Abstract
Purpose
To investigate the role of out-of-pocket cost supports through the Medicare Part D Low-Income Subsidy on disparities in breast cancer hormonal therapy persistence and adherence by race or ethnicity.
Methods
A nationwide cohort of women age ≥ 65 years with a breast cancer operation between 2006 and 2007 and at least one prescription filled for oral breast cancer hormonal therapy was identified from all Medicare D enrollees. The association of race or ethnicity with nonpersistence (90 consecutive days with no claims for a hormonal therapy prescription) and nonadherence (medication possession rate < 80%) was examined. Survival analyses were used to account for potential differences in age, comorbidity, or intensity of other treatments.
Results
Among the 25,111 women in the study sample, 77% of the Hispanic and 70% of the black women received a subsidy compared with 21% of the white women. By 2 years, 69% of black and 70% of Hispanic patients were persistent compared with 61% of white patients. In adjusted analyses, patients in all three unsubsidized race or ethnicity groups had greater discontinuation than subsidized groups (white patients: hazard ratio [HR], 1.83; 95% CI, 1.70 to 1.95; black patients: HR, 2.09; 95% CI, 1.73 to 2.51; Hispanic patients: HR, 3.00; 95% CI, 2.37 to 3.89). Racial or ethnic persistence disparities that were present for unsubsidized patients were not present or reversed among subsidized patients. All three subsidized race or ethnicity groups also had higher adherence than all three unsubsidized groups, although with the smallest difference occurring in black women.
Conclusion
Receipt of a prescription subsidy was associated with substantially improved persistence to breast cancer hormonal therapy among white, black, and Hispanic women and lack of racial or ethnic disparities in persistence. Given high subsidy enrollment among black and Hispanic women, policies targeted at low-income patients have the potential to also substantially reduce racial and ethnic disparities.
INTRODUCTION
Although breast cancer has historically been most common among white women, black women and women with low socioeconomic status have higher mortality from breast cancer. Between 2007 and 2011, the breast cancer mortality rate in black women was 31 per 100,000 compared with 22 per 100,000 for white women.1 Differences in health care and access, particularly in timely detection of cancer and treatment quality, seem to explain a substantial part of these mortality disparities.2,3 Socioeconomic status also affects health care quality and is strongly associated with poorer outcomes in nearly all cancers.3
Some disparities in breast cancer outcomes may be attributable to the high cost of breast cancer adjuvant hormonal (endocrine) therapy. Although adjuvant hormonal therapy for hormone-positive breast cancer reduces 15-year mortality by more than one third,4 one third to one half of patients do not take all doses,5-7 substantially reducing treatment effectiveness. Some studies8,9 show that nonadherence (defined as the number of doses taken out of the number of doses prescribed) and nonpersistence (early discontinuation) are substantially higher in black women5,10,11 and women with low incomes.10,11 Little is known about adherence or persistence among Hispanic women.12 Despite several recent studies that also link lack of insurance coverage or higher patient copays for the newer, more effective aromatase inhibitors with nonadherence or discontinuation,10,11,13 there has been little study of the effect of interventions that reduce patient out-of-pocket costs on disparities.
The Medicare Part D legislation’s Low-Income Subsidy program offers a unique opportunity to examine the effects of a policy targeting greater financial supports to low-income and low-asset patients on disparities in cancer care. Medicare D provides all enrolled patients with pharmaceutical coverage through privately administered plans with distinct Part D premiums, deductibles, and copays and a substantial coverage gap during which enrollees pay the full cost of medication. For many low-income and low–net worth patients, the federal government’s subsidy program eliminates or substantially reduces these additional premiums, deductibles, and copays and eliminates the coverage gap. Patients either are automatically enrolled onto this Medicare D Low-Income Subsidy program or apply for the subsidy by demonstrating low assets and income. We hypothesized that recipients of the Medicare D Low-Income Subsidy would have similar hormonal therapy persistence and adherence as patients who did not receive the subsidy. We further hypothesized that the differences in adherence for black and Hispanic women compared with white women seen in some earlier studies would be smaller among subsidized recipients.
METHODS
Study Sample
To identify a potentially eligible sample of women with breast cancer, we applied a previously validated claims-based prediction algorithm14 to administrative claims from the Medicare program. Women considered for inclusion were required to be age ≥ 65 years with a breast cancer operation identified by the algorithm in calendar years 2006 and 2007. To allow ascertainment of comorbidities and treatments, the women were also required to have been enrolled in Medicare Parts A and B and not enrolled in a Medicare Advantage plan for 12 months before breast cancer surgery and were required to be enrolled in a Medicare Prescription Drug Plan (stand-alone Part D plan) at the time of their cancer operation. All eligible women also had at least one prescription filled for oral breast cancer hormonal therapy with an aromatase inhibitor or tamoxifen through Medicare D within 1 year of breast cancer surgery.
Cohort members were further limited to women of white or black race or Hispanic ethnicity on the basis of Medicare file designation. Medicare race and ethnicity designations are initially self-reported at the time individuals apply for a Social Security number or replacement Social Security card. Because before 1994 people were limited to choices of white, black, or other, Medicare race and ethnicity information was edited by Medicare in 1997 on the basis of results of a survey of nearly 2.2 million people with Hispanic surnames and then, in 2005, with Asian and Hispanic surname-based imputation.15 The positive predictive value for white, black, and Hispanic race or ethnicity is now estimated at greater than 92%; because the positive predictive value is lower for other races and the total numbers of patients of other races were small,15 we excluded them from the study.
Measures of Prescription Use
Medicare D Prescription Drug Event files were used to determine patients’ pharmaceutical use. These files included variables for dispensed medications’ National Drug Code, the date and quantity dispensed, and medication charges paid to the pharmacy by the prescription drug plan and the beneficiary. Using the quantity of medication dispensed, we counted the number of pills of tamoxifen or any of the three aromatase inhibitors (all once-daily medications) received between the first prescription and July 2010, when generic aromatase inhibitors substantially altered patterns.10 Because a large number of participants received 90-day prescriptions, all pill counts were assessed by 90-day time periods. When a medication was dispensed before the previous prescription would have run out, the new prescription was assumed to start the day after the previous prescription should have ended, to account for stockpiled pills.16 Nonpersistence (or discontinuation) was defined as 90 consecutive days with no claims for a hormonal therapy prescription. Adherence was defined using a medication possession rate (MPR) for each quarter. Similar to other studies11,17,18 and consistent with a study of the association between aromatase inhibitors and mortality,19 patients with an MPR of less than 0.80 (or 80%) were defined as nonadherent. Patients who switched from one medication to another were considered to be persistent and adherent throughout the time period during which they possessed pills of any type.
The Medicare Part D Low-Income Subsidy
Medicare’s Low-Income Subsidy is recorded on Medicare files monthly with similar eligibility thresholds in all states and slight increases each year. A full subsidy is automatically provided to patients enrolled in Medicaid, a Medicare A/B Savings Program, or the Supplemental Security Income program and persons with income less than 135% of the federal poverty line with low assets (Appendix Table A1, online only). Other individuals with income less than 150% of the federal poverty line and low assets were eligible to apply for partial subsidies (Appendix Table A1).
Other Covariates
Other covariates included age at the time of surgery as recorded on Medicare files. Comorbidity score20 was based on the year before surgery, and total number of other medications was defined as the number of unique medications filled in the first quarter of hormonal therapy use. To account for expected reductions in adherence with increasing treatment duration,5,17 time since medication initiation was calculated. Socioeconomic status was estimated from US census data from the median per capita income and percentage of high school graduates among adults in the participant’s ZIP code.21,22 The Medicare D coverage gap (for unsubsidized patients) was defined as any month in which the patient had been in the coverage gap for at least the prior 60 days.
Analyses
Hormonal therapy persistence and adherence rates, as defined earlier, were computed for each 90-day period between the patient’s medication start date and July 2010. Descriptive statistics were used for demographics and other baseline variables, and differences in these variables by race or ethnicity were examined. Kaplan-Meier curves were first used to examine unadjusted differences in persistence by race or ethnicity, receipt of a subsidy, and the six subsidy-race combinations. Disenrollment from a Medicare D prescription drug plan (n = 389), death (n = 1,569), and end of study (n = 11,664) were treated as censoring events. In developing adjusted models, we tested whether predictor variables met the proportional hazards assumption. Because the assumption was not met for age, number of medications, and chemotherapy use, all models were stratified by these covariates. Race or ethnicity also violated the assumption, and there was an interaction between race or ethnicity and the Low-Income Subsidy. Therefore, we used a model with a time-dependent relationship between race or ethnicity and discontinuation, including the interaction term. Time axis was broken into the following three windows: 0 to 5 months, 5 to 35 months, and greater than 35 months after diagnosis.
In a second set of adjusted models, we also examined adjusted differences in 90-day average probability of adherence (MPR ≥ 80%) by race or ethnicity during time periods in which patients remained persistent with their medications. For these patients, we used logistic regression models with generalized estimating equations (Proc Genmod in SAS Version 9.3; SAS Institute, Cary, NC) to control for expected within-patient temporal correlation in adherence.
In sensitivity analyses, we first explored several different specifications for the Low-Income Subsidy variable. Few patients changed subsidy status during the study, so use of subsidy as a time-varying covariate would not have changed our results. In initial models, results did not differ for patients who had partial versus full subsidies (Appendix Table A1), so subsidy status was included as yes or no for all models. Finally, we explored how much any subsidy effect(s) on adherence could be attributed to elimination of the coverage gap.
RESULTS
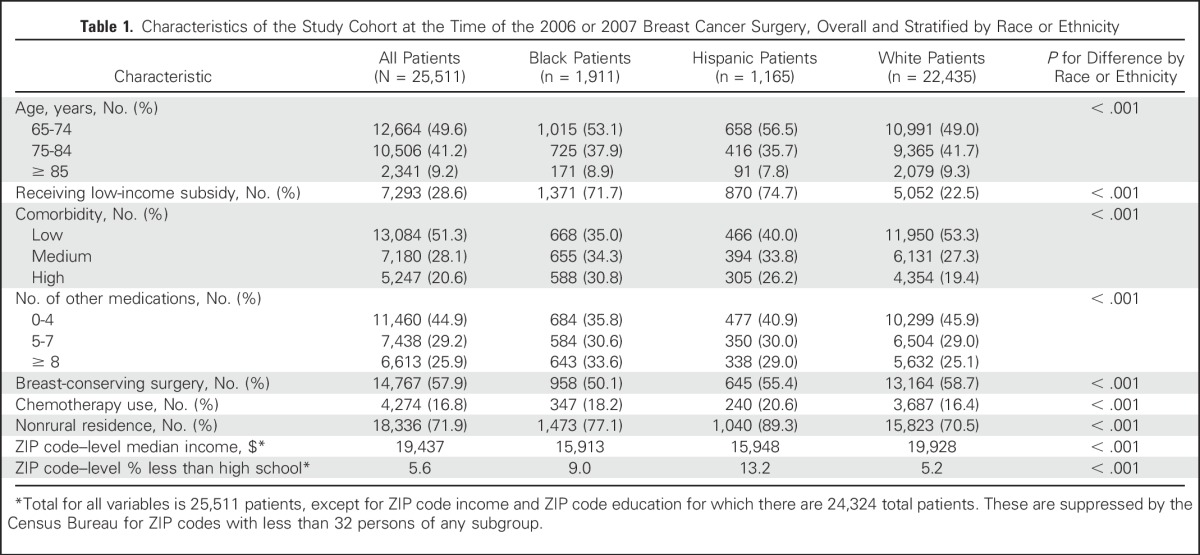
The cohort included 25,511 patients observed for a median of 2.2 years of hormonal therapy. Compared with both black and Hispanic women, non-Hispanic white women were older, had fewer comorbidities, and were more likely to reside in rural ZIP codes and ZIP codes with higher per-capita income and educational attainment (all P < .001; Table 1). Although 27% of the cohort overall received the Low-Income Subsidy, this varied substantially by race or ethnicity, with 77% of the Hispanic women and 70% of the black women receiving a subsidy, compared with 21% of the non-Hispanic white women.
Table 1.
Characteristics of the Study Cohort at the Time of the 2006 or 2007 Breast Cancer Surgery, Overall and Stratified by Race or Ethnicity
Persistence
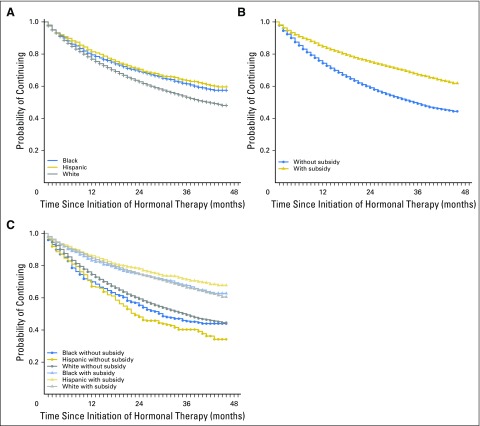
More than 77% of the total cohort persisted (continued) on hormonal therapy at 1 year after the initial prescription, and 64% persisted at 2 years. As shown in the first of the series of Kaplan-Meier curves (Fig 1A), in unadjusted analyses, black and Hispanic women were more persistent with medication (P < .001 by log-rank test) than white women throughout much of the study period, and by 2 years, 69% of black patients and 70% of Hispanic patients were persistent compared with 61% of white patients.
Fig 1.
Medication discontinuation among Medicare Part D enrollees who began hormonal therapy within 1 year of breast cancer surgery that occurred in 2006 or 2007. In all panels, the y-axis represents the proportion (or probability of) continuing with medication, and the x-axis represents months since initiation of hormonal therapy. (A) Unadjusted continuation (persistence) by race or ethnicity. (B) Unadjusted persistence by receipt of the Low-Income Subsidy. (C) Unadjusted persistence by six groups on the basis of receipt of subsidy and race or ethnicity. At 12 months, a total of 675 patients were censored (for death, disenrollment from Medicare, or study end); by 24 months, 1,624 patients were censored, and by 36 months, 7,858 patients were censored.
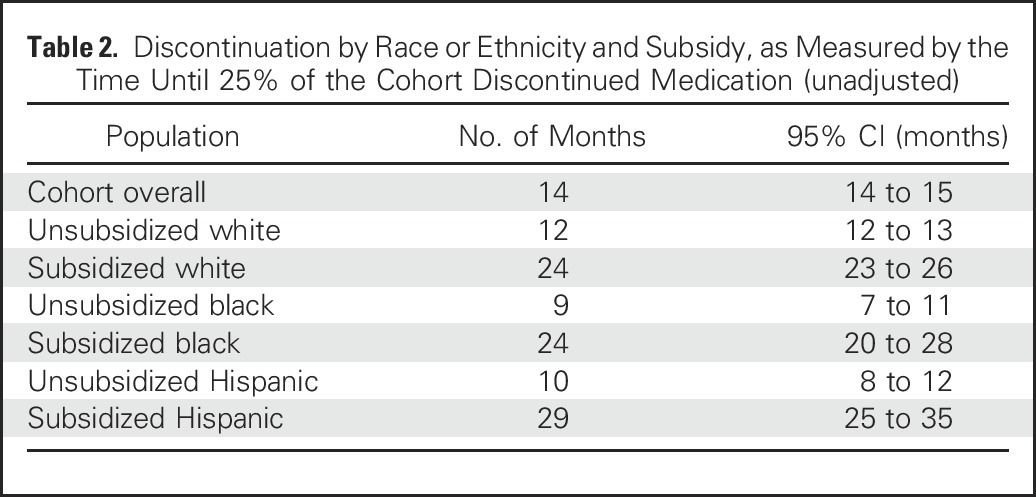
The differences in persistence by subsidy receipt were also large (Fig 1B). In analyses stratified by subsidy and race or ethnicity, subsidized women in all three race or ethnicity groups had higher persistence than unsubsidized women in any group (Fig 1C and Table 2). Table 2 lists, within each race by subsidy group, the times by which 25% of the group discontinued hormonal therapy. Within the unsubsidized group, 25% of white women discontinued therapy by 12 months, whereas the corresponding time period was 9 months for black women and 10 months for Hispanic women. Within the subsidized group, 25% discontinuation times were 24 months for white and black women and 29 months for Hispanic women.
Table 2.
Discontinuation by Race or Ethnicity and Subsidy, as Measured by the Time Until 25% of the Cohort Discontinued Medication (unadjusted)
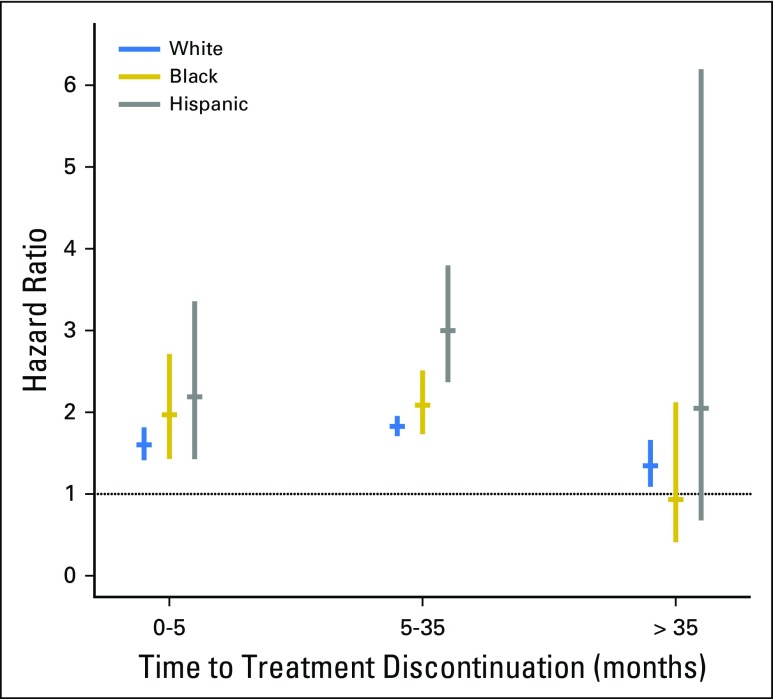
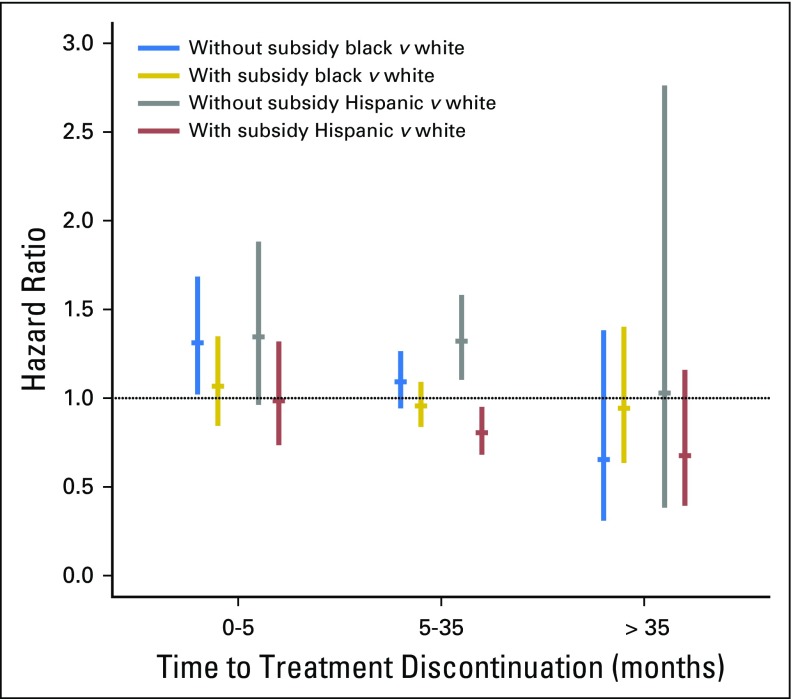
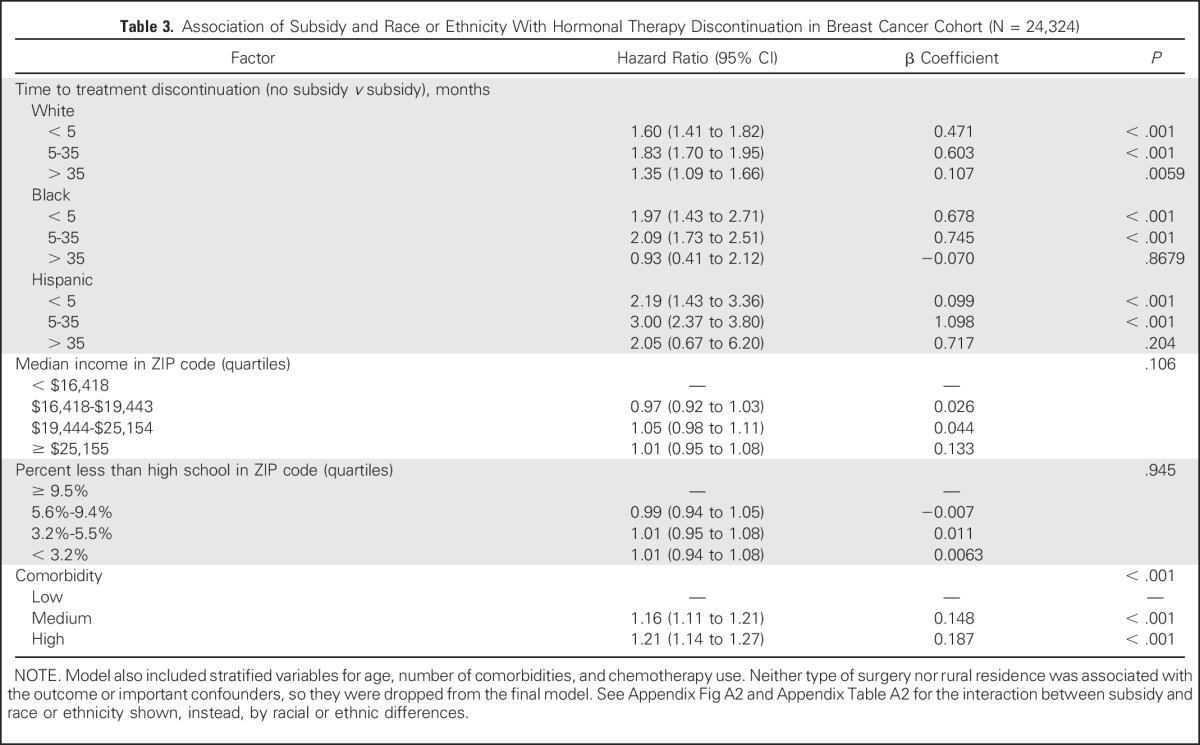
When analyses were adjusted for all other covariates including socioeconomic variables, not having a subsidy was associated with an increased likelihood (hazard) of discontinuation for women in all three race or ethnicity groups through 35 months, with a hazard ratio (HR) from 1.6 to 3.0 for all groups (Table 3 and Appendix Fig A1, online only). Furthermore, among unsubsidized women, there were statistically significant disparities in discontinuation (compared with white women) for black women during the first 5 months (HR, 1.31; 95% CI, 1.02 to 1.68) and for Hispanic women between 5 and 35 months (HR, 1.32; 95% CI, 1.10 to 1.58; Appendix Table A2 and Appendix Fig A2, online only). In contrast, among subsidized women, disparities were either not present or, for Hispanic women between 5 and 35 months (HR, 0.80; 95% CI, 0.68 to 0.95), actually reversed. Detailed description of results over time and differences between Hispanic and black women is provided in the Appendix (online only).
Table 3.
Association of Subsidy and Race or Ethnicity With Hormonal Therapy Discontinuation in Breast Cancer Cohort (N = 24,324)
Adherence
During the time periods in which patients persisted on hormonal therapy, there was no difference in the unadjusted probability of adherence (MPR ≥ 80%) by racial or ethnic group. However, the probability of adherence was substantially higher among subsidized than unsubsidized patients (odds ratio, 1.30; 95% CI, 1.25 to 1.35). Similar to the persistence analyses, both unadjusted comparisons of the six subsidy-race groups and an adjusted model (Appendix Table A3, online only) showed that subsidized women in all three race or ethnicity groups had higher adherence than all three unsubsidized groups. However, the size of the effect varied by race (P for interaction = .02), with the smallest effect among black women.
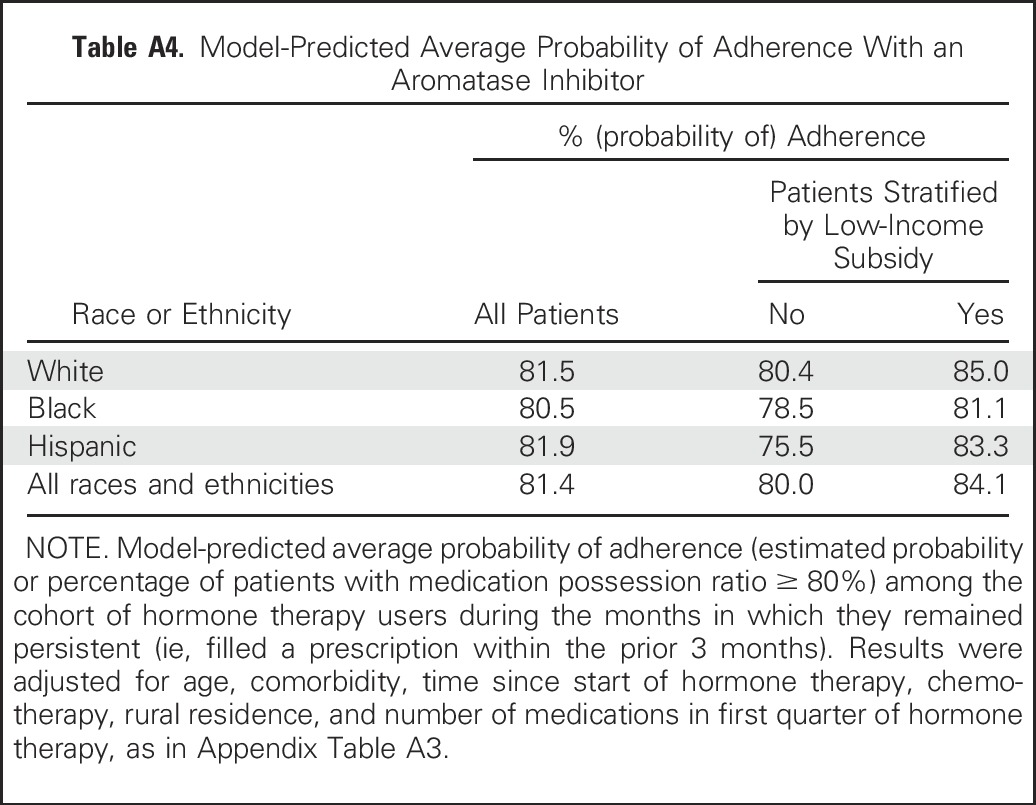
To further illustrate adherence differences between groups, we computed model-based (adjusted) probabilities of adherence. As shown in Appendix Table A4 (online only), an average of 80.0% of unsubsidized women were adherent, whereas 84.1% of subsidized women were adherent, but the differences between unsubsidized and subsidized women varied by race. Because the structure of the Medicare D program required unsubsidized patients to pay a much higher proportion of their medications once they passed a coverage gap (donut hole), we also examined potential differences in subsidy effects during time periods where patients were in the Medicare D coverage gap. The probability of adherence was 2.7% lower overall (ranging from 2.5% to 3.0% among the three race or ethnicity groups) for unsubsidized patients during the coverage gap.
DISCUSSION
In this national cohort of older Medicare enrollees with breast cancer, the majority of black and Hispanic women received the Medicare Part D Low-Income Subsidy. Unsubsidized women were 60% to 200% more likely to discontinue hormonal therapy in the first 35 months compared with the Low-Income Subsidy recipients of the same race or ethnicity. This was the case even after adjustment for socioeconomic status of their residence. In addition, there were racial and ethnic disparities in persistence among unsubsidized patients that were not present or were actually reversed among the subsidized patients.
Our study’s findings regarding the relatively large difference in persistence and adherence between subsidized and unsubsidized women are consistent with large reductions in out-of-pocket costs for aromatase inhibitors with the subsidy. In an earlier study in a similar Medicare D sample, we reported 90-day median out-of-pocket costs of $106 to $183 for unsubsidized patients on the aromatase inhibitor anastrozole compared with less than $10 for patients with a subsidy.10 In another breast cancer study that included commercially insured patients, persistence was 18% to 28% lower in patients who paid more than $90 for a 90-day medication supply. In survey studies, patients report that cancer treatments are large financial burdens,23 causing them to sell assets or declare bankruptcy. They also report delaying or forgoing medical care, including prescription refills.24,25
Our study adds to earlier work through our policy-relevant finding that the poor patients who received Medicare D subsidies had higher persistence than unsubsidized patients, who, on average, have substantially higher income and assets. There has been surprisingly little evidence in oncology that there are larger effects of cost-reducing policies for lower income than higher income patients,26 perhaps in part because such studies would have to contend with the potentially larger impact of small copays on low-income patients. For example, even copays of $1 to $3 have been shown to reduce use of and adherence to medications among Medicaid cohorts.27-29 Nonetheless, our findings regarding the subsidy benefits for patients overall are consistent with other recent studies in noncancer Medicare D populations, none of which reported results by race or ethnicity, in which subsidy recipients had higher adherence to medications for chronic conditions.17,30,31
This study’s findings regarding the presence of racial and ethnic disparities in persistence among unsubsidized patients and the absence of these disparities (or even, for Hispanic women, reversal) among low-income subsidized patients are, to our knowledge, novel. These findings suggest that income- and net worth–based subsidies can benefit patients of all races or ethnicities at least equally and may even benefit nonwhite (particularly Hispanic) women more. These findings are consistent with an earlier study, in which black women with commercial insurance were 24% less likely to be adherent than white women even after accounting for a number of nonfinancial factors, but when adjustment was made for net worth and copays, racial differences were no longer significant.32 Our study adds an important element to that earlier work, because their reports of an association between net worth and adherence and the relationships between race and net worth did not guarantee that low-income patients provided with a subsidy would adhere up to the level of higher income patients. Barriers such as poor transportation and lack of social capital play larger roles among populations with lower income and would not necessarily be improved with reductions in out-of-pocket costs.33 Furthermore, low-income and nonwhite patients have been shown to encounter more communication barriers and to be treated at lower quality hospitals.34 Despite these other potential barriers, our results regarding the lack of racial or ethnic persistence disparities among subsidized patients, coupled with the high enrollment in subsidies by patients of color, suggest that subsidies on the basis of economic status have the potential to reduce racial or ethnic disparities.
Our study had several limitations. The study was observational, and it is possible that factors other than costs differed between subsidy recipients and nonrecipients. However, on the basis of prior studies, most such factors (eg, transportation difficulties, greater sensitivities to small copays) would be expected to bias in the opposite direction of our findings. Although our adherence findings were generally similar to our findings for discontinuation, the smaller effect of the subsidy for black patients raises concerns that improvement for populations overall could actually increase some disparities. Given the importance of disparities in breast cancer, this should be explored further in future studies. Our cohort was identified through an initial prescription for an aromatase inhibitor, and we could not examine the previously described disparities in initial use.34 Although MPRs highly correlate with adherence rates,10 they are an imperfect proxy for actual use. We did not have information on either reasons for discontinuation or the stages of patients’ cancers. We have previously shown that nearly all of the patients are likely to have stage I to III disease given that patients with stage IV are less likely to have surgery.14 Finally, we were limited to ZIP code–level variables for socioeconomic status. Because our entitlement-identified patients actually had better persistence than a group made up of mostly wealthier patients, future studies should examine individual-level socioeconomic status to better understand the complex interactions between race, ethnicity, socioeconomic status, and medication use.
Our study supports the potential for policy interventions to improve equity in cancer outcomes. The substantially higher persistence and adherence among women of all three race or ethnicity groups enrolled onto the Medicare D Low-Income Subsidy should lead to further efforts to ensure all eligible women are enrolled. Furthermore, legislative and advocacy efforts should focus on lowering out-of-pocket costs for younger women. Given the high costs of oral oncologic and supportive medications, the impact on disparities of other initiatives to reduce out-of-pocket costs deserves urgent study.
Appendix
Persistence Models and Their Results
The association of persistence with race or ethnicity and income status was assessed in a survival model (Table 3) that used interaction terms between those variables. Therefore, the effect of the interaction term from this same model is shown as either a hazard ratio of discontinuation (without v with subsidy) for each race or ethnic group (Table 3 and Appendix Fig A1) or a hazard ratio between two racial or ethnic groups (eg, discontinuation hazards for blacks v whites) within each subsidy status (Appendix Fig A2 and Table A2).
Results for the discontinuation time periods of 0 to 5 and 5 to 35 months, as shown in these tables and figures, are similar to each other except for the differences noted in the main text. In summary, among women without a subsidy, black women were more likely than white women to discontinue treatment in months 0 to 5, and Hispanics were more likely to discontinue treatment during months 5 to 35. Among women with a subsidy, Hispanic women were less likely to discontinue treatment than white women during months 5 to 35. After 35 months, however, discontinuation differed by subsidy status only for white women (although CIs were large). Furthermore, after 35 months, there were no differences by race or ethnicity in discontinuation either in subsidized or unsubsidized women. Comparisons between black and Hispanic women for all time periods showed no clinically or statistically significant differences.
Fig A1.
Discontinuation hazard ratios for women without a subsidy versus women with a subsidy by race or ethnicity. The disparities in discontinuation, measured as a hazard ratio of discontinuation, for each race group in women with versus without a subsidy are shown. Results are derived from the model shown in Table 3. Because discontinuation was not proportional by race or ethnicity, results are shown for three time periods (see Methods for further details).
Fig A2.
Discontinuation hazard ratios for black and Hispanic women compared with white women by subsidy status. This disparities in discontinuation, measured as the comparison (hazard ratio) between race or ethnicity groups for women with and without subsidy, are shown. Results are derived from the adjusted model shown in Appendix Table A2. Results with CI crossing 1.0 are consistent with no statistically significant difference in discontinuation by race or ethnicity. Because discontinuation was not proportional by race or ethnicity, results are shown for three time periods (see Methods for further details).
Table A1.
Medicare Part D Low-Income Subsidy Eligibility Calculation for an Individual Enrollee or a Couple
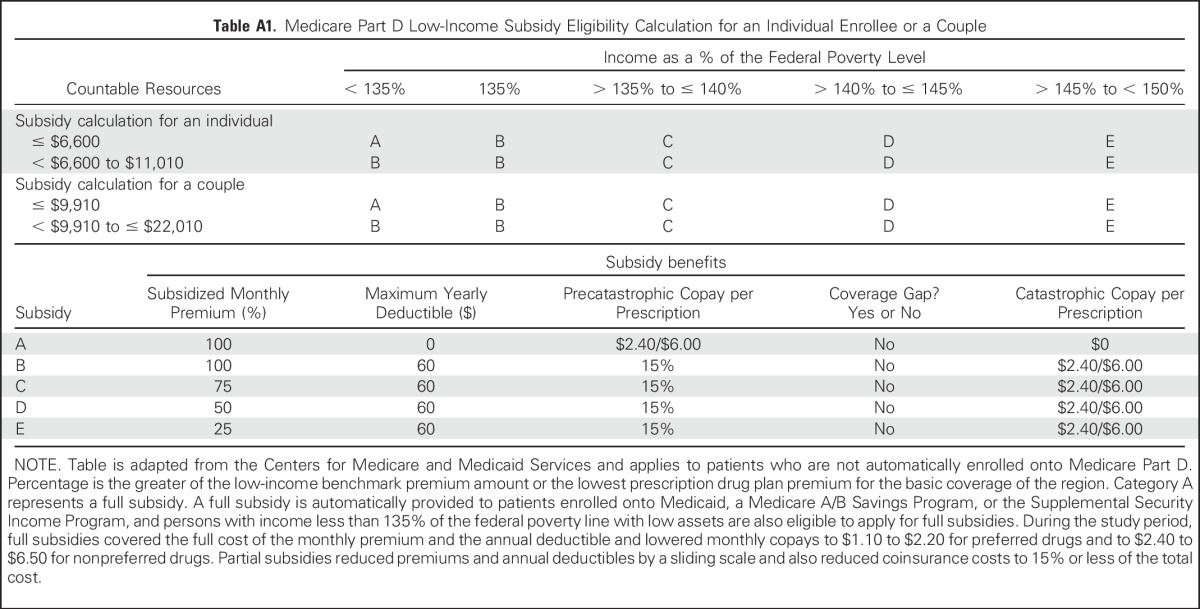
Table A2.
Disparities in Discontinuation by Race or Ethnicity
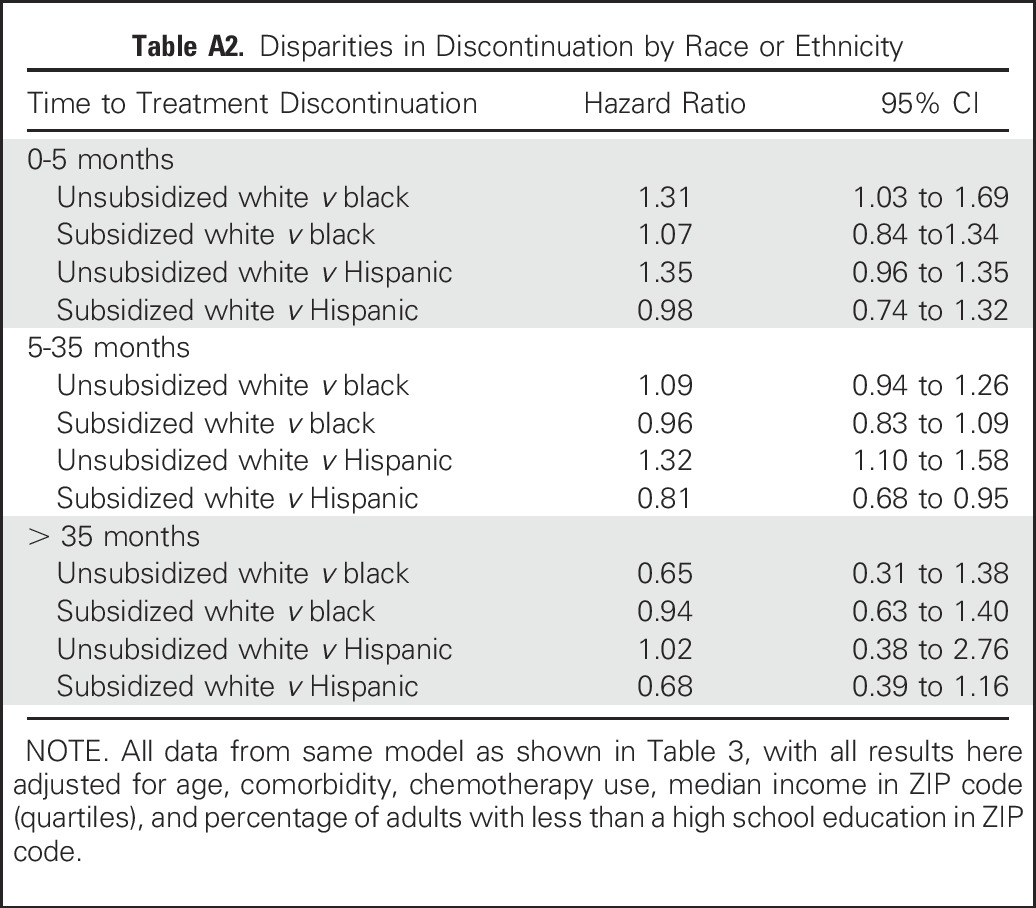
Table A3.
Factors Associated With Adherence Among a Cohort of Patients With Breast Cancer in 2006 and 2007 (N = 25,511)
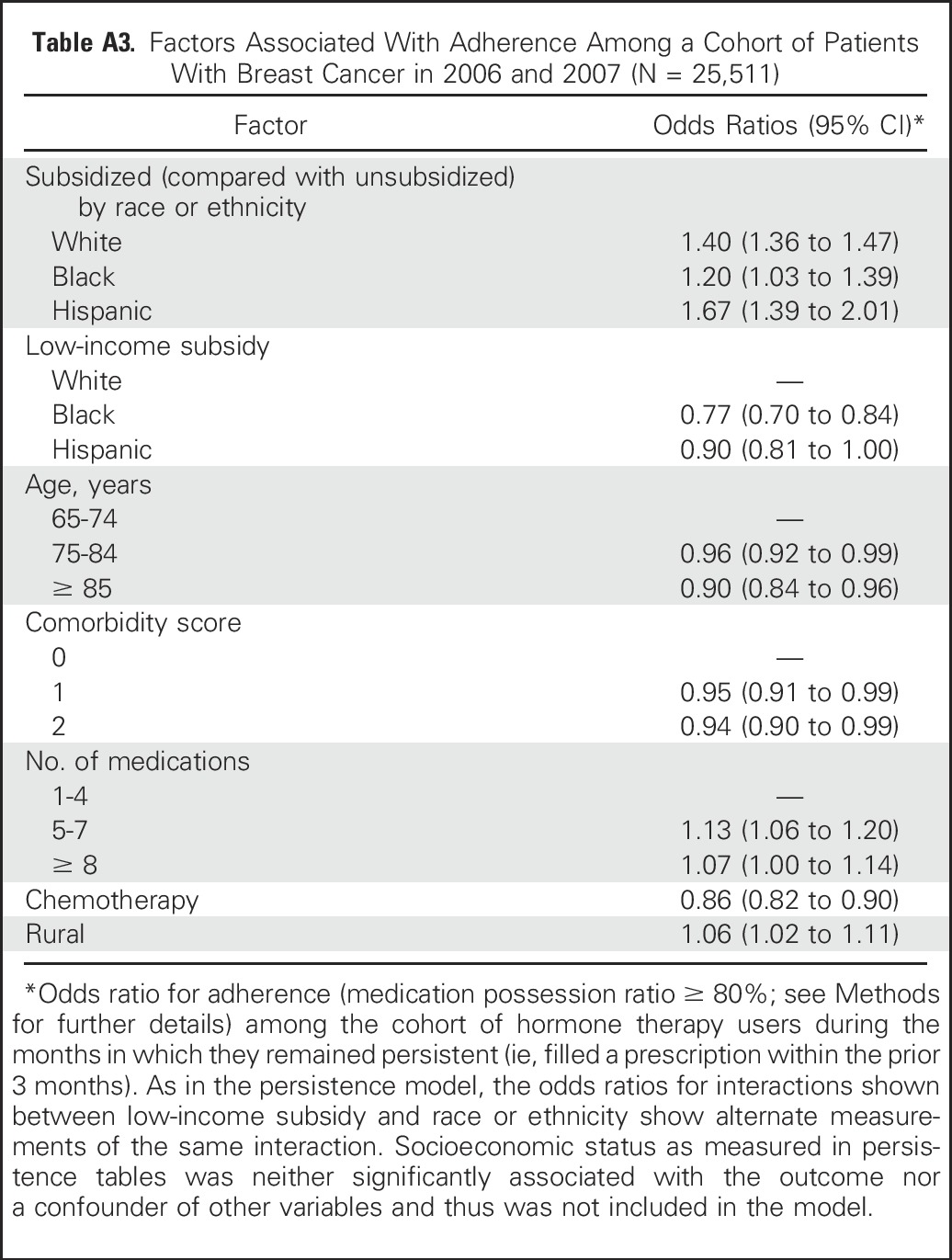
Table A4.
Model-Predicted Average Probability of Adherence With an Aromatase Inhibitor
Footnotes
Supported by the National Institutes of Health (Grant No. R01 CA127648 awarded to A.B.N. and Grant No. R01 CA170945 awarded to A.B.N. and Liliana E. Pezzin) and the American Cancer Society (Grant No. RSG-11-098-01-CPHPS awarded to J.M.N.).
Presented in part at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014; and the 3nd Annual ASCO Quality Care Symposium, Boston, MA, October 17-18, 2014.
Sponsors did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the article. J.M.N. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Alana Biggers, John Charlson, Ann B. Nattinger, Purushottam W. Laud, Joan M. Neuner
Financial support: Joan M. Neuner
Administrative support: Alicia J. Smallwood
Provision of study materials or patients: Joan M. Neuner
Collection and assembly of data: Alana Biggers, Joan M. Neuner
Data analysis and interpretation: Alana Biggers, Yushu Shi, John Charlson, Elizabeth C. Smith, Alicia J. Smallwood, Purushottam W. Laud
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Medicare D Subsidies and Racial Disparities in Persistence and Adherence With Hormone Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Alana Biggers
No relationship to disclose
Yushu Shi
No relationship to disclose
John Charlson
Research Funding: Eli Lilly (Inst), CytRx Corporation (Inst)
Elizabeth C. Smith
No relationship to disclose
Alicia J. Smallwood
No relationship to disclose
Ann B. Nattinger
No relationship to disclose
Purushottam W. Laud
No relationship to disclose
Joan M. Neuner
No relationship to disclose
REFERENCES
- 1.Kohler BA, Sherman RL, Howlader N, et al. Annual report to the nation on the status of cancer, 1975-2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J Natl Cancer Inst. 2015;107:djv048. doi: 10.1093/jnci/djv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandelblatt JS, Sheppard VB, Neugut AI. Black-white differences in breast cancer outcomes among older Medicare beneficiaries: Does systemic treatment matter? JAMA. 2013;310:376–377. doi: 10.1001/jama.2013.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society: Cancer Facts & Figures 2011. Atlanta, GA, American Cancer Society, 2011 [Google Scholar]
- 4.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Davies C, Godwin J, et al: Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 378:771-784, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 6.Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26:549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 7.Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99:215–220. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 8.Bhatta SS, Hou N, Moton ZN, et al. Factors associated with compliance to adjuvant hormone therapy in black and white women with breast cancer. Springerplus. 2013;2:356. doi: 10.1186/2193-1801-2-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Livaudais JC, Lacroix A, Chlebowski RT, et al. Racial/ethnic differences in use and duration of adjuvant hormonal therapy for breast cancer in the Women’s Health Initiative. Cancer Epidemiol Biomarkers Prev. 2013;22:365–373. doi: 10.1158/1055-9965.EPI-12-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuner JM, Kamaraju S, Charlson JA, et al. The introduction of generic aromatase inhibitors and treatment adherence among Medicare D enrollees. J Natl Cancer Inst. 2015;107:djv130. doi: 10.1093/jnci/djv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29:2534–2542. doi: 10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friese CR, Pini TM, Li Y, et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat. 2013;138:931–939. doi: 10.1007/s10549-013-2499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley CJ, Dahman B, Jagsi R, et al. Prescription drug coverage: Implications for hormonal therapy adherence in women diagnosed with breast cancer. Breast Cancer Res Treat. 2015;154:417–422. doi: 10.1007/s10549-015-3630-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nattinger AB, Laud PW, Bajorunaite R, et al. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39:1733–1749. doi: 10.1111/j.1475-6773.2004.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arday SL, Arday DR, Monroe S, et al. HCFA’s racial and ethnic data: Current accuracy and recent improvements. Health Care Financ Rev. 2000;21:107–116. [PMC free article] [PubMed] [Google Scholar]
- 16.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: Methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 17.Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 18.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Krieger N. Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geronimus AT, Bound J. Use of census-based aggregate variables to proxy for socioeconomic group: Evidence from national samples. Am J Epidemiol. 1998;148:475–486. doi: 10.1093/oxfordjournals.aje.a009673. [DOI] [PubMed] [Google Scholar]
- 23.Pezzin LE, O’Niel MB, Nattinger AB. The economic consequences of breast cancer adjuvant hormonal treatments. J Gen Intern Med. 2009 (suppl 2);24:S446–S450. doi: 10.1007/s11606-009-1079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsey S, Blough D, Kirchhoff A, et al. Washington State cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Aff (Millwood) 2013;32:1143–1152. doi: 10.1377/hlthaff.2012.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kent EE, Forsythe LP, Yabroff KR, et al. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119:3710–3717. doi: 10.1002/cncr.28262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: Associations with medication and medical utilization and spending and health. JAMA. 2007;298:61–69. doi: 10.1001/jama.298.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuart B, Zacker C. Who bears the burden of Medicaid drug copayment policies? Health Aff (Millwood) 1999;18:201–212. doi: 10.1377/hlthaff.18.2.201. [DOI] [PubMed] [Google Scholar]
- 28.Soumerai SB, Avorn J, Ross-Degnan D, et al. Payment restrictions for prescription drugs under Medicaid: Effects on therapy, cost, and equity. N Engl J Med. 1987;317:550–556. doi: 10.1056/NEJM198708273170906. [DOI] [PubMed] [Google Scholar]
- 29.Reeder CE, Nelson AA. The differential impact of copayment on drug use in a Medicaid population. Inquiry. 1985;22:396–403. [PubMed] [Google Scholar]
- 30.Riley GF, Warren JL, Harlan LC, et al. Endocrine therapy use among elderly hormone receptor-positive breast cancer patients enrolled in Medicare Part D. Medicare Medicaid Res Rev. 2011;1:E1–E26. doi: 10.5600/mmrr.001.04.a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P, McElligott S, Bergquist H, et al. Effect of the Medicare Part D coverage gap on medication use among patients with hypertension and hyperlipidemia. Ann Intern Med. 2012;156:776–784, W-263-W-269. doi: 10.7326/0003-4819-156-11-201206050-00004. [DOI] [PubMed] [Google Scholar]
- 32.Hershman DL, Tsui J, Wright JD, et al. Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. J Clin Oncol. 2015;33:1053–1059. doi: 10.1200/JCO.2014.58.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawachi I, Kennedy BP, Glass R. Social capital and self-rated health: A contextual analysis. Am J Public Health. 1999;89:1187–1193. doi: 10.2105/ajph.89.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu XC, Lund MJ, Kimmick GG, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30:142–150. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]