Abstract
The Oncology Grand Rounds series is designed to place original reports published in the Journal into clinical context. A case presentation is followed by a description of diagnostic and management challenges, a review of the relevant literature, and a summary of the authors’ suggested management approaches. The goal of this series is to help readers better understand how to apply the results of key studies, including those published in Journal of Clinical Oncology, to patients seen in their own clinical practice.
A 64-year-old woman with a history of hypertension and type 2 diabetes had been in her usual state of health until she developed symptoms of diarrhea, abdominal bloating, and discomfort in the midepigastrium. Evaluation with a contrast-enhanced abdominopelvic computed tomography (CT) scan demonstrated a mass in the pancreatic body that was approximately 3.1 cm × 2 cm × 2.1 cm in size with abutment of the portal vein–superior mesenteric vein confluence for less than 180°. The confluence was narrowed but without thrombosis. No tumor–vessel interface was noted at the superior mesenteric artery, celiac artery, or common hepatic artery. Several peripancreatic lymph nodes were observed that measured up to 11 mm × 5 mm. No evidence for distant spread of disease was identified. An upper endoscopy with endoscopic ultrasound was performed and fine-needle aspirates of the pancreas mass were positive for malignant cells that were consistent with adenocarcinoma. Chest CT scan without intravenous contrast demonstrated no evidence of metastatic disease. The patient came to the clinic to discuss management of her newly diagnosed malignancy.
CHALLENGES IN DIAGNOSIS AND MANAGEMENT
Pancreatic cancer is the third leading cause of cancer-related death in the United States.1 Surgical resection offers the only chance for cure, but less than 20% of patients present with early-stage disease amenable to surgery.2 Pancreatectomy is a complex surgical procedure best performed at a high-volume surgical center, where perioperative mortality has steadily declined.3 Even with pancreatectomy, 75% of patients with localized disease develop tumor recurrence as a result of occult metastatic disease, poor tolerance and ineffectiveness of adjuvant therapy, and presence of residual tumor cells at surgical resection margins.4,5
Marked regional variation is present in the management approach to localized pancreatic cancer. Use of neoadjuvant chemotherapy before surgical resection—an approach with growing utilization—is highlighted in the article by Mokdad and colleagues.6,7 Postoperative chemotherapy improves survival in patients with resected pancreatic cancer, but adjuvant treatment can be limited by postoperative complications, poor tolerance, and early disease recurrence. To improve compliance with systemic therapy, address occult micrometastatic disease earlier in the treatment course, and better select patients for surgical resection, an alternative approach of delivering therapy before resection has been advocated. Neoadjuvant therapy also has the potential benefit of reducing positive margin rates, particularly when tumors involve surrounding vascular structures. Nevertheless, evidence in favor of neoadjuvant therapy remains limited for the management of localized pancreatic cancer.
SUMMARY OF RELEVANT LITERATURE
Randomized trials have demonstrated improved survival in patients with localized pancreatic cancer who have received adjuvant treatment. Initially, the European Study Group for Pancreatic Cancer (ESPAC)-1 study randomly assigned patients after resection to receive adjuvant fluorouracil (5-FU) chemotherapy, chemoradiotherapy, neither treatment, or both treatments. After a median follow-up time of 47 months, the 5-year survival rate was 21% among patients who received chemotherapy compared with 8% among those who did not, which suggested an overall survival (OS) benefit with use of 6 months of adjuvant 5-FU.8 The clinical benefit of gemcitabine in the adjuvant setting was demonstrated in the phase III CONKO-001 trial (Charite Onkologie 001). In CONKO-001, 368 patients who underwent resection for pancreatic cancer were randomly assigned to receive 6 months of gemcitabine 1,000 mg/m2 on days 1, 8, and 15 of a 28-day cycle or observation. Patients who received gemcitabine achieved superior OS (median OS, 22.8 months v 20.2 months; P = .01) and improved 5-year survival rates (20.7% v 10.4%) compared with surgery only.9
Two additional studies have demonstrated similar efficacy for 5-FU and gemcitabine in the adjuvant setting. The ESPAC-3 study evaluated 1,088 patients who underwent resection and compared treatment with gemcitabine with 5-FU for 6 months and found no statistically significant difference in disease-free survival or OS between the two arms.10 RTOG 97-04 randomly assigned patients to 5-FU versus gemcitabine chemotherapy for 4 months, sandwiched around 5-FU–based chemoradiation. No statistically significant differences were noted in OS, which emphasized the role for either 5-FU or gemcitabine in the adjuvant setting.11 Given the efficacy of gemcitabine and 5-FU individually, the phase III ESPAC-4 trial compared gemcitabine and capecitabine (GEMCAP) with gemcitabine monotherapy in 732 patients with resected pancreatic cancer. ESPAC investigators reported at the 2016 ASCO Annual Meeting that median survival was 28 months for GEMCAP treatment compared with 25.5 months for gemcitabine monotherapy, with a similar adverse effect profile between the two treatment programs.12 The 5-year survival rate was estimated as 28.8% with GEMCAP versus 16.3% for gemcitabine monotherapy, which identified GEMCAP as a new adjuvant treatment option for patients with surgically resected pancreatic cancer. Several recently completed or ongoing trials have evaluated or are evaluating other treatment programs in the adjuvant setting, including gemcitabine plus nab-paclitaxel and FOLFIRINOX (5-FU, folinic acid, irinotecan, and oxaliplatin).13-16
The role of radiation therapy remains uncertain in patients with resectable pancreas cancer. Results of two small studies that were led by the Gastrointestinal Tumor Study Group and European Organization for Research and Treatment of Cancer suggest a potential benefit to concurrent chemoradiotherapy with 5-FU compared with observation alone in patients with resected cancer in the head of the pancreas.17,18 Likewise, multiple retrospective analyses support a benefit for adjuvant chemoradiotherapy.19 Conversely, the ESPAC-1 trial discussed above did not confirm a benefit to adjuvant chemoradiation8; however, the usefulness of these randomized studies is limited as a result of issues with study design, sample size, treatment implementation, and antiquated radiation delivery, including use of split-course fractionation. To address these issues, the ongoing RTOG 0848 study was initiated and randomly assigns patients to adjuvant fluoropyrimidine-based radiotherapy or observation after 6 months of gemcitabine-based chemotherapy.20 In addition, given the recent, promising results with respect to treatment response and tolerability of stereotactic body radiotherapy (SBRT), investigation of the role of SBRT in neoadjuvant treatment paradigms is ongoing.21,22 SBRT offers several clinical advantages over standard long-course chemoradiation, including short treatment times—3 to 5 days compared with 28 days with long-course chemoradiation—and less acute toxicity. However, these advantages are balanced against the potential for greater rates of chronic toxicities.
Although adjuvant chemotherapy has demonstrated improved survival, benefits of a neoadjuvant treatment approach have been suggested, including earlier treatment of occult micrometastatic disease, better tolerance of systemic therapy preoperatively, improved selection of patients for surgical resection without rapidly progressive disease, and lower rates of positive surgical resection margins. Multiple phase II studies have evaluated neoadjuvant regimens that were composed of either chemotherapy alone or concurrent chemotherapy and radiation.7,23 These studies demonstrated the feasibility of administering neoadjuvant chemoradiation with 5-FU or gemcitabine as radiosensitizing agents.24,25 In a randomized phase II study of neoadjuvant treatment versus upfront surgical resection, Golcher and colleagues26 demonstrated the feasibility of preoperative gemcitabine and cisplatin with concurrent radiation, but the study was underpowered to demonstrate this approach as being superior to upfront surgical resection. Most recently, a multi-institutional Alliance cooperative group study demonstrated the feasibility of FOLFIRINOX followed by chemoradiation as neoadjuvant therapy.27 Prospective studies,28-30 including the NEOPA trial31 of preoperative gemcitabine and radiation therapy and SWOG 1505,32 which is comparing perioperative FOLFIRINOX with perioperative gemcitabine and nab-paclitaxel, are underway.
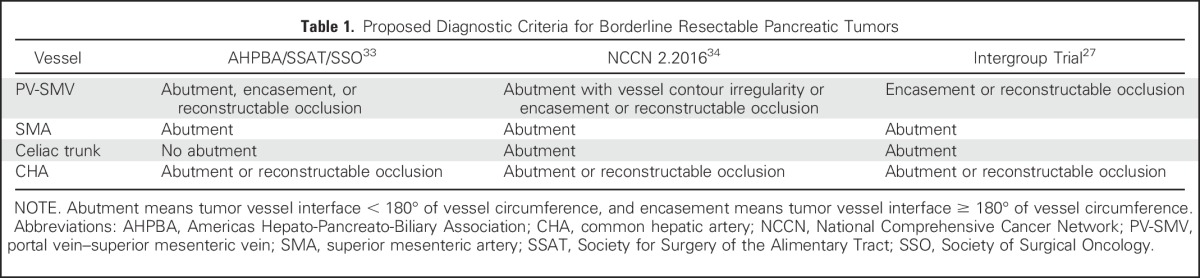
Of importance, positive surgical resection margins are common after pancreatectomy and remain a strong negative prognostic factor for patient survival.8,10 Therefore, a subset of patients with resectable pancreatic cancer is classified by consensus guidelines as having borderline-resectable disease, which is characterized by local tumor anatomy thought to confer a high risk for microscopically positive surgical margins with upfront surgical resection (Table 1).27,33,34 Although single-institution experience is accumulating,35 no studies have clearly demonstrated the benefit of neoadjuvant therapy, even in this subgroup of patients who are at high risk for recurrent disease as a result of local residual tumor cells and/or or more aggressive disease biology. Thus, at the current time, data to guide a specific chemotherapy program with or without radiation for the optimal treatment of these patients are limited. Future clinical trial enrollment will be critical for identifying an optimal treatment approach, and a large, multi-institutional phase II trial has been initiated to examine two treatment programs in patients with borderline-resectable disease (Alliance protocol A021101).
Table 1.
Proposed Diagnostic Criteria for Borderline Resectable Pancreatic Tumors
Analyzing patient data from the National Cancer Database between 2006 and 2012, Mokdad and colleagues asked whether patient survival differed between two groups of patients with stage I or stage II resected adenocarcinoma of the pancreatic head: those who received neoadjuvant treatment followed by resection and those who underwent upfront surgical resection with or without adjuvant therapy. The authors used propensity score matching to control for baseline differences in the two groups and demonstrated a modestly increased survival among patients who received neoadjuvant therapy.6 Although these findings are a valuable addition to the literature, inherent limitations of the nonrandomized retrospective design temper their immediate applicability to practice and, at the same time, further support the need for well-designed randomized studies that evaluate the neoadjuvant treatment approach.
SUGGESTED APPROACHES TO MANAGEMENT
In patients with nonmetastatic pancreatic cancer, the first consideration is whether radiographic evidence supports surgical resectability. This evaluation requires a staging work-up with a multiphasic pancreas-protocol abdomen and pelvis CT scan, together with measurement of serum cancer antigen 19-9 (CA 19-9) and consultation in a multidisciplinary pancreas clinic that is staffed by providers from gastroenterology, medical oncology, radiation oncology, radiology, and surgical oncology who have expertise in pancreatic cancer management.36 Patients with likely resectable or borderline-resectable disease would be considered future surgical candidates, although whether patients with unresectable disease as a result of vascular involvement should also be considered for future resection is an ongoing area of research.37 Patients with metastatic disease would not be considered surgical candidates, and chemotherapy remains the mainstay of current treatment programs for these patients.38
The National Comprehensive Cancer Network guidelines34 recommend that patients with resectable pancreatic adenocarcinoma without high-risk features undergo upfront surgical resection and receive adjuvant gemcitabine or fluoropyrimidine chemotherapy with or without chemoradiation, with neoadjuvant therapy recommended only when delivered as part of a clinical trial. For patients with high-risk features, which are defined as highly elevated serum CA 19-9, a large primary tumor, large regional lymph nodes, excessive weight loss, or extreme pain, neoadjuvant therapy with FOLFIRINOX or gemcitabine and nab-paclitaxel with or without concurrent radiation therapy may be considered. For borderline-resectable disease, the guidelines recommend neoadjuvant therapy with an approach that is similar to that for patients with resectable disease with high-risk features. As a result of a lack of evidence, specific guidance is not provided regarding duration and make-up of neoadjuvant therapy programs or whether further treatment should be administered postoperatively. In the recently published ASCO Clinical Practice Guideline for Potentially Curable Pancreatic Cancer, primary surgical resection is considered for patients with no metastases, appropriate performance status, and no radiographic interface between the primary tumor and mesenteric vasculature.2 Adjuvant therapy is recommended for all patients, whereas chemoradiation can be considered after 4 to 6 months of chemotherapy for patients who did not receive such therapy preoperatively and for those with positive margins or lymph nodes. Preoperative therapy is recommended for patients whose pancreas tumor demonstrates a radiographic interface with mesenteric vasculature or when distant disease is suspected but not clearly documented. Furthermore, the authors note that preoperative therapy may be offered as an alternate strategy for those patients who are being considered for upfront resection.
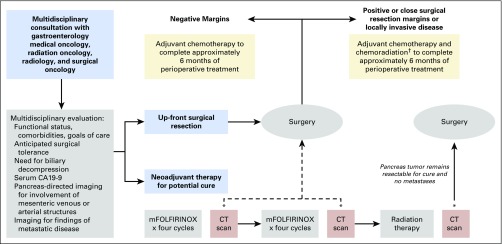
At Dana-Farber/Brigham and Women’s Cancer Center, patients with nonmetastatic pancreatic cancer are seen in a multidisciplinary clinic for consultation with providers from medical oncology, radiation oncology, radiology, gastroenterology, nutrition, and surgical oncology. The care team assesses patient functional status, comorbidities, and goals of care, evaluates for biliary obstruction requiring intervention, measures serum CA 19-9, and ensures appropriate pancreas-dedicated imaging to evaluate tumor involvement of adjacent vascular structures. For patients with adequate functional status, perceived ability to tolerate pancreatectomy, low serum CA 19-9—that is, < 10 times the upper limit of normal without biliary obstruction—and no abutment of mesenteric venous or arterial structures, upfront surgical resection is generally advised (Table 2 and Fig 1). Within 3 to 12 weeks of surgery, patients begin adjuvant chemotherapy with weekly gemcitabine or, alternatively, 5-FU and leucovorin. On the basis of the recently reported data from ESPAC-4, patients with excellent functional status are considered for adjuvant treatment with gemcitabine and capecitabine. Patients with a positive or close (< 1 mm) surgical resection margin or an otherwise locally invasive tumor receive long-course radiation concurrent with continuous infusion 5-FU or oral capecitabine after completing 5 months of adjuvant chemotherapy.
Table 2.
Classification of Nonmetastatic Pancreatic Cancer Used at Dana-Farber/Brigham and Women’s Cancer Center
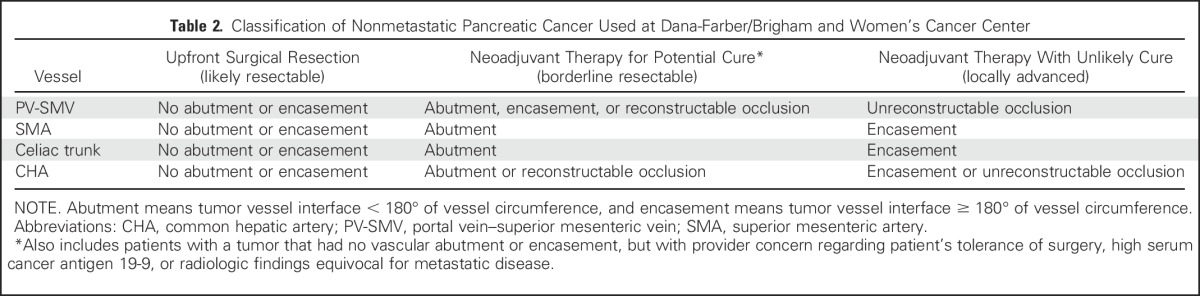
Fig 1.
General treatment algorithm for patients recommended for upfront surgical resection or neoadjuvant treatment followed by surgical resection at Dana-Farber/Brigham and Women’s Cancer Center. *Multidisciplinary consultation after each computed tomography (CT) scan to determine management plan regarding a continuation of the treatment algorithm versus a change in treatment approach, including potential surgical resection. †Adjuvant chemoradiation is pursued only for patients who did not receive radiation preoperatively. Close surgical resection margin is classified as ≤ 1 mm. mFOLFIRINOX, modified fluorouracil, folinic acid, irinotecan, and oxaliplatin.
Patients are treated neoadjuvantly if they have abutment of mesenteric vascular structures or worrisome features on multidisciplinary review, such as concern for poor surgical tolerance, high serum CA 19-9, or potential distant disease (Table 2 and Fig 1). If functional status permits, patients receive eight cycles of FOLFIRINOX administered every 2 weeks followed by radiation therapy, then surgical resection. For some patients, tolerability concerns lead to substitution of gemcitabine plus nab-paclitaxel for FOLFIRINOX. Radiation therapy is administered as long-course treatment with continuous infusion 5-FU (or oral capecitabine) or SBRT without concurrent chemotherapy. The goal for the total treatment course—neoadjuvant plus adjuvant—is for patients to receive 4 months of chemotherapy plus radiotherapy or 6 months of chemotherapy alone, primarily on the basis of the length of treatment programs evaluated in the adjuvant setting. When adjuvant chemotherapy is administered, it is generally a derivative of the neoadjuvant treatment program, depending on prior tolerance, recovery from surgery, and treatment response identified in the surgical resection specimen.
CLINICAL DISCUSSION
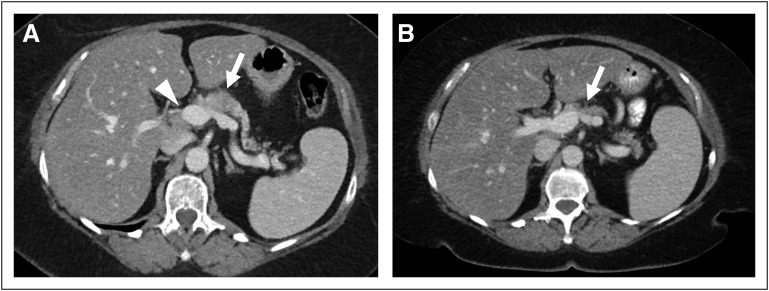
After her diagnosis, our patient was evaluated in the Dana-Farber/Brigham and Women’s Cancer Center pancreaticobiliary multidisciplinary clinic. Given the portal vein–superior mesenteric vein abutment identified on imaging (Fig 2), we recommended systemic therapy with modified FOLFIRINOX on an every-2-week cycle. After four, eight, and 12 cycles of therapy, restaging CT scans showed continued reduction in the size of the pancreatic mass. With this ongoing response, neoadjuvant radiation was deferred and the patient underwent a distal pancreatectomy that was notable for residual microscopic foci of adenocarcinoma staged as ypT3N1 with one of eight positive lymph nodes and negative resection margins. No additional therapy was administered after surgery. A surveillance program was initiated with interval clinic visits, serum CA 19-9 measurement, and imaging. The patient is now 3 years from initiation of neoadjuvant chemotherapy without evidence of disease recurrence.
Fig 2.
(A and B) Axial computed tomography (CT) images of the abdomen (A) before and (B) after neoadjuvant chemotherapy. (A) An initial diagnostic abdominopelvic CT scan demonstrated a hypodense mass in the proximal pancreatic body (arrow), with abutment of the portal vein–superior mesenteric vein confluence (arrowhead). (B) A follow-up abdominopelvic CT scan after 12 cycles of modified FOLFIRINOX (fluorouracil, folinic acid, irinotecan, and oxaliplatin) demonstrated a decrease in the size of the pancreatic body mass (arrow).
ACKNOWLEDGMENT
Supported by Burroughs Wellcome Fund CAMS and National Institutes of Health Grant No. KL2 TR001100 (to J.D.M.); National Institutes of Health, National Cancer Institute Grants No. U01 CA210171 and P50 CA127003, Department of Defense Grant No. CA130288, Lustgarten Foundation, Pancreatic Cancer Action Network, Celgene, Promises for Purple, Robert T. and Judith B. Hale Fund, Peter R. Leavitt Family Fund, Noble Effort Fund, and Wexler Family Fund (to B.M.W.). We thank the members of the pancreas cancer guidelines working group within the Gastrointestinal Cancer Center at Dana-Farber/Brigham and Women’s Cancer Center (DF/BWCC) for input into the treatment approaches illustrated in this manuscript, including Leona Doyle, MD, Charles Fuchs, MD, Jason Hornick, MD, Kunal Jajoo, MD, Harvey Mamon, MD, Neil Martin, MD, Douglas Rubinson, MD, Paul Shyn, MD, Richard Swanson, MD, and Matthew Yurgelun, MD. We also thank our past and current patients treated for pancreatic cancer who have shaped how care is delivered at DF/BWCC.
Footnotes
See accompanying article on page 515
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
When, What, and Why of Perioperative Treatment of Potentially Curable Pancreatic Adenocarcinoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Kimberly Perez
No relationship to disclose
Thomas E. Clancy
No relationship to disclose
Joseph D. Mancias
No relationship to disclose
Michael H. Rosenthal
Patents, Royalties, Other Intellectual Property: Patent on three-dimensional endoscope
Brian M. Wolpin
Research Funding: Celgene
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Khorana AA, Mangu PB, Berlin J, et al. Potentially curable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34:2541–2556. doi: 10.1200/JCO.2016.67.5553. [DOI] [PubMed] [Google Scholar]
- 3.Clancy TE. Surgery for pancreatic cancer. Hematol Oncol Clin North Am. 2015;29:701–716. doi: 10.1016/j.hoc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Ferrone CR, Pieretti-Vanmarcke R, Bloom JP, et al. Pancreatic ductal adenocarcinoma: Long-term survival does not equal cure. Surgery. 2012;152(suppl 1):S43–S49. doi: 10.1016/j.surg.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant therapy followed by resection versus upfront resection for resectable pancreatic cancer: A propensity score matched analysis. J Clin Oncol. 10.1200/JCO.2016.68.5081 [epub ahead of print on September 12, 2016] [DOI] [PubMed]
- 7.Tsai S, Evans DB. Therapeutic advances in localized pancreatic cancer. JAMA Surg. 2016;151:862–868. doi: 10.1001/jamasurg.2016.1113. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 9.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 10.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 11.Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–1326. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neoptolemos J, Palmer D, Ghaneh P, et al. ESPAC-4: A multicenter, international, open-label randomized controlled phase III trial of adjuvant combination chemotherapy of gemcitabine (GEM) and capecitabine (CAP) versus monotherapy gemcitabine in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol. 2016;34 (abstr LBA4006) [Google Scholar]
- 13.Sinn M, Liersch T, Gellert K, et al. CONKO-005: Adjuvant therapy in R0 resected pancreatic cancer patients with gemcitabine plus erlotinib versus gemcitabine for 24 weeks—A prospective randomized phase III study. J Clin Oncol. 2015;33 doi: 10.1200/JCO.2017.72.6463. (abstr 4007) [DOI] [PubMed] [Google Scholar]
- 14. ClinicalTrials.gov Immunotherapy study for surgically resected pancreatic cancer. https://clinicaltrials.gov/ct2/show/NCT01072981?term=phase+III+algenpantucel-L&rank=2.
- 15.Tempero MA, Cardin DB, Biankin A, et al. APACT: A phase 3 randomized, open-label, multicenter trial evaluating the use of adjuvant nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with surgically resected ductal pancreatic adenocarcinoma. J Clin Oncol. 2014;32:5s. (abstr TPS4162) [Google Scholar]
- 16.NCIC Trials Group PA.6 and Pancreatic Cancer Canada. https://clinicaltrials.gov/ct2/show/NCT01526135?term=James+Biagi&rank=2.
- 17.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: Phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999;230:776–782, discussion 782-784. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: The Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–990. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ClinicalTrials.gov Gemcitabine hydrochloride with or without erlotinib hydrochloride followed by the same chemotherapy regimen with or without radiation therapy and capecitabine or fluorouracil in treating patients with pancreatic cancer that has been removed by surgery. https://clinicaltrials.gov/ct2/show/NCT01013649?term=rtog+0848&rank=1.
- 21.Goodman KA. Stereotactic body radiation therapy for pancreatic cancer. Cancer J. 2016;22:290–295. doi: 10.1097/PPO.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 22.Myrehaug S, Sahgal A, Russo SM, et al. Stereotactic body radiotherapy for pancreatic cancer: Recent progress and future directions. Expert Rev Anticancer Ther. 2016;16:523–530. doi: 10.1586/14737140.2016.1168698. [DOI] [PubMed] [Google Scholar]
- 23.Franke AJ, Rosati LM, Pawlik TM, et al. The role of radiation therapy in pancreatic ductal adenocarcinoma in the neoadjuvant and adjuvant settings. Semin Oncol. 2015;42:144–162. doi: 10.1053/j.seminoncol.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–1339. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 25.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 26.Golcher H, Brunner TB, Witzigmann H, et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: Results of the first prospective randomized phase II trial. Strahlenther Onkol. 2015;191:7–16. doi: 10.1007/s00066-014-0737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz MH, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX treatment followed by capecitabine-based chemoradiation for borderline resectable pancreatic cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg. 2016;151:e161137. doi: 10.1001/jamasurg.2016.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. ClinicalTrials.gov Adjuvant versus neoadjuvant plus adjuvant chemotherapy in resectable pancreatic cancer. https://clinicaltrials.gov/ct2/show/NCT01314027?term=NCT01314027&rank=1.
- 29. ClinicalTrials.gov . Gemcitabine, cisplatin, epirubicin, and capecitabine in treating patients with stage I-II resectable pancreatic cancer (PACT-15) https://clinicaltrials.gov/ct2/show/NCT01150630?term=NCT01150630&rank=1. [Google Scholar]
- 30. ClinicalTrials.gov . Randomized multicenter phase II/III study with adjuvant gemcitabine versus neoadjuvant/adjuvant FOLFIRINOX for resectable pancreas carcinoma. https://clinicaltrials.gov/ct2/show/NCT02172976?term=NCT02172976&rank=1. [Google Scholar]
- 31.Tachezy M, Gebauer F, Petersen C, et al. Sequential neoadjuvant chemoradiotherapy (CRT) followed by curative surgery vs. primary surgery alone for resectable, non-metastasized pancreatic adenocarcinoma: NEOPA—A randomized multicenter phase III study ( NCT01900327, DRKS00003893, ISRCTN82191749) BMC Cancer. 2014;14:411. doi: 10.1186/1471-2407-14-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.SWOG Protocol abstract: S1505—A randomized phase II study of perioperative mFOLFIRINOX versus gemcitabine/nab-paclitaxel as therapy for resectable pancreatic adenocarcinoma. http://swog.org/Visitors/ViewProtocolDetails.asp?ProtocolID=2295.
- 33.Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: Expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 34.National Comprehensive Cancer Network Pancreatic adenocarcinoma (version 2.2016) https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
- 35.Schwarz L, Katz MH. Diagnosis and management of borderline resectable pancreatic adenocarcinoma. Hematol Oncol Clin North Am. 2015;29:727–740. doi: 10.1016/j.hoc.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Pawlik TM, Laheru D, Hruban RH, et al. Evaluating the impact of a single-day multidisciplinary clinic on the management of pancreatic cancer. Ann Surg Oncol. 2008;15:2081–2088. doi: 10.1245/s10434-008-9929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–17. doi: 10.1097/SLA.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubinson DA, Wolpin BM. Therapeutic approaches for metastatic pancreatic adenocarcinoma. Hematol Oncol Clin North Am. 2015;29:761–776. doi: 10.1016/j.hoc.2015.04.012. [DOI] [PubMed] [Google Scholar]