Abstract
Purpose
Multiple agents for advanced non–small-cell lung cancer (NSCLC) have been approved in the past decade, but little is known about their use and associated spending and survival.
Methods
We used SEER-Medicare data for elderly patients with a new diagnosis of advanced-stage NSCLC and were treated with antineoplastic agents between 2000 and 2011 (N = 22,163). We estimated the adjusted percentage of patients who received each agent, days while on treatment, survival, and spending in the 12 months after diagnosis.
Results
During the 12-year study period, a marked shift in treatment occurred along with a rapid adoption of pemetrexed (39.2%), erlotinib (20.3%), and bevacizumab (18.9%) and a decline in paclitaxel (38.7%), gemcitabine (17.0%), and vinorelbine (5.7%; all P < .05). The average total days on therapy increased by 5 days (from 103 to 108 days). Patients who received bevacizumab, erlotinib, or pemetrexed had the longest treatment durations on average (approximately 146 days v 75 days for those who did not receive these agents). Approximately 44% of patients received antineoplastic agents in the last 30 days of life throughout the study period. Acute inpatient spending declined (from $29,376 to $23,731), whereas outpatient spending increased 23% (from $37,931 to $46,642). Median survival gains of 1.5 months were observed.
Conclusion
Considerable shifts in the treatment of advanced-stage NSCLC occurred along with modest gains in survival and total Medicare spending. More precise outcome information is needed to inform value-based treatment decisions for advanced-stage NSCLC.
INTRODUCTION
Non–small-cell lung cancer (NSCLC) is the leading cause of cancer death in the United States. There will be an estimated 224,390 new cases of lung cancer diagnosed in 2016 and approximately 158,000 deaths,1 causing considerable morbidity and mortality. Two thirds of all NSCLC cases are diagnosed at age 65 years or older.1 Nearly 60% of NSCLC cases are metastatic at diagnosis, and the 5-year relative survival rate for metastatic disease is 4.2%.2
Although the Food and Drug Administration approved several new agents, including biologic targeted agents, prognosis for advanced-stage NSCLC is poor. In a study of US population–based data from more than two decades ago (1994 to 1999), patients who received chemotherapy had a median survival of 8 months relative to 5 months for those who did not receive chemotherapy.3 These gains come with toxicities and medical care costs. Older patients, who comprise the majority with advanced-stage NSCLC, experience greater toxicity rates than younger patients.4-6 Moreover, the cost-effectiveness of newer agents, such as bevacizumab, is $560,000 per quality-adjusted life-year.7 Pemetrexed has an incremental cost-effectiveness ratio of $122,371 per life-year gained compared with observation with best supportive care.8 Erlotinib added to standard chemotherapy regimens has a cost-effectiveness ratio of $95,000 per quality-adjusted life-year relative to standard regimens alone.9
With the use of data from linked SEER-registry Medicare claims files, this study evaluated infusion and oral antineoplastic agents prescribed for newly diagnosed advanced-stage NSCLC over a 12-year period. These longitudinal, population-based data allowed us to observe corresponding changes in survival and spending over time. Use, survival, and spending trends of antineoplastic agents provide insight into the value of these therapies and offer a glimpse of the shape of future treatment trends in advanced NSCLC within the context of escalating medical care costs.
METHODS
Data
We used linked SEER registry-Medicare claims data.10 The SEER registries are population based and ascertain all incident cancers that occur in defined geographic areas that include 28% of the US population. For each patient, the SEER record contains demographic data, month and year of diagnosis, cancer site, and stage at diagnosis. The Medicare data include date of death (if applicable) and claims for beneficiaries with fee-for-service coverage. All claims include dates of service and codes for diagnoses and procedures from the International Classification of Diseases, Ninth Revision, Clinical Modification; Healthcare Common Procedure Coding System; or National Drug Code. Each claim includes Medicare payments and patient deductibles and copayments.
Sample
We selected all beneficiaries age 65 years and older with a metastatic first and only primary NSCLC diagnosis from 2000 through 2011 and treated with an antineoplastic agent defined as either chemotherapy or molecularly targeted therapies bevacizumab or erlotinib (N = 22,163). The observation period was 12 months after the month of diagnosis for the analysis of treatment and spending. The inclusion of a 12-month assessment period captures most treatment of most patients, given that the median survival was 9.2 months in 2010. Patients were excluded if they had more than one cancer, the month of diagnosis was unknown, or if they were identified through death certificate or autopsy. We selected patients continuously enrolled in Medicare Parts A and B fee-for-service coverage because services were not reported while beneficiaries were enrolled in managed care plans. In addition, we excluded 126 beneficiaries who had no payments associated with their Medicare claims.
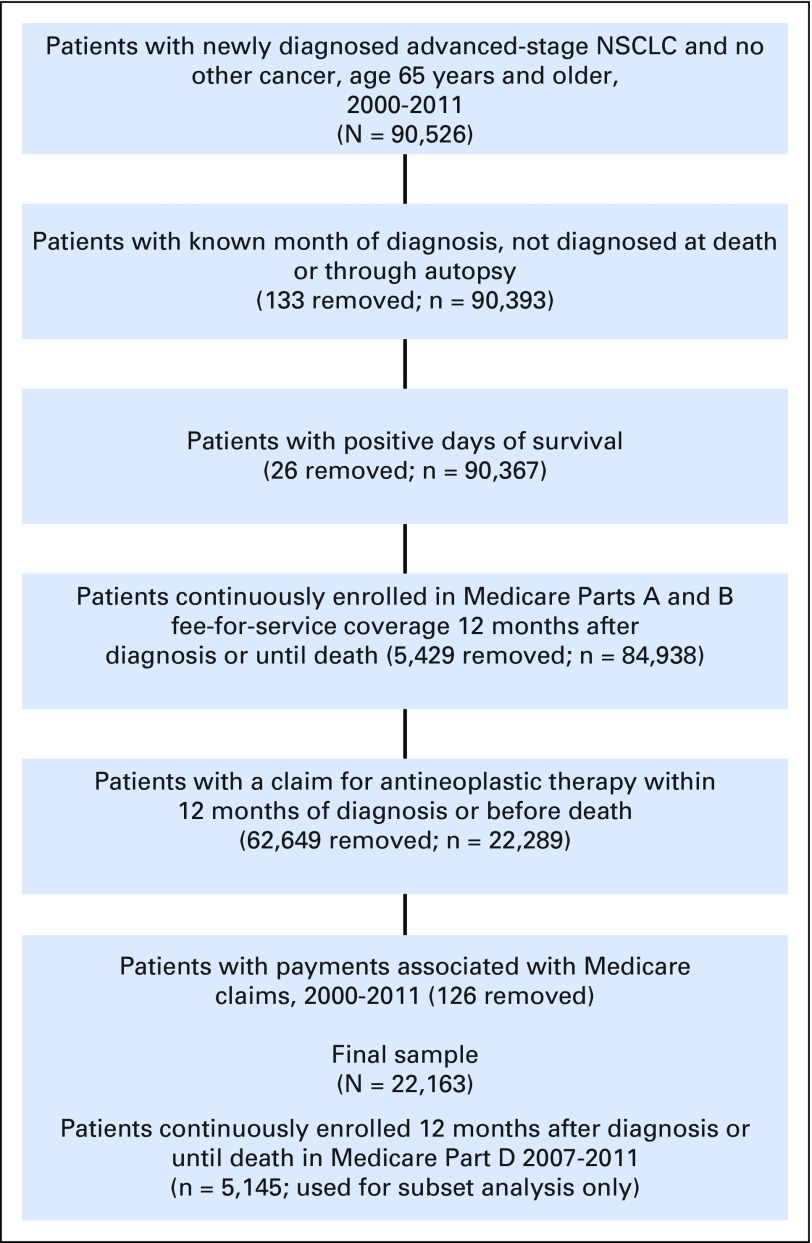
Erlotinib is an oral agent used to treat advanced-stage NSCLC and is not covered under Medicare Part B, the data source for infusion agents. Therefore, we extracted claims from Part D data for patients enrolled in the Medicare Prescription Drug Coverage program. Enrollment is voluntary and applies only to the subset of beneficiaries who enroll. These data were available starting in 2007. Within the advanced NSCLC sample from 2007 to 2011, 5,145 patients were enrolled in Medicare Part D and met the study inclusion and exclusion criteria. Figure 1 shows the sample selection process.
Fig 1.
Sample derivation. Newly diagnosed advanced-stage non–small-cell lung cancer (NSCLC) in patients age 65 years and older treated with antineoplastic agents, SEER-Medicare, 2000 to 2011.
Antineoplastic Agents
We identified antineoplastic agents and reported trends in the percentage of patients who received these agents (ie, cisplatin, carboplatin, paclitaxel, docetaxel, vinorelbine, gemcitabine, etoposide, irinotecan, pemetrexed, bevacizumab, erlotinib). Dates on the claims allowed us to determine the start and stop dates for specific drugs; however, we could not identify combinations given the varied timing for administering specific drugs in a regimen (Data Supplement).
We also estimated the average number of days patients received any antineoplastic agent. Similarly, we estimated the average number of days between the last antineoplastic administration to death and the percentage of patients who received treatment in the last 30 days of life.
Medical Care Spending
To estimate the average spending per patient, we used the amount paid by Medicare.10-12 We added patient copayments and deductibles to the Medicare claims to estimate total spending. All claims were included in the estimates because all patients had advanced disease and received an antineoplastic agent, which lent confidence that most claims were related to cancer. Spending is presented separately for patients who are enrolled in Medicare Part D. No information about the use of oral agents is available for patients without Part D coverage. We deconstructed spending estimates by acute inpatient stays and outpatient care, which encompasses outpatient services, including infusions and physician claims found in the National Claims History file. These data reveal how other aspects of care are altered by the adoption of new agents. Medicare payments were standardized using the Consumer Price Index–All Urban Consumers. We report all estimates in 2012 dollars.
Statistical Analysis
Treatment utilization.
We used separate multivariable logistic regression analyses to estimate probabilities of receiving each of 11 types of treatment and used linear regression to estimate length of treatment by year of diagnosis and adjusted for patient sex, age, race, months of survival, and registry. We report results as predicted marginals, which are calculated by averaging the estimated probabilities of receiving treatment (or mean treatment days) under the assumption that the entire cohort received a diagnosis in each calendar year.13 Predicted marginals standardize outcomes to the entire study sample for covariance imbalance in calendar years13,14 and can be interpreted as percentages for logistic models and means for linear models. Predicted marginals were estimated by using the SUDAAN procedures PROC LOGISTIC (RLOGIST) for dichotomous outcomes and PROC REGRESS for continuous outcomes (RTI International, Research Triangle Park, NC). Models were adjusted for categorical covariates year of diagnosis, 5-year age-group at diagnosis, sex, ethnicity, and SEER registry. Statistically significant trends were determined by using the Wald test at P < .05.
Spending.
We used a two-part model to first estimate the probability of positive spending and then the mean spending per patient for those who had positive spending. We used multivariable gamma regression analyses with a log link (SAS PROC NLMIXED; SAS Institute, Cary, NC).
Survival.
We estimated unadjusted median and mean survival by year and report survival for a 24-month period after diagnosis. Because follow-up information was incomplete in 2013, we included cases diagnosed in 2000 to 2010. The last observation date was December 31, 2012. Statistical significance of changes in survival was determined from the log-rank test. Mortality was measured as all-cause mortality. A prior study reported that among patients with advanced lung cancer, 92.3% have cancer as the cause of death on their death certificate.14 We estimated a multivariable Cox proportional hazards regression model by using the SAS PROC PHREG procedure to adjust hazard ratios (HRs) for patient sex, age, ethnicity, and registry. Use and spending during the study years varied little by year; therefore, we report findings for the first year of the study (2000), the intermediate years (2004 and 2007), and 2011. All years analyzed are reported for survival estimates.
RESULTS
Descriptive Statistics
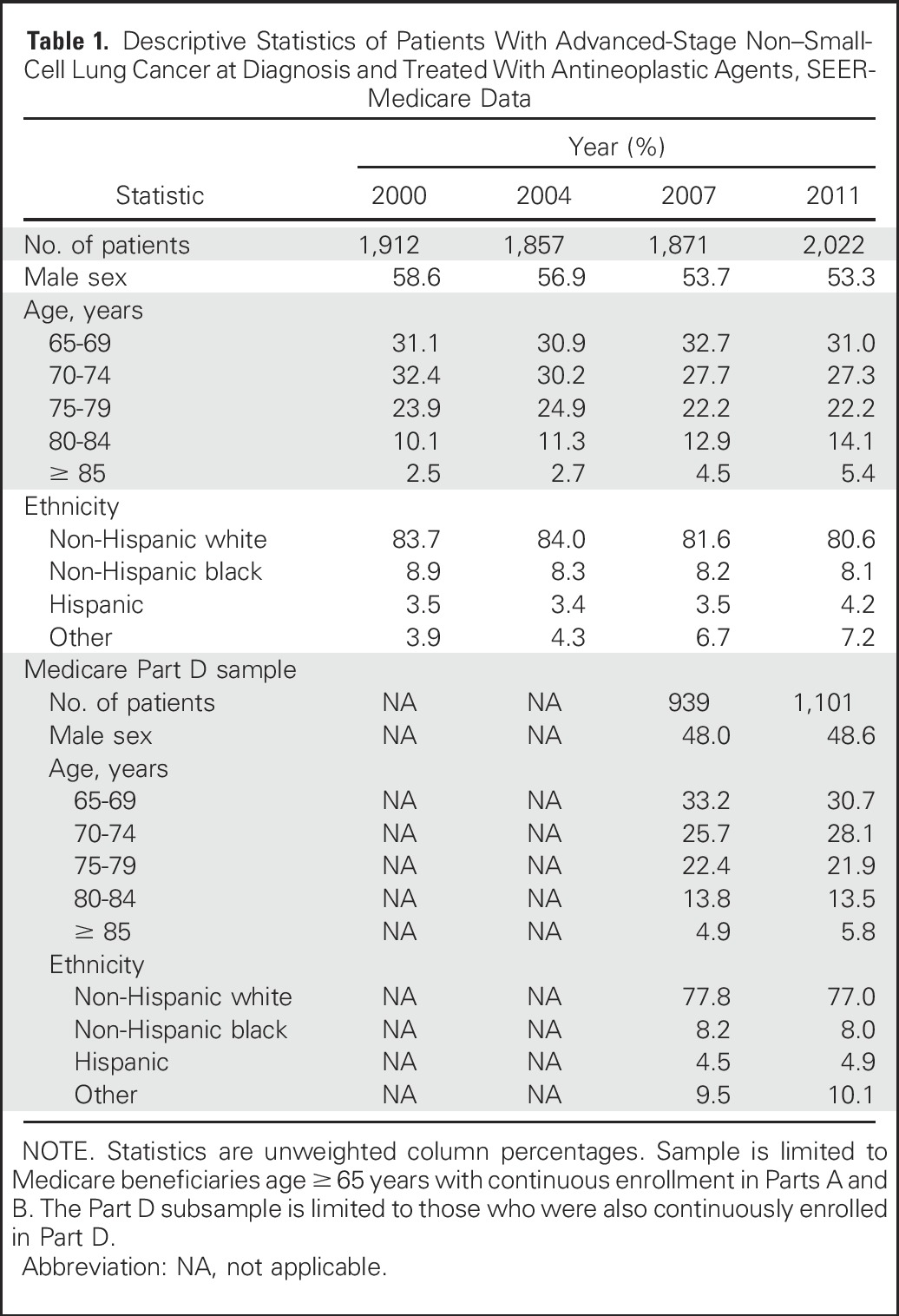
Table 1 lists the characteristics of the sample. Slightly more than one half of the patients were male. Most patients were younger than age 80 years, with a trend toward increasingly more patients in older age-groups during the later years of the study. Approximately 80% of patients were non-Hispanic whites. Age and ethnicity distributions were similar for the subset of patients with Medicare Part D coverage and the full sample, although a smaller percentage was male.
Table 1.
Descriptive Statistics of Patients With Advanced-Stage Non–Small-Cell Lung Cancer at Diagnosis and Treated With Antineoplastic Agents, SEER-Medicare Data
Treatment Patterns
Between 2000 and 2011, several agents declined in usage, including paclitaxel (58% to 39%), gemcitabine (32% to 17%), and vinorelbine (24% to 6%; Table 2). The percentage of patients who received cisplatin, docetaxel, irinotecan, and etoposide remained about the same. The percentage of patients who received carboplatin increased slightly (67% to 74%), and carboplatin was the most commonly prescribed agent for advanced-stage NSCLC in all years. Newer agents such as pemetrexed, bevacizumab, and erlotinib were rapidly adopted.
Table 2.
Antineoplastic Treatment and Survival Among Patients With Advanced-Stage Non–Small-Cell Lung Cancer and Treated With Antineoplastic Agents, 12 Months After Diagnosis, SEER-Medicare Data
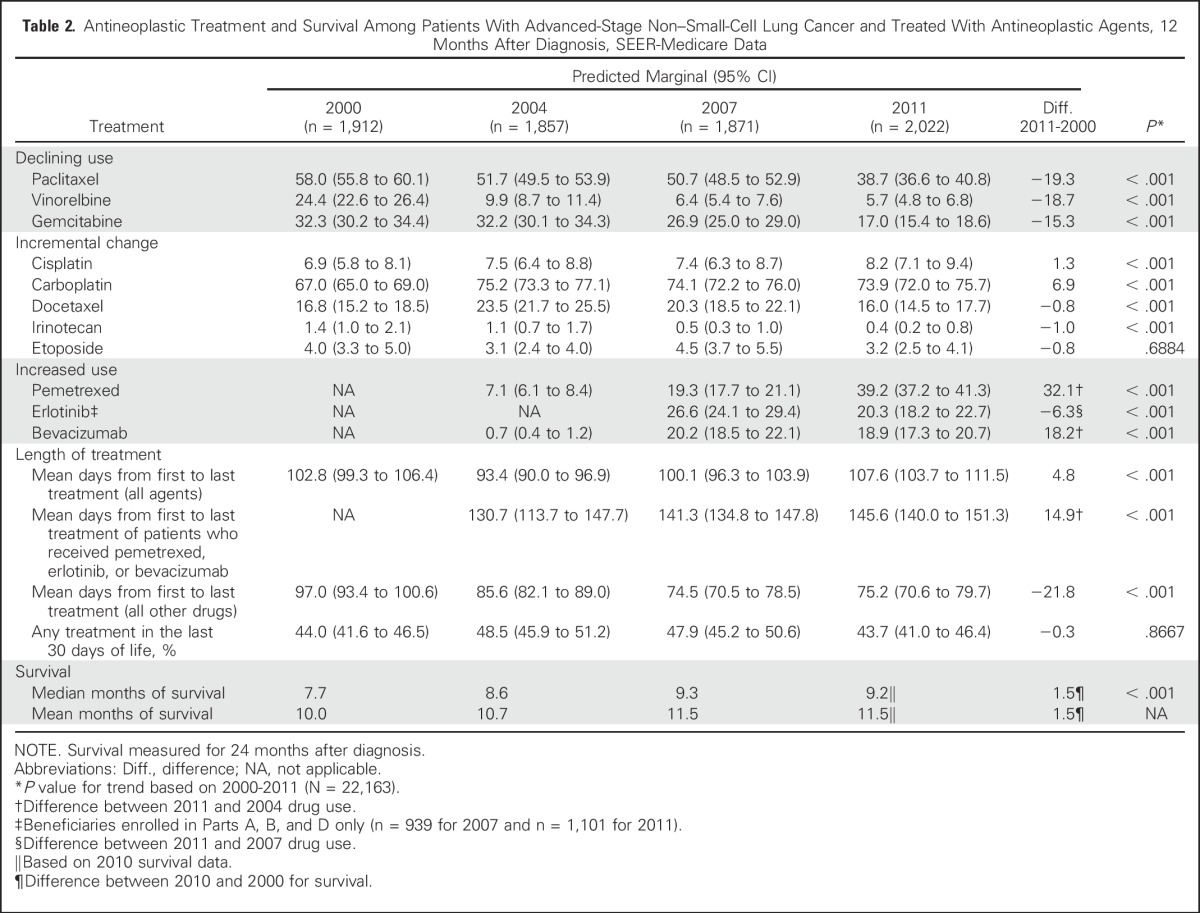
In 2000, the average number of days of chemotherapy treatment was 103. In 2011, the average number of days of chemotherapy or combination of chemotherapy and a targeted agent was 108. The average treatment duration for patients who received pemetrexed, bevacizumab, or erlotinib was 146 days in 2011. In contrast, the average number of days for patients who did not received these treatments was 75 in 2011. The percentage of patients who received treatment in the last 30 days of life remained unchanged (approximately 44%).
Spending
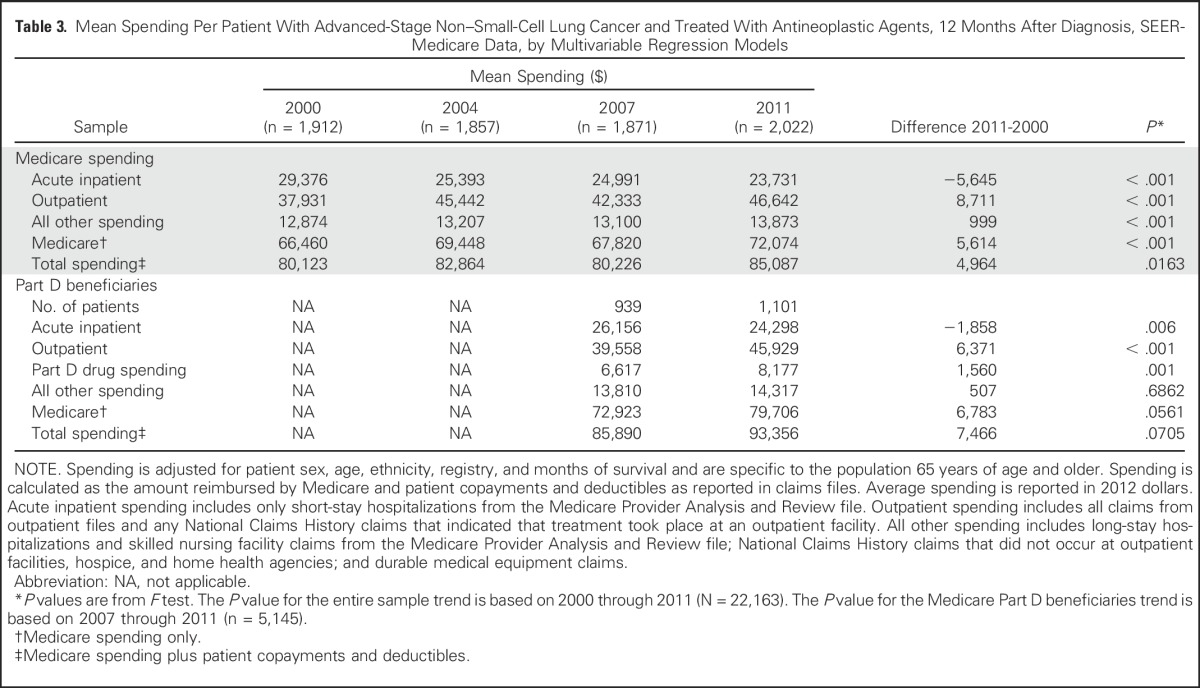
Table 3 lists the adjusted mean Medicare and total spending per patient per year from the multivariable regression models. Medicare and total spending increased modestly from $66,460 in 2000 to $72,074 in 2011 and from $80,123 in 2000 to $85,087 in 2011, respectively. With the increased use of targeted agents, the expectation was that Medicare spending would have risen more substantially. However, increases in outpatient spending were offset by decreases in inpatient use. The total average acute inpatient spending decreased from $29,376 in 2000 to $23,731 in 2011, a decrease of > $5,000 per patient. In contrast, outpatient spending increased from $37,931 in 2000 to $46,642 in 2011, which reflects an increase of 23%. For the subset of patients with Medicare Part D prescription drug coverage, Part D total gross drug spending increased from $6,617 in 2007 to $8,177 in 2011, which reflects a 24% increase. Part D beneficiaries had nearly a 9% increase in total Medicare spending from 2007 to 2011; this increase is marginally statistically significant (P = .07).
Table 3.
Mean Spending Per Patient With Advanced-Stage Non–Small-Cell Lung Cancer and Treated With Antineoplastic Agents, 12 Months After Diagnosis, SEER-Medicare Data, by Multivariable Regression Models
Survival
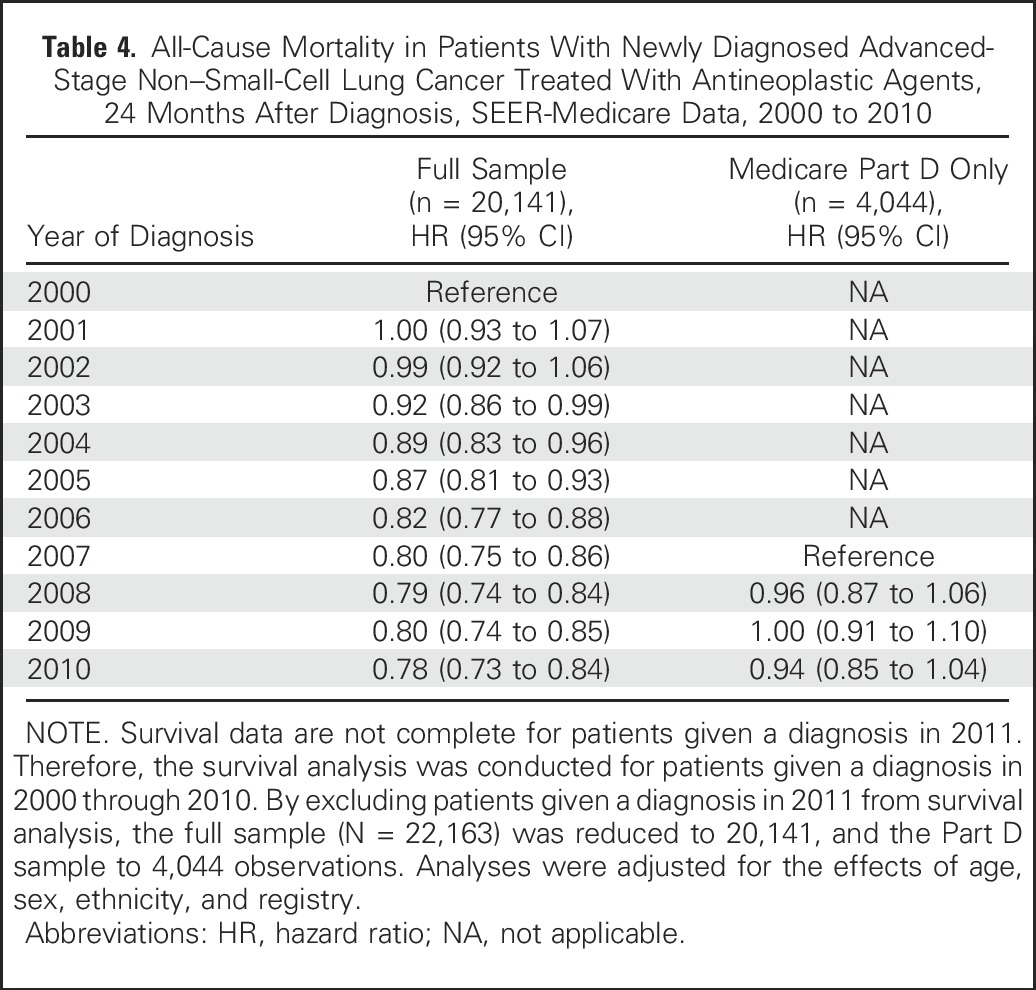
Table 4 lists adjusted HRs for patients in the full sample and patients with Medicare Part D coverage. Starting in 2003, statistically significant survival gains were observed relative to the reference year 2000 (HR, 0.92; 95% CI, 0.86 to 0.99). For 2010, we report an HR of 0.78 (95% CI, 0.73 to 0.84) relative to survival in 2000. Adjusted HRs for Part D beneficiaries were not statistically significantly different from 2007. Although adjusted HRs for the full sample are statistically significant relative to year 2000, the absolute gain in survival was small. As listed in Table 2, median survival in 2000 was 7.7 months, and in 2010 it was 9.2 months, an increase of 1.5 months (P < .001).
Table 4.
All-Cause Mortality in Patients With Newly Diagnosed Advanced-Stage Non–Small-Cell Lung Cancer Treated With Antineoplastic Agents, 24 Months After Diagnosis, SEER-Medicare Data, 2000 to 2010
DISCUSSION
We examined trends in antineoplastic agents used to treat advanced-stage NSCLC in elderly Medicare fee-for-service patients for a 12-year period. Several findings have clinical and policy relevance. First, a major shift has occurred in the agents used to treat advanced NSCLC, and this shift is accompanied by increased treatment duration. Second, median survival increased modestly. Finally, with the use of newer, more-expensive agents, one would have expected medical spending to increase substantially, but instead, spending rose only slightly over the study period; this was largely due to offsets in inpatient use.
Older drugs declined in use, whereas newer agents, such as pemetrexed, erlotinib, and bevacizumab (all approved in 2004), were rapidly adopted to treat patients with metastatic NSCLC. Along with the adoption of these agents came a marked increase in treatment duration. Patients treated with newer drugs averaged approximately 146 days on therapy, which is approximately 5 weeks longer than the duration for those who did not receive these agents. Longer treatment durations are associated with these agents as well as increased cumulative toxicities,15,16 increased spending, and modest survival benefits.17,18 Because these treatments are used with greater frequency and as first-line therapy, we can expect treatment durations to extend in the future.
A pattern that remained unchanged is the length of time patients are treated before death and the percentage of patients treated within the last 30 days of life. Almost one half (44%) of patients received chemotherapy or a targeted agent within the last 30 days of life, regardless of year of diagnosis. Current practice is to treat patients until evidence of disease progression, after which time patients may live only a few weeks.
In 2000, the median survival was 7.7 months. In a prior analysis of SEER-Medicare data (2006 through 2009), Langer et al19 reported that patients treated with bevacizumab had a median survival of 10.5 months relative to the 8.5 months of survival in patients not treated with bevacizumab. Erlotinib added to regimens that included bevacizumab modestly increased survival in patients with epidermal growth factor receptor mutations, but survival benefits are not observed in patients without these mutations.20,21 The trend toward value-based care underscores the need for information to help patients and physicians to decide whether to pursue treatments that may offer modest survival benefit in exchange for toxicities and significant medical expenditures. Patients with advanced-stage NSCLC face a daunting challenge because their mean and median survival times are shorter than those for patients with other types of metastatic disease, such as breast and colorectal cancer.
Spending on outpatient care increased 23% from 2000 to 2011. Outpatient spending increases were due to use of more-expensive agents and longer therapy duration. The monthly costs of $5,551, $6,374, and $5,231 for bevacizumab, pemetrexed, and erlotinib, respectively, contrasted with older agents, such as cisplatin ($454 per month) and vinblastine ($586/month).22 Nationally, it is estimated that there will be 224,390 new cases of advanced NSCLC in 2016, of which 85% are NSCLC and 70% of those are metastatic (n = 133,512).1 Using estimates from our study that predicted the number of patients taking antineoplastic agents, we expect monthly drug spending to be nearly $36 million, $87 million, and $37 million for those treated with bevacizumab, pemetrexed, and erlotinib, respectively, not including the cost of other agents and supportive medications associated with multidrug regimens. Increasingly, Medicare beneficiaries are facing higher rates of coinsurance, leading to higher out-of-pocket costs for patients and their families.23
Total medical spending remained somewhat stable over the study period for the full sample, which is notable given the marked increase in the use of bevacizumab, pemetrexed, and erlotinib. The stability of total spending for advanced-stage NSCLC treatment reflects a reduced use of inpatient hospitalizations. Were drug spending more modest, the reduced hospital use could have resulted in a savings to the Medicare program for this highly prevalent disease. However, bevacizumab and pemetrexed are among the 10 most expensive drugs covered by Medicare Part B, with total annual payments approaching $882 million.24 Total Medicare spending increased for patients with Part D coverage by approximately 9%. However, spending on drugs increased by 24% from 2007 to 2011. Erlotinib, with 2014 monthly costs > $5,200,22 was the fourth leading oral oncologic drug sold in the United States in 2013.25
This study has limitations. Although we used the most recently available SEER-Medicare data, the last observation year for diagnosis was 2011, and the last year for vital status was 2013. We do not have information on patients’ functional status, quality of life, or toxicities and morbidity associated with treatment. This information would lend insight into the value of treatments received for particular patient profiles. We do not estimate spending beyond 12 months. Therefore, total spending is incomplete for patients who survived beyond 12 months. However, the median survival was 9.2 months in 2010; therefore, we are comfortable that we captured spending for the majority of the sample. We also do not have information about variation in the practice patterns and outcomes that may result from regional difference or the types of facilities that deliver care. Research has demonstrated that regional Medicare spending variations are not associated with survival difference for patients with advanced-stage NSCLC.26 Finally, information about oral agents such as erlotinib is only available for the subset of patients enrolled in Medicare Part D. Therefore, the generalizability of the estimates for erlotinib use is unknown, although in this study, patient characteristics were similar for those enrolled in Part D and the remainder of the Medicare sample.
Between 2000 and 2011, major changes occurred in the agents used to treat advanced-stage NSCLC. Along with these changes came an increase in treatment duration and a modest increase in survival and spending. Taken together, we believe that these findings are an excellent example of the constellation of characteristics that make value-based decisions in health care so challenging. Value-based decisions, as promoted by ASCO,27 urge providers and patients to consider clinical benefit, toxicities, and costs in their treatment decisions.24 In advanced NSCLC, only a subset of patients will realize benefit, and many patients will experience toxicities that reduce their quality of life. Moreover, patient responsibility for total spending is substantial (approximately $13,000 in 2011 alone). To make a rational value-based decision to try new agents for advanced NSCLC, more patient-level data on the likelihood of benefit, toxicities, and conditions (eg, comorbidities) that could alter these outcomes are needed. This evidence becomes more critical as new agents are rapidly approved. The Food and Drug Administration added 15 new cancer drugs and biologics in 2015,28 and spending for new, expensive agents has been rising more rapidly than the other components of cancer care cost.29 New specialty drugs, such as those used to treat cancer, have been a major driver in the rapid increase in prescription drug expenditures.30 Patients with cancer and their families bear a significant portion of the financial burden. In a national survey, approximately one quarter of families who experienced a cancer death within the last year reported that the cost of care was a major financial burden; one third used all or most of their savings.31 For these reasons, an understanding of how practice patterns for highly prevalent cancers such as advanced-stage NSCLC are changing over time and how these changes affect medical care costs and affect patient outcomes is important. More evidence is needed to weigh the benefit of these agents against their costs and the possibility of savings with lower prices and lower inpatient use.
AUTHOR CONTRIBUTIONS
Conception and design: Cathy J. Bradley, K. Robin Yabroff, Angela B. Mariotto, Joan L. Warren
Collection and assembly of data: Angela B. Mariotto, Christopher Zeruto
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Antineoplastic Treatment of Advanced-Stage Non–Small-Cell Lung Cancer: Treatment, Survival, and Spending (2000 to 2011)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Cathy J. Bradley
No relationships to disclose
K. Robin Yabroff
No relationships to disclose
Angela B. Mariotto
No relationships to disclose
Christopher Zeruto
No relationships to disclose
Quyen Tran
No relationships to disclose
Joan L. Warren
No relationships to disclose
REFERENCES
- 1.American Cancer Society Key statistics for lung cancer. http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-key-statistics
- 2.National Cancer Institute: SEER stat fact sheets: Lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html
- 3.Ramsey SD, Howlader N, Etzioni RD, et al. Chemotherapy use, outcomes, and costs for older persons with advanced non-small-cell lung cancer: Evidence from Surveillance, Epidemiology and End Results-Medicare. J Clin Oncol. 2004;22:4971–4978. doi: 10.1200/JCO.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Rocha Lima CM, Herndon JE, II, Kosty M, et al. Therapy choices among older patients with lung carcinoma: An evaluation of two trials of the Cancer and Leukemia Group B. Cancer. 2002;94:181–187. doi: 10.1002/cncr.10174. [DOI] [PubMed] [Google Scholar]
- 5.Chrischilles EA, Pendergast JF, Kahn KL, et al. Adverse events among the elderly receiving chemotherapy for advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:620–627. doi: 10.1200/JCO.2009.23.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gridelli C, Balducci L, Ciardiello F, et al. Treatment of elderly patients with non-small-cell lung cancer: Results of an International Expert Panel Meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer. 2015;16:325–333. doi: 10.1016/j.cllc.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Goulart B, Ramsey S. A trial-based assessment of the cost-utility of bevacizumab and chemotherapy versus chemotherapy alone for advanced non-small cell lung cancer. Value Health. 2011;14:836–845. doi: 10.1016/j.jval.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Klein R, Wielage R, Muehlenbein C, et al. Cost-effectiveness of pemetrexed as first-line maintenance therapy for advanced nonsquamous non-small cell lung cancer. J Thorac Oncol. 2010;5:1263–1272. doi: 10.1097/JTO.0b013e3181e15d16. [DOI] [PubMed] [Google Scholar]
- 9.Bradbury PA, Tu D, Seymour L, et al. Economic analysis: Randomized placebo-controlled clinical trial of erlotinib in advanced non-small cell lung cancer. J Natl Cancer Inst. 2010;102:298–306. doi: 10.1093/jnci/djp518. [DOI] [PubMed] [Google Scholar]
- 10.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40(suppl):IV-55–IV-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 11.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yabroff KR, Warren JL, Banthin J, et al. Comparison of approaches for estimating prevalence costs of care for cancer patients: What is the impact of data source? Med Care 47S64–S69.2009suppl [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graubard BI, Korn EL. Predictive margins with survey data. Biometrics. 1999;55:652–659. doi: 10.1111/j.0006-341x.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- 14.Lund JL, Harlan LC, Yabroff KR, et al. Should cause of death from the death certificate be used to examine cancer-specific survival? A study of patients with distant stage disease. Cancer Invest. 2010;28:758–764. doi: 10.3109/07357901003630959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JO, Kim SW, Ahn JS, et al. Phase III trial of two versus four additional cycles in patients who are nonprogressive after two cycles of platinum-based chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007;25:5233–5239. doi: 10.1200/JCO.2007.10.8134. [DOI] [PubMed] [Google Scholar]
- 16.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 17.Socinski MA, Baggstrom MQ, Hensing TA. Duration of therapy in advanced, metastatic non-small-cell lung cancer. Clin Adv Hematol Oncol. 2003;1:33–38. [PubMed] [Google Scholar]
- 18.Soon YY, Stockler MR, Askie LM, et al. Duration of chemotherapy for advanced non-small-cell lung cancer: A systematic review and meta-analysis of randomized trials. J Clin Oncol. 2009;27:3277–3283. doi: 10.1200/JCO.2008.19.4522. [DOI] [PubMed] [Google Scholar]
- 19.Langer C, Ravelo A, Hazard SJ, et al. Comparison of survival and hospitalization rates between Medicare patients with advanced NSCLC treated with bevacizumab-carboplatin-paclitaxel and carboplatin-paclitaxel: A retrospective cohort study Lung Cancer 86350-3572014 [DOI] [PubMed] [Google Scholar]
- 20.Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): An open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi T, Ando M, Asami K, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA) J Clin Oncol. 2014;32:1902–1908. doi: 10.1200/JCO.2013.52.4694. [DOI] [PubMed] [Google Scholar]
- 22.Memorial Sloan Kettering Cancer Center Cancer drug costs for a month of treatment at initial Food and Drug Administration approval. https://www.mskcc.org/sites/default/files/node/25097/documents/120915-drug-costs-table.pdf
- 23.Kaiser Health News: Coinsurance trend means seniors likely to face higher out-of-pocket drug costs, report says, 2016. http://khn.org/news/coinsurance-trend-means-seniors-likely-to-face-higher-out-of-pocket-drug-costs-report-says
- 24.American Society of Clinical Oncology: The State of Cancer Care in America: 2016. http://www.asco.org/research-progress/reports-studies/cancer-care-america-2016#/message-ascos-president [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FiercePharma: Top 10 best-selling cancer drugs of 2013. http://www.fiercepharma.com/special-reports/top-10-best-selling-cancer-drugs-2013
- 26.Brooks GA, Li L, Sharma DB, et al. Regional variation in spending and survival for older adults with advanced cancer. J Natl Cancer Inst. 2013;105:634–642. doi: 10.1093/jnci/djt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnipper LE, Davidson NE, Wollins DS, et al. American Society of Clinical Oncology Statement: A conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33:2563–2577. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration: Hematology/oncology (cancer) approvals & safety notifications. http://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm279174.htm
- 29.Politico. Health care spending again accelerating. http://www.politico.com/story/2015/07/health-care-spending-hike-prediction-120740
- 30.Centers for Medicare & Medicaid Services: National Health Expenditure Projections 2014-2024. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-reports/NationalHealthExpendData/Downloads/Proj2015.pdf
- 31.Cagle JG, Carr DC, Hong S, et al: Financial burden among US households affected by cancer at the end of life. Psychooncology, 25:919-926, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]