Abstract
Purpose
There is substantial concern surrounding affordability of orally administered anticancer therapies, particularly for Medicare beneficiaries. We examined rates of initiation and adherence to tyrosine kinase inhibitors (TKIs) among Medicare beneficiaries with chronic myeloid leukemia (CML) with and without cost-sharing subsidies. We selected TKIs given their effectiveness and strong indication for use among patients diagnosed with CML.
Patients and Methods
Using SEER-Medicare data, we identified individuals diagnosed with CML from 2007 to 2011. We used Cox proportional hazards regression to assess time from diagnosis to TKI initiation. We used generalized estimating equations to examine treatment initiation within 180 days and TKI adherence among initiators. We defined adherence as at least 80% of days covered during the 6 months after TKI initiation.
Results
Among 393 individuals diagnosed with CML from 2007 to 2011, 68% initiated TKI treatment within 180 days after diagnosis. In multivariate analysis, individuals with cost-sharing subsidies, younger age, lower comorbidity, and later year of diagnosis were significantly more likely to initiate TKIs. Among TKI initiators, 61% were adherent; adherence was lower for individuals age 80 years or older versus 66 to 69 years.
Conclusion
Only 68% of Medicare beneficiaries with CML initiated TKI therapy within 6 months of diagnosis. Delayed initiation among individuals without cost-sharing subsidies suggests that out-of-pocket costs may be a barrier to timely initiation of therapy among individuals diagnosed with CML.
INTRODUCTION
There has been a dramatic increase in the number of orally administered oncology treatments in recent years.1,2 With this transition away from office-based infusions, it is important to identify potential gaps in adherence to therapies, because the risks for primary nonadherence (never filling) and secondary nonadherence (discontinuing or using less supply than expected) increase when patients are obtaining drugs outside of the infusion center. There is a broad literature documenting nonadherence to medications across many conditions, including some cancers.3-5 However, most prior work examining medication adherence among patients with cancer has examined use of relatively affordable treatments, such as endocrine therapy,6 or use among privately insured individuals with relatively generous prescription drug coverage.7-9 Furthermore, because of the limitations of available data, most population-based studies of medication adherence have evaluated use among patients who had initiated therapy and have not considered factors associated with never starting therapy. Given the expense of newer oral oncologic treatments (most of which are priced at ≥ $10,000 per month), out-of-pocket costs for initiating therapy may be high and could act as a barrier to starting treatment.10
In the United States, nearly all adults older than age 65 years qualify for enrollment in Medicare, the national insurance program. On reaching Medicare eligibility, beneficiaries are enrolled in hospital coverage at no charge, and they have the option to purchase coverage for outpatient medical and prescription drug benefits separately. Recent work has demonstrated that individuals insured through the Medicare drug benefit program (ie, Part D) may face out-of-pocket costs of nearly $3,000 when initiating tyrosine kinase inhibitors (TKIs),10,11 which potentially limits access to treatments.12,13 This high upfront cost is a result of the Medicare Part D benefit design, which requires patients to pay a higher proportion of medication costs until they reach a catastrophic spending threshold ($4,850 out of pocket in 2016), after which patients pay 5% of the approximate $11,000 monthly drug costs, or $550. Studying patients with chronic myeloid leukemia (CML) can provide insight into the impact of expected out-of-pocket spending on use of oral anticancer medications among individuals insured through Medicare prescription drug plans. Specifically, the average age at diagnosis of CML is 64 years (Medicare eligibility begins at age 65 years), TKIs are highly recommended for individuals with Philadelphia chromosome–positive disease (nearly all of CML diagnoses), and individuals using these therapies are expected to take them for a long period of time. In addition, clinical guidelines recommend initiating a TKI immediately after a diagnosis of CML.14,15 Finally, low adherence to TKI therapy can decrease response to treatment, which can result in patients requiring stem-cell transplantation, worse clinical outcomes, and potentially shorter life expectancy.16
The objectives of this study were to estimate rates of TKI initiation among Medicare beneficiaries diagnosed with CML between 2007 and 2011 and to evaluate factors associated with initiation of and adherence to TKIs. Given the critical role of TKIs for patients with CML, it is important to understand whether cost sharing or other patient- or provider-level factors act as a barrier to treatment use.
PATIENTS AND METHODS
Study Sample and Data Sources
We used SEER-Medicare linked data for this study. The SEER-Medicare data combine data obtained from cancer registries covering 28% of the US population and Medicare administrative claims for patients age older than 65 years who are included in the registry and enrolled in traditional (ie, fee-for-service) Medicare plans.17,18 The linked data source broadly represents the health care experiences of older adults in the United States who are diagnosed with incident cancers and who are insured through traditional fee-for-service Medicare plans. Importantly, claims for individuals enrolled in Medicare health maintenance organizations are available (approximately 30% of Medicare beneficiaries in the United States as of 2015).19
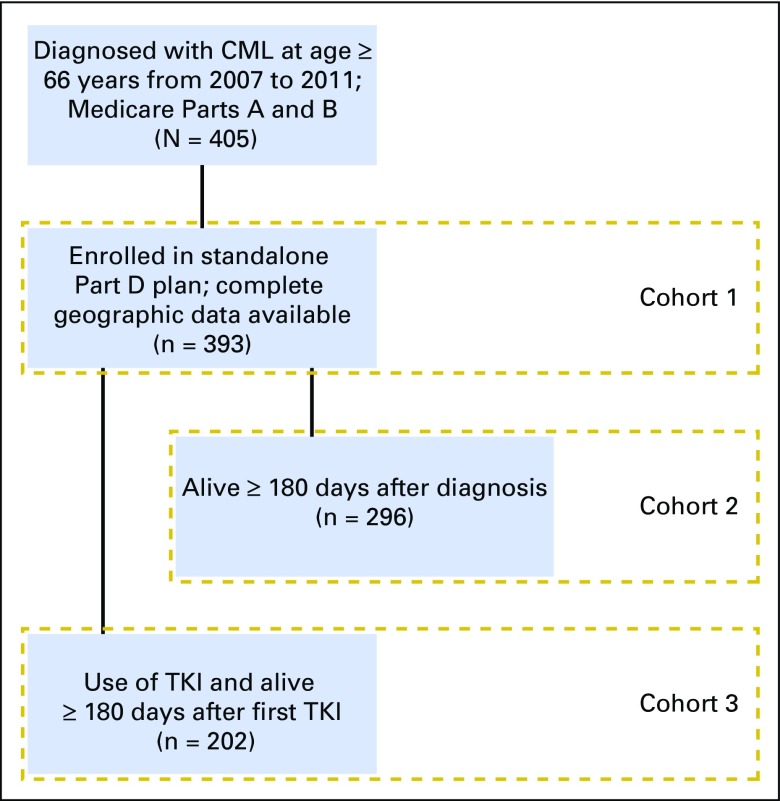
We identified individuals age 66 years or older with a diagnosis of Philadelphia chromosome–positive CML (International Classification of Diseases for Oncology [third edition] code 9863 or 9875) from 2007 through 2011 who were continuously enrolled in Medicare inpatient and outpatient medical coverage for 1 year before diagnosis (N = 405). We excluded individuals who were not continuously enrolled in a prescription drug plan (Part D) after diagnosis through death or through 1 year after initiation of treatment or who had missing values for geographic factors (census tract poverty level and urbanicity; n = 12), resulting in a cohort of 393 patients (cohort 1; Fig 1) for the analysis focused on time to TKI initiation. For evaluating any use of a TKI within 180 days after diagnosis we further restricted our sample to individuals alive during the full 180-day follow-up period (n = 296; cohort 2; Fig 1). Finally, for examining adherence to TKIs, we restricted analysis to individuals who ever initiated a TKI and were alive for 180 days after their first TKI fill (n = 202; cohort 3; Fig 1).
Fig 1.
CONSORT diagram of populations included in analyses. TKI, tyrosine kinase inhibitor.
TKI Initiation
We identified TKIs available for treating CML during our study period: imatinib, dasatinib, and nilotinib. We evaluated treatment initiation in two ways. First, we evaluated rates of and time to TKI initiation after CML diagnosis (diagnosis date was estimated as the first day of the month of diagnosis) among the full cohort, treating death as a competing risk. Second, we evaluated initiation within 180 days of diagnosis among those who survived for at least 180 days after diagnosis to avoid including individuals who may have been close to death and thus were less likely to benefit from therapy.
TKI Adherence
Among individuals who initiated TKIs, we used the days of supply and dispensing date to create a supply diary for whether a person had prescription drug supply available during each day of follow-up. Because patients may switch from one TKI to another if they are intolerant to one TKI, all TKIs were treated as interchangeable, so if a person had overlapping supply of more than one TKI (eg, he or she filled a prescription for nilotinib before exhausting his or her supply of imatinib), then use of the second medication was assumed to start the day after the end of the prior fill. We summarized the proportion of days covered (PDC) by dividing the number of days covered during the 180-day period after TKI initiation by 180 days. Individuals were considered adherent if their PDC was greater than 80%.
Covariates
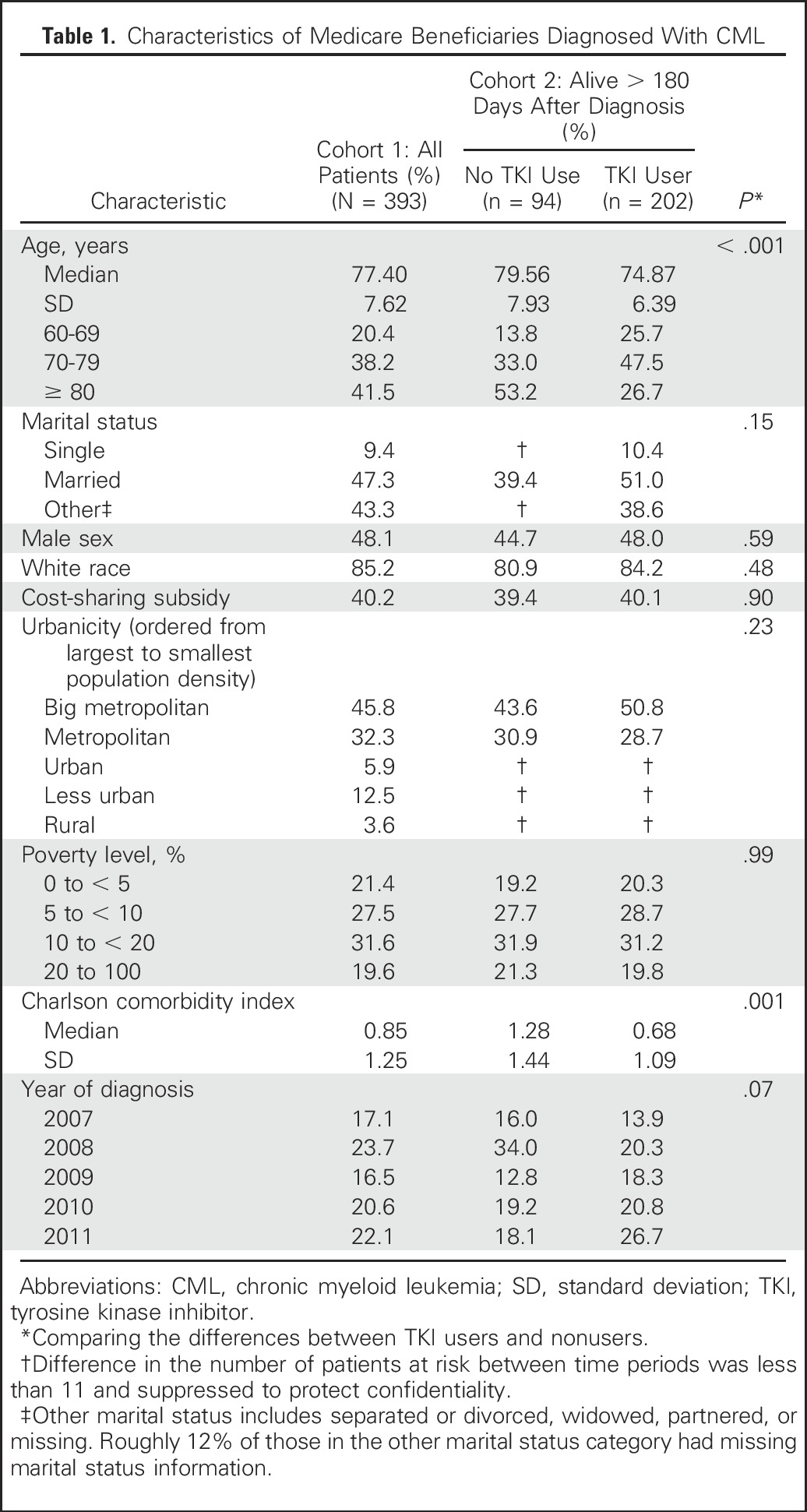
Covariates included age at diagnosis, marital status, race, comorbidity (measured during the year before diagnosis using the Klabunde adaptation of the Charlson comorbidity index20), year of diagnosis, poverty level (based on census tract of residence from US Census data), and urbanicity. Individuals were classified as receiving cost-sharing subsidies if they were eligible for both Medicaid (US health insurance program for low-income individuals) and Medicare or if they had a low-income subsidy for Medicare Part D drug costs, either of which would result in significantly lower out-of-pocket costs for beneficiaries. Because out-of-pocket expenses for drugs were similar for both groups, we combined these groups and identified them as receiving cost-sharing subsidies. Variables were categorized as listed in Table 1.
Table 1.
Characteristics of Medicare Beneficiaries Diagnosed With CML
Statistical Analysis
Time to initiation of TKIs.
To determine factors associated with TKI initiation, we tested unadjusted differences using a Wilcoxon rank sum test. Our adjusted analysis used multivariable Cox proportional hazards regression and allowed for competing risks resulting from death. From these models, we present adjusted hazard ratios (HRs) with bootstrap-estimated 95% CIs.
Treatment initiation and adherence.
Among the patients who survived for 180 days or longer, we estimated the risk of not initiating treatment within 180 days of diagnosis. We also estimated the risk of being nonadherent to therapy among the subset of patients who initiated treatment. For both models, we used generalized estimating equations with log links, Poisson distributions, and robust SEs,21,22 and we included all covariates listed in Covariates section. Adjusted risk ratios (RRs) with bootstrapped 95% CIs were estimated from each model. Statistical significance was assumed as P less than .05. The study protocol was considered exempt by the University of North Carolina Institutional Review Board.
RESULTS
Characteristics of the 393 individuals included in our cohort are listed in Table 1. The mean age at diagnosis was 77 years (standard deviation, 7.6), with more than 40% of the population being older than age 80 years. The majority of the population was white (85%), and 47% were married. Approximately 40% of individuals were classified as receiving cost-sharing subsidies.
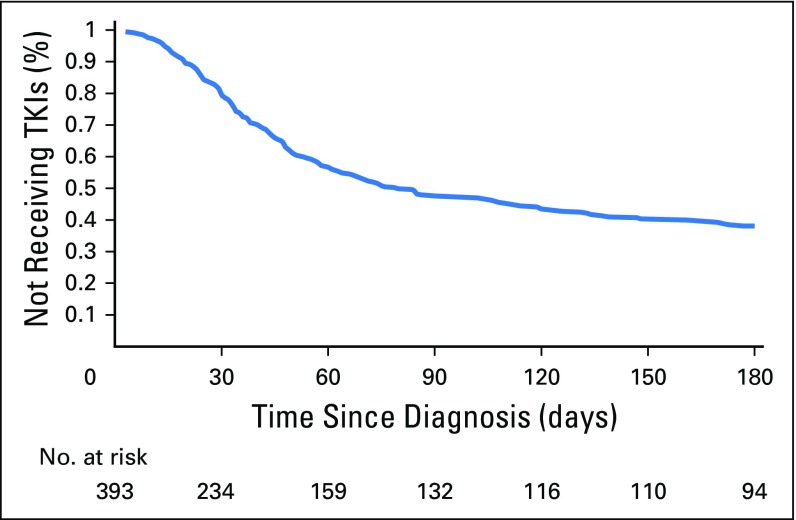
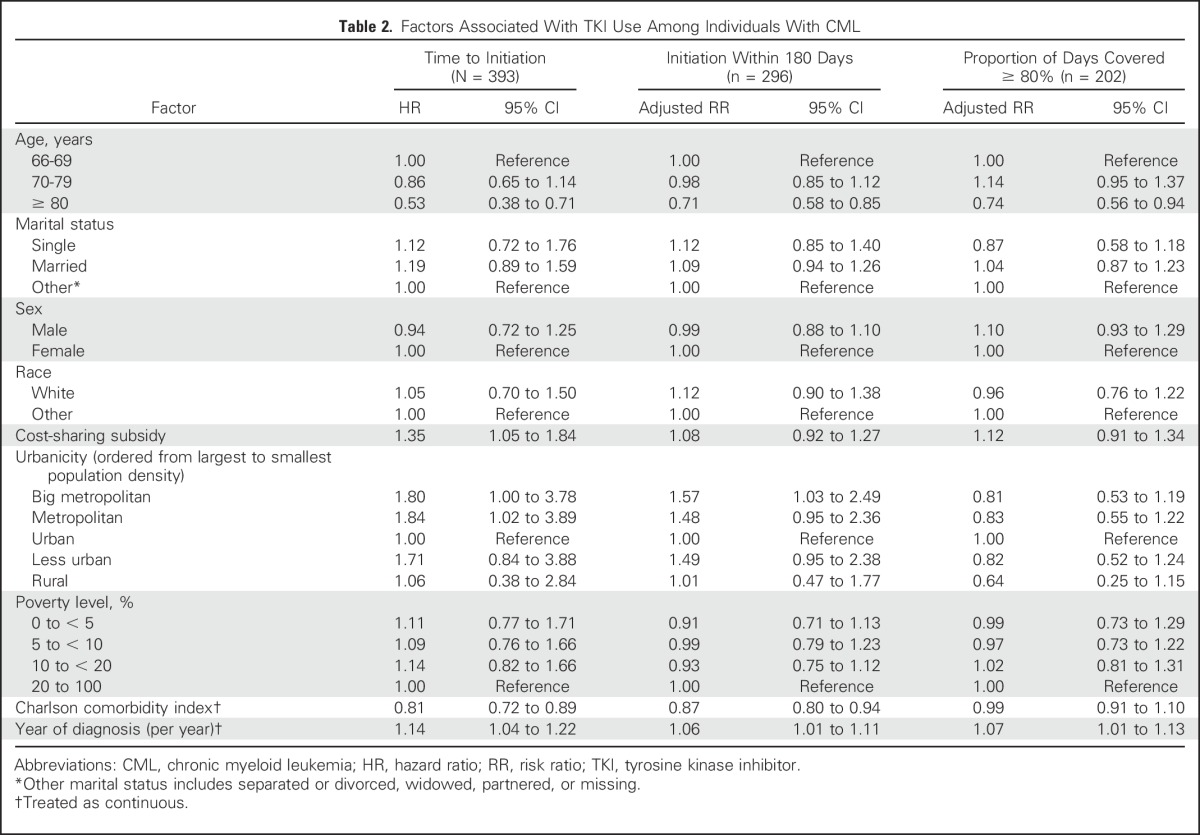
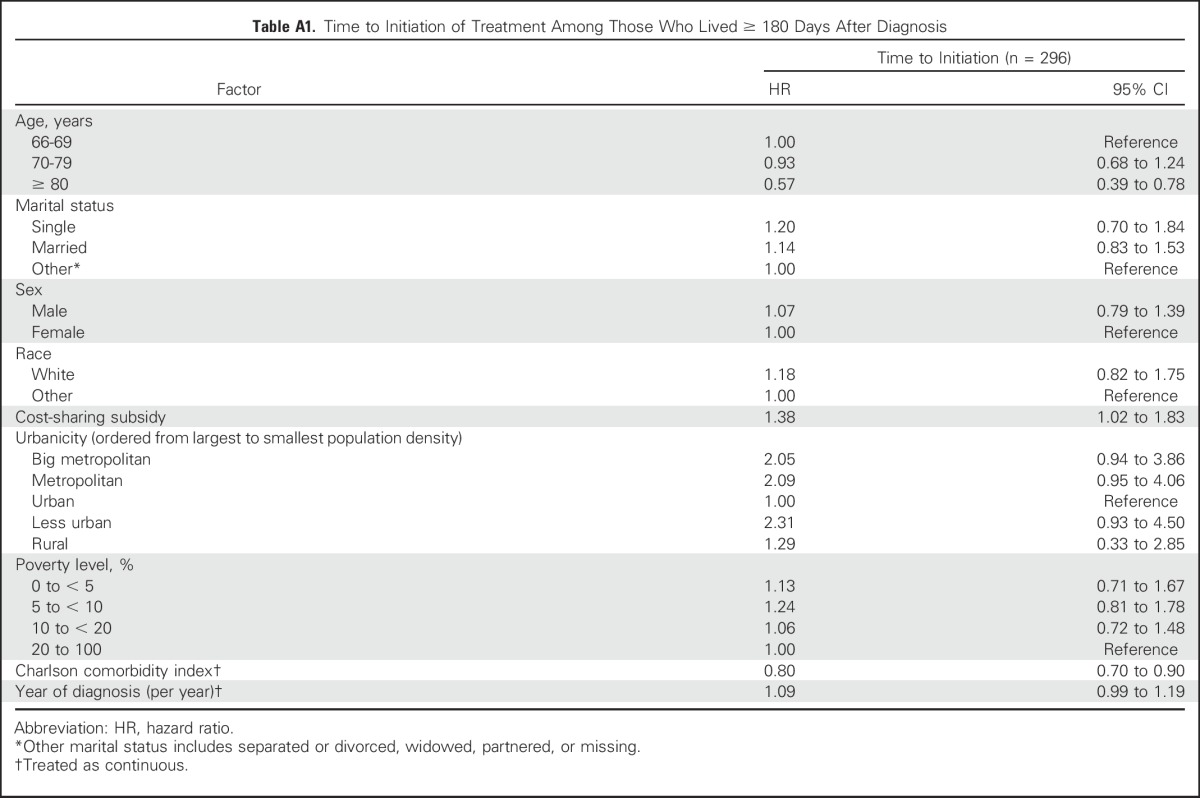
The unadjusted time to TKI initiation within 180 days after diagnosis is presented in Figure 2. The median time to initiation among initiators was 75 days. Factors associated with earlier treatment initiation were receipt of cost-sharing subsidies (HR, 1.35; 95% CI, 1.05 to 1.84), being diagnosed in more recent years (HR, 1.14; 95% CI, 1.04 to 1.22), and living in a big metropolitan area (HR, 1.80; 95% CI, 1.00 to 3.78) or metropolitan area (HR, 1.84; 95% CI, 1.02 to 3.89) compared with an urban area. Those with higher levels of comorbidity (HR, 0.81; 95% CI, 0.72 to 0.89) and those age older than 80 versus younger than 70 years (HR, 0.53; 95% CI, 0.38 to 0.72) experienced later initiation of TKIs (Table 2). Analyses were repeated among the cohort of patients who lived through 180 days after diagnosis, and results were consistent with the primary analysis (Appendix Table A1, online only).
Fig 2.
Unadjusted time to tyrosine kinase inhibitor (TKI) initiation among Medicare beneficiaries with chronic myeloid leukemia.
Table 2.
Factors Associated With TKI Use Among Individuals With CML
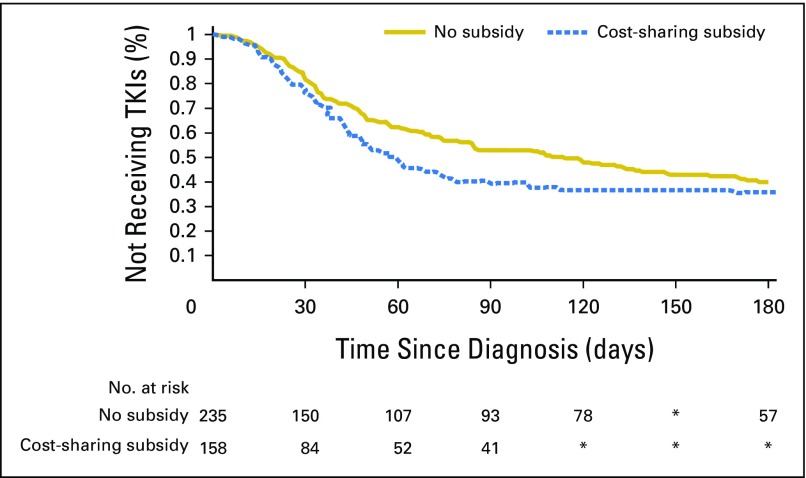
Because of the difference observed between those who did and did not receive cost-sharing subsidies, we examined the unadjusted difference in time to initiation of TKIs (Fig 3). We found the median time to initiation of treatment after CML diagnosis was 58 days among individuals who received cost-sharing subsidies and 108 days among beneficiaries who did not receive cost-sharing subsidies (P = .04).
Fig 3.
Unadjusted time to tyrosine kinase inhibitor (TKI) initiation among Medicare beneficiaries with chronic myeloid leukemia by receipt of cost-sharing subsidy. (*) Difference in the number of patients at risk between time periods was less than 11 and suppressed to protect confidentiality.
Initiation of TKIs Within 180 Days
Overall, 68.2% of patients with newly diagnosed CML initiated TKI therapy within 180 days after diagnosis. When restricting to patients who were alive during the full 180-day follow-up period, we found that year of diagnosis (adjusted RR, 1.06; 95% CI, 1.00 to 1.11) and living in a large metropolitan area versus an urban area were associated with increased use of TKIs (adjusted RR, 1.57; 95% CI, 1.03 to 2.49), and age older than 80 years compared with younger than age 70 years was associated with a reduced use of TKIs (adjusted RR, 0.71; 95% CI, 0.58 to 0.85; Table 2). When looking at use at 180 days, in contrast with the time-to-event analysis, the effect of cost-sharing subsidies on TKI initiation was not statistically significant (adjusted RR, 1.08; 95% CI, 0.92 to 1.27).
TKI Adherence
Among individuals who ever initiated TKIs, 61% were adherent (PDC > 80%) to therapy during the first 180 days post–TKI initiation. When examining factors associated with adherence, individuals age older than 80 years compared with those younger than age 70 years (adjusted RR, 0.74; 95% CI, 0.56 to 0.94) were less likely to adhere to therapy, and year of diagnosis was associated with higher adherence (adjusted RR, 1.07; 95% CI, 1.01 to 1.13; Table 2). No other patient factors were associated with adherence.
DISCUSSION
Medicare beneficiaries’ use of TKIs was far lower than expected, with approximately 30% of patients with newly diagnosed CML not initiating treatment within 6 months of diagnosis. Low-income beneficiaries who received cost-sharing subsidies for prescription drugs seemed to initiate treatment sooner than Medicare beneficiaries without cost-sharing subsidies. However, when restricting to those patients who lived for at least 180 days after diagnosis, there was no longer a statistically significant difference between individuals with and without copay subsidies for the probability of staring a TKI or in adherence among those who had initiated TKI treatment. Furthermore, we found that individuals diagnosed who were older than age 80 years (> 40% of the cohort) were less likely to initiate treatment and were less adherent to treatments.
During the study period of 2007 to 2011, there were three US Food and Drug Administration–approved treatments for CML (imatinib, dasatinib, and nilotinib). Imatinib was the only TKI approved for first-line use between 2007 and 2010, with the other TKIs approved for first-line use starting in 2010. Although these newer products were more expensive than imatinib at the time they were approved, estimated out-of-pocket spending for 1 year of therapy for patients using one of these drugs long term in 2010 would have been similarly high across products, ranging from $9,062 to $10,083.10 Furthermore, in multivariable adjusted models, we found that the time to TKI initiation was faster and adherence was higher across more recent years of our study, suggesting that changes in recommendations for first-line treatment over time did not negatively affect use of TKIs.
Prior work evaluating older patients’ ability to manage high health care costs has shown that roughly half of the elderly population has $13,800 or less in liquid assets available, and this amount decreases as patients age.23 Assuming a list price of $10,000 for a standard dose or supply of drug, Medicare beneficiaries without cost-sharing subsidies are expected to pay close to $3,000 to initiate their first month of therapy with a TKI and $550 for their remaining fills over 1 calendar year once they reach the catastrophic phase of their plan benefit (with 5% coinsurance for each fill after that point). It is possible that patients without prescription drug cost-sharing subsidies may delay initiating treatment as they work to obtain funds to cover these high upfront costs.10,24 Additionally, these individuals may also delay treatment initiation in the hope of receiving financial assistance from a patient assistance program or foundation. This may explain the longer time to initiation among individuals who were not receiving cost-sharing subsides. Specifically, differences in initiation between patients with and without cost-sharing subsidies were largest in the early postdiagnosis period and narrowed over time (Fig 3). Our findings are consistent with recent work that has also found low overall use of TKIs within the Medicare population and more generally that prescriptions with copays more than $500 are four times as likely to never be picked up from a pharmacy when compared with oral oncologics with lower copays.25
This study has several limitations. First, we compared TKI use and adherence among patients with and without cost-sharing subsidies. Historically, individuals with cost-sharing subsidies have had lower rates of initiation of therapy and similar or lower adherence to medications than individuals without such support.26-28 This may understate our findings, resulting in a conservative estimate of the impact of cost sharing in this setting. Additionally, we were unable to determine reasons for delaying or not initiating treatments, nor were we able to account for physician or patient preferences regarding use of TKIs. However, there are few, if any, clinical reasons for patients to delay initiation, and guidelines encourage a trial of TKIs among all individuals with CML. Furthermore, we have no reason to believe that there would be differences between patients’ need for therapy on the basis of their eligibility for cost-sharing assistance. Nevertheless, additional studies that collect information from patients and physicians will be important to understand barriers to initiation. Another limitation of this study is our reliance on International Classification of Diseases for Oncology (third edition) codes to identify patients with Philadelphia chromosome–positive CML. However, because nearly all CML cases are associated with the Philadelphia chromosome,29,30 this limitation is unlikely to have affected our findings. There are no validation studies that have empirically tested if this code completely captures all cases. Another limitation of this study is that less than 1% of patients who did not receive cost-sharing subsidies had low expected out-of-pocket spending at the time of initiating TKI therapy, and thus, we were unable to assess whether the relatively lower expected spending among this group led to higher TKI initiation rates. Furthermore, we were not able to discern if patients were receiving free samples, which may have delayed their observed start date. Finally, we were limited by the small number of individuals who were newly diagnosed with CML during our time period and by the large proportion of patients diagnosed at older ages (≥ 80 years) and who died within the study period. Although the SEER registries capture all incident cancers in areas covering 28% of the US population, we were limited to studying older adults who were insured by the fee-for-service Medicare program and enrolled in a Medicare prescription drug benefit plan. Although our cohort was relatively large for an incident CML cohort, we had relatively low power for some analyses.
With the recent loss of patent protection for imatinib (2016), there is anticipation that generic entry will result in lower prices. Recent research has suggested that in the United States, the price of specialty oral cancer drugs typically falls by roughly 50% to 75% after generic entry (price reductions of approximately 75% have been estimated for imatinib in the Canadian market).31-33 If prices for generic imatinib fall by 75% from the branded product price to approximately $2,500 per month, patients will still face high out-of-pocket spending of at least $125 per month, which may continue to inhibit access to TKIs.7 Even in light of imatinib losing patent protection, we believe our general finding that high cost sharing may result in delays in initiation of these life-saving medications remains valid for this and other cancers for which orally administered drugs are recommended.
In summary, we found that approximately 40% of older adults with CML did not initiate TKIs within 180 days after their diagnosis. Furthermore, we found that individuals with cost-sharing subsidies initiated TKIs sooner than those without cost-sharing subsidies; however, among TKI users, adherence was similar between these groups. Our findings highlight important gaps in TKI use among Medicare beneficiaries with CML and suggest that high cost sharing may result in delays in initiation of these life-saving medications.
ACKNOWLEDGMENT
We acknowledge the efforts of the National Cancer Institute Applied Research Program; the Centers for Medicare and Medicaid Office of Research, Development and Information; Information Management Services; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Appendix
Table A1.
Time to Initiation of Treatment Among Those Who Lived ≥ 180 Days After Diagnosis
Footnotes
Supported by the Comparative Effectiveness Research Strategic Initiative of University of North Carolina (UNC) Clinical and Translational Science Award No. UL1TR001111 (database infrastructure funding); the UNC School of Medicine; the National Institutes of Health Building Interdisciplinary Research Careers in Women’s Health K12 Program and North Carolina Translational and Clinical Sciences Institute Grant No. UL1TR001111 (S.B.D.); the Royster Society of Fellows at UNC Chapel Hill (A.N.W.); and National Cancer Institute Grant No. K24CA181510 (N.L.K.).
This study used the linked SEER-Medicare database. The interpretation and reporting of these data, as well as the content of the article, are the sole responsibility of the authors. The article content does not necessarily represent the official views of the National Institutes of Health. The ideas and opinions expressed herein are those of the authors, and endorsement is not intended nor should be inferred by the Department of Public Health, the National Cancer Institute, or their contractors and subcontractors.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
See accompanying editorial on page 4307
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Stacie B. Dusetzina
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Factors Associated With Tyrosine Kinase Inhibitor Initiation and Adherence Among Medicare Beneficiaries With Chronic Myeloid Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Aaron N. Winn
No relationship to disclose
Nancy L. Keating
No relationship to disclose
Stacie B. Dusetzina
No relationship to disclose
REFERENCES
- 1.Lotvin AM, Shrank WH, Singh SC, et al. Specialty medications: Traditional and novel tools can address rising spending on these costly drugs. Health Aff (Millwood) 2014;33:1736–1744. doi: 10.1377/hlthaff.2014.0511. [DOI] [PubMed] [Google Scholar]
- 2.Conti RM, Fein AJ, Bhatta SS. National trends in spending on and use of oral oncologics, first quarter 2006 through third quarter 2011. Health Aff (Millwood) 2014;33:1721–1727. doi: 10.1377/hlthaff.2014.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res Treat. 2012;134:459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 5.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27:1218–1224. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 6.Neuner JM, Kamaraju S, Charlson JA, et al. The introduction of generic aromatase inhibitors and treatment adherence among Medicare D enrollees. J Natl Cancer Inst. 2015;107:djv130. doi: 10.1093/jnci/djv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dusetzina SB, Winn AN, Abel GA, et al. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32:306–311. doi: 10.1200/JCO.2013.52.9123. [DOI] [PubMed] [Google Scholar]
- 8.Gotay C, Dunn J. Adherence to long-term adjuvant hormonal therapy for breast cancer. Expert Rev Pharmacoecon Outcomes Res. 2011;11:709–715. doi: 10.1586/erp.11.80. [DOI] [PubMed] [Google Scholar]
- 9.Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20:431–436. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 10.Dusetzina SB, Keating NL. Mind the gap: Why closing the doughnut hole is insufficient for increasing Medicare beneficiary access to oral chemotherapy. J Clin Oncol. 2016;34:375–380. doi: 10.1200/JCO.2015.63.7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faden RR, Chalkidou K, Appleby J, et al. Expensive cancer drugs: A comparison between the United States and the United Kingdom. Milbank Q. 2009;87:789–819. doi: 10.1111/j.1468-0009.2009.00579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weingart SN, Brown E, Bach PB, et al. NCCN Task Force report: Oral chemotherapy. J Natl Compr Canc Netw. 2008;6(suppl 3):S1–S14. [PubMed] [Google Scholar]
- 13.Bach PB. Limits on Medicare’s ability to control rising spending on cancer drugs. N Engl J Med. 2009;360:626–633. doi: 10.1056/NEJMhpr0807774. [DOI] [PubMed] [Google Scholar]
- 14.Baccarani M, Pileri S, Steegmann JL, et al. Chronic myeloid leukemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii72–vii77. doi: 10.1093/annonc/mds228. [DOI] [PubMed] [Google Scholar]
- 15.Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2016 update on diagnosis, therapy, and monitoring. Am J Hematol. 2016;91:252–265. doi: 10.1002/ajh.24275. [DOI] [PubMed] [Google Scholar]
- 16.Jabbour EJ, Kantarjian H, Eliasson L, et al. Patient adherence to tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Am J Hematol. 2012;87:687–691. doi: 10.1002/ajh.23180. [DOI] [PubMed] [Google Scholar]
- 17.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: Content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl):IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute SEER-Medicare linked database. http://healthcaredelivery.cancer.gov/seermedicare.
- 19.Kaiser Family Foundation . Fact Sheet: Medicare Advantage. http://files.kff.org/attachment/Fact-Sheet-Medicare-Advantage. [Google Scholar]
- 20.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 22.Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol. 2011;174:984–992. doi: 10.1093/aje/kwr183. [DOI] [PubMed] [Google Scholar]
- 23.Knickman JR, Hunt KA, Snell EK, et al. Wealth patterns among elderly Americans: Implications for health care affordability. Health Aff (Millwood) 2003;22:168–174. doi: 10.1377/hlthaff.22.3.168. [DOI] [PubMed] [Google Scholar]
- 24.Banegas MP, Guy GP, Jr, de Moor JS, et al. For working-age cancer survivors, medical debt and bankruptcy create financial hardships. Health Aff (Millwood) 2016;35:54–61. doi: 10.1377/hlthaff.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streeter SB, Schwartzberg L, Husain N, et al. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. J Oncol Pract. 2011;7(suppl):46s–51s. doi: 10.1200/JOP.2011.000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couto JE, Panchal JM, Lal LS, et al. Geographic variation in medication adherence in commercial and Medicare part D populations. J Manag Care Spec Pharm. 2014;20:834–842. doi: 10.18553/jmcp.2014.20.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yun H, Safford MM, Brown TM, et al. Statin use following hospitalization among Medicare beneficiaries with a secondary discharge diagnosis of acute myocardial infarction. J Am Heart Assoc. 2015;4:e001208. doi: 10.1161/JAHA.114.001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuart B, Davidoff A, Erten M, et al. How Medicare Part D benefit phases affect adherence with evidence-based medications following acute myocardial infarction. Health Serv Res. 2013;48:1960–1977. doi: 10.1111/1475-6773.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 30.Quintás-Cardama A, Cortes JE. Chronic myeloid leukemia: Diagnosis and treatment. Mayo Clin Proc. 2006;81:973–988. doi: 10.4065/81.7.973. [DOI] [PubMed] [Google Scholar]
- 31.Padula WV, Larson RA, Dusetzina SB, et al. Cost-effectiveness of tyrosine kinase inhibitor treatment strategies for chronic myeloid leukemia in chronic phase after generic entry of imatinib in the United States. J Natl Cancer Inst. 2016;108:djw003. doi: 10.1093/jnci/djw003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Conti RM, Padula WV, Larson RA. Changing the cost of care for chronic myeloid leukemia: The availability of generic imatinib in the USA and the EU. Ann Hematol. 2015;94(suppl 2):S249–S257. doi: 10.1007/s00277-015-2319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelsall C. Generic imatinib cost-effective first-line treatment for CML. http://www.healio.com/hematology-oncology/leukemia/news/in-the-journals/%7B6ed7ed48-4741-4bd6-ace9-50578005de32%7D/generic-imatinib-cost-effective-first-line-treatment-for-cml. [Google Scholar]