Abstract
Purpose
Cancer-related cognitive impairment is an important problem for patients with breast cancer, yet its trajectory is not fully understood. Some previous cancer-related cognitive impairment research is limited by heterogeneous populations, small samples, lack of prechemotherapy and longitudinal assessments, use of normative data, and lack of generalizability. We addressed these limitations in a large prospective, longitudinal, nationwide study.
Patients and Methods
Patients with breast cancer from community oncology clinics and age-matched noncancer controls completed the Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) at prechemotherapy and postchemotherapy and at a 6-month follow-up as an a priori exploratory aim. Longitudinal models compared FACT-Cog scores between patients and controls at the three assessments and adjusted for age, education, race, menopausal status, and baseline reading ability, anxiety, and depressive symptoms. A minimal clinically important difference cutoff determined percentages of impairment over time.
Results
Of patients, 581 patients with breast cancer (mean age, 53 years; 48% anthracycline-based regimens) and 364 controls (mean age, 53 years) were assessed. Patients reported significantly greater cognitive difficulties on the FACT-Cog total score and four subscales from prechemotherapy to postchemotherapy compared with controls as well as from prechemotherapy to 6-month follow-up (all P < .001). Increased baseline anxiety, depression, and decreased cognitive reserve were significantly associated with lower FACT-Cog total scores. Treatment regimen, hormone, or radiation therapy was not significantly associated with FACT-Cog total scores in patients from postchemotherapy to 6-month follow-up. Patients were more likely to report a clinically significant decline in self-reported cognitive function than were controls from prechemotherapy to postchemotherapy (45.2% v 10.4%) and from prechemotherapy to 6-month follow-up (36.5% v 13.6%).
Conclusion
Patients with breast cancer who were treated in community oncology clinics report substantially more cognitive difficulties up to 6 months after treatment with chemotherapy than do age-matched noncancer controls.
INTRODUCTION
Cancer-related cognitive impairment (CRCI) is an important and prevalent problem for survivors of and patients with breast cancer and it includes problems with memory, executive function, attention, and processing speed.1 CRCI can be related to disease, surgery, chemotherapy, radiation, hormone therapy, and immunotherapy.2-5 CRCI negatively impacts quality of life (QOL).6-9 Although several studies have assessed CRCI in cancer populations via objective neuropsychological testing and self-report assessments, the majority of these studies have relied on small sample sizes, included heterogeneous disease and treatment groups, included patients from academic medical centers, lacked pretreatment chemotherapy assessments, and used normative control data.1,7,8
Assessing the patient’s perspective is an important aspect of CRCI, particularly because some neuropsychological tests cannot detect CRCI complaints. Patient-reported outcomes (PROs) are ideal because of the lack of practice effects and clinical adaptability. The Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog)10,11 is a validated PRO that was created specifically to assess cognitive challenges identified by patients with cancer.
By using the FACT-Cog in an a priori exploratory aim analysis, we investigated the impact of cancer and chemotherapy on perceived CRCI in female patients with breast cancer in the largest prospective, longitudinal nationwide study in community oncology clinics to date and compared results with age- and gender-matched controls recruited and assessed at similar times to patients.
We hypothesized that self-reported cognitive difficulties, that is, perceived cognitive impairment, assessed via FACT-Cog total score, would be more prevalent among patients with breast cancer than in a noncancer control group; that cognitive difficulties would persist longitudinally for patients with breast cancer but not for noncancer controls; and that factors including age, education, race, menopausal status, and psychological symptoms at baseline would be associated with persistent cognitive difficulties12-14 and that, by adequately controlling for these variables, we would observe significant and persistent cognitive complaints. We also hypothesized that patients who received anthracyclines—thought to be cardiotoxic and neurotoxic15-18—would lead to more cognitive complaints than in those who received nonanthracycline regimens.
PATIENTS AND METHODS
Study Design
We conducted a nationwide, multicenter, prospective longitudinal study that examined the impact of chemotherapy on cognitive function in female patients with breast cancer who received chemotherapy at community oncology clinics via the University of Rochester Cancer Center National Cancer Institute Community Oncology Research Program (NCORP) Research Base. We recruited an age-matched noncancer control group for longitudinal comparisons with patients. Controls were obtained from the same source population as the patients; the NCORP clinic from which the patient was recruited was also responsible for recruiting the control within 2 months of accruing the patient. Controls could be family members or friends of patients, or unrelated. NCORP is a unique collaboration between researchers and community physicians to address research questions regarding patients treated in community-based health care systems.
Institutional review boards at the University of Rochester Cancer Center NCORP Research Base and each of the 22 NCORP sites approved the study before participants enrolled. Coordinators completed study-specific training. Measures were completed at three assessments: within 7 days before chemotherapy (prechemotherapy baseline; assessment 1), within 4 weeks after chemotherapy completion (postchemotherapy; assessment 2), and 6 months after assessment 2 (6-month follow-up; assessment 3). Controls completed study assessments within the same time windows as patients. Once 367 patients and controls were recruited, we recruited additional patients to specifically address questions regarding chemotherapy regimen differences on cognitive function.
Study Participants
Patients with breast cancer must have a diagnosis of invasive breast cancer (stage I to IIIC), be scheduled to begin a course of chemotherapy, not be scheduled to receive concurrent radiation with chemotherapy, and not have metastatic disease. Both patients and controls must: be chemotherapy naïve, have a life expectancy > 10 months, be able to speak English, be age ≥ 21 years, not be currently hospitalized or have been hospitalized within the last year for a psychiatric illness, not be diagnosed with a neurodegenerative disease, not have any CNS disease, for example, a movement disorder, and not be pregnant. Each noncancer control participant was within 5 years of the age of the patient with breast cancer. All participants provided informed consent before completing study requirements.
Measures
Clinical and demographic information was collected by coordinators. Treatment regimens for patients with breast cancer were dichotomized into anthracycline-containing or non–anthracycline-containing regimens. Participants self-identified their race and ethnicity. Perceived cognitive function was assessed by using FACT-Cog, version 2, a well-validated measure developed by Wagner and colleagues10 to address cognitive complaints related to CRCI, and was completed at the clinic location. FACT-Cog examines a wide range of self-reported cognitive functioning domains, including perceived cognitive impairment (PCI), perceived cognitive abilities (PCA), impact of cognitive impairment on QOL, and cognitive impairment perceived by others, in addition to an overall cognitive function score, which is the sum of the four subscales. Smaller values on these scales imply greater cognitive difficulties. A 1/2 standard deviation as a possible cutoff for a minimal clinically important difference (MCID) has been identified for this measure.19 Reading ability—a proxy for cognitive reserve—was assessed by the Wide Range Achievement Test, 4th Edition (WRAT-4) reading subscale.20 Anxiety was assessed with the Spielberger State/Trait Anxiety Inventory State score (form Y-1).21 Depressive symptoms were captured via an item from the Multidimensional Fatigue Symptom Inventory22 in which patients responded to the statement “I feel depressed” using a scale that ranged from “not at all” to “very much.”
Statistical Analyses
The overall goal of this study was to longitudinally assess cognitive function in patients with breast cancer who received chemotherapy compared with age-matched controls. This is an exploratory analysis of a tertiary study aim that assessed longitudinal changes in cognitive function by using the FACT-Cog. The primary and secondary study aims were to assess cognitive function by objective cognitive measures. Those analyses are still ongoing and they will be reported in a separate article. All available data were used herein.
Descriptive analyses.
For comparison of baseline characteristics for patients and controls, t tests were used for continuous variables and χ2 tests were used for categorical variables.
Means and standard deviations were tabulated for all FACT-Cog total scores and subscale scores at each assessment. Group comparisons of change over time were assessed with Welch t tests and were expressed as effect sizes. We calculated percentages of improvement, decline, or no change on the basis of a 1/2 standard deviation MCID19 over time from assessment 1 to assessment 2, assessment 2 to assessment 3, and assessment 1 to assessment 3. In our study, the MCID was a decrease of ≥ 13.8 points in FACT-Cog using the standard deviation for controls at baseline.
Longitudinal analyses.
We used linear mixed models to compare the trajectories of FACT-Cog scores of patients versus controls over the three assessments and adjusted for important baseline factors. The linear mixed model fixed effects were time (assessments 1, 2, and 3 treated as nominal), group (patient or control), group by time interaction, and adjustment variables age, education (less than high school, high school/GED, college/graduate), race (black, white, other), menopausal status, and prechemotherapy cognitive reserve, anxiety, and depressive symptoms. Subject-specific mean cognitive function score was the random effect and was independent of residual error. We also tested the impact of treatment regimen (anthracycline or nonanthracycline), hormone therapy from assessment 2 to assessment 3 and radiation treatment from assessment 2 to assessment 3 in a separate model for patients only while adjusting for the same characteristics as above. Maximum likelihood estimation was used for these models, significance testing was based on F tests, and marginal adjusted means were used to explore the trajectories. The marginal means for each time, split by group, are listed in Appendix Table A1 (online only). All participants who completed the baseline assessment were included in these longitudinal analyses (intent to treat).
Missing data.
χ2 tests were used to assess whether significant differences in dropout existed between groups. We used a logistic regression model to identify any demographic characteristics that might lead to dropout, and the only one found was a higher dropout rate for blacks versus whites (P = .003). Hence, race was included in the above models. Under the missing at random assumption, the linear mixed modeling method will yield unbiased estimates and standard errors.23 Not being able to rule out missing not at random (MNAR), however, we used a pattern-mixture method24 to evaluate the sensitivity of our results to MNAR, and they were robust across a number of extreme MNAR situations.
Computations were performed by using R (version 3; The R Foundation, Vienna, Austria) and SAS (SAS/STAT User’s Guide, Version 9.4; SAS Institute, Cary, NC) as appropriate. MNAR sensitivity analysis was performed by using SAS PROCs MI and MIANALYZE. P = .05 was used to assess statistical significance.
RESULTS
Baseline Characteristics
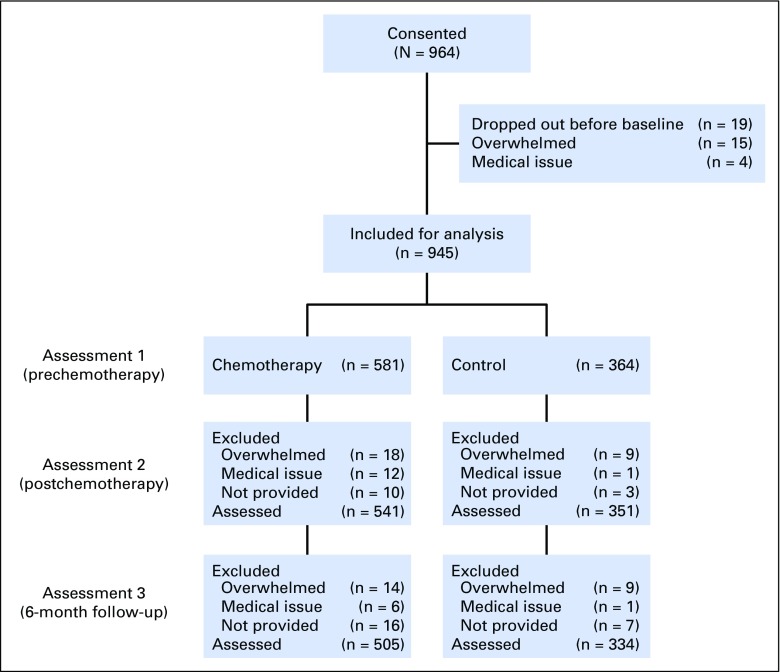
Of participants, 964 consented to the study. Of the 945 who were included for analysis, 505 patients with breast cancer who received chemotherapy and 334 noncancer controls provided data for all three assessments (Fig 1). After assessment 1, 6.7% of patients with breast cancer and 3.5% of controls dropped out. The difference in dropout rate between groups did not reach statistical significance. After assessment 2, 6.0% of patients with breast cancer and 4.6% of controls dropped out, and this difference also was not statistically significant.
Fig 1.
CONSORT diagram.
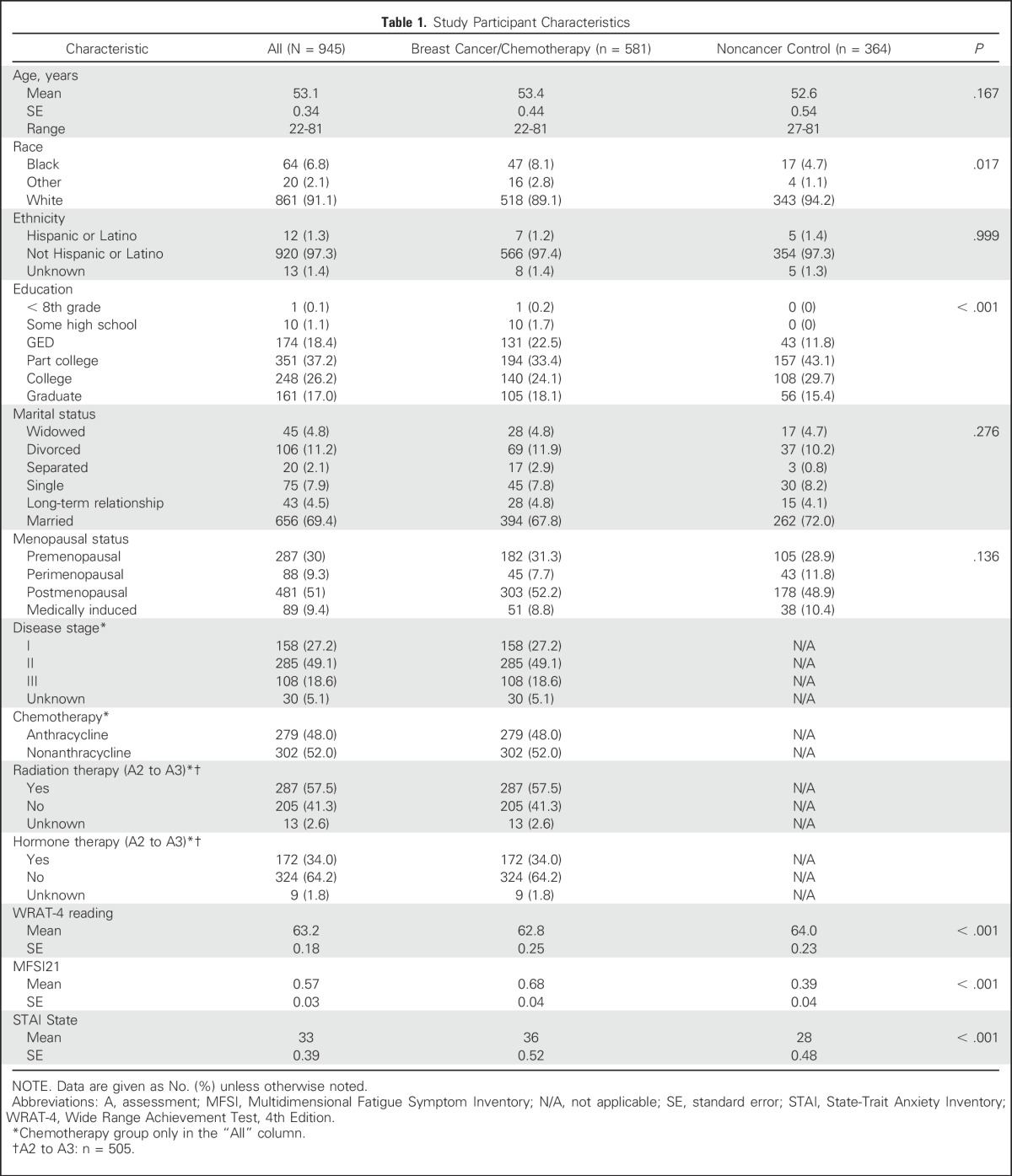
Groups were balanced on age, ethnicity, and marital status (Table 1). Participants were fairly balanced on education, except there were more high school–educated patients compared with controls (P ≤ .001). The population included 9% nonwhite participants with more blacks in the breast cancer group than in the noncancer control group (P = .017). Controls also had higher WRAT-4 general reading ability scores (P < .001). Chronbach α values for FACT-Cog were .95 and .93 at baseline for patients and controls, respectively.
Table 1.
Study Participant Characteristics
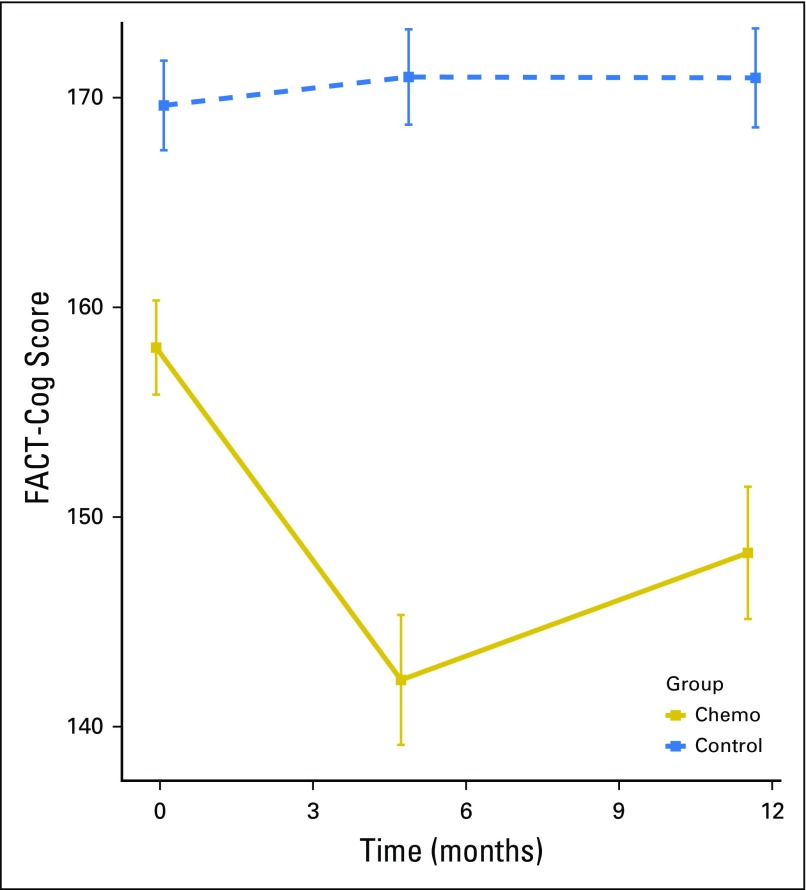
Before any chemotherapy, FACT-Cog scores were lower in patients with breast cancer compared with controls (Fig 2; P < .001). When adjusting for covariates, overall difference between the two groups remained a trend in the same direction (P = .071), with higher age (P = .009), black race (P = .034), lower WRAT-4 reading score, higher anxiety, and higher depressive symptoms (all P < .001) predictive of lower FACT-Cog scores at baseline, whereas education was not predictive (P = .809).
Fig 2.
Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) total scores in patients with breast cancer and controls prechemotherapy, postchemotherapy, and 6 months after chemotherapy. Smaller values imply greater cognitive deficit. Patients reported significant decline, that is, greater perceived difficulty, in FACT-Cog score after chemotherapy and 6 months after chemotherapy. Assessment 1 is prechemotherapy (0 months), assessment 2 is postchemotherapy (4.8 months), and assessment 3 is 6 months after assessment 2 (11.5 months). Controls are assessed at the same time intervals as patients. Scores represent mean and 95% CIs, not adjusted for multiplicity.
Longitudinal Changes in Cognitive Function From Pre- to Postchemotherapy, Postchemotherapy to 6-Month Follow-Up, and Prechemotherapy to 6-Month Follow-Up
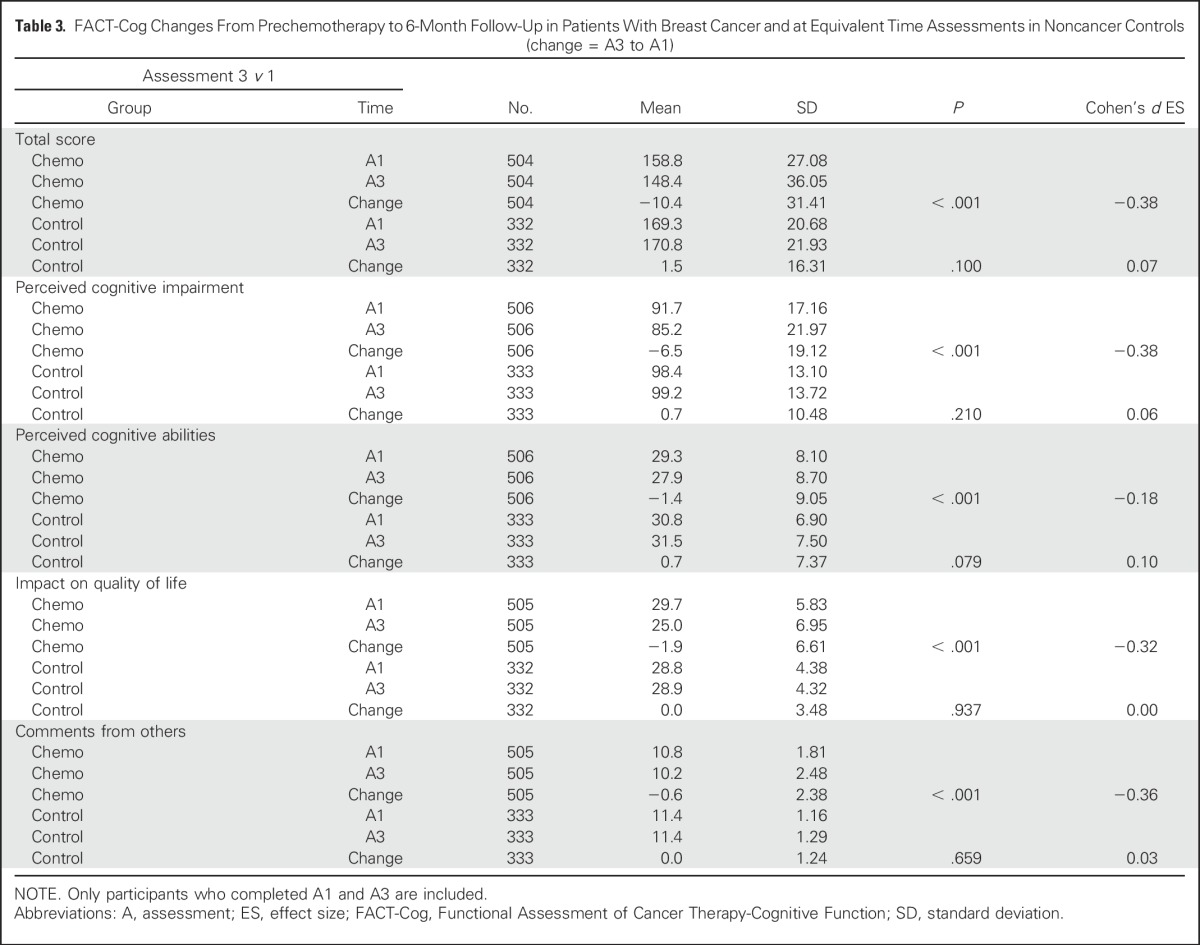
Patients with breast cancer reported statistically significant greater cognitive complaints represented by declines on the FACT-Cog total score and subscale scores of PCI, PCA, impact of cognitive impairment on QOL, and perceived cognitive impairment by others from prechemotherapy to postchemotherapy over time and compared with controls (all P < .001). Patients with breast cancer declined on FACT-Cog scores and the scores of controls did not change. The Cohen’s d effect sizes (ESs) for perceived cognitive difficulty of the patients ranged from 0.23 to 0.62 for total score, PCI, PCA, impact on QOL, and comments from others (Table 2). Reported cognitive difficulties in patients with breast cancer improved slightly from postchemotherapy to 6-months follow-up (a median of 11.5 months from prechemotherapy), whereas corresponding changes in mean scores of controls were small and did not reach statistical significance. Domains that significantly improved were total score (ES = 0.15; P < .001; Appendix Table A2, online only), PCI (ES = 0.19; P < .001), and comments from others (ES = 0.14; P < .001). Whereas most cognitive domains improved over time, all subscales and the total impairment score remained significantly below baseline at 6 months postchemotherapy (ES range = 0.18 to 0.38; Table 3). Mean control changes were small and not statistically significant for any domain.
Table 2.
FACT-Cog Changes From Prechemotherapy to Postchemotherapy in Patients With Breast Cancer and at Equivalent Time Assessments in Noncancer Controls (change = A2 to A1)
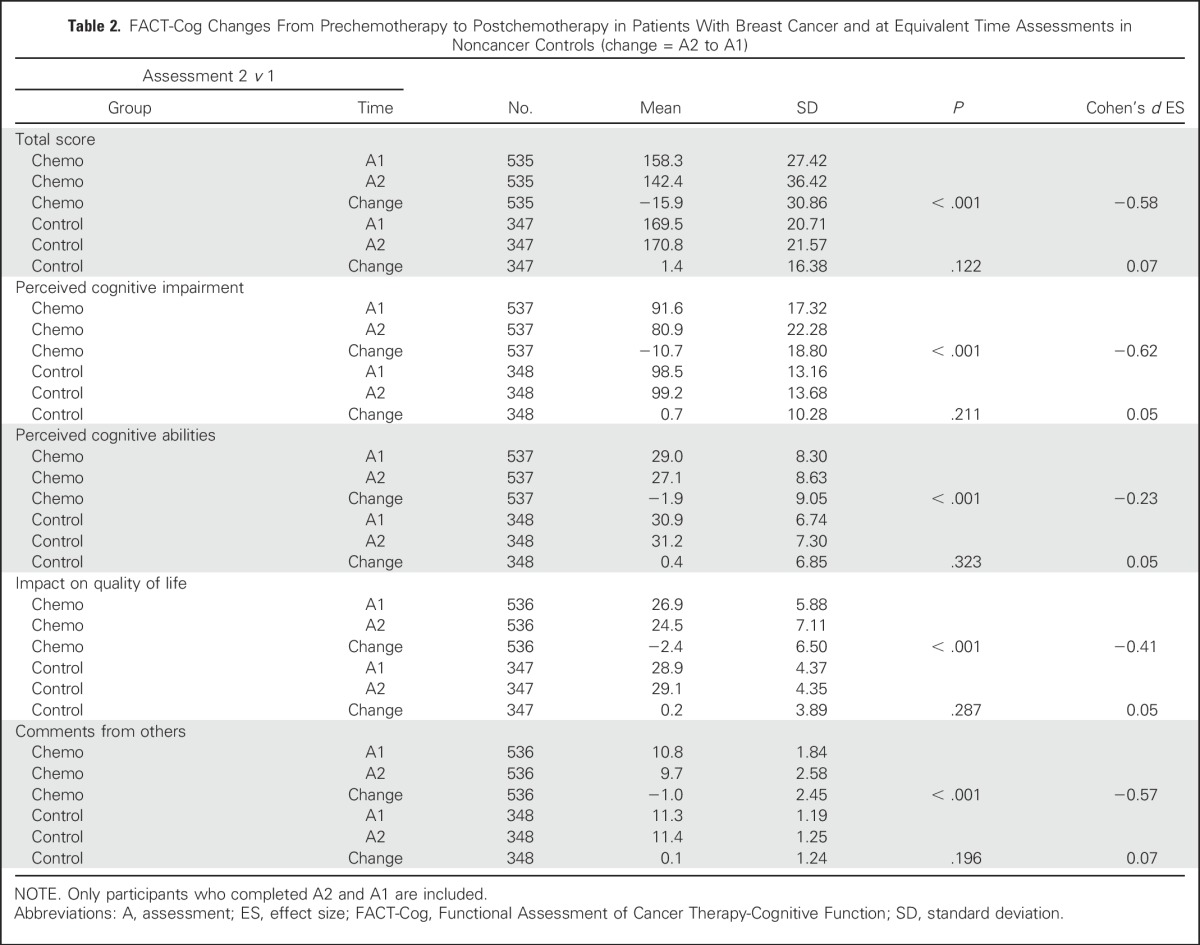
Table 3.
FACT-Cog Changes From Prechemotherapy to 6-Month Follow-Up in Patients With Breast Cancer and at Equivalent Time Assessments in Noncancer Controls (change = A3 to A1)
Prevalence of Clinically Meaningful Perceived Cognitive Impairment Over Time
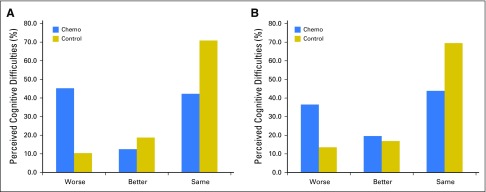
Patients with breast cancer had more clinically meaningful and statistically significant perceived cognitive decline, that is, they reported greater difficulty over time, in the FACT-Cog total score than did controls from prechemotherapy to postchemotherapy using a 1/2 standard deviation cutoff as previously reported.19 In patients with breast cancer, 45.2% reported a perceived decline in FACT-Cog scores compared with 10.4% of controls (P < .001; Fig 3). From postchemotherapy to 6-months follow-up, 18.4% of patients with breast cancer reported clinically meaningful perceived decline in FACT-Cog scores compared with 11.5% in controls. From prechemotherapy to 6-months follow-up, which represented almost 1 year later, 36.5% of patients with breast cancer reported a decline in FACT-Cog scores compared with 13.6% of controls (P < .001).
Fig 3.
Prevalence of overall perceived cognitive difficulties via Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog) total score from (A) prechemotherapy to postchemotherapy and (B) prechemotherapy to 6-month follow-up. Better is defined as an increase of ≥ 13.8 points in FACT-Cog, and Worse is defined as a decrease of ≥ 13.8 points.
Longitudinal Changes in Cognitive Function Controlling for Covariates and Predictors of FACT-Cog Outcomes
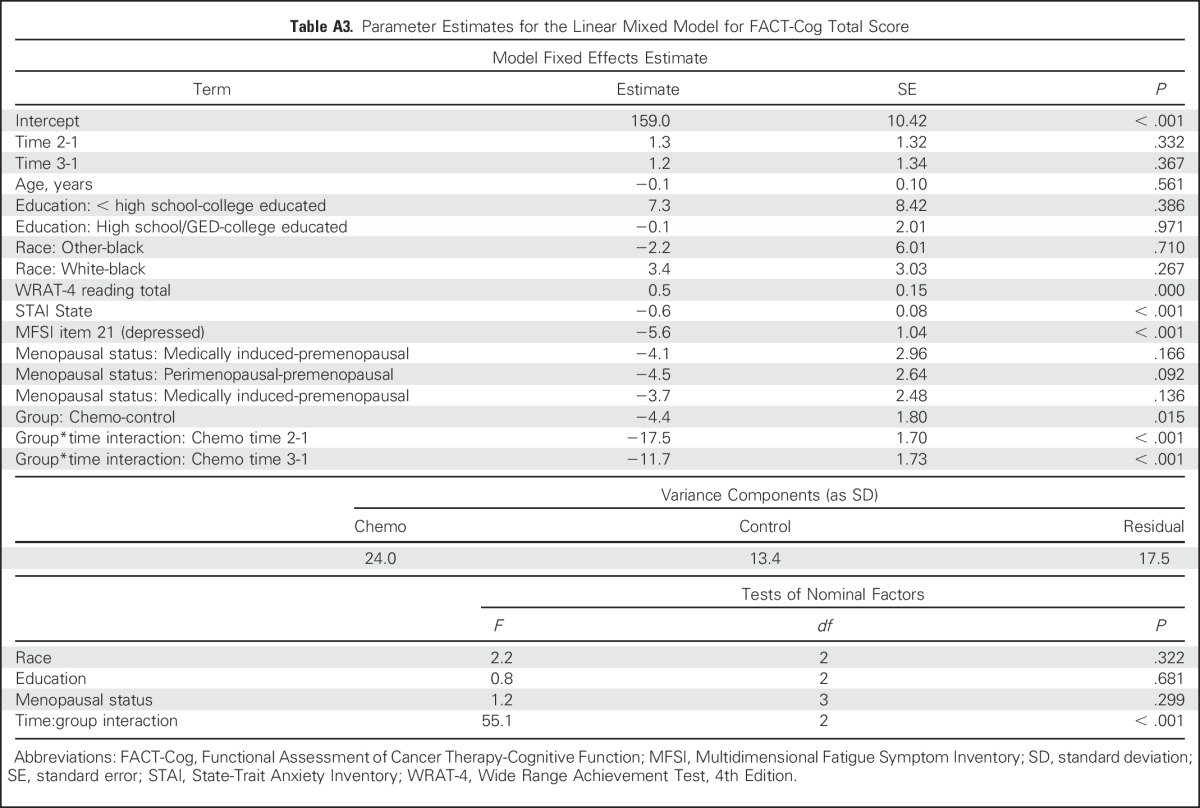
When compared with controls—using linear mixed models, controlling for important prechemotherapy characteristics—patients with breast cancer, on average, reported greater declines on all FACT-Cog domains from prechemotherapy to postchemotherapy (all P < .001), and scores significantly improved from postchemotherapy to 6-month follow-up on FACT-Cog total scores, PCI scale, and comments from others (all P < .001; Appendix Tables A1 and A3, online only). Important predictors of overall perceived cognitive difficulties via FACT-Cog total score included lower WRAT-4 reading score, higher anxiety, and higher depression (all P < .001). Predictors of impairment on the PCI scale included higher anxiety and higher depression (P < .001) and perimenopausal status and postmenopausal status (P = .022 and .014, respectively). Predictors of impairment on the PCA scale included younger age (P = .032), race (whites did better than blacks; P < .001), lower WRAT-4 reading score (P < .001), and higher anxiety (P < .001). Higher anxiety and depression were associated with impairments on the impact on QOL domain and the comments from others domain (P < .001). Perimenopausal status was associated with a lower QOL domain score (P = .025) compared with premenopausal status. In a separate model for patients with breast cancer only, no statistically significant differences in mean FACT-Cog total scores between treatment regimens, radiation therapy, or hormonal therapy were observed (all P > .50).
DISCUSSION
By using a well-validated measure of perceived cognitive function, our results from the largest study to date show that self-reported cognitive impairment, indicative of CRCI, is a substantial and pervasive problem for patients with breast cancer who received chemotherapy. The clinically and statistically significant self-reported cognitive decline among patients with breast cancer was 36.5% from prechemotherapy to 6 months after chemotherapy completion (11.5 months from prechemotherapy); prevalence was 13.6% in controls assessed at the same times. Few large studies, if any, have systematically used FACT-Cog to assess this aspect of CRCI in patients who received chemotherapy in a well-controlled longitudinal study design. Our focus on patients who were treated in community oncology clinics is also novel and important.
Our findings are in agreement with similar studies. By using a clinically relevant cutoff, our findings show a prevalence of perceived cognitive impairment in patients with breast cancer that is similar to that found in other studies using global deficit scores from neuropsychological tests.25 Our study suggests that perceived CRCI is a complex multifactorial problem for patients with breast cancer and that it is likely that a combination of demographic, medical, and psychological factors plays a role in predisposing someone to CRCI. Before any chemotherapy, patients reported lower FACT-Cog scores than did controls; however, we found this effect was influenced by age, race, cognitive reserve, and higher anxiety and depressive symptoms, as our results became a trend after including these covariates. It is also not known what effect the disease itself may have on CRCI, nor that of other symptoms, such as fatigue, which may contribute to baseline function. Increased baseline levels of anxiety and depressive symptoms, lower baseline cognitive reserve, and perimenopausal or postmenopausal status were predictive of perceived cognitive impairment and may represent mechanisms that could contribute to the development and exacerbation of CRCI. Although distinct processes from CRCI, managing anxiety and depression during chemotherapy may lessen CRCI and its impact on QOL. One other study suggests that cognitive reserve may be an important factor in conferring risk of longer-term CRCI. Ahles et al26 found that age and pretreatment cognitive reserve predicted longitudinal processing speed performance in chemotherapy-treated patients with breast cancer. We did not find a significant effect of education in our study, which underscores the importance of cognitive reserve as opposed to educational status on cognitive impairment.
Younger age and the black race were predictive of problems with perceived cognitive abilities, in addition to anxiety and cognitive reserve. It could be that younger adults are more aware of problems with cognitive abilities than are older adults. Evidence suggests that blacks are more likely to develop cognitive impairments than whites, and further studies focused on CRCI in blacks are warranted to discern contributing factors, for example, comorbid conditions. Baseline anxiety and depression were the only significant predictors of both QOL and comments from others. It is possible that these factors are most important in determining the overall impact of cognitive impairment on QOL as well as how others, for example, caregivers, view the patient’s cognitive function. Whereas research demonstrates that anthracycline agents are more inflammatory27,28 and neurotoxic18 than nonanthracyclines, our study shows patients being treated with either regimen experience CRCI similarly. In addition, those who received hormone therapies and/or radiation therapy after chemotherapy did not experience CRCI statistically differently from those who did not receive these modalities.
A limitation of this research is that we do not know the longer-term impact of CRCI assessed by FACT-Cog. We are currently observing a small subset of these patients and controls for 2 years post-treatment. This study is an exploratory aim analysis and we are still analyzing the longitudinal objective neuropsychological assessments (primary and secondary aims), which are to be reported elsewhere. More research is needed to address moderating and mediating effects of anxiety, depression, and other factors, particularly depression as our measure was limited. Whereas our results are generalizable, studies more that fully evaluate CRCI in older and younger patients with cancer are warranted, as well as investigation of minority populations.
Despite these limitations, there are multiple strengths to our study. This is the largest study to date to longitudinally assess perceived CRCI. This, to our knowledge, is the first nationwide study and also the first to assess perceived CRCI in community oncology clinic patients. We used a well-controlled study design using age-matched controls that were observed for the same length of time as patients with breast cancer to control for aging effects. We used a longitudinal study design with a prechemotherapy assessment and we also had adequate sample size to control for and assess multiple covariates. FACT-Cog is a well-validated PRO that encompasses many facets of CRCI; using FACT-Cog, we obtained prevalence results similar to those of studies using objective cognitive tests. Many studies have used this measure in a cross-sectional design. Ours is one of the few to assess CRCI in a longitudinal fashion. We have also expanded knowledge about the relationship between CRCI and its impact on QOL and comments from others. We had excellent retention, which reduced our chance of bias. Our results indicate perceived CRCI is a substantial problem for breast cancer survivors.
ACKNOWLEDGMENT
We thank the participants in this study and all staff at the University of Rochester Cancer Center National Cancer Institute (NCI) Community Clinical Oncology Research Program (NCORP) Research Base and our NCORP affiliate sites who recruited and observed participants. We thank the National Cancer Institute Clinical Community Oncology Program (CCOP) and NCORP programs for their funding and support of this project. We also thank Susan Rosenthal for her critical review of this manuscript. The following CCOP/NCORPs participated in this study: Central Illinois, Columbus, Cancer Research Consortium of West Michigan, Dayton, Delaware, Grand Rapids, Greenville, Hematology-Oncology Associates of Central New York, Kalamazoo, Kansas City, Marshfield, Metro Minnesota, Nevada, North Shore, Pacific Cancer Research Consortium, Southeast Cancer Control Consortium, Southeast Clinical Oncology Research Consortium, Upstate Carolina, Virginia Mason, Wichita, Wisconsin NCORP, and Western Oncology Research Consortium.
Appendix
Table A1.
Adjusted Means in Longitudinal Mixed Model Analyses
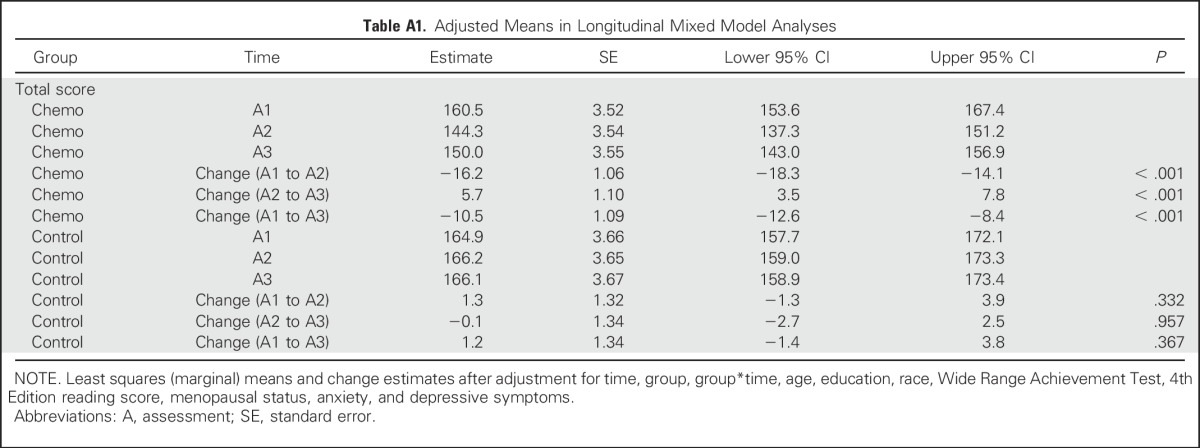
Table A2.
FACT-Cog Changes From Postchemotherapy to 6-Month Follow-Up in Patients With Breast Cancer and at Equivalent Time Assessments in Noncancer Controls (change = A3 to A2)
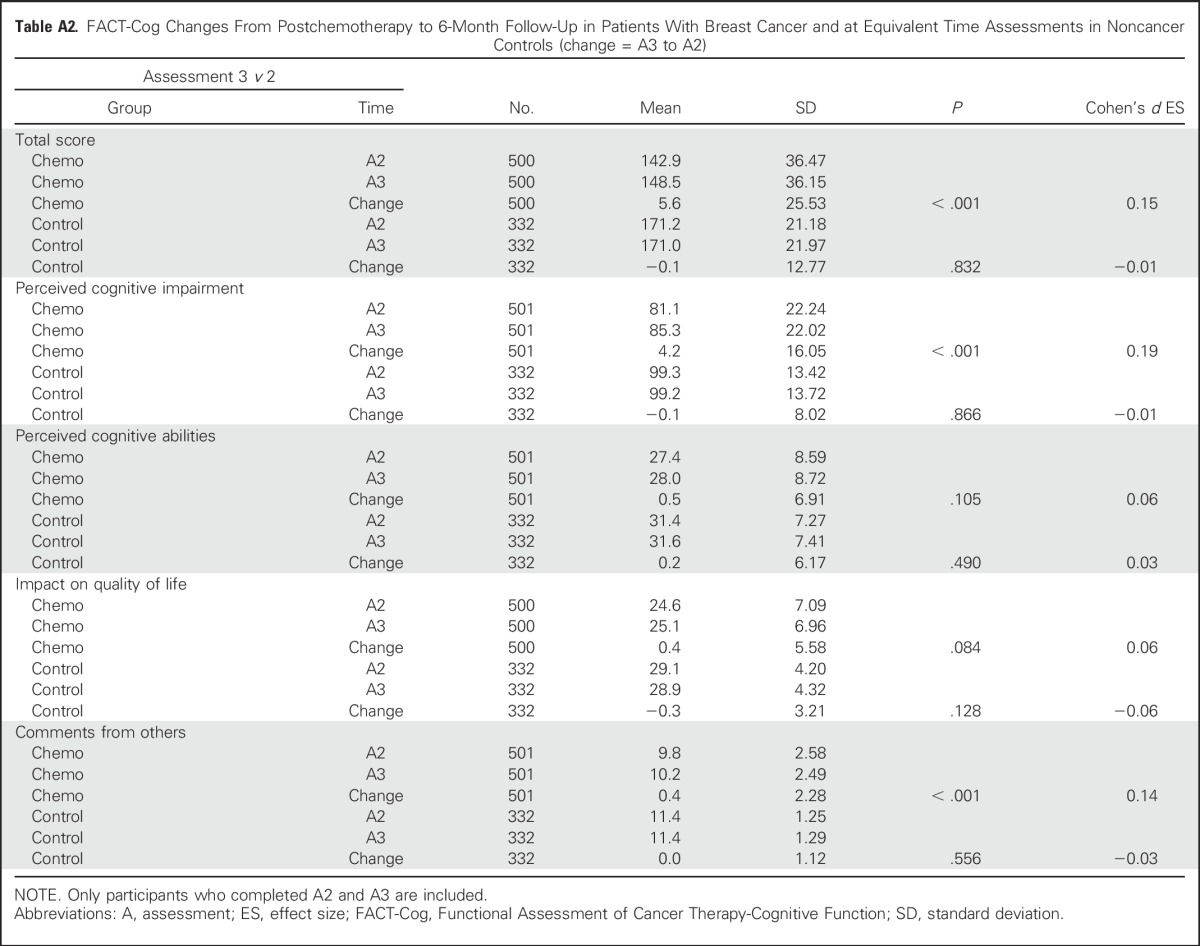
Table A3.
Parameter Estimates for the Linear Mixed Model for FACT-Cog Total Score
Footnotes
Supported by National Cancer Institute Grants No. U10CA037420, U10CA037420 Supplement, UG1CA189961, K07CA1688, and R25CA102618.
Presented in part at the 2015 American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 29-June 2, 2015.
Clinical trial information: NCT01382082.
See accompanying Editorial on page 482
AUTHOR CONTRIBUTIONS
Conception and design: Michelle C. Janelsins, Charles E. Heckler, Christine A. Ambrosone, Gary R. Morrow
Financial support: Michelle C. Janelsins, Gary R. Morrow
Administrative support: Michelle C. Janelsins
Provision of study materials or patients: Michelle C. Janelsins, Kelley L. Young, Lora R. Weiselberg, Jerry W. Mitchell, Alison K. Conlin
Collection and assembly of data: Michelle C. Janelsins, Charles E. Heckler, Joseph J. Guido, Kelley L. Young, Alison K. Conlin, Lora R. Weiselberg, Jerry W. Mitchell
Data analysis and interpretation: Michelle C. Janelsins, Charles E. Heckler, Luke J. Peppone, Charles Kamen, Karen M. Mustian, Supriya G. Mohile, Allison Magnuson, Ian R. Kleckner, Joseph J. Guido, Tim A. Ahles
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared With Age-Matched Controls: An Analysis From a Nationwide, Multicenter, Prospective Longitudinal Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Michelle C. Janelsins
No relationship to disclose
Charles E. Heckler
No relationship to disclose
Luke J. Peppone
Consulting or Advisory Role: Butler Evergeen
Charles Kamen
No relationship to disclose
Karen M. Mustian
No relationship to disclose
Supriya G. Mohile
Consulting or Advisory Role: Seattle Genetics
Allison Magnuson
Travel, Accommodations, Expenses: American Society of Clinical Oncology
Ian R. Kleckner
Consulting or Advisory Role: Janssen Pharmaceuticals
Research Funding: Janssen Pharmaceuticals
Joseph J. Guido
No relationship to disclose
Kelley L. Young
No relationship to disclose
Alison K. Conlin
Consulting or Advisory Role: Celgene
Lora R. Weiselberg
No relationship to disclose
Jerry W. Mitchell
Employment: Ohio Oncology, Novartis (I)
Stock or Other Ownership: Novartis (I)
Christine A. Ambrosone
No relationship to disclose
Tim A. Ahles
No relationship to disclose
Gary R. Morrow
No relationship to disclose
REFERENCES
- 1.Janelsins MC, Kesler SR, Ahles TA, et al. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102–113. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganz PA, Petersen L, Castellon SA, et al. Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: An observational cohort study. J Clin Oncol. 2014;32:3559–3567. doi: 10.1200/JCO.2014.56.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender CM, Merriman JD, Gentry AL, et al. Patterns of change in cognitive function with anastrozole therapy. Cancer. 2015;121:2627–2636. doi: 10.1002/cncr.29393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahles TA. Cognitive changes associated with cancer and cancer treatment. Semin Oncol Nurs. 2013;29:229–231. doi: 10.1016/j.soncn.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Joly F, Giffard B, Rigal O, et al. Impact of cancer and its treatments on cognitive function: Advances in research from the Paris International Cognition and Cancer Task Force Symposium and update since 2012. J Pain Symptom Manage. 2015;50:830–841. doi: 10.1016/j.jpainsymman.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Reid-Arndt SA, Yee A, Perry MC, et al. Cognitive and psychological factors associated with early posttreatment functional outcomes in breast cancer survivors. J Psychosoc Oncol. 2009;27:415–434. doi: 10.1080/07347330903183117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley CJ, Neumark D, Bednarek HL, et al. Short-term effects of breast cancer on labor market attachment: Results from a longitudinal study. J Health Econ. 2005;24:137–160. doi: 10.1016/j.jhealeco.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Wefel JS, Lenzi R, Theriault RL, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer. 2004;100:2292–2299. doi: 10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 9.Myers JS. Chemotherapy-related cognitive impairment: The breast cancer experience. Oncol Nurs Forum. 2012;39:E31–E40. doi: 10.1188/12.ONF.E31-E40. [DOI] [PubMed] [Google Scholar]
- 10.Wagner L, Sweet J, Butt Z., et al. Measuring patient self-reported cognitive function: Development of the Functional Assessment of Cancer Therapy-Cognitive Function instrument. J Support Oncol. 2009;7:W32–W39. [Google Scholar]
- 11.Jacobs SR, Jacobsen PB, Booth-Jones M, et al. Evaluation of the functional assessment of cancer therapy cognitive scale with hematopoietic stem cell transplant patients. J Pain Symptom Manage. 2007;33:13–23. doi: 10.1016/j.jpainsymman.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Chou YH, Chen NK, Madden DJ. Functional brain connectivity and cognition: Effects of adult age and task demands. Neurobiol Aging. 2013;34:1925–1934. doi: 10.1016/j.neurobiolaging.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stein J, Luppa M, Luck T, et al. The assessment of changes in cognitive functioning: Age-, education-, and gender-specific reliable change indices for older adults tested on the CERAD-NP battery: Results of the German Study on Ageing, Cognition, and Dementia in Primary Care Patients (AgeCoDe) Am J Geriatr Psychiatry. 2012;20:84–97. doi: 10.1097/JGP.0b013e318209dd08. [DOI] [PubMed] [Google Scholar]
- 14.Matthews F, Marioni R, Brayne C. Examining the influence of gender, education, social class and birth cohort on MMSE tracking over time: A population-based prospective cohort study. BMC Geriatr. 2012;12:45. doi: 10.1186/1471-2318-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy AC, Tolnay E, Nagykálnai T, et al. Cardiotoxicity of anthracycline in young breast cancer female patients: The possibility of detection of early cardiotoxicity by TDI. Neoplasma. 2006;53:511–517. [PubMed] [Google Scholar]
- 16.Ho E, Brown A, Barrett P, et al. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow-up of asymptomatic breast cancer survivors: A speckle tracking echocardiographic study. Heart. 2010;96:701–707. doi: 10.1136/hrt.2009.173997. [DOI] [PubMed] [Google Scholar]
- 17.Steinherz LJ, Steinherz PG, Tan CT, et al. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266:1672–1677. [PubMed] [Google Scholar]
- 18.Kesler SR, Blayney DW. Neurotoxic effects of anthracycline- vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol. 2016;2:185–192. doi: 10.1001/jamaoncol.2015.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheung YT, Foo YL, Shwe M, et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: Cognitive function (FACT-Cog) in breast cancer patients. J Clin Epidemiol. 2014;67:811–820. doi: 10.1016/j.jclinepi.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4 (WRAT4). Lutz, FL: Psychological Assessment Resources; [Google Scholar]
- 21.Maruish ME (ed): Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI), in The Use of Psychological Testing for Treatment Planning and Outcomes Assessment (ed 2). Mahwah, NJ, Lawrence Erlbaum Associates, 1999, pp 993-1021 [Google Scholar]
- 22.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: Use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 23.Little RJA, Rubin DB. Statistical Analysis With Missing Data (ed 2) Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 24.van Buuren S. Flexible Imputation of Missing Data. Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 25.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J Clin Oncol. 2012;30:3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. J Clin Oncol. 2010;28:4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janelsins MC, Mustian KM, Palesh OG, et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: Implications for cognitive impairment research. Support Care Cancer. 2012;20:831–839. doi: 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tangpong J, Cole MP, Sultana R, et al. Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol Dis. 2006;23:127–139. doi: 10.1016/j.nbd.2006.02.013. [DOI] [PubMed] [Google Scholar]