGE- and TEMPO-treated sections are provided to illustrate the single-channel properties of ENaC. In Figure 4B, we provide a dot plot of eight independent observations showing an increase in ENaC activity, measured as NPo. ENaC NPo increased from 0.12 ± 0.05 to 0.53 ± 0.16 (P = 0.025) with hAGE challenge, and was reduced to 0.10 ± 0.03 (P = 0.013) when TEMPO was added, demonstrating that hAGEs activate ENaC through an oxidant-mediated signaling pathway. Together, Figures 3 and 4 show that hAGEs stimulate an increase in the open probability of the ENaC in all cells comprising the alveolar epithelium. Based on these observations, it is likely that net salt and water absorption will occur in the alveolar surface fluid in the presence of AGEs.
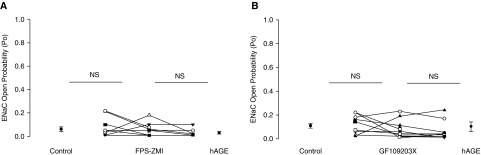
T2 cells treated with FPS-ZM1, a selective RAGE inhibitor (30), further support the hypothesis that hAGEs activate ENaC through RAGE signaling in the lung. Figure 5A shows that hAGE failed to induce an increase in ENaC activity in T2 cells pretreated with FPS-ZM1. The dot plot summary of eight independent observations shows that ENaC Po did not significantly change after the addition of FPS-ZM1 nor hAGEs in T2 cells. Specifically, the measured open probabilities were 0.06 ± 0.02, 0.06 ± 0.02 and 0.03 ±0.01, respectively. After showing that hAGEs stimulate ENaC via activation of RAGE, we evaluated the signaling pathway through which hAGEs act. To start, we evaluated the role of PKC on hAGE-induced regulation of ENaC activity by using a PKC inhibitor, GF109203. In Figure 5B, we provide a dot plot graph of eight independent observations showing that T2 cells pretreated with GF109203X failed to respond to hAGE treatment. This study indicates that the stimulatory effect of hAGEs on ENaC activity is mediated via a PKC isoform.
Figure 5.
Inhibition of RAGE and protein kinase C (PKC) signaling attenuates hAGE-induced activation of ENaC. (A) Results of eight independent observations shown on dot plot with ENaC open probability (Po) on the y axis. Inhibition of RAGE signaling using FPS-ZM1 attenuated the effect of hAGEs on ENaC activity (ENaC Po: 0.06 ± 0.02 to 0.06 ± 0.02, and then to 0.03 ± 0.01). (B). Results of eight independent observations shown on dot plot with ENaC Po on the y axis. Inhibition of PKC signaling using GF109203X attenuated the effect of hAGEs on ENaC activity (ENaC Po: 0.11 ± 0.03 to 0.09 ± 0.03, and then to 0.11 ± 0.06). NS, nonsignificant.
hAGEs Activate ENaC Activity via PKC Activation of gp91phox Production of ROS in the Lung
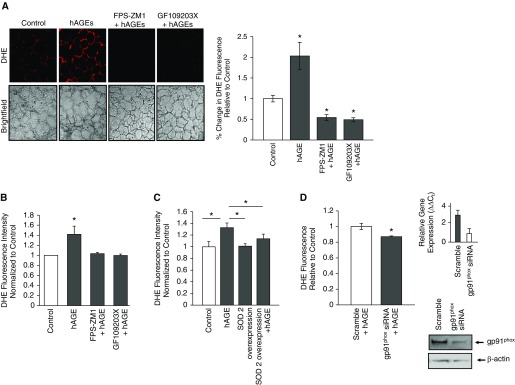
PKC has been shown to regulate Nox enzyme activity (20, 31). However, it is not clear whether hAGEs plays an important role in ROS production via PKC signaling in the alveolar epithelium. We therefore treated rat lung slices with 2 μg of hAGEs alone or in the presence of 1 μM FPS-ZM1, a selective RAGE inhibitor (30), or 10 μM GF109203X (a PKC inhibitor). ROS production was determined using confocal microscopy and the fluorescent properties of dihydroethidium, which reacts with ROS. hAGEs significantly increased ROS production in lung tissue slices, whereas inhibition of RAGE or PKC signaling pathways abrogated hAGE-induced ROS production (Figure 6A). To illustrate that PKC signaling is important in hAGE-induced ROS production, we provide representative images of GF109203X-treated lung tissue with a challenge of hAGEs. A challenge with hAGEs did not affect ROS production compared with controls, which demonstrates that PKC signaling is required for subsequent ROS production. Because PKC and Nox isoforms are ubiquitously expressed, we also assessed ROS production in T2 cells challenged with hAGEs (Figure 6B). hAGEs increased ROS production in isolated alveolar T2 cells through RAGE-dependent signaling. In addition, a challenge with hAGEs had no effect on ROS production in the setting of PKC signaling inhibition.
Figure 6.
Challenge with hAGEs increased gp91phox–dependent reactive oxygen species (ROS) production in lung tissue slices and isolated primary alveolar type 2 cells. (A) Representative lung tissue slices challenged with hAGEs with and without FPS-ZM1, a RAGE inhibitor, or with and without GF109203X, a PKC inhibitor, and quantification of fluorescence signal shown in the bar graph. hAGEs significantly increased ROS production. Inhibition of RAGE and PKC signaling significantly decreased ROS production compared with samples treated with hAGEs alone. *P < 0.05. (B) Isolated alveolar type 2 cells treated with hAGEs with and without FPS-ZM1 show a significant increase in ROS production as measured by dihydroethidium (DHE) fluorescence. *P < 0.05. (C). SOD2 was ectopically overexpressed in rat isolated alveolar type 2 cells using adeno-associated viral construct, and then the cells were challenged with hAGEs, and ROS production measured using DHE fluorescence. Overexpression of SOD2 significantly attenuated hAGE-induced ROS production.*P < 0.05. (D) Knockdown of gp91phox significantly attenuated hAGE-induced ROS production in isolated alveolar type 2 cells. gp91phox small interfering (si) RNA efficiency was assessed by evaluating gene and protein expression; efficiency was approximately 70%. *P < 0.05.
We also overexpressed the antioxidant enzyme, SOD, to implicate hAGE-regulated production of ROS. Isolated rat primary alveolar T2 cells were transduced with adeno-associated viral SOD2 vector and then exposed to hAGEs. Ectopic expression of SOD2 in T2 cells significantly reduced measureable dihydroethidium fluorescence, indicating that hAGEs indeed increase ROS production (Figure 6C). Next, we evaluated the effects of knocking down gp91phox on hAGE-induced ROS production using small interfering (si) RNA (Figure 6D). Figure 6D shows that gp91phox protein expression decreased roughly 20% after siRNA inhibition of transcript. More importantly, knockdown of gp91phox significantly attenuated hAGE-induced ROS production, thus suggesting that gp91phox is an important source of hAGE-induced ROS production.
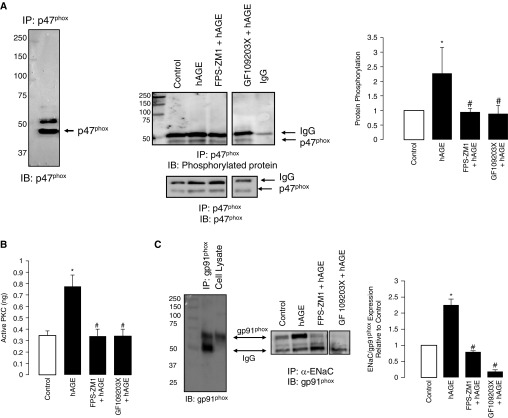
The Nox 2 isoform consists of cytosolic regulator subunits (p47phox, p60phox, and p67phox) that, when activated, translocate to interact with gp91phox, the catalytic domain, to produce superoxide. Studies show that PKC phosphorylation of p47phox is involved in the activation of the cytosolic subunits (20, 32). We tested the effects of hAGEs on the phosphorylation of p47phox. In Figure 7A, we show the specificity of anti-p47phox antibody by immunoprecipitating for p47phox protein, followed by Western blot analysis for p47phox. To evaluate the effects of hAGE on p47phox phosphorylation, we treated rat primary isolated T2 cells with hAGEs with or without FPS-ZM1 (a RAGE inhibitor) or GF109203X (a PKC inhibitor). Immunoprecipitated p47phox was then probed for its phosphorylated form using a pan-phosphorylation antibody, and protein expression normalized to total p47phox protein. Treatment with hAGEs significantly increased p47phox phosphorylation. Inhibition of RAGE signaling and PKC activity decreased p47phox phosphorylation, suggesting that RAGE is upstream of PKC phosphorylation of p47phox. To provide further evidence, we measured PKC activity in the presence of hAGE and inhibitors. Figure 7B shows that hAGEs significantly increased PKC activity in T2 cells, whereas FPS-ZM1 attenuated hAGE-mediated effects. As expected, Figure 7B shows that GX109203X attenuated hAGE activation of PKC activity, verifying that PKC signaling occurs downstream of RAGE ligation.
Figure 7.
Challenge with hAGEs increased phosphorylation of p47phox in isolated primary alveolar type 2 cells. (A) Western blot of immunoprecipitated p47phox protein that was immunoblotted for p47phox, showing molecular weight of approximately 47 kD; the slightly larger molecular weight band is IgG. Representative Western blot of immunoprecipitated p47phox protein immunoblotted for phosphorylated protein and normalized to total protein (lower panel) under control, hAGE, FPS-ZM1 plus hAGE, and GF109203X plus hAGE treatment conditions. Quantification of blot shows that hAGE treatment significantly increased 47phox phosphorylation, whereas treatment with FPS-ZM1 and GF109203X attenuated the effects of hAGE. (B) Active PKC of isolated primary type 2 cells was determined under control, hAGE, FPS-ZM1 plus hAGE, and GF109203X plus hAGE treatment. hAGE treatment significantly increased PKC activity, an effect that was abrogated with RAGE and PKC inhibition. (C) The left panel is an immunocontrol of immunoprecipitated gp91phox and cell lysate (both from alveolar type 2 cells) immunoblotted for gp91phox. The right panel is immunoprecipitated α-ENaC protein that was then immunoblotted for gp91phox (nicotinamide adenine dinucleotide phosphate [NADPH] oxidases [Nox] 2) under control, hAGE, FPS-ZM1 plus hAGE, and GF109203X plus hAGE treatment conditions. Bar graph of Western blots showing that hAGEs increased coimmunoprecipitation of ENaC-Nox2, an effect that was attenuated when treated with either FPS-ZM1 or GF109203X. *P < 0.05 compared with control, #P < 0.05 compared with hAGEs.
PKC has been shown to play an important role in regulating both Nox and ENaC activity (20, 25). Because we have previously shown that gp91phox is a critical regulator of lung ENaC activity (28, 29), we next determined whether RAGE regulation of PKC activity regulates Nox2 activation of lung ENaC. In Figure 7C we provide a Western blot of immunoprecipitated a-ENaC protein that was immunoblotted for gp91phox. Interestingly, a challenge with hAGEs increased α-ENaC–gp91phox coimmunoprecipitation, and inhibition of RAGE and PKC signaling significantly attenuated the effects of hAGE on α-ENaC–gp91phox coimmunoprecipitation. Together, these data support our hypothesis that PKC–gp91phox signaling is involved in RAGE regulation of ENaC.
Physiological Relevance of hAGEs in Promoting Alveolar Fluid Clearance In Vivo
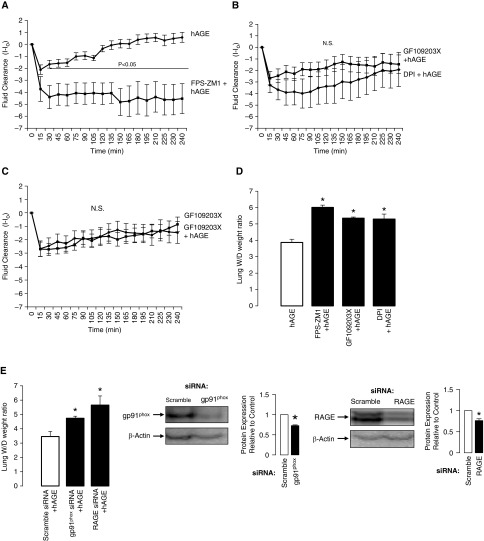
Using real-time X-ray imaging (fluoroscopy), we are able to determine the response of spontaneously breathing mice after a tracheal instillation of hAGEs alone or in combination with 1 μM FPS-ZM1, 10 μM GX109203X, or 10 μM diphenyliodonium, a general Nox inhibitor, delivered in a saline solution at 5 μl/g body weight. Mouse lung receiving hAGEs coupled with RAGE inhibition severely attenuated lung fluid clearance (Figure 8A). A significant difference in lung fluid clearance occurred within 30 minutes of the instillation, and persisted through the experimental procedure (P < 0.05). We observed no difference in the rate of lung fluid clearance between PKC and Nox2-inhibited mice challenged with hAGEs (Figure 8B). In Figure 8C, we show that lung fluid clearance in mice that received the PKC inhibitor was not different than that in mice receiving the PKC inhibitor with a challenge with hAGEs, demonstrating that hAGE-induced lung fluid clearance requires ligand–receptor (AGE–RAGE) binding to activate PKC signaling.
Figure 8.
Instillation of hAGEs promotes lung fluid clearance via RAGE–PKC–Nox2 signaling. Line graphs depicting changes in lung fluid clearance in mice receiving a tracheal challenge, with y axis representing lung fluid clearance (I − Io), where I represents fluid volume at a respective point in time, and Io is fluid volume at the first X-ray exposure. (A) hAGEs versus FPS-ZM1 plus hAGEs, (B) GF109203X plus hAGEs versus diphenyliodonium (DPI) plus hAGEs, (C) GF109203X versus GF109203X plus hAGEs. More positive values represent greater fluid clearance. n = 10 per group. N.S., nonsignificant. (D) Lung wet-to-dry weight ratios from mouse lung instilled with hAGEs, FPS-ZM1 plus hAGEs, GF109203X plus hAGEs, and DPI plus hAGEs (*P < 0.05; n = 5 mice/group). (E). Lung wet-to-dry weight ratios from C57Bl/6 mice given a tracheal instillation of scramble, RAGE, or gp91phox siRNA, and then (24 h later) challenged with hAGEs (*P < 0.05; n = 5 mice/group). Western blots of siRNA instilled lung probed for gp91phox or RAGE; siRNA decreased protein expression roughly 30% (n = 3).
We validated our findings using the radiographic technique against traditional lung wet-to-dry weight ratios, and observed that inhibition of RAGE, PKC, and Nox signaling significantly attenuated the effect of hAGEs on lung fluid clearance (Figure 8D). In Figure 8E, we knocked down gp91phox and RAGE protein expression, and then challenged the lung with hAGEs. Knockdown of gp91phox and RAGE significantly attenuated the effect of hAGEs on lung wet-to-dry weight ratios, further supporting the hypothesis that RAGE regulates lung fluid balance via PKC-gp91phox signaling to lung ENaC.
Discussion
Studies show that RAGE is highly expressed in the lung and differentially expressed in T1 cells compared with T2 cells (33). The role of RAGE in the physiology of the lung and in the pathogenesis of lung disease has garnered considerable attention recently. Studies have shown that RAGE plays a role in cellular differentiation, as well as spreading of adherent cells in culture (34–36). However, the role of RAGE in regulating lung fluid balance is unknown. In the current study, we sought to provide mechanistic insight into how RAGE regulates lung fluid balance, and have made several observations warranting additional discussion.
Effect of hAGEs on Biophysical Properties of ENaC
We have previously reported the effects of a variety of agonists on the biophysical properties of ENaC—in particular, the number of inserted channels and the molecular characteristics of these channels (19, 28, 29, 37, 38). ENaC channels are classified based on their selectivity for Na+ over other cations (e.g., K+), with HSC channels being selective for Na+ (Na+:K+, >40:1), and NSC channels being less discriminate (Na+:K+, 1:1). In the current study, we observed an increase in HSC channel activity in T1 and T2 cells when challenged with hAGEs (see point amplitude histograms, Figures 3D and 4C). NSC channel activity was observed, but the preponderance of channels were HSC channels. We did not observe a significant increase in the number of active channels; rather, the main effect was an increase in open probability (see Figures 3 and 4).
RAGE and Its Isoforms Regulate Lung Fluid Balance
RAGE functions to amplify and perpetuate the inflammatory response. A key feature of inflammation is edema, so it is not surprising that RAGE may play a critical, if not pivotal, role in the regulation of lung fluid balance. This has significant implications for a variety of acute and chronic lung diseases in which lung fluid balance is altered.
We and others have evaluated the effects of cigarette smoke on ion transport in airway epithelial cells, and we have reported an increase in ENaC activity when alveolar epithelial cells are challenged with an aqueous form of cigarette smoke (19, 39). In addition, studies show an increase in the expression of RAGE protein in the airway and distal lung of smokers and those with COPD (11, 12). Studies show that systemic levels of sRAGE are deficient in individuals with COPD and, in the current study, we show that sRAGE levels are significantly lower in the BAL fluid of smokers, an observation that is coupled with lower volumes of ELF. sRAGE consists of two isoforms: an endogenously secreted form (esRAGE) and a cleaved form (cRAGE). esRAGE is produced through alternative splicing, whereas cRAGE occurs through proteolytic cleavage from matrix metalloproteinases (40). Collectively, the effect of esRAGE and cRAGE results in ligation with RAGE ligands to reduce proinflammatory signaling at the microenvironmental level. However, studies have not consistently discriminated between the two forms of RAGE, and have instead reported the aggregate of the two (sRAGE). In addition, we show that hAGE, a RAGE ligand, increases ENaC activity through oxidant-mediated signaling to ultimately impact lung fluid clearance. Collectively, these data suggest that RAGE and its decoy signal, sRAGE, may play an important role in maintaining ELF volume.
AGEs: Predictive Biomarkers for Lung Disease?
The concentration of AGEs in smokers, who are predisposed to developing COPD, is significantly higher than in nonsmokers (41). There is also an inverse relationship between AGE and sRAGE expression level in the BAL fluid of smokers and nonsmokers. Furthermore, individuals with high levels of sRAGE expression (nonsmokers) had measurably higher ELF volumes. The important implication from these observations is that elevated AGE expression in smokers could lead to dry lung disorders, whereas sRAGE expression in nonsmokers can protect the lungs from deleterious effects of RAGE activation. Indeed, numerous studies have shown that administration of sRAGE can alleviate the harmful effects of RAGE ligands. Specifically, sRAGE has been shown to slow tumor growth (42), suppress diabetic atherosclerosis (43), reverse vascular hyperpermeability in diabetic rats (44), and reduce plaque formation in a mouse model of Alzheimer’s disease (45).
Mechanistic Insight into RAGE and ENaC
Understanding the regulation of ENaC is important to developing improved therapies for acute and chronic lung disease. This is perhaps most visibly apparent in cystic fibrosis and ARDS, two distinct disease processes at polar ends of a spectrum of lung fluid balance. In addition, it is important to understand ion transport within the context of the specific pathology under investigation. In this study, we sought to obtain mechanistic insight into the regulation of ENaC through activation of RAGE signaling. This is highly relevant to many lung diseases, as RAGE-driven inflammation is a key feature.
Our findings are in line with other reports of RAGE activation of PKC (46–48). In the lung, all major isoforms of PKC are present. Data from our present study indicate that the stimulatory effect of hAGEs on ENaC activity is mediated by a Ca2+-independent PKC, as classical PKC signaling is responsible for mediating inhibitory effects of Ca2+ on ENaC (49, 50). Results from our studies suggest that the stimulatory effects of hAGE (and subsequent PKC activation) results in activation of Nox via phosphorylation of the p47phox subunit. In line with this observation, several PKC isoforms (Ca2+ dependent and independent) have been shown to phosphorylate p47phox (reviewed in Ref. 25).
We used a nonspecific PKC inhibitor to demonstrate the importance of p47phox phosphorylation in the regulation of ENaC via gp91phox-generated ROS. However, more specific phosphopeptide mapping of p47phox showed that serines 303, 304, 315, 320, 328, 359, 370, and 379 are all targets of PKC-α, -βII, and -δ (31). In addition to GF109203X, a nonspecific PKC inhibitor, we performed fluid clearance studies using Ro-31-8425 at concentrations specific for the PKCβII subunit (data not shown). Inhibition of PKCβII paralleled the effects of RAGE inhibition, suggesting that the PKCβII subunit is critical for RAGE signaling.
Looking Forward, the Prognostic Role of sRAGE and RAGE Inhibition in Lung Disease
The role of sRAGE as a prognostic tool or biomarker has gained attention. Multiple studies describe an increase in serum sRAGE in individuals with COPD (41). To our knowledge, this is the first study to evaluate sRAGE in the BAL fluid of smokers. Interestingly, we observed a reduction in sRAGE levels in the BAL fluid of smokers compared with other studies that evaluated serum levels. We observed a similar pattern, despite assaying the BAL fluid. As for the utility of sRAGE from either the BAL or serum and its utility in the early detection, staging, or monitoring for disease progression, it remains unclear which form is most predictive, and will require larger randomized controlled trials to address the issue.
RAGE inhibition may prove to be an important therapeutic target in the management of acute and chronic lung disease. Given the many signaling pathways regulated by RAGE, acute inhibition of RAGE may prove to be highly effective in the management of lung fluid balance, which would have implications for cystic fibrosis, COPD, and ARDS. However, it is unclear if delivery of sRAGE or inhibition of RAGE signaling would be therapeutic. Furthermore, the utility of RAGE inhibition in acute and chronic diseases may require a less direct approach. Studies show that certain therapeutic drug classes, including statins (51), angiotensin-converting enzyme inhibitors (52), angiotensin II type 1 receptor blockers (53), and thiazolidinediones (54), either increase expression of sRAGE or decrease expression of the active membrane-bound form. This raises the possibility of these agents being used in chronic airway disease to ameliorate the effects of RAGE signaling. However, more work is needed to evaluate the effectiveness of these agents in the treatment of the RAGE axis in chronic lung disease.
Conclusions
In the current study, we observed a reduction in the volume of ELF in smokers compared with nonsmokers that is coupled with increased AGEs and lower levels of sRAGE, suggesting that RAGE signaling may play a critical role in the regulation of lung fluid balance. Single-channel patch clamp analysis revealed that the addition of AGEs increased ENaC activity through an oxidant-mediated process to ultimately affect lung fluid clearance. Mechanistically, we observed that activation of RAGE signaling activated ENaC through PKC-dependent phosphorylation of p47phox. These data argue that RAGE plays a critical role in the regulation of the tissue microenvironment, and may serve as a critical feature in the maintenance of appropriate lung fluid balance.
Acknowledgments
Acknowledgments
The authors acknowledge Douglas McCarty, University of Cincinnati, for the adeno-associated viral construct, and the technical assistance of Matthew Goodson.
Footnotes
This work was supported by a Parker B. Francis Fellowship and by University Research Committee/Atlanta Clinical and Translational Science Institute grant that is supported by the National Center for Advancing Translation Sciences of the National Institutes of Health under award number ULTR000454 (C.A.D.).
Author Contributions: conception and design—C.A.D., L.A.B., and M.N.H.; conducted experiments—C.A.D., L.H.K., N.M.J.; analysis and interpretation—C.A.D., L.H.K., N.M.J., and M.N.H.; drafting the manuscript for important intellectual content—C.A.D. and M.N.H.; reviewed manuscript—C.A.D., L.H.K., N.M.J., L.A.B., and M.N.H.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0002OC on June 30, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt AM, Stern DM. Receptor for AGE (RAGE) is a gene within the major histocompatibility class III region: implications for host response mechanisms in homeostasis and chronic disease. Front Biosci. 2001;6:D1151–D1160. doi: 10.2741/schmidt. [DOI] [PubMed] [Google Scholar]
- 4.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;126:332–337. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- 5.Fehrenbach H, Kasper M, Tschernig T, Shearman MS, Schuh D, Muller M. Receptor for advanced glycation endproducts (RAGE) exhibits highly differential cellular and subcellular localisation in rat and human lung. Cell Mol Biol (Noisy -le-grand) 1998;44:1147–1157. [PubMed] [Google Scholar]
- 6.Herold K, Moser B, Chen Y, Zeng S, Yan SF, Ramasamy R, Emond J, Clynes R, Schmidt AM. Receptor for advanced glycation end products (RAGE) in a dash to the rescue: inflammatory signals gone awry in the primal response to stress. J Leukoc Biol. 2007;82:204–212. doi: 10.1189/jlb.1206751. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 8.Ostendorp T, Leclerc E, Galichet A, Koch M, Demling N, Weigle B, Heizmann CW, Kroneck PM, Fritz G. Structural and functional insights into RAGE activation by multimeric S100B. EMBO J. 2007;26:3868–3878. doi: 10.1038/sj.emboj.7601805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 10.Fukuchi Y. The aging lung and chronic obstructive pulmonary disease: similarity and difference. Proc Am Thorac Soc. 2009;6:570–572. doi: 10.1513/pats.200909-099RM. [DOI] [PubMed] [Google Scholar]
- 11.Boschetto P, Campo I, Stendardo M, Casimirri E, Tinelli C, Gorrini M, Ceconi C, Fucili A, Potena A, Papi A, et al. Plasma sRAGE and N-(carboxymethyl) lysine in patients with CHF and/or COPD. Eur J Clin Invest. 2013;43:562–569. doi: 10.1111/eci.12079. [DOI] [PubMed] [Google Scholar]
- 12.Sukkar MB, Wood LG, Tooze M, Simpson JL, McDonald VM, Gibson PG, Wark PA. Soluble RAGE is deficient in neutrophilic asthma and COPD. Eur Respir J. 2012;39:721–729. doi: 10.1183/09031936.00022011. [DOI] [PubMed] [Google Scholar]
- 13.Eaton DC, Helms MN, Koval M, Bao HF, Jain L. The contribution of epithelial sodium channels to alveolar function in health and disease. Annu Rev Physiol. 2009;71:403–423. doi: 10.1146/annurev.physiol.010908.163250. [DOI] [PubMed] [Google Scholar]
- 14.Matalon S, O’Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol. 1999;61:627–661. doi: 10.1146/annurev.physiol.61.1.627. [DOI] [PubMed] [Google Scholar]
- 15.Stewart AP, Haerteis S, Diakov A, Korbmacher C, Edwardson JM. Atomic force microscopy reveals the architecture of the epithelial sodium channel (ENaC) J Biol Chem. 2011;286:31944–31952. doi: 10.1074/jbc.M111.275289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao RZ, Nie HG, Su XF, Han DY, Lee A, Huang Y, Chang Y, Matalon S, Ji HL. Characterization of a novel splice variant of δ ENaC subunit in human lungs. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1262–L1272. doi: 10.1152/ajplung.00331.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji HL, Zhao RZ, Chen ZX, Shetty S, Idell S, Matalon S. δ ENaC: a novel divergent amiloride-inhibitable sodium channel. Am J Physiol Lung Cell Mol Physiol. 2012;303:L1013–L1026. doi: 10.1152/ajplung.00206.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hummler E, Barker P, Beermann F, Gatzy J, Verdumo C, Boucher R, Rossier BC. Role of the epithelial sodium channel in lung liquid clearance. Chest. 1997;111(6 suppl):113S. doi: 10.1378/chest.111.6_supplement.113s. [DOI] [PubMed] [Google Scholar]
- 19.Downs CA, Kreiner LH, Trac DQ, Helms MN. Acute effects of cigarette smoke extract on alveolar epithelial sodium channel activity and lung fluid clearance. Am J Respir Cell Mol Biol. 2013;49:251–259. doi: 10.1165/rcmb.2012-0234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dekker LV, Leitges M, Altschuler G, Mistry N, McDermott A, Roes J, Segal AW. Protein kinase C-beta contributes to NADPH oxidase activation in neutrophils. Biochem J. 2000;347:285–289. [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy MM, Quinton PM. ENaC activity requires CFTR channel function independently of phosphorylation in sweat duct. J Membr Biol. 2005;207:23–33. doi: 10.1007/s00232-005-0798-8. [DOI] [PubMed] [Google Scholar]
- 22.Shimkets RA, Lifton R, Canessa CM. In vivo phosphorylation of the epithelial sodium channel. Proc Natl Acad Sci USA. 1998;95:3301–3305. doi: 10.1073/pnas.95.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Windhager EE, Frindt G. Role of Ca++ as a regulator of Na+ permeability in epithelia. Prog Clin Biol Res. 1984;168:71–75. [PubMed] [Google Scholar]
- 24.Cosentino-Gomes D, Rocco-Machado N, Meyer-Fernandes JR. Cell signaling through protein kinase C oxidation and activation. Int J Mol Sci. 2012;13:10697–10721. doi: 10.3390/ijms130910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, Lane PH, Pollock JS, Carmines PK. PKC-dependent superoxide production by the renal medullary thick ascending limb from diabetic rats. Am J Physiol Renal Physiol. 2009;297:F1220–F1228. doi: 10.1152/ajprenal.00314.2009. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Lane PH, Pollock JS, Carmines PK. Protein kinase C–dependent NAD(P)H oxidase activation induced by type 1 diabetes in renal medullary thick ascending limb. Hypertension. 2010;55:468–473. doi: 10.1161/HYPERTENSIONAHA.109.145714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Downs CA, Helms MN. Regulation of ion transport by oxidants. Am J Physiol Lung Cell Mol Physiol. 2013;305:L595–L603. doi: 10.1152/ajplung.00212.2013. [DOI] [PubMed] [Google Scholar]
- 28.Downs CA, Trac DQ, Kreiner LH, Eaton AF, Johnson NM, Brown LA, Helms MN. Ethanol alters alveolar fluid balance via NADPH oxidase (NOX) signaling to epithelial sodium channels (ENaC) in the lung. PLoS One. 2013;8:e54750. doi: 10.1371/journal.pone.0054750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodson P, Kumar A, Jain L, Kundu K, Murthy N, Koval M, Helms MN. NADPH oxidase regulates alveolar epithelial sodium channel activity and lung fluid balance in vivo via O−2 signaling. Am J Physiol Lung Cell Mol Physiol. 2012;302:L410–L419. doi: 10.1152/ajplung.00260.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, et al. A multimodal RAGE-specific inhibitor reduces amyloid β–mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 32.Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol. 2004;173:5730–5738. doi: 10.4049/jimmunol.173.9.5730. [DOI] [PubMed] [Google Scholar]
- 33.Dahlin K, Mager EM, Allen L, Tigue Z, Goodglick L, Wadehra M, Dobbs L. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol. 2004;31:309–316. doi: 10.1165/rcmb.2003-0423OC. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt AM, Hori O, Chen JX, Li JF, Crandall J, Zhang J, Cao R, Yan SD, Brett J, Stern D. Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice: a potential mechanism for the accelerated vasculopathy of diabetes. J Clin Invest. 1995;96:1395–1403. doi: 10.1172/JCI118175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt AM, Weidman E, Lalla E, Yan SD, Hori O, Cao R, Brett JG, Lamster IB. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: a potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res. 1996;31:508–515. doi: 10.1111/j.1600-0765.1996.tb01417.x. [DOI] [PubMed] [Google Scholar]
- 36.Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006;323:475–488. doi: 10.1007/s00441-005-0069-0. [DOI] [PubMed] [Google Scholar]
- 37.Downs CA, Kriener LH, Yu L, Eaton DC, Jain L, Helms MN. β-adrenergic agonists differentially regulate highly selective and nonselective epithelial sodium channels to promote alveolar fluid clearance in vivo. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1167–L1178. doi: 10.1152/ajplung.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Downs C, Kumar A, Kreiner LHJNM, Helms MN. H2O2 regulates lung epithelial sodium channel (ENaC) via ubiquitin-like protein Nedd8. J Biol Chem. 2013;288:8136–8145. doi: 10.1074/jbc.M112.389536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26:533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bucciarelli LG, Wendt T, Rong L, Lalla E, Hofmann MA, Goova MT, Taguchi A, Yan SF, Yan SD, Stern DM, et al. RAGE is a multiligand receptor of the immunoglobulin superfamily: implications for homeostasis and chronic disease. Cell Mol Life Sci. 2002;59:1117–1128. doi: 10.1007/s00018-002-8491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu L, Ma L, Nicholson LF, Black PN. Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD. Respir Med. 2011;105:329–336. doi: 10.1016/j.rmed.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, Tanji N, Lu Y, Lalla E, Fu C, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 43.Park JY, Kim HK, Chung YE, Kim SW, Hong SK, Lee KU. Incidence and determinants of microalbuminuria in Koreans with type 2 diabetes. Diabetes Care. 1998;21:530–534. doi: 10.2337/diacare.21.4.530. [DOI] [PubMed] [Google Scholar]
- 44.Wautier JL, Zoukourian C, Chappey O, Wautier MP, Guillausseau PJ, Cao R, Hori O, Stern D, Schmidt AM. Receptor-mediated endothelial cell dysfunction in diabetic vasculopathy: soluble receptor for advanced glycation end products blocks hyperpermeability in diabetic rats. J Clin Invest. 1996;97:238–243. doi: 10.1172/JCI118397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho HJ, Son SM, Jin SM, Hong HS, Shin DH, Kim SJ, Huh K, Mook-Jung I. RAGE regulates BACE1 and Abeta generation via NFAT1 activation in Alzheimer’s disease animal model. FASEB J. 2009;23:2639–2649. doi: 10.1096/fj.08-126383. [DOI] [PubMed] [Google Scholar]
- 46.Curran CS, Bertics PJ. Human eosinophils express RAGE, produce RAGE ligands, exhibit PKC-delta phosphorylation and enhanced viability in response to the RAGE ligand, S100B. Int Immunol. 2011;23:713–728. doi: 10.1093/intimm/dxr083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grotterød I, Maelandsmo GM, Boye K. Signal transduction mechanisms involved in S100A4-induced activation of the transcription factor NF-kappaB. BMC Cancer. 2010;10:241. doi: 10.1186/1471-2407-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y, Wang S, Feng L, Zhu Q, Xiang P, He B. Blockade of PKC-beta protects HUVEC from advanced glycation end products induced inflammation. Int Immunopharmacol. 2010;10:1552–1559. doi: 10.1016/j.intimp.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Frindt G, Windhager EE. Regulatory role of calcium in sodium transport. Prog Clin Biol Res. 1984;164:403–406. [PubMed] [Google Scholar]
- 50.Frindt G, Lee CO, Yang JM, Windhager EE. Potential role of cytoplasmic calcium ions in the regulation of sodium transport in renal tubules. Miner Electrolyte Metab. 1988;14:40–47. [PubMed] [Google Scholar]
- 51.Brun-Buisson C, Doyon F, Carlet J, Dellamonica P, Gouin F, Lepoutre A, Mercier JC, Offenstadt G, Régnier B French ICU Group for Severe Sepsis. Incidence, risk factors, and outcome of severe sepsis and septic shock in adults: a multicenter prospective study in intensive care units. JAMA. 1995;274:968–974. [PubMed] [Google Scholar]
- 52.Paletas K, Sailer X, Rizeq L, Dimitriadi A, Koliakos G, Kaloyianni M. Angiotensin-II–dependent NHE1 activation in human monocytes. J Am Soc Hypertens. 2008;2:173–181. doi: 10.1016/j.jash.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Herrera M, Silva GB, Garvin JL, Angiotensin II. Angiotensin II stimulates thick ascending limb superoxide production via protein kinase C(α)–dependent NADPH oxidase activation. J Biol Chem. 2010;285:21323–21328. doi: 10.1074/jbc.M110.109157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marx N, Walcher D, Ivanova N, Rautzenberg K, Jung A, Friedl R, Hombach V, de Caterina R, Basta G, Wautier MP, et al. Thiazolidinediones reduce endothelial expression of receptors for advanced glycation end products. Diabetes. 2004;53:2662–2668. doi: 10.2337/diabetes.53.10.2662. [DOI] [PubMed] [Google Scholar]