Abstract
Purpose
Observational study evidence has associated overweight/obesity with decreased survival in women with breast cancer and with several other cancers. Although full-scale, definitive weight loss adjuvant intervention trials with cancer end points remain to be conducted, a number of randomized controlled trials have evaluated weight loss interventions in survivors of cancer in women. Findings from these trials in breast, endometrial, and ovarian cancer are reviewed.
Methods
A systematic review of randomized controlled clinical trials evaluating weight loss interventions was updated (for studies published 2013 to 2016), and clinical trials registers were searched for ongoing trials.
Results
Six new randomized trials in breast cancer survivors and two randomized trials in endometrial cancer survivors were identified. Evidence from these trials and the 10 earlier randomized trials in female cancer survivors provide support for the feasibility of recruiting women closer to the cancer diagnosis and efficacy for achieving weight loss, in particular with telephone-based interventions, and have identified the challenge of achieving significant weight loss in African American cancer survivors and of maintaining weight loss in any cancer survivor group. Seven ongoing randomized trials are evaluating the influence of weight loss interventions on cancer end points (five in breast cancer, one in ovarian cancer, and one in endometrial cancer).
Conclusion
After a decade of preliminary studies, ongoing randomized, controlled clinical trials will potentially provide definitive assessment of whether weight loss can improve breast cancer clinical outcome. Longer-term interventions (> 2 years’ duration) may be needed to optimize weight loss maintenance and any potential benefits on cancer end points.
INTRODUCTION
Obesity has been associated with adverse survival outcome in women with early-stage breast cancer in a series of observational studies1-3 beginning > 40 years ago, with a considerable body of supportive evidence available by 2002.4 In 2006, findings from the randomized Women’s Intervention Nutrition Study provided support for a potential influence of weight control on breast cancer outcome.5 In this trial, which enrolled 2,437 postmenopausal women with early-stage breast cancer, fat intake and body weight decreased in the dietary group, and a significant improvement in relapse-free survival was seen.5 In 2009, a National Cancer Institute–sponsored conference outlined the clinical trial rationale for studies of weight control and breast cancer outcome.6 The most recent meta-analysis of observational studies reported an increased risk of total mortality of 20% to 40% and breast cancer mortality of 25% to 35% in obese breast cancer survivors compared with their healthy-weight counterparts (on the basis of weight prediagnosis and within 12 months postdiagnosis).1 Against this background of decades of accumulating information regarding the association between obesity and breast cancer adverse outcome, definitive trial evidence from weight loss intervention trials in breast cancer survivors remains lacking. Finally, in 2015 recommendations for obesity clinical trials in cancer survivors were put forward in an ASCO Statement.7
In 2014, Reeves and colleagues8 published a systematic review of weight loss intervention trials in women with breast cancer including studies reported up until June 2013. Ten randomized controlled trials evaluating behaviorally based weight loss interventions in women with breast cancer were identified9-18 and are summarized here. These trials recruited relatively small samples, ranging from 2411,16 to 1029 women with early-stage breast cancer. One study was limited to a subgroup of breast cancer survivors, broadly defined as those with estrogen receptor (ER) –positive tumors,18 and two exclusively recruited African American or Hispanic women.11,12 Five studies reported mean time since diagnosis at baseline, with this ranging from 3.5 to 5.6 years.10-12,14,18
Interventions varied considerably in their behavioral targets, delivery, and duration. Six trials targeted both diet and physical activity,10-15 with the remaining four addressing dietary intake only. Most interventions were delivered by face-to-face sessions; however, three included intervention arms delivered primarily via telephone,10,11,13 and one included combined telephone and face-to-face sessions.14 Six studies evaluated interventions of ≤ 6 months’ duration,12,14-18 and the remaining four studies evaluated interventions of ≥ 12 months.9-11,13 Weight loss was the primary outcome reported in all studies. Statistically significant weight loss was observed in most intervention arms, with six studies achieving clinically meaningful mean weight loss of at least 5% of initial body weight.9,10,13,14,17,18
Various other outcomes were assessed in most of these trials; however, most were underpowered to detect these. Six measured clinical biomarkers,10,12-15,18,19 with relatively consistent changes seen in reductions in LDL cholesterol and glucose in groups losing weight. Five trials reported on insulin pathways, adipokines, and inflammatory markers, all factors associated with breast cancer progression.20,21 In two trials, where weight losses of > 5% were seen, reductions in insulin levels and insulin resistance were found.10,18,19 C-reactive protein was measured in three trials,12,15,18 with a nonstatistically significant reduction of 7% to 9% in the one trial with mean weight loss > 5%.18
Despite the relatively small sample sizes and heterogeneous interventions, these trials provided promising evidence that weight loss is safe and feasible to achieve (although of variable magnitude) in women diagnosed with breast cancer. However, few studies aimed to recruit women close to their breast cancer diagnosis. Furthermore, these studies are limited by their general focus on short-term weight loss and weight change as the primary outcome. There is little rationale to suggest that weight loss interventions and strategies, well-established to be effective in similar age women without cancer, would differ in their effectiveness in women with breast cancer or that the impact on metabolic and inflammatory biomarkers would differ. The real need is to determine whether effective weight loss interventions can be implemented in women with breast cancer closer to diagnosis, when they could potentially influence the higher recurrence risk seen in the first years after a breast cancer diagnosis.22,23
The systematic review by Reeves and colleagues8 identified a number of ongoing trials, aimed to address novel research questions and outcomes in powered trials in breast cancer survivors. The aim of this systematic review was to update the evidence on weight loss interventions published over the previous 3 years and to identify ongoing trials with cancer end points. Because obesity has been associated with incidence24-26 and poorer outcomes27-30 for women diagnosed with other female cancers, such as ovarian and endometrial cancer, we also sought to review the recent evidence on weight loss interventions in these populations.
METHODS
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,31 we conducted a structured search of PubMed (from July 1, 2013) and Web of Science (from January 1, 2013) to July 14, 2016. Titles and abstracts were searched using the following search strategy: (intervention OR program OR trial) AND (RCT OR random* OR control OR arm) AND (cancer AND (breast OR endometri* OR uter* OR ovar*) AND (weight loss OR weight-loss OR weight management OR weight control OR weight change). Titles and abstracts and full texts, where needed, were screened independently by M.M.R. and a research assistant, with any disagreements resolved by discussion. For inclusion, the publication had to report on results of a randomized trial evaluating a behaviorally based weight loss intervention (calorie restriction with or without exercise/physical activity) in women diagnosed with breast, endometrial, or ovarian cancer. Data were extracted by the research assistant and independently reviewed by M.M.R. Risk of bias was also assessed, consistent with the previous review.8
A search of two clinical trials registers (ClinicalTrials.gov and the International Standard Randomized Controlled Trial Number Registry, www.isrctn.com) was also conducted to identify ongoing randomized trials of weight loss interventions in women with breast, endometrial, or ovarian cancer. Those reporting on cancer end points were of particular interest, with those reporting on efficacy or intermediate outcomes also identified.
RESULTS
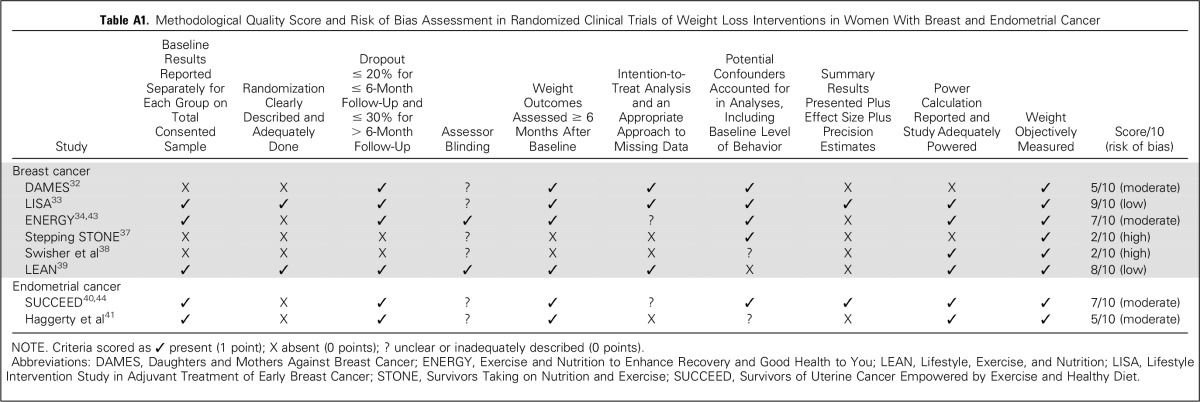
The search identified 311 records, of which 10 publications (from eight individual studies) were considered eligible (Fig 1).32-41 Six were conducted in breast cancer survivors and two in endometrial cancer survivors (Table 1). Risk of bias was considered high in two studies,37,38 moderate in four,32,34,40,41 and low in two33,39 (Appendix Table A1, online only).
Fig 1.
Preferred reporting items for systematic reviews and meta-analyses flow of literature search.
Table 1.
Recent Randomized Controlled Trials Evaluating Weight Loss Interventions

Completed Randomized Controlled Trials
Breast cancer.
Among the six trials, sample size ranged from 2237 to 692,34 with two larger-scale trials.33,34 All trials included women with early-stage invasive breast cancer, with two also including stage 0 disease.32,39 One trial recruited women with ER-positive tumors treated with letrozole,33 one trial recruited only women with triple-negative breast cancer,38 and one trial exclusively recruited African American women.37 Women were recruited on average 9.4 months33 up to 4 to 5 years postdiagnosis.37 All of the trials evaluated interventions targeting diet and exercise; however, the method of delivery and duration differed considerably.
The two larger-scale trials are discussed in detail, with the remaining trials summarized briefly first. The Daughters and Mothers Against Breast Cancer (DAMES) trial aimed to recruit a unique population, namely mother-daughter dyads.32 The intervention was delivered via mailed materials over 12 months. Dyads were randomly assigned to receive individually tailored materials, team-tailored materials, or standardized materials (control). Compared with control (−0.9 kg), 12-month weight change was significantly greater in survivors in the individually tailored group (−3.8 kg) but not statistically different in the team-tailored group (−2.1 kg). Although the overall retention rate in this trial was high (90%), recruitment of mother-daughter dyads proved difficult.
The Stepping STONE (Survivors Taking on Nutrition and Exercise) study randomly assigned 31 African American women diagnosed with stage I to III breast cancer on average 1.7 years before to a 12-week, culturally tailored intervention or usual care.37 The intervention included alternating once-weekly group sessions (delivered by nutritionist and exercise physiologist) and individual telephone counseling sessions (peer-delivered by trained survivors). Change in weight in the intervention group was minimal, with no significant difference between groups, highlighting the previously identified challenge of achieving clinically meaningful weight loss in this population.11,12,42
Swisher and colleagues38 randomly assigned 28 women with triple-negative breast cancer (ER/progesterone receptor/human epidermal growth factor receptor 2 [HER2] –negative), on average 4 to 5 years postdiagnosis, to a 12-week intervention or control. The intervention included an exercise component involving three supervised exercise sessions plus two unsupervised sessions per week. In comparison, the dietary component of the intervention included only two face-to-face sessions with a dietician (a month apart), with the focus primarily on decreasing caloric intake from fat by 200 kcal/day. No statistically significant difference in weight was observed, although sample size was small (−3.0 kg v −0.4 kg). Importantly, the intervention group observed statistically significant and clinically meaningful improvements in quality of life.
The Lifestyle Exercise and Nutrition (LEAN) study randomly assigned 100 women with stage 0 to III breast cancer (on average 2.9 years postdiagnosis) to one of three study arms: face-to-face counseling, telephone counseling, or control.39 The two intervention arms received the same lifestyle intervention (500 kcal/day energy deficit, < 25% energy from fat, predominantly plant-based diet, 150 minutes per week of moderate-intensity activity) and same number of contacts (11 sessions tapered over 6 months) but differed in the method of delivery. Intervention adherence was slightly higher in the face-to-face counseling group (88% v 71% attending at least 80% of sessions), although no statistically significant difference in weight loss at 6 months was observed between the two intervention groups (−6.4% v −5.4%, P = .46), and both were significantly greater than usual care (−1.7%).
Lifestyle Intervention Study in Adjuvant Treatment of Early Breast Cancer trial.
The Lifestyle Intervention Study in Adjuvant Treatment of Early Breast Cancer (LISA) trial33 was a multicenter, randomized controlled trial evaluating a telephone-delivered lifestyle intervention versus brief, mail-based intervention, with participants recruited from 16 Canadian and four US centers. The trial aimed to examine whether the weight loss intervention could favorably influence clinical outcome (ie, disease-free survival) in overweight and obese postmenopausal women with early-stage breast cancer as an addition to conventional cancer management. However, the trial was terminated after 338 randomizations (of a planned 2,150) when funding was lost. Eligible participants were postmenopausal women with early-stage, ER-positive breast cancer (body mass index [BMI], 24 to 40 kg/m2) receiving letrozole. At study entry, participants were on average 9.4 months postdiagnosis, with a mean age of 61 years and mean BMI of 31 kg/m2.
The lifestyle intervention was delivered over 2 years, with up to 19 telephone calls on a tapered schedule. The intervention was designed to achieve 10% weight loss through a reduction in calories (500 to 1,000 kcal/day deficit) and fat (20% of calories); increased intakes of fruits, vegetables, and grains; and increasing to 150 to 200 minutes per week of moderate-intensity aerobic physical activity (primarily walking). Overall, 81.1% of scheduled calls were completed by intervention participants, with 62% completing all 19 calls. Study retention at 24-month follow-up was 78.1%.
Mean weight loss was significantly (P < .001) greater in the lifestyle intervention group than the mail-based intervention group at all follow-up time points (−5.5% v −0.7% at 12 months; −3.6% v −0.4% at 24 months), although a slight regain in weight was observed at 24 months. Physical activity levels were significantly greater in the lifestyle intervention versus mail-based intervention over the 24-month follow-up, although changes in dietary intake were less consistently observed. Despite early termination of recruitment, participants continue to be followed postintervention for clinical outcomes.
Exercise and Nutrition to Enhance Recovery and Good Health to You trial.
The Exercise and Nutrition to Enhance Recovery and Good Health to You (ENERGY) trial34 was designed as a vanguard trial to inform a full-scale, definitive trial powered for cancer end points. The vanguard trial aimed to establish effectiveness of the intervention for achieving and maintaining weight loss and improvements in quality of life in overweight/obese breast cancer survivors. Women with stage I to III breast cancer and BMI of 25 to 45 kg/m2 were recruited from four US sites on average 2 years post primary treatment completion and randomly assigned to weight loss intervention or control groups. The intervention was delivered primarily by face-to-face group sessions (26 sessions over 12 months on tapered frequency), with telephone calls or emails (24 to 38 in total over 2 years) and tailored newsletters (quarterly from 6 to 24 months), designed to achieve 7% weight loss through reduction in calories (500 to 1,000 kcal/day deficit) and increasing physical activity (60 minutes per day moderate-intensity planned exercise and two to three sessions per week resistance exercise).43 Study retention at 24 months was 84.8%. Intervention adherence was not reported.
Significantly greater weight loss was seen in the intervention group compared with controls at 12 and 24 months (−6.0% v −1.5% and −3.7% v −1.3%, respectively), albeit with some regain in weight observed at 24 months. Importantly, multivariable analysis showed that, in addition to intervention assignment, age was the only other predictor of maintained weight loss at 24 months, with no weight loss seen at 24 months in women in the intervention arm age 30 to 44 years. A significant difference in new medical conditions diagnosed in the intervention group versus the control group at 12-month follow-up was observed (19.6% v 32.2%, P < .001), but there was no difference at 24-month follow-up (26.2% v 22.0%, P = .27).36 Furthermore, a beneficial effect of the intervention versus control on vitality and physical function (the primary quality-of-life outcomes) was observed only at 6 months, but it diminished over time.35 Of concern were depressive symptoms, which increased in the intervention group and were statistically significantly higher than in the control group at 24 months.35 Because no minimum clinically important difference has been established for the depressive symptom tool used (Centre for Epidemiologic Studies Depression Scale), it is difficult to determine if the relatively small effect size observed (approximately 0.2) is clinically meaningful. Further understanding of this finding is warranted, with assessment of depressive symptomology an important outcome for future trials.
A limitation of the ENERGY trial is the large reliance on face-to-face intervention delivery over the first year. Face-to-face contacts are costly (both from provider and patient perspective), thus affecting wider-scale implementation and upscale.
Endometrial cancer.
The two trials in endometrial cancer survivors are summarized in Table 1. The Survivors of Uterine Cancer Empowered by Exercise and Health Diet (SUCCEED) trial40 randomly assigned 75 overweight and obese women with stage I or II endometrial cancer (on average 20.7 months postdiagnosis) to a 12-month intervention or usual care. The intervention was delivered primarily via group face-to-face sessions in the first 6 months, with support provided via newsletters and telephone/e-mail in the second 6 months. A significant difference in weight change between groups was observed at 12 months (−3.0% v +1.4%).44 Short-term (3- and 6-month) within- and between-group improvements in some domains of quality of life were observed.40
Haggerty and colleagues41 recruited 16 women (BMI > 30 kg/m2) with stage I to III endometrial cancer and four women with endometrial hyperplasia, who were randomly assigned to one of two weight loss interventions. The telephone intervention group received up to 20 telephone calls over the 6-month intervention (once weekly for the first 16 weeks and then biweekly) and were provided with a WiFi scale for once-weekly recording of weight. The text message intervention group received between three and five text messages once daily focused on intervention messages. Diet and physical activity targets were the same between the two interventions. Mean weight change was reported for the 90% of participants who lost weight, suggesting greater weight loss with the telephone-delivered, compared with the text message–based, intervention (median, 7.6% v −4.1%).
Ongoing Randomized Trials
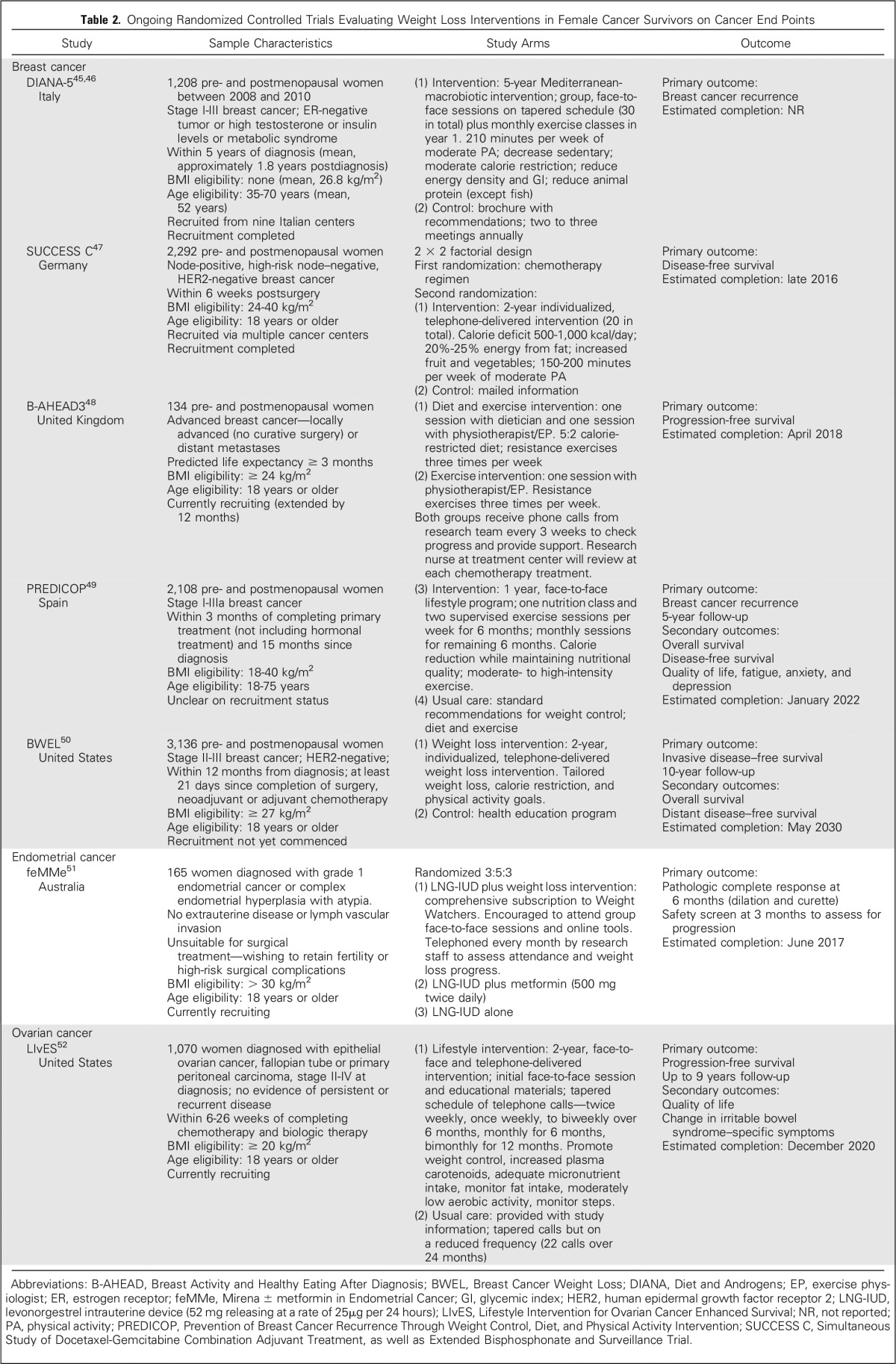
A search of two clinical trials registries identified 22 active trials in breast cancer survivors, four active trials in endometrial cancer survivors (one completed in June 2016), and one active trial in ovarian cancer survivors. Of the 22 current breast cancer trials, six were single-arm feasibility and efficacy trials, 13 were randomized trials with weight or intermediate outcomes (eg, biomarkers) as primary outcomes, and three were randomized trials with cancer end points as primary outcomes. These three trials, along with the ongoing Diet and Androgens (DIANA)-5 and Simultaneous Study of Docetaxel-Gemcitabine Combination adjuvant treatment, as well as Extended Bisphosphonate and Surveillance (SUCCESS C) trials identified in the previous review,8 are summarized in Table 2.45-52 One of the endometrial cancer trials and the ovarian cancer trial are also examining cancer end points (Table 2). Of the remaining endometrial cancer trials, one is a single-arm trial, and two are randomized trials with weight, body composition, and biomarker primary outcomes.
Table 2.
Ongoing Randomized Controlled Trials Evaluating Weight Loss Interventions in Female Cancer Survivors on Cancer End Points
Breast cancer.
DIANA-5.
The DIANA-5 study is evaluating the influence of a 5-year lifestyle intervention on breast cancer events.45 In total, 1,208 pre- and postmenopausal women with early-stage breast cancer at high risk for recurrence (ER-negative, metabolic syndrome, high testosterone, or high insulin) were recruited approximately 1.8 years postdiagnosis (between 2008 and 2010). On the basis of Mediterranean and macrobiotic diet principles, the intervention promotes increasing moderate physical activity, delivered via group face-to-face sessions including cooking classes. The primary outcome is breast cancer recurrence. Preliminary results on weight change (n = 778) at one year (−2.4 kg v −1.0 kg) suggest a relatively small between-group difference.46 It is unclear when the outcomes from the full trial will be available.
SUCCESS C.
The SUCCESS C trial, with a 2 × 2 factorial design, is addressing research questions regarding taxane chemotherapy and the influence of a lifestyle intervention on prognosis of early-stage breast cancer.47 More than 2,000 pre- and postmenopausal women with HER2-negative, node-positive, or high-risk node-negative disease (BMI, 24 to 40 kg/m2) are potentially eligible to be randomly assigned to a 2-year lifestyle intervention or control condition after chemotherapy. The intervention is delivered via telephone (up to 20 calls), with a focus on reducing energy (500 to 1,000 kcal/day deficit) and fat intake (20% to 25% of energy) and increasing moderate physical activity (150 to 200 minutes per week). The primary outcome is disease-free survival, with trial completion expected in late 2016.
Breast Activity and Healthy Eating After Diagnosis 3 trial.
The Breast Activity and Healthy Eating After Diagnosis 3 (B-AHEAD3) trial48 is aiming to recruit 134 pre- and postmenopausal women diagnosed with advanced breast cancer undergoing chemotherapy. Eligible participants have a prognosis > 3 months and BMI ≥ 24 kg/m2. Participants are randomly assigned to a resistance exercise program or resistance exercise plus intermittent fasting diet (5:2 diet). The primary trial outcome is progression-free survival, with chemotherapy toxicity, quality of life, fatigue, and body composition as secondary outcomes. Recruitment was extended by 12 months, with trial completion expected by early 2018.
Prevention of Breast Cancer Recurrence Through Weight Control, Diet, and Physical Activity Intervention trial.
The Prevention of Breast Cancer Recurrence Through Weight Control, Diet, and Physical Activity Intervention (PREDICOP) trial49 aims to evaluate a 12-month lifestyle intervention versus usual care on breast cancer recurrence over 5-year follow-up. The trial aims to recruit 2,108 pre- and postmenopausal Spanish women diagnosed with stage I to IIIa breast cancer (BMI, 18 to 40 kg/m2). The intervention is delivered face to face, with once-weekly nutrition classes and supervised exercise sessions, with reduced frequency in the second 6 months. Recruitment status of the trial is unclear, with the registry indicating that the trial is due for completion in 2022.
Breast Cancer Weight Loss study.
The Breast Cancer Weight Loss (BWEL) study50 will enroll almost 3,200 pre- and postmenopausal women with stage II or III HER2-negative breast cancer (BMI ≥ 27 kg/m2) from clinical centers and practices in the United States and Canada to determine whether weight loss will improve breast cancer outcome. Participants will be randomly assigned to a 2-year weight loss intervention or control condition. The intervention will be centralized and telephone based, designed to increase exercise and reduce calories, with the inclusion of a variety of Fitbit devices and programs (including individual fitness trackers and a Fitbit smart scale that links to a mobile dashboard allowing individuals to monitor their progress). Recruitment is scheduled to begin in the second half of 2016. The primary outcome is invasive disease–free survival, with follow-up over 10 years.
Endometrial cancer.
Mirena ± Metformin Trial for Endometrial Cancer.
The Mirena ± Metformin Trial for Endometrial Cancer51 (feMMe) is recruiting 165 women (BMI ≥ 30 kg/m2) diagnosed with grade 1 endometrial cancer or complex endometrial hyperplasia with atypia (precancerous). This trial is evaluating nonsurgical interventions in women who want to retain fertility or those with a high risk of complications. The trial will compare an intrauterine device (IUD) alone, IUD plus metformin, and IUD plus weight loss on the primary outcome of pathologic complete response at 6-month follow-up. Participants in the weight loss arm will be provided with a comprehensive subscription to Weight Watchers, including attendance at group sessions and online tools. The trial is due for completion in June 2018.
Ovarian cancer.
Lifestyle Intervention for Ovarian Cancer Enhanced Survival trial.
The Lifestyle Intervention for Ovarian Cancer Enhanced Survival (LIvES) trial will recruit 1,070 women (BMI ≥ 20 kg/m2) diagnosed with epithelial ovarian cancer or fallopian tube or primary peritoneal carcinoma (stage II to IV at diagnosis but with no evidence of persistent or recurrent disease) after completion of chemotherapy.52 Participants are randomly assigned to receive a 2-year lifestyle intervention (primarily delivered via telephone) or usual care, with the primary outcome of progression-free survival. Completion of the trial is expected by the end of 2020.
DISCUSSION
Weight control and lifestyle (diet and physical activity) changes are recommended as an important part of survivorship care, particularly for breast cancer survivors.53 This is despite definitive clinical trial evidence on the impact of weight control on cancer outcomes. As noted by Goodwin,54 this evidence is important for patients and clinicians to place value on such recommendations but also for health funders to pay for such programs. This review identified a growing number of ongoing and planned definitive trials in breast and ovarian cancer aiming to address this evidence gap.
The biggest challenge for these trials is how to maintain participants’ motivation and engagement once intervention contact ceases, to maximize weight loss maintenance. Both the LISA and ENERGY trials have shown that even with intervention contact over a 2-year period, small weight regain began after 12 months. This pattern of weight regain is not unique to cancer survivors and has been observed in noncancer groups.55 Lessons can also be learned from primary prevention (Women’s Health Initiative Dietary Modification trial)56,57 and secondary prevention (Women’s Intervention Nutrition Study)5,58 dietary intervention trials in breast cancer, where, after long-term dietary interventions of 5.6 and 8.3 years, respectively,5,56 suggested dietary intervention effects on breast cancer outcomes rapidly attenuated in the postintervention follow-up when nutritionist contact ended, despite the long duration of the dietary intervention.
BWEL, LIvES, and SUCCESS C trials are implementing 2-year interventions, whereas the PREDICOP trial intervention is for only 1 year. The DIANA-5 trial included a 5-year intervention; however, preliminary data after 1 year46 suggest that weight loss from this intervention may not be adequate to alter obesity-associated physiology and tumor microenvironment.54 There is the opportunity with the BWEL trial, which is yet to commence recruitment, to learn from the broader weight loss maintenance literature and identify additional strategies and likely extend intervention contact, to optimize weight loss maintenance and potential benefits on breast cancer outcomes.
The studies identified in the current and the prior review8 provide support for the feasibility, safety, and efficacy in achieving weight loss in breast and endometrial cancer survivors. However, these and ongoing trials have largely assumed a one-size-fits-all approach in terms of intervention delivery, targets, and content, which is unlikely to be appropriate or most effective. In the ENERGY trial, for example, significant differences in intervention effectiveness were seen depending on participants’ age. A more personalized approach to weight loss and lifestyle interventions may be needed. Further research in this area is warranted and can be informed by evidence in noncancer populations. Post hoc analysis of ongoing trials to explore outcomes by subgroups (eg, breast cancer subtype, ethnic group) would also be informative.
In this competitive research funding environment, future research in this field needs to be strategic. Additional single-arm trials assessing feasibility or short-term randomized trials assessing efficacy are not necessary. As the effectiveness of centralized interventions by telephone are established59 and evidence on technology-driven strategies (internet, smart phones, and wearables) continues to mount, such interventions are scalable and provide an opportunity for implementation in full-scale trials with cancer outcome end points using pragmatic clinical trial study designs including cost-effectiveness analyses.
In conclusion, after a decade of preliminary studies, randomized controlled clinical trials are now underway that will potentially provide definitive assessment on whether weight loss can improve clinical outcome in female cancer survivors. However, findings from these trials are still a number of years away. Evidence to support the translation of effective weight loss intervention programs into wider-scale implementation is needed so that they can be offered as part of routine survivorship care.
ACKNOWLEDGMENT
We thank the Women’s Health Initiative investigators, staff, and trial participants for their work on the Women’s Health Initiative Dietary Modification trial discussed in this review and for their outstanding dedication and commitment. We also thank Zoe Thomson and Caroline Terranova (PhD candidates) for research assistant support with the literature search.
Appendix
Table A1.
Methodological Quality Score and Risk of Bias Assessment in Randomized Clinical Trials of Weight Loss Interventions in Women With Breast and Endometrial Cancer
Footnotes
The Women’s Health Initiative program is supported by the National Heart, Lung, and Blood Institute through contracts No. N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221; and by American Institute for Cancer Research Grant No. 30210-01 (R.T.C.). M.M.R. is supported by a fellowship from the Australian National Breast Cancer Foundation.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Rowan T. Chlebowski
Financial support: Rowan T. Chlebowski
Administrative support: Rowan T. Chlebowski
Provision of study materials or patients: Rowan T. Chlebowski
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Weight Loss Randomized Intervention Trials in Female Cancer Survivors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Rowan T. Chlebowski
Honoraria: Novartis, Genentech
Consulting or Advisory Role: Novartis, Genentech, Novo Nordisk, Amgen, Pfizer, Genomic Health
Speakers' Bureau: Novartis, Genentech
Research Funding: National Institutes of Health/National Cancer Institute
Marina M. Reeves
No relationship to disclose
REFERENCES
- 1.Chan DS, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer: Systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 3.Abe R, Kumagai N, Kimura M, et al. Biological characteristics of breast cancer in obesity. Tohoku J Exp Med. 1976;120:351–359. doi: 10.1620/tjem.120.351. [DOI] [PubMed] [Google Scholar]
- 4.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 5.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 6.Ballard-Barbash R, Hunsberger S, Alciati MH, et al. Physical activity, weight control, and breast cancer risk and survival: Clinical trial rationale and design considerations. J Natl Cancer Inst. 2009;101:630–643. doi: 10.1093/jnci/djp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ligibel JA, Alfano CM, Hershman D, et al. Recommendations for obesity clinical trials in cancer survivors: American Society of Clinical Oncology statement. J Clin Oncol. 2015;33:3961–3967. doi: 10.1200/JCO.2015.63.1440. [DOI] [PubMed] [Google Scholar]
- 8.Reeves MM, Terranova CO, Eakin EG, et al. Weight loss intervention trials in women with breast cancer: A systematic review. Obes Rev. 2014;15:749–768. doi: 10.1111/obr.12190. [DOI] [PubMed] [Google Scholar]
- 9.de Waard F, Ramlau R, Mulders Y, et al. A feasibility study on weight reduction in obese postmenopausal breast cancer patients. Eur J Cancer Prev. 1993;2:233–238. doi: 10.1097/00008469-199305000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Djuric Z, DiLaura NM, Jenkins I, et al. Combining weight-loss counseling with the weight watchers plan for obese breast cancer survivors. Obes Res. 2002;10:657–665. doi: 10.1038/oby.2002.89. [DOI] [PubMed] [Google Scholar]
- 11.Djuric Z, Mirasolo J, Kimbrough L, et al. A pilot trial of spirituality counseling for weight loss maintenance in African American breast cancer survivors. J Natl Med Assoc. 2009;101:552–564. doi: 10.1016/s0027-9684(15)30940-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenlee HA, Crew KD, Mata JM, et al. A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity (Silver Spring) 2013;21:65–76. doi: 10.1002/oby.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris MN, Swift DL, Myers VH, et al. Cancer survival through lifestyle change (CASTLE): A pilot study of weight loss. Int J Behav Med. 2013;20:403–412. doi: 10.1007/s12529-012-9234-5. [DOI] [PubMed] [Google Scholar]
- 14.Mefferd K, Nichols JF, Pakiz B, et al. A cognitive behavioral therapy intervention to promote weight loss improves body composition and blood lipid profiles among overweight breast cancer survivors. Breast Cancer Res Treat. 2007;104:145–152. doi: 10.1007/s10549-006-9410-x. [DOI] [PubMed] [Google Scholar]
- 15.Scott E, Daley AJ, Doll H, et al. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long-term prognosis after early-stage breast cancer: A randomized controlled trial. Cancer Causes Control. 2013;24:181–191. doi: 10.1007/s10552-012-0104-x. [DOI] [PubMed] [Google Scholar]
- 16.Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer–related lymphedema. Cancer. 2007;110:1868–1874. doi: 10.1002/cncr.22994. [DOI] [PubMed] [Google Scholar]
- 17.Shaw C, Mortimer P, Judd PA. Randomized controlled trial comparing a low-fat diet with a weight-reduction diet in breast cancer–related lymphedema. Cancer. 2007;109:1949–1956. doi: 10.1002/cncr.22638. [DOI] [PubMed] [Google Scholar]
- 18.Thomson CA, Stopeck AT, Bea JW, et al. Changes in body weight and metabolic indexes in overweight breast cancer survivors enrolled in a randomized trial of low-fat vs. reduced carbohydrate diets. Nutr Cancer. 2010;62:1142–1152. doi: 10.1080/01635581.2010.513803. [DOI] [PubMed] [Google Scholar]
- 19.Jen KL, Djuric Z, DiLaura NM, et al. Improvement of metabolism among obese breast cancer survivors in differing weight loss regimens. Obes Res. 2004;12:306–312. doi: 10.1038/oby.2004.38. [DOI] [PubMed] [Google Scholar]
- 20.Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: Weight of the evidence. J Clin Oncol. 2011;29:4–7. doi: 10.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- 21.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: Local and systemic mechanisms. Annu Rev Med. 2015;66:297–309. doi: 10.1146/annurev-med-050913-022228. [DOI] [PubMed] [Google Scholar]
- 22.Cheng L, Swartz MD, Zhao H, et al. Hazard of recurrence among women after primary breast cancer treatment--a 10-year follow-up using data from SEER-Medicare. Cancer Epidemiol Biomarkers Prev. 2012;21:800–809. doi: 10.1158/1055-9965.EPI-11-1089. [DOI] [PubMed] [Google Scholar]
- 23.Cossetti RJ, Tyldesley SK, Speers CH, et al. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. J Clin Oncol. 2015;33:65–73. doi: 10.1200/JCO.2014.57.2461. [DOI] [PubMed] [Google Scholar]
- 24. Anderson AS, Key TJ, Norat T, et al: European code against cancer 4th edition: Obesity, body fatness and cancer. Cancer Epidemiol 39:S34-45, 2015 (suppl 1) [DOI] [PubMed] [Google Scholar]
- 25.Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixon SC, Nagle CM, Thrift AP, et al. Adult body mass index and risk of ovarian cancer by subtype: A Mendelian randomization study. Int J Epidemiol. 2016;45:884–895. doi: 10.1093/ije/dyw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arem H, Chlebowski R, Stefanick ML, et al. Body mass index, physical activity, and survival after endometrial cancer diagnosis: Results from the Women’s Health Initiative. Gynecol Oncol. 2013;128:181–186. doi: 10.1016/j.ygyno.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arem H, Irwin ML. Obesity and endometrial cancer survival: A systematic review. Int J Obes. 2013;37:634–639. doi: 10.1038/ijo.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagle CM, Dixon SC, Jensen A, et al. Obesity and survival among women with ovarian cancer: Results from the Ovarian Cancer Association Consortium. Br J Cancer. 2015;113:817–826. doi: 10.1038/bjc.2015.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Protani MM, Nagle CM, Webb PM. Obesity and ovarian cancer survival: A systematic review and meta-analysis. Cancer Prev Res (Phila) 2012;5:901–910. doi: 10.1158/1940-6207.CAPR-12-0048. [DOI] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demark-Wahnefried W, Jones LW, Snyder DC, et al. Daughters and Mothers Against Breast Cancer (DAMES): Main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer. 2014;120:2522–2534. doi: 10.1002/cncr.28761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodwin PJ, Segal RJ, Vallis M, et al. Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: The LISA trial. J Clin Oncol. 2014;32:2231–2239. doi: 10.1200/JCO.2013.53.1517. [DOI] [PubMed] [Google Scholar]
- 34.Rock CL, Flatt SW, Byers TE, et al. Results of the Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial: A behavioral weight loss intervention in overweight or obese breast cancer survivors. J Clin Oncol. 2015;33:3169–3176. doi: 10.1200/JCO.2015.61.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demark-Wahnefried W, Colditz GA, Rock CL, et al. Quality of life outcomes from the Exercise and Nutrition Enhance Recovery and Good Health for You (ENERGY)-randomized weight loss trial among breast cancer survivors. Breast Cancer Res Treat. 2015;154:329–337. doi: 10.1007/s10549-015-3627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sedjo RL, Flatt SW, Byers T, et al. Impact of a behavioral weight loss intervention on comorbidities in overweight and obese breast cancer survivors. Support Care Cancer. 2016;24:3285–3293. doi: 10.1007/s00520-016-3141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheppard VB, Hicks J, Makambi K, et al. The feasibility and acceptability of a diet and exercise trial in overweight and obese black breast cancer survivors: The Stepping STONE study. Contemp Clin Trials. 2016;46:106–113. doi: 10.1016/j.cct.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swisher AK, Abraham J, Bonner D, et al. Exercise and dietary advice intervention for survivors of triple-negative breast cancer: Effects on body fat, physical function, quality of life, and adipokine profile. Support Care Cancer. 2015;23:2995–3003. doi: 10.1007/s00520-015-2667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrigan M, Cartmel B, Loftfield E, et al. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: The Lifestyle, Exercise, and Nutrition (LEAN) study. J Clin Oncol. 2016;34:669–676. doi: 10.1200/JCO.2015.61.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarroll ML, Armbruster S, Frasure HE, et al. Self-efficacy, quality of life, and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): A randomized controlled trial. Gynecol Oncol. 2014;132:397–402. doi: 10.1016/j.ygyno.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Haggerty AF, Huepenbecker S, Sarwer DB, et al. The use of novel technology-based weight loss interventions for obese women with endometrial hyperplasia and cancer. Gynecol Oncol. 2016;140:239–244. doi: 10.1016/j.ygyno.2015.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stolley MR, Sharp LK, Oh A, et al. A weight loss intervention for African American breast cancer survivors, 2006. Prev Chronic Dis. 2009;6:A22. [PMC free article] [PubMed] [Google Scholar]
- 43.Rock CL, Byers TE, Colditz GA, et al. Reducing breast cancer recurrence with weight loss, a vanguard trial: The Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial. Contemp Clin Trials. 2013;34:282–295. doi: 10.1016/j.cct.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Gruenigen V, Frasure H, Kavanagh MB, et al. Survivors of uterine cancer empowered by exercise and healthy diet (SUCCEED): A randomized controlled trial. Gynecol Oncol. 2012;125:699–704. doi: 10.1016/j.ygyno.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 45.Villarini A, Pasanisi P, Traina A, et al. Lifestyle and breast cancer recurrences: The DIANA-5 trial. Tumori. 2012;98:1–18. doi: 10.1177/030089161209800101. [DOI] [PubMed] [Google Scholar]
- 46. Pasanisi P, Villarini A, Gargano G, et al: A randomized controlled trial of diet, physical activity and breast cancer recurrences: The Diana-5 study. Eur J Cancer 48:S283-S284, 2012 (suppl 5) [Google Scholar]
- 47.Rack B, Andergassen U, Neugebauer J, et al. The German SUCCESS C study: The first European lifestyle study on breast cancer. Breast Care (Basel) 2010;5:395–400. doi: 10.1159/000322677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. International Standard Randomized Controlled Trial Number Registry: A randomised phase II trial of intermittent energy restriction and resistance exercise in women receiving chemotherapy for advanced breast cancer. http://www.isrctn.com/ISRCTN12841416.
- 49. ClinicalTrials.gov: Prevention of breast cancer recurrence through weight control, diet, and physical activity intervention (PREDICOP). https://clinicaltrials.gov/show/NCT02035631.
- 50. doi: 10.1038/s41523-017-0040-8. ClinicalTrials.gov: Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (BWEL study). https://clinicaltrials.gov/show/NCT02750826. [DOI] [PMC free article] [PubMed]
- 51.Hawkes AL, Quinn M, Gebski V, et al. Improving treatment for obese women with early stage cancer of the uterus: Rationale and design of the levonorgestrel intrauterine device ± metformin ± weight loss in endometrial cancer (feMME) trial. Contemp Clin Trials. 2014;39:14–21. doi: 10.1016/j.cct.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 52. ClinicalTrials.gov: Can diet and physical activity modulate ovarian, fallopian tube and primary peritoneal cancer progression-free survival? https://clinicaltrials.gov/show/NCT00719303.
- 53.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. J Clin Oncol. 2016;34:611–635. doi: 10.1200/JCO.2015.64.3809. [DOI] [PubMed] [Google Scholar]
- 54.Goodwin PJ. Obesity and breast cancer outcomes: How much evidence is needed to change practice? J Clin Oncol. 2016;34:646–648. doi: 10.1200/JCO.2015.64.7503. [DOI] [PubMed] [Google Scholar]
- 55.Look AHEAD Research Group Eight-year weight losses with an intensive lifestyle intervention: The look AHEAD study. Obesity (Silver Spring) 2014;22:5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–642. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 57.Thomson CA, Van Horn L, Caan BJ, et al. Cancer incidence and mortality during the intervention and postintervention periods of the Women’s Health Initiative dietary modification trial. Cancer Epidemiol Biomarkers Prev. 2014;23:2924–2935. doi: 10.1158/1055-9965.EPI-14-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chlebowski RT, Blackburn GL, Hoy MK, et al: Survival analyses from the Women’s Intervention Nutrition Study (WINS) evaluating dietary fat reduction and breast cancer outcome. J Clin Oncol 26, 2008 (suppl; abstr 522) [Google Scholar]
- 59.Reeves MM, Whelan M, Brackenridge C, et al. Effectiveness of telephone-delivered interventions for achieving weight loss in overweight and obese adults: A meta-analysis. Obes Facts. 2013;641(suppl 1) [Google Scholar]