Abstract
Increased vascular permeability contributes to life-threatening pathological conditions, such as acute respiratory distress syndrome. Current treatments for sepsis-induced pulmonary edema rely on low–tidal volume mechanical ventilation, fluid management, and pharmacological use of a single angiogenic or chemical factor with antipermeability activity. However, it is becoming clear that a combination of multiple angiogenic/chemical factors rather than a single factor is required for maintaining stable and functional blood vessels. We have demonstrated that mouse platelet-rich plasma (PRP) extract contains abundant angiopoietin (Ang) 1 and multiple other factors (e.g., platelet-derived growth factor), which potentially stabilize vascular integrity. Here, we show that PRP extract increases tyrosine phosphorylation levels of Tunica internal endothelial cell kinase (Tie2) and attenuates disruption of cell–cell junctional integrity induced by inflammatory cytokine in cultured human microvascular endothelial cells. Systemic injection of PRP extract also increases Tie2 phosphorylation in mouse lung and prevents endotoxin-induced pulmonary edema and the consequent decreases in lung compliance and exercise intolerance resulting from endotoxin challenge. Soluble Tie2 receptor, which inhibits Ang-Tie2 signaling, suppresses the ability of PRP extract to inhibit pulmonary edema in mouse lung. These results suggest that PRP extract prevents endotoxin-induced pulmonary edema mainly through Ang-Tie2 signaling, and PRP extract could be a potential therapeutic strategy for sepsis-induced pulmonary edema and various lung diseases caused by abnormal vascular permeability.
Keywords: lung, vascular permeability, platelet-rich plasma extract, Tunica internal endothelial cell kinase, angiopoietin
Clinical Relevance
We have demonstrated that platelet-rich plasma (PRP) extract up-regulates Tunica internal endothelial cell kinase activity and prevents LPS-induced vascular leakage in mouse lungs. PRP extract could be a potential therapeutic approach for sepsis-induced acute respiratory distress syndrome as well as other diseases caused by abnormal vascular permeability.
Uncontrolled vascular leakage contributes to various life-threatening pathological conditions, such as acute respiratory distress syndrome (ARDS). In ARDS, excess fluid leaks out of the alveolar capillaries, causing pulmonary edema, which leads to impairment of alveolar gas exchange and refractory hypoxemia (1). Almost half of patients with severe sepsis develop ARDS, and the mortality rate of sepsis-induced ARDS is higher than 60% (2). Furthermore, the long-term quality of life of patients who survive ARDS is adversely affected even after recovery from the acute phase (3). Despite a great amount of effort, most of the current pharmacological approaches for treating sepsis-induced pulmonary edema, which rely on the use of a single antipermeability factor, such as activated protein C (4), are not very successful. Because it has been recognized that multiple angiogenic and growth factors are required for formation and stabilization of capillary blood vessels (5, 6), new approaches using combinations of multiple factors will likely improve current pharmacological treatments for sepsis-induced ARDS.
In addition to coagulation factors, platelets release many bioactive angiogenic factors, including platelet-derived epidermal growth factor, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), basic fibroblast growth factor, angiopoietins (Angs), and transforming growth factor (TGF)-β, and enhance angiogenesis, tissue regeneration, and wound healing (7, 8). Thus, platelet-rich plasma (PRP), which contains combinations of various platelet-derived chemical factors, has been extensively used in the orthopedic and periodontal fields (9). In addition, platelets and platelet-derived factors are known to maintain endothelial barrier functions in the lung (10, 11). Recently, we have reported that PRP extract from mouse whole blood contains abundant Ang1, which stabilizes blood vessels and maintains vascular integrity, and lower amounts of other angiogenic factors (e.g., PDGF, VEGF) (12). The Ang-Tunica internal endothelial cell kinase (Tie2) pathway contributes to the pathogenesis of endotoxin-induced lung injury (13–15) and bronchopulmonary dysplasia (16, 17), a neonatal lung injury accompanied by an increase in lung vascular permeability (18). Thus, PRP extract may control vascular permeability through Ang-Tie2 signaling.
In this study, we have demonstrated that PRP extract attenuates the disruption of cell–cell junctional integrity induced by the inflammatory cytokine, TNFα, in capillary endothelial cells in vitro and prevents endotoxin-induced pulmonary edema in mouse lung through Ang-Tie2 signaling. Although Ang1 is known to prevent sepsis-induced ARDS in various animal models (13–15), there are several limitations, including: (1) technical difficulty and high costs of preparing clinically relevant amounts of Ang1 protein; and (2) safety issues in cell-based Ang1 delivery or use of viral constructs to deliver therapeutically relevant amounts of Ang1 inside the body. In contrast, PRP extract, which has already been applied clinically in the orthopedic and periodontal fields, is generated autologously from peripheral blood by a simple method, can be stored stably at −80°C (12), and has little immunological reaction and no risk of unnecessary pathogen transfer. However, the mechanism and effects of PRP extract on pulmonary edema have not been tested as a therapeutic strategy for lung injury. This study demonstrates a novel role for PRP extract as a promising therapeutic approach for sepsis-induced ARDS and various human diseases caused by abnormal vascular permeability.
Materials and Methods
Materials
Anti-CD31 and anti-vascular endothelial (VE)-cadherin monoclonal antibodies were from Transduction Laboratories (Lexington, KY). Anti–β-actin monoclonal antibody was from Sigma (St. Louis, MO). Anti-Tie2 monoclonal antibody was from Upstate (Lake Placid, NY). Anti–phospho-Tie2 (Tyr992) antibody was from R&D Systems (Minneapolis, MN). LPS and TNF-α were from Sigma. Recombinant Ang1 and soluble Tie2 receptor were from R&D (Minneapolis, MN). Human lung microvascular endothelial (L-HMVE) cells (Lonza, Walkersville, MD) were cultured as described previously (13, 15, 16).
Preparation of PRP Extract
The in vivo animal study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was reviewed and approved by the Animal Care and Use Committee of Boston Children’s Hospital (protocol numbers 13-10-2526R, 10-12-1810R). PRP extract was prepared as described previously (12). Briefly, CD1 (8–12 wk of age; Charles River Laboratory, Wilmington, MA) mice were anesthetized with Ketamine (intraperitoneal injection) and whole blood was collected via cardiac puncture. The blood was anticoagulated with 3% acid-citrate dextrose (1/10 vol) and centrifuged at 100 × g for 15 minutes at room temperature. PRP (middle layer of the tube) was collected and sonicated using an ultrasonic water bath sonicator for 30 seconds to release the factors from the concentrated platelets. Each sample was centrifuged at 10,000 × g for 10 minutes and the clear, cell-free supernatant was collected and stored at −80°C as PRP extract. As a control vehicle, protein concentration–matched mouse serum was used.
Gene Knockdown
Gene knockdown was performed using the RNA interference technique (19). small interfering RNA (siRNA) for human Tie2 was a smart pool siRNA from Dharmacon (Lafayette, CO) (16). Cells were transfected with 30 nM of siRNA duplexes using Silentfect (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions, and were used for each assay after incubation for 48 hours (16, 19). As a control, siRNA duplex with irrelevant sequence (QIAGEN, Germantown, MD) was used.
Cell Analysis Methods
L-HMVE cells were cultured for 12 hours and immunostaining was performed with VE-cadherin antibody (13, 15, 20). Monolayer junction formation was analyzed using a confocal Leica SP2 microscope (Leica, Buffalo Grove, IL) (15, 20). Endothelial cell permeability was determined by measuring leakage of FITC-labeled BSA (Sigma) through the monolayer of L-HMVE cells, as described previously (13, 15). FITC-albumin (final concentration, 1 mg/ml) was added to the luminal chamber for 6 hours, and samples were taken from both the luminal and abluminal chamber for fluorometric analysis. Where indicated, vehicle, TNF-α (20 ng/ml), PRP extract (1:250, vol/vol), or soluble Tie2 receptor (1 μg/ml) was added to the luminal chamber with FITC-labeled BSA. Fluorescence readings were converted with the use of a standard curve to albumin concentration. These concentrations were used to determine the permeability coefficient of albumin, as described previously (13, 15).
In Vivo Pulmonary Vascular Permeability Assay
Mice (8–12 wk old) were treated with LPS (2.5 mg/kg, intraperitoneal) and lung vascular permeability was assessed 24 hours after injection (13, 15, 20). The lung vascular permeability was measured using Evans blue dye or low–molecular weight (LMW), fluorescently labeled dextran (MW 4,000; sigma) leakage (13, 15, 20). Evans blue dye was extracted from the lung by incubation with formamide (70°C for 24 h) and the absorbance of extracted dye was measured at 620 nm. Dextran leakage was quantified using a macro designed for National Institutes of Health’s ImageJ software that counts colored pixels between thresholds selected to minimize background, yielding a percentage of total image area, which was then normalized to vessel density, with each parameter analyzed independently (15).
Mouse lung wet:dry weight ratio was used to measure lung water accumulation after LPS injection (14). Lung wet weight was determined immediately after removal of the right lung. Lung dry weight was determined after the lung had been dried in an oven at 50°C for 24 hours. The wet:dry ratio was calculated by dividing the wet weight by the dry weight.
Bronchoalveolar lavage (BAL) fluid was collected by infusing 0.9% NaCl in two separate 0.5-ml aliquots. The fluid was recovered by gentle suction and placed on ice for immediate processing. An aliquot of the BAL fluid was processed immediately for differential cell counts by performing cytospin preparations and staining with modified Wright-Giemsa stain (Diff-Quik; American Scientific Products, McGaw Park, IL) (15, 20). Separately, protein concentration of the supernatant of BAL fluid was measured by bicinchoninic acid protein assay kit (Bio-Rad).
Histological Analysis
Lungs were processed and embedded in optimal cutting temperature compound, as previously described (5). Serial step sections, 10 μm in thickness, were taken along the longitudinal axis of the lobe. Lungs were analyzed using hematoxylin and eosin (H&E) staining (15, 16, 20). For decellularization of the lung, we perfused the lung with decellularization buffer (0.5% SDS/water) through the right ventricle for 1 hour; we then cryosectioned and analyzed the collagen structure by Picrosirius red staining and observed the structure using a light microscope (17, 20).
Measurement of Static Lung Compliance
Static lung compliance was evaluated 1 week after LPS challenge, as described previously (21–24). Briefly, mice were anesthetized with ketamine (100 mg/kg, intraperitoneal) and placed in a chamber containing 100% oxygen to deflate the lung. A catheter (20-gauge blunt needle) was inserted into the trachea and the chest wall and diaphragm were opened carefully to avoid damage to the lungs. The cannula was connected to a three-way stopcock, which was connected to a silicon pressure sensor (Freescale Semiconductor, Austin, TX). The pressure transducer was calibrated with an H2O manometer before each measurement. The pressure transducer output signal was direct current–coupled, sampled at 20 Hz, and displayed in scrolling mode at 1 second per division with a Tektronix digital oscilloscope Model TBS 1,022 (Tektronix, Beaverton, OR). The lungs were inflated with air in 0.1-ml increments every 10 seconds to a maximal pressure of 30 mm H2O and then deflated in stepwise fashion. Airway opening pressure and lung volume curves were generated for each treatment. Lung compliance was determined by calculating the slope of the curve.
Exercise Capacity
Mice were run according to a predetermined protocol, and we assessed the ability of untrained mice to run for distance (15). Mice were initially acclimated to the treadmill environment for 30 minutes. For warm-up and for further familiarization with treadmill running, mice were required to run at a relatively easy pace of 10 m/min for 30 minutes. Then, the speed of the treadmill was increased to 20 m/min, and we recorded the exercise duration and distance mice could run until exhaustion. Exhaustion was defined operationally as the time at which a mouse was unable, or refused, to maintain its running speed despite encouragement by mild electrical stimulation.
Statistical Analysis
All phenotypic analysis was performed by masked observers unaware of the identity of experimental groups. All statistical data were analyzed using GraphPad Prism V5.0 (GraphPad Software, Inc., La Jolla, CA). Error bars (SEM) and P values were determined from the results of at least three independent experiments. The ANOVA with post hoc Student’s t test was used for analysis of statistical significance.
Results
PRP Extract Preserves Endothelial Cell–Cell Junctional Integrity In Vitro
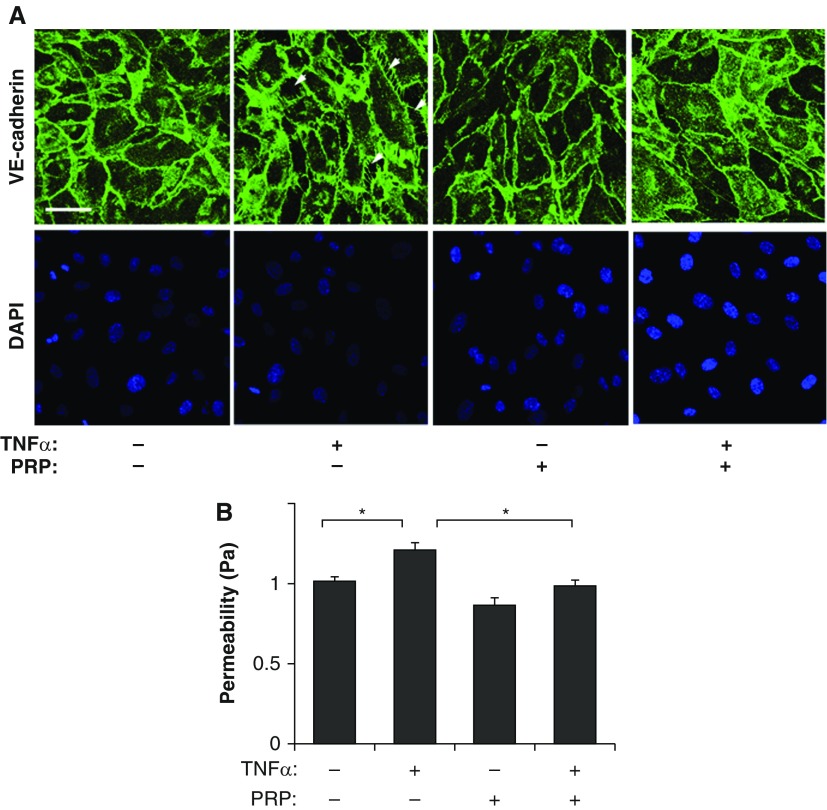
We have recently reported that mouse PRP extract contains abundant Ang1, an antipermeability factor, which preserves cell–cell junctional integrity and stabilizes blood vessel structure (12, 13, 15). Thus, we first explored whether PRP extract controls vascular barrier function in cultured L-HMVE cells. VE-cadherin–containing cell–cell junctions, which resist traction forces generated by the actin cytoskeleton, control vascular permeability (13, 15, 20). Immunocytochemical analysis revealed that cell–cell junctions were well developed in control L-HMVE cells, whereas they were disrupted in L-HMVE cells treated with the inflammatory cytokine, TNF-α (20 ng/ml), and this effect was attenuated by treatment with PRP extract (1:250, vol/vol) (Figure 1A). Consistent with our previous report (13, 15), TNF-α increased vascular permeability by 1.2-fold, as measured by quantitating the flux of fluorescently labeled albumin across the endothelial cell monolayer cultured in a Transwell chamber in vitro, and PRP extract suppressed increases in vascular permeability induced by TNF-α to basal levels (Figure 1B). These results suggest that PRP extract prevents disruption of endothelial cell–cell junctions induced by TNF-α in vitro.
Figure 1.
Platelet-rich plasma (PRP) extract attenuates disruption of endothelial cell–cell junctional integrity in human lung microvascular endothelial (L-HMVE) cells treated with TNF-α in vitro. (A) Immunofluorescence micrographs showing cell–cell junction structure detected by VE-cadherin staining in L-HMVE cells treated with TNF-α, PRP extract, or both in combination (scale bar, 50 μm). 4′,6-Diamidino-2-phenylindole (DAPI) staining shows the nucleus of each cell. Arrowheads show the regions where cell–cell junctions are disrupted. As a control, cells were treated with protein concentration–matched mouse serum. (B) Graph showing endothelial permeability in L-HMVE cells treated with TNF-α, PRP extract, or both in combination (*P < 0.05). Permeability (Pa) values are expressed as percentage of control cells. Error bars represent SEM of three independent experiments.
PRP Extract Preserves Cell–Cell Junctional Integrity through Ang-Tie2 Signaling
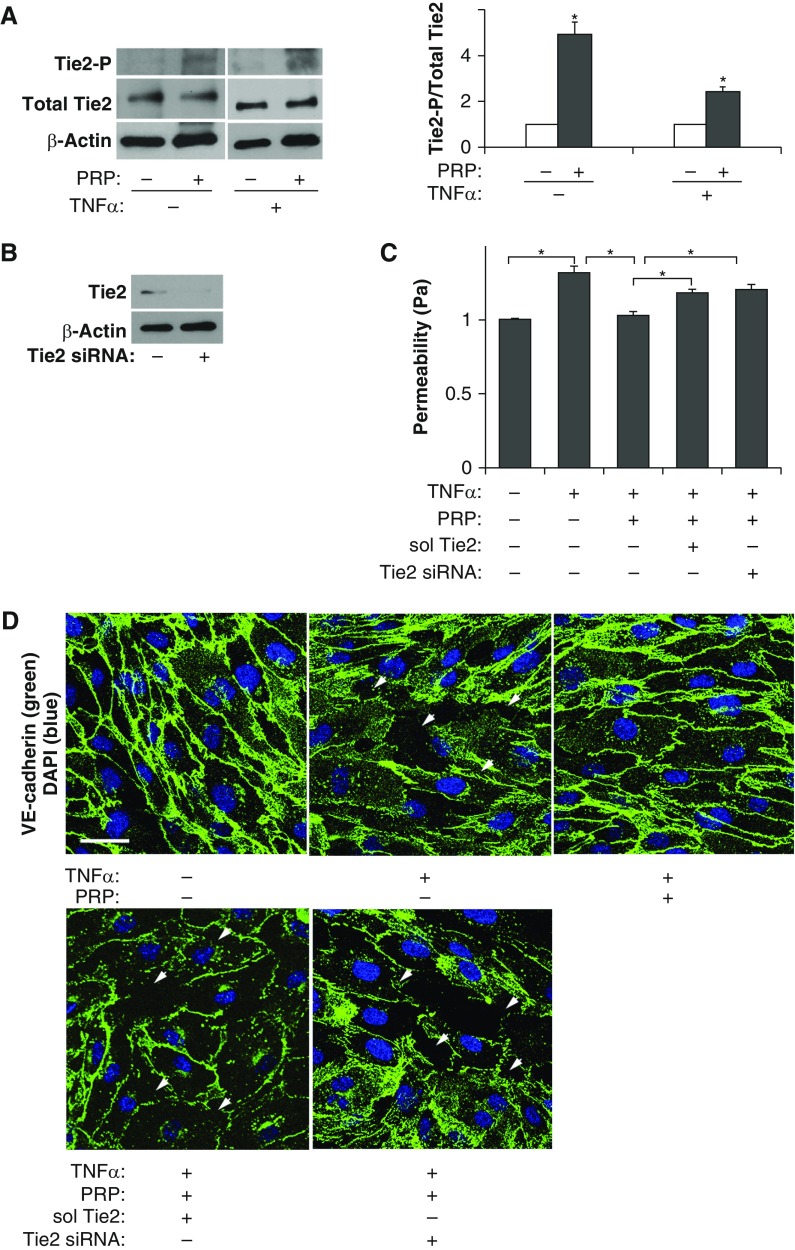
PRP extract contains abundant Ang1 (12), and recombinant Ang1 stabilizes endothelial cell–cell junctional integrity through tyrosine phosphorylation of its receptor, Tie2 (13). Therefore, we next examined whether PRP extract induces Tie2 phosphorylation in L-HMVE cells. When L-HMVE cells were incubated with PRP extract (1:250, vol/vol) or protein concentration–matched mouse serum for 30 minutes, PRP extract increased the levels of Tie2 phosphorylation by 4.9-fold (Figure 2A). Similar effects were also observed in TNF-α (20 ng/ml)–treated L-HMVE cells; Tie2 phosphorylation levels were 2.4-fold higher in L-HMVE cells treated with TNF-α in combination with PRP extract (Figure 2A). To further examine whether PRP extract preserves cell–cell junctional integrity in TNF-α–treated L-HMVE cells through Ang-Tie2 signaling, we treated cells with soluble Tie2 (1 μg/ml), which blocks Ang-Tie2 signaling, or siRNA against Tie2, which decreased the protein expression of Tie2 in L-HMVE cells (Figure 2B) (12, 16). PRP extract suppressed TNF-α–induced disruption of cell–cell junctions (Figure 2D) and decreased permeability (Figure 2C) in L-HMVE cells, whereas these antipermeability abilities of PRP extract were attenuated by treating cells with soluble Tie2 or Tie2 siRNA (Figures 2C and 2D). These results suggest that PRP extract exerts its antipermeability effects through Ang-Tie2 signaling in L-HMVE cells in vitro.
Figure 2.
PRP extract suppresses disruption of endothelial cell–cell junctional integrity in L-HMVE cells treated with TNF-α through angiopoietin (Ang)–Tunica internal endothelial cell kinase (Tie2) signaling. (A) Immunoblots showing tyrosine-phosphorylated Tie2, total Tie2, and β-actin protein levels in L-HMVE cells treated with PRP extract or in combination with TNF-α. Quantitative results (ratio of phosphorylated Tie2 to total Tie2) were normalized to cells treated with protein concentration–matched mouse serum (*P < 0.05). (B) Immunoblots showing Tie2 and β-actin protein levels in L-HMVE cells treated with Tie2 small interfering RNA (siRNA) or siRNA with irrelevant sequences. (C) Graph showing endothelial permeability in L-HMVE cells treated with TNF-α, PRP extract, or in combination with soluble Tie2 receptor (sol Tie2) or Tie2 siRNA (*P < 0.05). Pa values are expressed as percentage of control, untreated cells. Error bars represent SEM of three independent experiments. (D) Immunofluorescence micrographs showing cell–cell junction structure detected by VE-cadherin staining in L-HMVE cells treated with TNF-α, PRP extract, or in combination with soluble Tie2 receptor (sol Tie2) or Tie2 siRNA (scale bar, 35 μm). DAPI staining shows the nucleus of each cell. Arrowheads show the regions where cell–cell junctions are disrupted. As a control, cells were treated with protein concentration–matched mouse serum.
PRP Extract Prevents Endotoxin-Induced Vascular Leakage in Mouse Lung In Vivo
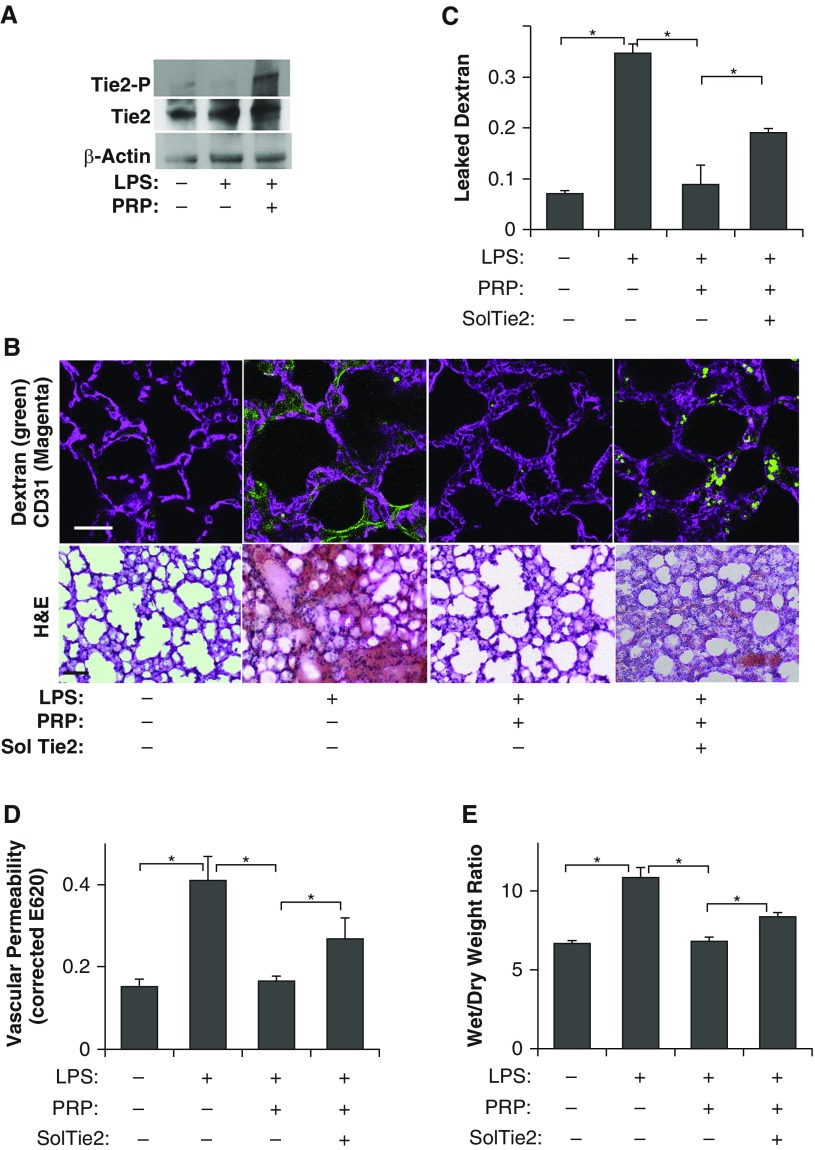
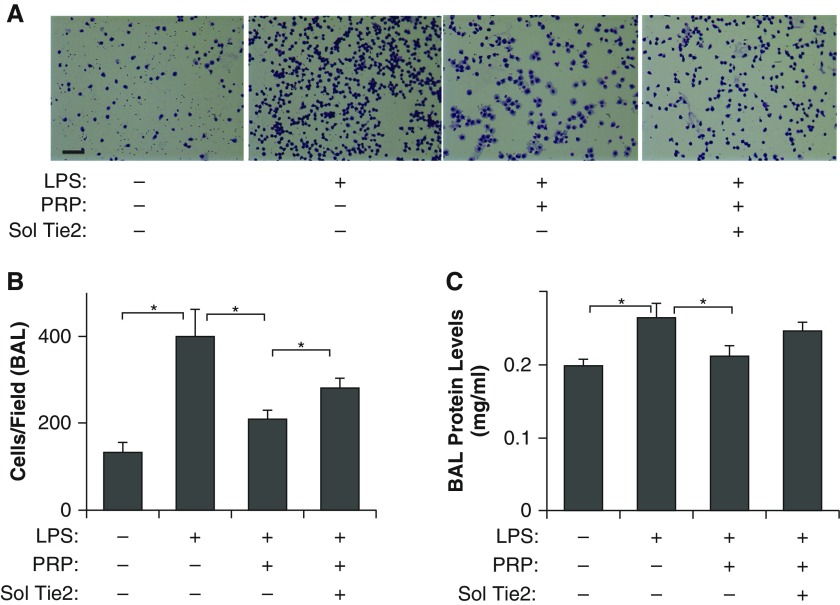
To determine whether PRP extract suppresses lung vascular leakage in endotoxin-induced lung injury in vivo, we treated adult mice with the endotoxin, LPS (2.5 mg/kg, intraperitoneal), which induces the development of pulmonary edema and ARDS in humans with sepsis, and is widely accepted as a physiological animal model for sepsis-induced ARDS (13, 15, 20). Consistent with in vitro results, PRP extract (20 μl, intraperitoneal) up-regulates tyrosine phosphorylation levels of Tie2 in LPS-treated mouse lungs (Figure 3A). To determine whether PRP extract prevents endotoxin-induced increase in pulmonary vascular permeability in vivo, we measured vascular permeability in mouse lungs by measuring leakage of fluorescently labeled LMW dextran (Figures 3B and 3C) or Evans blue dye (Figure 3D) (13, 15, 20). Systemic LPS treatment increased lung vascular permeability 4.9- and 2.6-fold 24 hours after the treatment compared with untreated control mice when measured using fluorescent-labeled LMW dextran and Evans blue dye leakage, respectively (Figures 3B–3D). Consistent with in vitro results, the LPS-induced increase in lung vascular permeability was significantly suppressed in PRP extract–treated mice (Figures 3B–3D). We also confirmed the results by performing H&E staining of lung sections and measuring wet:dry lung weight ratio. The alveolar septa were thickened and protein and inflammatory cells leaked into the alveolar spaces in the H&E–stained, LPS-treated lung sections, and wet:dry lung weight ratio was higher in the mouse lungs treated with LPS for 1 day compared with control untreated lungs, whereas cotreatment with PRP extract reversed these effects (Figures 3B and 3E). The number of immune cells that migrated through the endothelial barrier into the BAL fluid also increased by threefold in the LPS-treated mice, whereas this effect was attenuated in mice cotreated with PRP extract (Figures 4A and 4B). Total protein levels in the BAL fluid also increased by 1.3-fold 1 day after LPS challenge, and this protein leakage was inhibited by cotreatment with PRP extract (Figure 4C). These results suggest that PRP extract suppresses endotoxin-induced vascular leakage and proinflammatory effects in mouse lungs. Importantly, Ang depletion using soluble Tie2 receptor (2 μg, intraperitoneal) suppressed the antipermeability effects of PRP extract in mouse lung (Figures 3B–3E), and increased the number of immune cells and level of protein leakage into the BAL fluid in vivo (Figures 4A–4C), suggesting that Ang-Tie2 signaling contributes to the antipermeability effects of PRP extract and mediates the suppression of endotoxin-induced lung vascular permeability in vivo.
Figure 3.
PRP extract prevents vascular leakage in endotoxin-induced mouse lung injury through Ang-Tie2 signaling. (A) Immunoblots showing tyrosine-phosphorylated Tie2, total Tie2, and β-actin protein levels in mouse lungs treated with LPS or in combination with PRP extract. (B) Immunofluorescence micrographs showing low–molecular weight (LMW), fluorescently labeled dextran leakage (green) and blood vessel structures (CD31 staining, magenta) in mouse lungs treated with LPS, PRP extract, or in combination with soluble Tie2 (sol Tie2) for 1 day (top, scale bar, 25 μm). Micrographs showing the hematoxylin and eosin (H&E)–stained mouse lungs treated with LPS, PRP extract, or in combination with soluble Tie2 (sol Tie2) for 1 day (bottom, scale bar, 25 μm). (C) Graph showing the leaked dextran density normalized to vessel density in mouse lungs treated with LPS, PRP extract, or in combination with soluble Tie2 for 1 day (n = 7, mean ± SEM; *P < 0.05). (D) Lung vascular permeability detected by Evans blue dye leakage in mice treated with LPS, PRP extract, or in combination with soluble Tie2 for 1 day (n = 10, mean ± SEM; *P < 0.05). (E) Wet:dry lung weight ratio of mouse lungs treated with LPS, PRP extract, or in combination with soluble Tie2 for 1 day (n = 7, mean ± SEM; *P < 0.05).
Figure 4.
PRP extract decreases leakage of cells and protein in bronchoalveolar lavage (BAL) fluid in endotoxin-induced mouse lung injury. (A) Micrographs showing the immune cells migrated through the endothelial barrier into the alveolar space (BAL fluid) in the mouse lungs treated with LPS, PRP extract, or in combination with soluble Tie2 (sol Tie2) for 1 day, detected by Wright-Giemsa staining (scale bar, 50 μm). (B) Graph showing the immune cell count in the BAL fluid from the mouse lungs treated with LPS, PRP extract, or in combination with soluble Tie2 for 1 day (n = 8, mean ± SEM; *P < 0.05). (C) Protein concentration in BAL fluid from the mouse lungs treated with LPS, PRP extract, or in combination with soluble Tie2 for 1 day (n = 7, mean ± SEM; *P < 0.05).
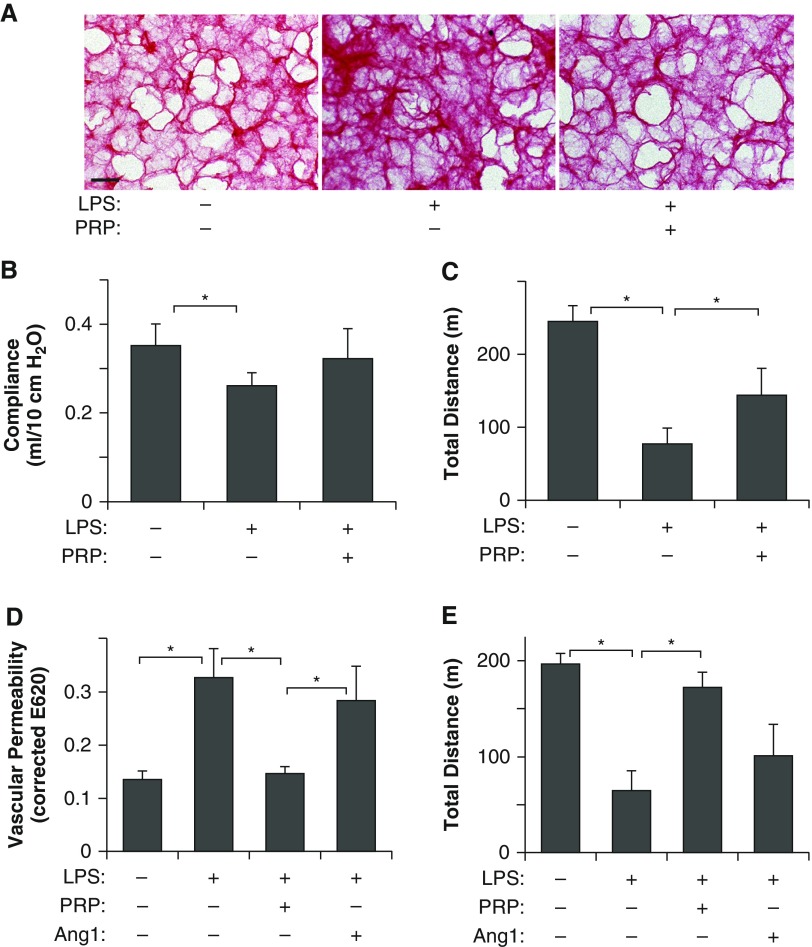
It has been reported that the long-term quality of life of patients who survive acute-phase ARDS is severely affected (3). Extracellular matrix (ECM) structure and lung tissue mechanics are known to change after lung injury and contribute to impairment of lung function (17, 20). Characterization of collagen structure, a major ECM component in the lung, using picrosirius red staining, which detects fibrillar collagens, on the decellularized lung sections (17), revealed that thickness and density of collagen fibers increased in the lung sections of LPS-treated mice compared with those in control, untreated lungs, and collagen structures were restored by PRP treatment (Figure 5A). The mean static lung compliance 1 week after LPS challenge was also significantly lower compared with control, untreated mice, and cotreatment with PRP extract inhibited this reduction (Figure 5B). As a more physiological assay, we also analyzed exercise capacity by measuring the total distance mice were able to run using a rodent treadmill exercise protocol, and found that the running distance was decreased by 71% in LPS-treated mice 1 week after LPS challenge, whereas cotreatment with PRP extract suppressed the exercise intolerance induced by LPS (Figure 5C). These results indicate that PRP extract prevents impairment of physiological lung function after the acute phase of endotoxin-induced lung injury. Importantly, although Ang1 is the major component of PRP extract, which suppressed the endotoxin-induced increase in vascular permeability, equivalent concentration of recombinant Ang1 (1 ng, intraperitoneal) alone failed to suppress lung vascular leakage and exercise intolerance in LPS-treated mice (Figures 5D and 5E). These results suggest that, although Ang1-Tie2 signaling is a major pathway through which PRP extract stabilizes blood vessels and preserves vascular integrity in the lungs, combination with other angiogenic factors in PRP extract may be necessary to prevent acute lung injury.
Figure 5.
PRP extract recovers physiological lung function in endotoxin-induced mouse lung injury. (A) Micrographs showing the picrosirius red–stained decellularized mouse lung sections treated with LPS or in combination with PRP extract for 1 day and assessed 7 days after LPS challenge (scale bar, 25 μm). (B) The mean static lung compliance of mice treated with LPS or in combination with PRP extract for 1 day and assessed 7 days after LPS challenge (n = 7, mean ± SEM; *P < 0.05). (C) Exercise capacity of mice treated with LPS or in combination with PRP extract for 1 day and assessed 7 days after LPS challenge by total running distance according to a rodent treadmill exercise protocol (n = 10, mean ± SEM; *P < 0.05). (D) Lung vascular permeability detected by Evans blue dye leakage in mice treated with LPS or in combination with PRP extract or Ang1 for 1 day (n = 10, mean ± SEM; *P < 0.05). (E) Exercise capacity of mice treated with LPS or in combination with PRP extract or Ang1 for 1 day and assessed 7 days later by total running distance according to a rodent treadmill exercise protocol (n = 10, mean ± SEM; *P < 0.05).
Discussion
Tightly regulated vascular permeability is critical to maintaining lung function, whereas deregulated vascular permeability contributes to the pathogenesis of acute lung injury and ARDS (1, 13). Here, we show that PRP extract, which contains abundant Ang1, a potent endogenous antipermeability factor, and lower amounts of other angiogenic factors (12), controls lung vascular permeability by altering Ang-Tie2 signaling. PRP extract increases tyrosine phosphorylation levels of Tie2 and attenuates disruption of cell–cell junctional integrity induced by the inflammatory cytokine, TNF-α, in human microvascular endothelial cells in vitro. PRP extract also prevents endotoxin-induced acute pulmonary edema, chronic impairment of lung function, and exercise intolerance in mice in vivo, whereas soluble Tie2 receptor, which inhibits Ang-Tie2 signaling, suppresses the antipermeability effects of PRP extract in mouse lung. These results suggest that PRP extract prevents endotoxin-induced pulmonary edema mainly through Ang-Tie2 signaling, and that PRP extract will be a potential therapeutic intervention for sepsis-induced ARDS and other diseases associated with abnormal vascular permeability.
Mouse PRP extract contains abundant Ang1 and lower amounts of other angiogenic factors (e.g., PDGF), which potentially stabilize vascular integrity and decrease vascular permeability (12). Although PRP extract suppressed the endotoxin-induced increase in lung vascular permeability, the equivalent concentration of recombinant Ang1 alone could not reproduce the effects of the extract. This may be because other angiogenic factors in the extract, such as VEGF and PDGF, may potentially interact with the Ang-Tie2 system and enhance its antipermeability effects (25, 26). In fact, it is recognized that a combination of multiple angiogenic factors, rather than a single factor, is preferred for maintenance of stable and functional blood vessels (5, 6). For example, VEGF, a well known vascular permeability factor, is one of the components of PRP extract (12). Although an excess amount of VEGF causes pulmonary edema (27), physiological levels of VEGF expression have protective effects on alveolar epithelium after lung injury (28), and lower VEGF levels in the plasma are associated with a higher mortality rate in patients with ARDS (29). VEGF activates tyrosine phosphorylation of Tie2 in endothelial cells (26), and may positively modulate Ang1-Tie2 signaling to enhance endothelial barrier function (30). Furthermore, PRP also contains various other growth factors, such as keratinocyte growth factor and epidermal growth factor, that stimulate lung repair after damage by endotoxin (31, 32). Thus, multiple factors contained in PRP extract may interact with each other to prevent LPS-induced pulmonary edema in vivo.
It is known that Ang1 and Ang2 bind to a common receptor, Tie2, antagonize each other, and control blood vessel maturation and stabilization (15, 33); Ang1 stabilizes blood vessel formation (13, 33, 34), whereas Ang2 destabilizes the blood vessel structure and increases vascular permeability in lung injury (14). Although Ang1 is the major component of PRP extract generated by our protocol, PRP extract also contains a lower amount of antagonistic Ang2 (12). Thus, the addition of either soluble Tie2 receptor, which traps both Ang1 and Ang2, or Tie2 siRNA may inhibit the destabilizing effects of Ang2 in PRP extract on endotoxin-treated lungs, resulting in only partial suppression of the antipermeability effects of PRP extract in vitro and in vivo.
It has been reported that adenoviral- or cell-based Ang1 delivery (35, 36), or injection of recombinant Ang1 (13), prevents LPS-induced acute lung injury in the animal model. However, viral delivery of Ang1 may not be plausible in human patients. In addition, cell-based Ang1 delivery requires the generation of genetically modified mesenchymal stem cells (36), and large-scale production of recombinant Ang1 is costly and technically challenging because of aggregation and insolubility of the protein (37). PRP extract, which contains not only Ang1, but multiple other factors required for preventing pulmonary edema, is generated from autologous peripheral blood by a simple method, can be preserved stably at −80°C for a long period (12), and can be optimized by adding extra recombinant factors or by modifying the preparation method. Given these observations, PRP extract could be a good therapeutic tool for treating patients with sepsis-induced ARDS, and disease-targeted customized PRP extract may expand applications of PRP extract to various other diseases caused by abnormal vascular permeability.
Although platelets enhance angiogenesis and integrate lung vasculature (11), platelets also contribute to neutrophil-mediated lung injury (38). Platelet-derived adhesion molecules, such as P-selectin, enhance the interaction between platelets and neutrophils and exaggerate lung injury (39). Direct interaction of platelets with neutrophils and monocytes and communication between the endothelium, neutrophils, and platelets appear to cause vascular injury (40). In fact, it has been reported that platelet depletion reduces lung injury in mouse models (41). However, our results reveal that the extract from platelets stabilizes blood vessel integrity and prevents pulmonary edema in a mouse endotoxin-induced lung injury model. Consistently, it has been reported that platelets release various factors, such as sphingosine-1-phosphate (42), that promote endothelial barrier function (43). Platelet-derived soluble factors and the interactions of platelets with other cellular components in the lung may have distinct roles in maintenance of vascular integrity and pathogenesis of ARDS.
In addition to soluble angiogenic factors, changes in ECM mechanics (stiffness) control angiogenesis (17, 19) and lung vascular permeability (20). In fact, ECM stiffness increases in endotoxin-treated lungs (20), tumor tissues (44, 45), and fibrotic lungs (46), in which microvessels are leaky. Because ECM stiffness controls angiogenesis through Tie2 signaling in neonatal lung developmental disorders (e.g., bronchopulmonary dysplasia) (17), and Ang-Tie2 signaling is involved in destabilization of blood vessels in various diseases in which tissue stiffness is changed (13, 17, 47), in addition to chemical regulators, it appears that physical changes in ECM mechanics regulate lung vascular permeability through Ang-Tie2 signaling. Given that platelet-derived soluble factors inhibit sepsis-induced ARDS by inhibiting macrophage-dependent inflammation (48), and that immune cells play important roles in remodeling of ECM structures in the lung (49, 50), PRP extract may prevent LPS-induced pulmonary edema by preserving lung ECM structures as well.
In summary, we have demonstrated that PRP extract up-regulates Tie2 activity and prevents LPS-induced vascular leakage in mouse lungs. PRP extract could be a potential therapeutic approach for sepsis-induced ARDS as well as other diseases caused by abnormal vascular permeability.
Acknowledgments
Acknowledgments
The authors thank D. Ingber for technical help and helpful discussion. They also thank the neurodevelopmental behavioral core at Boston Children’s Hospital for the analysis of exercise capacity.
Footnotes
This work was supported by funds from American Heart Association (A.M.), American Brain Tumor Association (A.M.), Boston Children’s Hospital Faculty Career Development Fellowship (T.M.), Department of Defense grant BC074986, and National Institutes of Health (P30 HD18655, Children's Hospital Boston Intellectual and Developmental Disabilities Research Center).
Author Contributions: T.M. and A.M. conceived and designed the experiments; T.M., A.J., E.J., and A.M. performed the experiments; T.M. and A.M. analyzed the data; T.M. and A.M. contributed reagents/materials/analysis tools; A.M. and T.M. wrote the paper.
Originally Published in Press as DOI: 10.1165/rcmb.2014-0076OC on June 24, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spragg RG, Bernard GR, Checkley W, Curtis JR, Gajic O, Guyatt G, Hall J, Israel E, Jain M, Needham DM, et al. Beyond mortality: future clinical research in acute lung injury. Am J Respir Crit Care Med. 2010;181:1121–1127. doi: 10.1164/rccm.201001-0024WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Murakami K, Okajima K, Uchiba M, Johno M, Nakagaki T, Okabe H, Takatsuki K. Activated protein c attenuates endotoxin-induced pulmonary vascular injury by inhibiting activated leukocytes in rats. Blood. 1996;87:642–647. [PubMed] [Google Scholar]
- 5.Anisimov A, Tvorogov D, Alitalo A, Leppanen VM, An Y, Han EC, Orsenigo F, Gaal EI, Holopainen T, Koh YJ, et al. Vascular endothelial growth factor–angiopoietin chimera with improved properties for therapeutic angiogenesis. Circulation. 2013;127:424–434. doi: 10.1161/CIRCULATIONAHA.112.127472. [DOI] [PubMed] [Google Scholar]
- 6.Benest AV, Salmon AH, Wang W, Glover CP, Uney J, Harper SJ, Bates DO. VEGF and angiopoietin-1 stimulate different angiogenic phenotypes that combine to enhance functional neovascularization in adult tissue. Microcirculation. 2006;13:423–437. doi: 10.1080/10739680600775940. [DOI] [PubMed] [Google Scholar]
- 7.Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pintucci G, Froum S, Pinnell J, Mignatti P, Rafii S, Green D. Trophic effects of platelets on cultured endothelial cells are mediated by platelet-associated fibroblast growth factor-2 (FGF-2) and vascular endothelial growth factor (VEGF) Thromb Haemost. 2002;88:834–842. [PubMed] [Google Scholar]
- 9.Everts PA, Knape JT, Weibrich G, Schonberger JP, Hoffmann J, Overdevest EP, Box HA, van Zundert A. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38:174–187. [PMC free article] [PubMed] [Google Scholar]
- 10.Ho-Tin-Noe B, Demers M, Wagner DD. How platelets safeguard vascular integrity. J Thromb Haemost. 2011;9:56–65. doi: 10.1111/j.1538-7836.2011.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weyrich AS, Zimmerman GA. Platelets in lung biology. Annu Rev Physiol. 2013;75:569–591. doi: 10.1146/annurev-physiol-030212-183752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mammoto T, Jiang A, Jiang E, Mammoto A. Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvasc Res. 2013;89:15–24. doi: 10.1016/j.mvr.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, Ingber DE, Sukhatme VP. Angiopoietin-1 requires p190RhoGAP to protect against vascular leakage in vivo. J Biol Chem. 2007;282:23910–23918. doi: 10.1074/jbc.M702169200. [DOI] [PubMed] [Google Scholar]
- 14.Parikh SM, Mammoto T, Schultz A, Yuan HT, Christiani D, Karumanchi SA, Sukhatme VP. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mammoto T, Jiang E, Jiang A, Lu Y, Juan AM, Chen J, Mammoto A. Twist1 controls lung vascular permeability and endotoxin-induced pulmonary edema by altering Tie2 expression. PLoS One. 2013;8:e73407. doi: 10.1371/journal.pone.0073407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mammoto T, Chen J, Jiang E, Jiang A, Smith LE, Ingber DE, Mammoto A. Lrp5 regulates development of lung microvessels and alveoli through the angiopoietin-Tie2 pathway. PLoS One. 2012;7:e41596. doi: 10.1371/journal.pone.0041596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mammoto T, Jiang E, Jiang A, Mammoto A. ECM structure and tissue stiffness control postnatal lung development through the Lrp5-Tie2 signaling system. Am J Respir Cell Mol Biol. 2013;49:1009–1018. doi: 10.1165/rcmb.2013-0147OC. [DOI] [PubMed] [Google Scholar]
- 18.Groneck P, Gotze-Speer B, Oppermann M, Eiffert H, Speer CP. Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics. 1994;93:712–718. [PubMed] [Google Scholar]
- 19.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mammoto A, Mammoto T, Kanapathipillai M, Yung CW, Jiang E, Jiang A, Lofgren K, Gee EPS, Ingber DE. Control of lung vascular permeability and endotoxin-induced pulmonary edema by changes in extracellular matrix mechanics. Nat Commun. 2013;4:1759. doi: 10.1038/ncomms2774. [DOI] [PubMed] [Google Scholar]
- 21.Akinbi HT, Breslin JS, Ikegami M, Iwamoto HS, Clark JC, Whitsett JA, Jobe AH, Weaver TE. Rescue of SP-B knockout mice with a truncated SP-B proprotein: function of the C-terminal propeptide. J Biol Chem. 1997;272:9640–9647. doi: 10.1074/jbc.272.15.9640. [DOI] [PubMed] [Google Scholar]
- 22.Arkovitz MS, Garcia VF, Szabo C, McConnell K, Bove K, Wispe JR. Decreased pulmonary compliance is an early indicator of pulmonary oxygen injury. J Surg Res. 1997;67:193–198. doi: 10.1006/jsre.1996.4980. [DOI] [PubMed] [Google Scholar]
- 23.Matthew E, Warden G, Dedman J. A murine model of smoke inhalation. Am J Physiol Lung Cell Mol Physiol. 2001;280:L716–L723. doi: 10.1152/ajplung.2001.280.4.L716. [DOI] [PubMed] [Google Scholar]
- 24.Duan Y, Learoyd J, Meliton AY, Leff AR, Zhu X. Inhibition of Pyk2 blocks lung inflammation and injury in a mouse model of acute lung injury. Respir Res. 2012;13:4. doi: 10.1186/1465-9921-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, et al. Angiopoietin-2 differentially regulates angiogenesis through Tie2 and integrin signaling. J Clin Invest. 2012;122:1991–2005. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh H, Milner CS, Aguilar Hernandez MM, Patel N, Brindle NP. Vascular endothelial growth factor activates the Tie family of receptor tyrosine kinases. Cell Signal. 2009;21:1346–1350. doi: 10.1016/j.cellsig.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 27.Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, Crystal RG. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am J Respir Cell Mol Biol. 2000;22:657–664. doi: 10.1165/ajrcmb.22.6.3779. [DOI] [PubMed] [Google Scholar]
- 28.Kunig AM, Balasubramaniam V, Markham NE, Morgan D, Montgomery G, Grover TR, Abman SH. Recombinant human VEGF treatment enhances alveolarization after hyperoxic lung injury in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2005;289:L529–L535. doi: 10.1152/ajplung.00336.2004. [DOI] [PubMed] [Google Scholar]
- 29.Zhai R, Gong MN, Zhou W, Thompson TB, Kraft P, Su L, Christiani DC. Genotypes and haplotypes of the VEGF gene are associated with higher mortality and lower VEGF plasma levels in patients with ARDS. Thorax. 2007;62:718–722. doi: 10.1136/thx.2006.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Findley CM, Cudmore MJ, Ahmed A, Kontos CD. VEGF induces Tie2 shedding via a phosphoinositide 3-kinase/Akt dependent pathway to modulate Tie2 signaling. Arterioscler Thromb Vasc Biol. 2007;27:2619–2626. doi: 10.1161/ATVBAHA.107.150482. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Li C, Gao X, Liang Z, Yu L, Li Y, Xiao X, Chen L. Keratinocyte growth factor gene delivery via mesenchymal stem cells protects against lipopolysaccharide-induced acute lung injury in mice. PLoS One. 2013;8:e83303. doi: 10.1371/journal.pone.0083303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang B, Huang W, Han J, Liang Z. Study of the role of epidermal growth factor on lung fluid transport in rabbits with acute lung injury caused by endotoxin. Exp Ther Med. 2012;4:611–614. doi: 10.3892/etm.2012.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saharinen P, Bry M, Alitalo K. How do angiopoietins tie in with vascular endothelial growth factors? Curr Opin Hematol. 2010;17:198–205. doi: 10.1097/MOH.0b013e3283386673. [DOI] [PubMed] [Google Scholar]
- 34.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 35.Witzenbichler B, Westermann D, Knueppel S, Schultheiss HP, Tschope C. Protective role of angiopoietin-1 in endotoxic shock. Circulation. 2005;111:97–105. doi: 10.1161/01.CIR.0000151287.08202.8E. [DOI] [PubMed] [Google Scholar]
- 36.Mei SH, McCarter SD, Deng Y, Parker CH, Liles WC, Stewart DJ. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho CH, Kammerer RA, Lee HJ, Steinmetz MO, Ryu YS, Lee SH, Yasunaga K, Kim KT, Kim I, Choi HH, et al. Comp-Ang1: a designed angiopoietin-1 variant with nonleaky angiogenic activity. Proc Natl Acad Sci USA. 2004;101:5547–5552. doi: 10.1073/pnas.0307574101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthay MA, Zemans RL. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol. 2011;6:147–163. doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet–neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheiermann C, Kunisaki Y, Jang JE, Frenette PS. Neutrophil microdomains: linking heterocellular interactions with vascular injury. Curr Opin Hematol. 2010;17:25–30. doi: 10.1097/MOH.0b013e328333d2a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Looney MR, Nguyen JX, Hu Y, Van Ziffle JA, Lowell CA, Matthay MA. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119:3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.English D, Welch Z, Kovala AT, Harvey K, Volpert OV, Brindley DN, Garcia JG. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 2000;14:2255–2265. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- 43.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by EDG-dependent cytoskeletal rearrangement. J Clin Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu P, Takai K, Weaver VM, Werb Z.Extracellular matrix degradation and remodeling in development and disease Cold Spring Harb Perspect Biol 20113pii: a005058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu F, Mih JD, Shea BS, Kho AT, Sharif AS, Tager AM, Tschumperlin DJ. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol. 2010;190:693–706. doi: 10.1083/jcb.201004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu B, Guo P, Fang Q, Tao HQ, Wang D, Nagane M, Huang HJ, Gunji Y, Nishikawa R, Alitalo K, et al. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci USA. 2003;100:8904–8909. doi: 10.1073/pnas.1533394100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiang B, Zhang G, Guo L, Li XA, Morris AJ, Daugherty A, Whiteheart SW, Smyth SS, Li Z. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat Commun. 2013;4:2657. doi: 10.1038/ncomms3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Noble PW, Jiang D. Matrix regulation of lung injury, inflammation, and repair: the role of innate immunity. Proc Am Thorac Soc. 2006;3:401–404. doi: 10.1513/pats.200604-097AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnston LK, Rims CR, Gill SE, McGuire JK, Manicone AM. Pulmonary macrophage subpopulations in the induction and resolution of acute lung injury. Am J Respir Cell Mol Biol. 2012;47:417–426. doi: 10.1165/rcmb.2012-0090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]