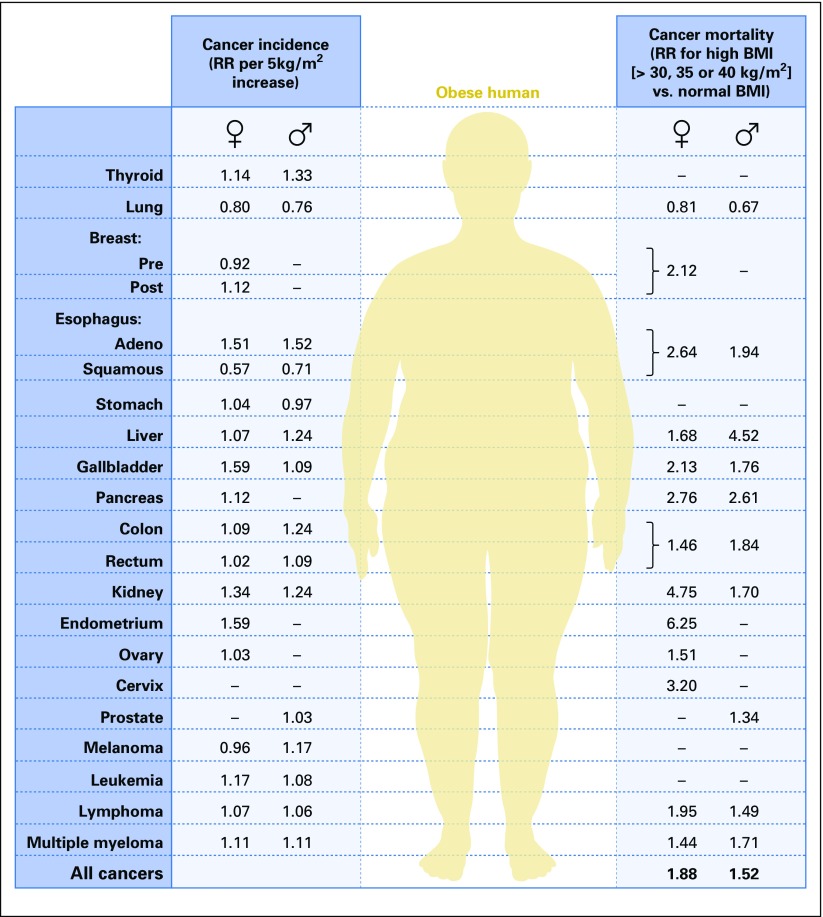
The association of obesity with cancer, increasingly recognized in both lay and medical communities and underscored by the ongoing obesity epidemic, has stimulated a large body of research and led to calls for programs to minimize any potential impact of obesity on cancer. Calle et al1 described the association of body mass index (BMI) with cancer mortality in the prospective American Cancer Society Cohort Study of > 900,000 American adults. After 16 years of follow-up, individuals with a BMI (BMI = weight [kg]/height [m]2) of at least 40 kg/m2 had an increased cancer mortality (relative risk [RR], 1.52; 95% CI, 1.13 to 2.05 for men and RR, 1.62; 95% CI, 1.40 to 1.87 for women). Higher mortality risk was reported for most cancers for both sexes, apart from lung cancer. For men, the highest RRs were identified for cancer of the liver (RR, 4.52; 95% CI, 2.94 to 6.94) and stomach (RR, 1.94; 95% CI, 1.21 to 3.13) and for women, cancer of the uterus (RR, 6.25; 95% CI, 3.75 to 10.42), kidney (RR, 4.75; 95% CI, 2.50 to 9.04), pancreas (RR, 2.76; 95% CI, 1.74 to 4.36) and breast (RR, 2.12; 95% CI, 1.41 to 3.19). Given the patterns of overweight and obesity in the United States in 2003, it was estimated that overweight and obesity accounted for 14% of all cancer deaths in men and 20% of all cancer deaths in women.
Subsequently, Renehan et al,2 with a meta-analysis of studies of BMI and cancer incidence, associated obesity with higher risk of several cancers. For example, in men, for each 5-kg/m2 increase in BMI, the RR of esophageal adenocarcinoma was 1.52 (95% CI, 1.33 to 1.74), colon cancer 1.24 (95% CI, 1.20 to 1.28), and multiple myeloma 1.11 (95% CI, 1.05 to 1.18); in women, the RR of endometrial cancer was 1.59 (95% CI, 1.50 to 1.68), gallbladder cancer 1.59 (95% CI, 1.02 to 2.47), esophageal adenocarcinoma 1.51 (95% CI, 1.31 to 1.74), and postmenopausal breast cancer 1.12 (95% CI, 1.08 to 1.16). Associations between BMI and breast cancer risk were somewhat stronger in the Asia Pacific area. Key results of these two studies are summarized in Figure 1.3
Fig 1.
Associations of obesity with cancer incidence and mortality. BMI, body mass index; RR, relative risk. Reprinted with permission.3
The International Association for Research on Cancer recently updated findings on body fatness and cancer risk and concluded that evidence supported “a cancer-preventive effect of the absence of excess body fatness”4 (using BMI as a proxy for body fatness) for cancers of: corpus uteri (RR, 7.1; 95% CI, 6.3 to 8.1), esophagus (adenocarcinoma; RR, 4.8; 95% CI, 3.0 to 7.7), gastric cardia (RR, 1.8; 95% CI, 1.3 to 2.5), liver (RR, 1.8; 95% CI, 1.6 to 2.1), renal cell (RR, 1.8; 95% CI, 1.7 to 1.9), pancreas (RR, 1.5; 95% CI, 1.2 to 1.8), meningioma (RR, 1.5; 95% CI, 1.3 to 1.8), multiple myeloma (RR, 1.5; 95% CI, 1.2 to 2.0), colon/rectum (RR, 1.3; 95% CI, 1.3 to 1.4), gallbladder (RR, 1.3; 95% CI, 1.2 to 1.4), postmenopausal breast (RR, 1.1; 95% CI, 1.1 to 1.2), ovary (RR, 1.1; 95% CI, 1.1 to 1.2), and thyroid (RR, 1.1; 95% CI, 1.0 to 1.1). The framing of findings in relation to avoidance of body fatness represents recognition that the potential impact of weight loss in obese individuals has not been convincingly demonstrated.
Arnold et al5 calculated the population-attributable fraction (PAF: the proportion of cancers potentially avoidable if the obesity-cancer association is causal) for obesity and cancer risk worldwide. They estimated that 3.6% of all new cancers in adults in 2012 (approximately 481,000 cases) could be attributed to high BMI. The PAF was higher in women (5.4% v 1.9% in men), reflecting the strong associations of BMI with cancer of the body of the uterus, postmenopausal breast cancer, and colon cancer. The PAF was also higher in more-well-developed countries, possibly reflecting a greater prevalence of obesity and obesity-associated cancer. It was estimated that, had BMI remained at levels observed in 1982 (the beginning of the obesity epidemic), approximately 25% (118,000 cases) of high BMI–related cancer seen in 2012 could have been averted.
This special issue of Journal of Clinical Oncology extends the prior focus on obesity and cancer incidence to explore potential impacts of obesity once cancer has been diagnosed. This includes associations with prognosis, impact on treatment, potential biologic mechanisms, approaches to weight loss, and the ASCO Obesity Initiative.
Jiralerspong and Goodwin6 review evidence linking obesity to breast cancer prognosis, the cancer site with arguably the strongest evidence base, and conclude that “obesity is associated with a 35 to 40% increased risk of breast cancer recurrence and death”6 and that evidence is strongest for hormone receptor–positive breast cancer. Evidence linking obesity to poor outcome in triple-negative and human epidermal growth factor receptor 2–positive breast cancers was less consistent. Also discussed were ongoing randomized clinical trials of lifestyle-based weight loss interventions as well as the generic diabetes drug metformin (which improves many aspects of obesity-associated physiology) on breast cancer outcomes.
Brown and Meyerhardt7 examined obesity and gastrointestinal (GI) cancer associations and cite “mounting evidence associating overweight and/or obesity with worsened prognosis in multiple GI cancers, including esophageal, gastric, hepatocellular, pancreatic, and colorectal.”7 They discuss potential contributions of components of energy balance, including diet and physical activity, to cancer prognosis; the relationship between sarcopenic obesity and GI cancer prognosis; and the potential for alterations in energy balance to adversely affect prognosis in metastatic cancers.
Onstad et al8 examined obesity and endometrial cancer associations and highlight the extremely strong association between obesity and endometrial cancer risk, likely contributing to increasing endometrial cancer risk in younger women. Mechanistic pathways, including unopposed estrogen, insulin and insulin-like growth factor-1 signaling hyperactivity, and inflammation, are discussed. They also review potential interventions, including lifestyle and surgical approaches as well as metformin. Finally, they discuss the impact of obesity on management in women with endometrial cancer.
Yang et al9 focus on other cancers for which the greatest evidence is available (prostate, hematologic [including multiple myeloma and leukemia], and renal cancers). The authors conclude that higher BMI is associated with a higher risk of developing advanced prostate cancer and prostate cancer mortality and is also associated with poor survival in many hematologic malignancies, including multiple myeloma and leukemia. Although observational studies associate obesity with higher renal cancer risk, in retrospective observational studies obesity may be associated with improved renal cancer outcome.
Authors of these four articles also discuss lifestyle recommendations (diet, physical activity, weight management) for patients with cancer, as well as the role of intervention studies in ascertaining the potential benefits of weight loss/lifestyle change. However, the observational nature of the existing evidence is insufficient to allow conclusions to be drawn regarding the impact of lifestyle change on cancer outcomes.
Chlebowski and Reeves10 focus on ongoing and completed randomized weight loss intervention clinical trials in cancer populations, updating a prior systematic review that had identified 10 breast cancer trials. These studies, along with six more recently reported breast cancer trials and two in patients with endometrial cancer, establish feasibility of recruitment of cancer survivors into such trials and the efficacy of the lifestyle interventions, particularly telephone-based interventions, in achieving weight loss of sufficient magnitude to provide a valid test of impact on cancer outcomes. These trials also identified challenges in maintenance of weight loss. These results, taken together with findings from clinical trials targeting breast cancer with fat intake reduction,11-13 suggest long-term lifestyle interventions may be needed to optimize effects against cancer. Encouragingly, a total of five ongoing trials of weight loss in patients with breast cancer with cancer end points as well as two ongoing trials in gynecologic cancer (one endometrial, one ovarian) were identified. Many of these trials are adequately powered to identify weight loss influence on cancer outcomes, with some results expected in late 2016. This important paper underscores the recognition of the need for rigorous testing of the hypothesis that weight loss in overweight and obese patients with cancer will improve outcomes. After a decade of preliminary studies, randomized controlled clinical trials are now underway that can potentially provide definitive assessment of that question.
Shlomai et al14 address the many factors linking diabetes with cancer, including their associated therapies. Diabetes is strongly linked to obesity, and diabetes-associated physiologic changes may contribute to the cancer-obesity association. The association of diabetes with a higher mortality risk from several cancers, including pancreatic, hepatobiliary, breast, endometrial, and colorectal, is outlined along with potential mechanistic links, including hyperinsulinemia. Also reviewed are associations of diabetes pharmacotherapy with cancer, notably the potential for a protective effect of metformin, a generic drug that is currently under evaluation in a large number of clinical trials in cancer populations. The authors provide reassurance that there is “no definitive evidence of a mitogenic effect of antidiabetic therapeutics.”14 Also addressed is the impact of cancer therapy on the development of insulin resistance, hyperglycemia, and diabetes, with a focus on glucocorticoids, hormone therapies such as androgen-deprivation therapy, phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin inhibitors, and insulin-like growth factor-1–targeted therapy; these issues are of major clinical relevance for practicing oncologists.
Three articles delve into potential biologic mechanisms underlying the obesity-cancer association. These articles address mechanisms from different perspectives, recognizing that obesity is associated with a broad range of interrelated changes in adipose tissue, systemic physiology, and cellular metabolism, and it is likely that multiple mechanisms contribute simultaneously to the obesity-cancer association, with different mechanisms having different relative contributions in different settings.
Lohmann et al15 discuss the contribution of obesity-associated systemic physiology to the obesity-cancer link. They review insulin resistance, discussing molecular mechanisms that stimulate the growth of cancer cells as well as observational evidence linking insulin resistance to increased cancer risk and poor cancer outcomes. Two obesity-associated adipokines (leptin and adiponectin) seem to have differing associations with cancer, leptin being potentially associated with increased risk and worse prognosis and adiponectin having opposite associations. Finally, potential contributions of reproductive hormones to female cancers (breast, gynecologic) are discussed. Therapeutic options for reversing some of the physiologic changes potentially implicated in the obesity-cancer link are also reviewed.
Iyengar et al16 discuss the potential role of the tumor microenvironment and inflammation. Their focus is on the impact of excess adiposity on adipose tissue function, adipocyte death, and low-grade inflammation, and they link these changes to some of the physiologic changes discussed by Lohmann et al.15 Also addressed are the obesity-associated alterations in tumor microenvironment and research that associates localized inflammation, manifest as crown-like structures (dying adipocytes surrounded by inflammatory cells), with poor prognosis in breast and tongue cancer. They review molecular mechanisms linking white adipose tissue inflammation to cancer development and progression. Finally outlined are potential interventions to reduce adipose inflammation, including weight loss, bariatric surgery, and anti-inflammatory medications.
Hopkins et al17 discuss obesity as a state of chronic caloric excess, focusing on nutrient homeostasis, cancer metabolism, and potential contributions of excess insulin, glucose, lipids, and fatty acids to cancer. They state, “By chronically activating metabolic signaling cascades, the obese state lowers the barrier for oncogenic transformation by driving cell growth, proliferation, and resisting apoptosis.”17
The emergence of a strong biologic basis for the obesity-cancer association as discussed in these three papers is supportive of a causal association of obesity with cancer and is supportive of development of potential interventions (lifestyle, pharmacologic) that could impact cancer outcomes. An effect of these interventions would need to be confirmed in randomized trials powered to investigate effects on cancer outcomes to support recommendations that weight loss may improve cancer outcomes.
Two articles discuss management of the obese patient with cancer and of obesity in general. Renehan et al18 focus on management of the obese patient with cancer, discussing increased risks of toxicity, particularly after surgery, as well as chemotherapy dosing in the obese patient. They also discuss weight gain during chemotherapy, a problem that may be most relevant in the setting of breast cancer. Alamuddin et al19 review accepted approaches to weight loss using lifestyle interventions, pharmacotherapy, and bariatric surgery and outline realistic expectations for weight loss (for example, 6% to 8% weight loss for a 6-month, high-intensity lifestyle intervention) and associated improvements in obesity-related comorbidities. They review the contributions of dietary change and physical activity to weight loss, the common problem of gradual weight regain, medications currently approved by the Food and Drug Administration for weight management, as well as indications for and outcomes of bariatric surgery. This review is a must read for clinical oncologists who seek a greater understanding of modern-day approaches to management of obesity.
Finally, Ligibel and Wollins20 discuss the ASCO Obesity Initiative, an initiative developed in response to growing recognition of an association of obesity with cancer risk and outcome. Many oncologists will be familiar with this initiative; this article discusses progress to date and outlines plans to expand activities through collaborations both in and outside the field of cancer.
The obesity-cancer field has advanced tremendously in the past decade, and there is increasing evidence of an adverse association between obesity and cancer risk and outcomes. There is also an emerging understanding of the biology that underlies an obesity-cancer link and interest in intervening to break this link to lower cancer risk and improve cancer outcomes. However, current evidence linking obesity to cancer risk and outcomes is observational, and the modest associations commonly seen could be due to bias and/or confounding; convincing evidence that any negative impact of obesity on cancer can be reversed through weight loss or through targeting of potential biologic/physiologic mechanisms is lacking. Although avoidance of overweight and obesity (as discussed by the International Association for Research on Cancer group)4 would reasonably also avoid obesity related to cancer risk, it is uncertain whether weight loss in overweight and/or obese individuals will mitigate potential effects of obesity on cancer risk or cancer outcomes. Because obesity may lead to nonreversible cancer precursors or more aggressive, harder-to-treat cancers, and because weight loss interventions typically lead to modest weight loss (rather than attainment of normal weight), it remains to be demonstrated that weight loss can lower risk and/or improve outcomes.20
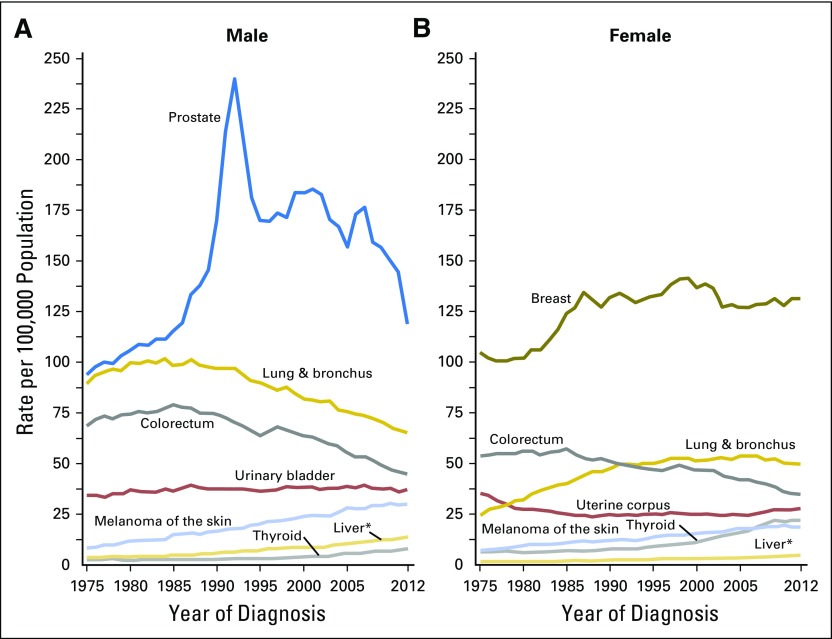
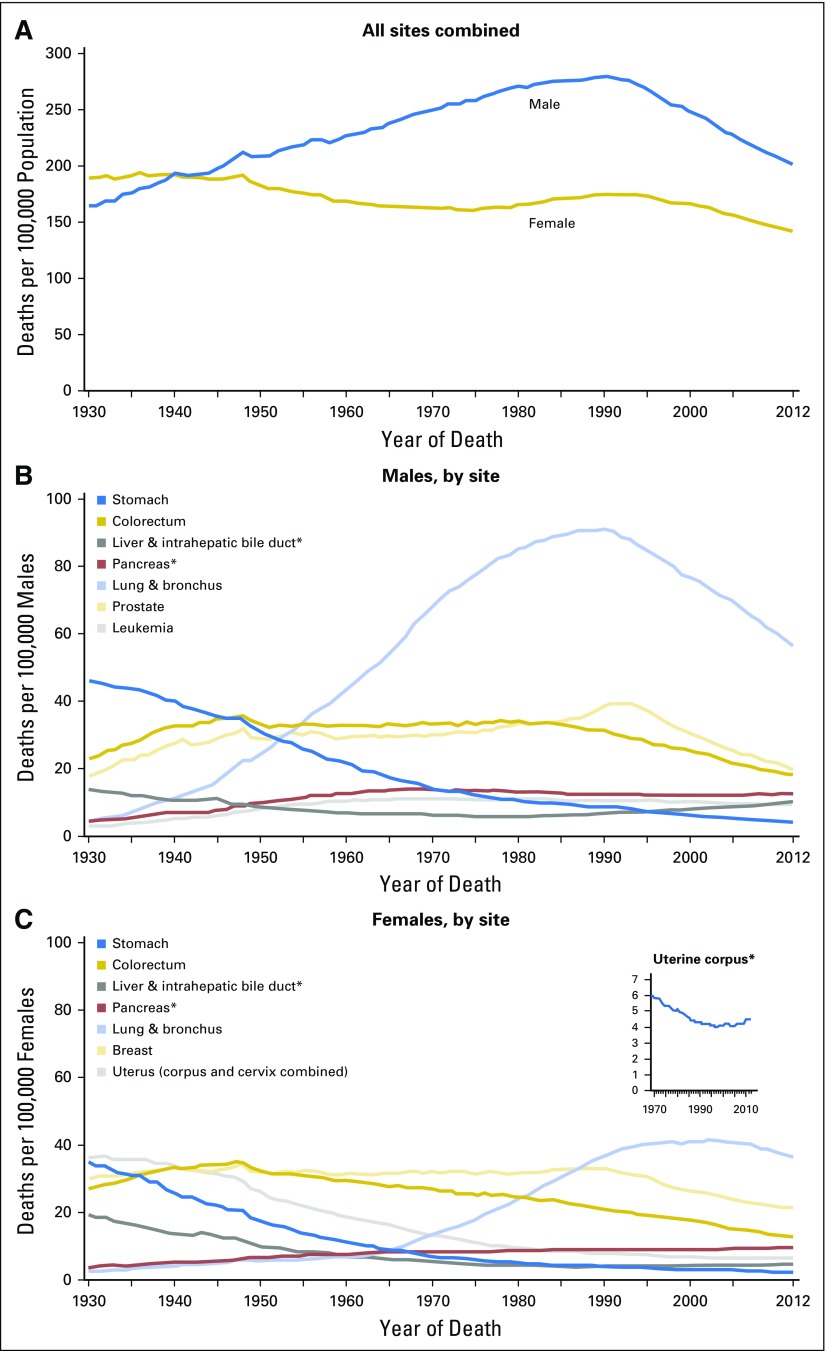
The obesity epidemic is now three decades old. During that time, there has been an overall increase in incidence of both male and female cancer in the United States, related mainly to the smoking-associated epidemic of lung cancer. However, there is no clear evidence that obesity has led to a substantial increase in cancer incidence or negatively affected cancer outcomes in the United States over this period (Figs 2 and 3).21 For example, incidence of endometrial and colorectal cancer, both obesity associated, have not increased in women, and colorectal cancer incidence in men has fallen. This lack of concordance between population trends in obesity versus cancer incidence and outcome may reflect a noncausal relationship. Alternatively, the lag time between obesity exposure and influence on cancer incidence or mortality may not yet have been reached, in which case major future increases may be seen. In addition, the relatively modest associations seen for obesity and cancer incidence and outcome may complicate detection of obesity impacts on cancer incidence and outcome in the face of changes in other factors, such as declines in menopausal hormone therapy, implementation of screening programs, and advances in cancer therapy. If treatment advances have, to some extent, negated adverse effects of obesity, it could be anticipated that reductions in obesity might result in even better cancer outcomes.
Fig 2.
Cancer statistics, 2016. Trends in incidence rates for selected cancers by sex, United States, 1975 to 2012. Reprinted with permission.21
Fig 3.
Cancer statistics, 2016. Trends in death rates (A) overall, and for selected sites for (B) males and (C) females, United States, 1930 to 2012. Reprinted with permission.21
The obesity-cancer association is a major clinical concern in oncology. New research, including randomized controlled clinical intervention trials of weight loss and pharmacologic targeting of obesity-associated physiology (for example, metformin), is underway, incorporating correlative research to identify subjects who benefit from the interventions and elucidate underlying biologic mechanisms of intervention effects. Additional randomized clinical intervention trials are needed to provide definitive evidence on the potential benefits of reversing obesity and its associated physiologic and biologic changes in patients with cancer. In parallel to these clinical trials, basic research is needed to enhance understanding of the biology of the obesity-cancer link. At a population level, it will be important to minimize the obesity epidemic, not only to lower cancer risk and improve outcomes but also to lower treatment toxicity and reduce comorbidity in cancer populations.
ACKNOWLEDGMENT
Supported by The Breast Cancer Research Foundation and Hold’Em for Life Translating Discoveries into Breast Cancer Cures (P.J.G.); and the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services Contracts No. N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221 (R.T.C.). This report was also funded by the American Institute for Cancer Research Grant No. 30210-01.
AUTHOR CONTRIBUTIONS
Financial support: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Obesity and Cancer: Insights for Clinicians
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Pamela J. Goodwin
Consulting or Advisory Role: Novo Nordisk
Rowan T. Chlebowski
Consulting or Advisory Role: Novartis, Pfizer, Genentech, Novo Nordisk, Genomic Health, Amgen
Speakers’ Bureau: Novartis
REFERENCES
- 1.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 2.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin PJ, Stambolic V. Impact of the obesity epidemic on cancer. Annu Rev Med. 2015;66:281–296. doi: 10.1146/annurev-med-051613-012328. [DOI] [PubMed] [Google Scholar]
- 4.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer: Viewpoint of the IARC Working Group. N Engl J Med. 2016;375:794–798. doi: 10.1056/NEJMsr1606602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: A population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiralerspong S, Goodwin PJ. Obesity and breast cancer prognosis: Evidence, challenges, and opportunities. J Clin Oncol. 2016;34:4203–4216. doi: 10.1200/JCO.2016.68.4480. [DOI] [PubMed] [Google Scholar]
- 7.Brown JC, Meyerhardt JA. Obesity and energy balance in GI cancer. J Clin Oncol. 2016;34:4217–4224. doi: 10.1200/JCO.2016.66.8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onstad MA, Schmandt RE, Lu KH. Addressing the role of obesity in endometrial cancer risk, prevention, and treatment. J Clin Oncol. 2016;34:4225–4230. doi: 10.1200/JCO.2016.69.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang L, Drake BF, Colditz GA. Obesity and other cancers. J Clin Oncol. 2016;34:4231–4237. doi: 10.1200/JCO.2016.68.4837. [DOI] [PubMed] [Google Scholar]
- 10.Chlebowski RT, Reeves MM. Weight loss randomized intervention trials in female cancer survivors. J Clin Oncol. 2016;34:4238–4248. doi: 10.1200/JCO.2016.69.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chlebowski RT, Blackburn GL, Thomson CA, et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women’s Intervention Nutrition Study. J Natl Cancer Inst. 2006;98:1767–1776. doi: 10.1093/jnci/djj494. [DOI] [PubMed] [Google Scholar]
- 12.Chlebowski RT, Blackburn GL, Hoy MK, et al. Survival analyses from the Women’s Intervention Nutrition Study (WINS) evaluating dietary fat reduction and breast cancer outcome. J Clin Oncol. 2008;26:522. [Google Scholar]
- 13.Thomson CA, Van Horn L, Caan BJ, et al. Cancer incidence and mortality during the intervention and postintervention periods of the Women’s Health Initiative dietary modification trial. Cancer Epidemiol Biomarkers Prev. 2014;23:2924–2935. doi: 10.1158/1055-9965.EPI-14-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. doi: 10.1200/JCO.2016.67.4044. Shlomai G, Neel B, LeRoith D, et al: Type 2 diabetes mellitus and cancer: The role of pharmacotherapy. J Clin Oncol 34:4261-4269, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. doi: 10.1200/JCO.2016.69.6187. Lohmann AE, Goodwin PJ, Chlebowski RT, et al: Association of obesity-related metabolic disruptions with cancer risk and outcome. J Clin Oncol 34:4249-4255, 2016. [DOI] [PubMed] [Google Scholar]
- 16. doi: 10.1200/JCO.2016.67.4283. Iyengar NM, Gucalp A, Dannenberg AJ, et al: Obesity and cancer mechanisms: Tumor microenvironment and inflammation. J Clin Oncol 34:4270-4276, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. doi: 10.1200/JCO.2016.67.9712. Hopkins BD, Goncalves MD, Cantley LC: Obesity and cancer mechanisms: Cancer metabolism. J Clin Oncol 34:4277-4283, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. doi: 10.1200/JCO.2016.69.1899. Renehan AG, Harvie M, Cutress RI, et al: How to manage the obese patient with cancer. J Clin Oncol 34:4284-4294, 2016. [DOI] [PubMed] [Google Scholar]
- 19.Alamuddin N, Bakizada Z, Wadden TA. Management of obesity. J Clin Oncol. 2016;34:4295–4305. doi: 10.1200/JCO.2016.66.8806. [DOI] [PubMed] [Google Scholar]
- 20. doi: 10.1200/JCO.2016.67.4051. Ligibel JA, Wollins D: The American Society of Clinical Oncology obesity initiative: Rationale, progress, and future directions. J Clin Oncol 34:4256-4260, 2016. [DOI] [PubMed] [Google Scholar]
- 21.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]