Abstract
In Gram-negative bacteria, the biogenesis of β-barrel outer membrane proteins is mediated by the β-barrel assembly machinery (BAM) complex. Over the past decade, structural and functional studies have collectively contributed to advancing our understanding of the BAM complex structure and function; however the exact mechanism remains elusive. In this Progress article, we discuss recent structural studies that have revealed that the accessory proteins may regulate essential unprecedented conformational changes within the core component BamA during function. We also review the mechanistic insights that have been gained from structural data, mutagenesis studies, molecular dynamics simulations, and explore two emerging models for BAM-mediated OMP biogenesis in bacteria.
ToC
In this Progress article, Buchanan and colleagues discuss recent structural studies that have advanced our understanding of the structure of the fully assembled β-barrel assembly machinery (BAM) complex and the interactions between the individual components. They also review the mechanistic insights that have been gained and explore two emerging models for BAM-mediated OMP biogenesis in bacteria.
Transmembrane proteins consist of either an α-helical fold or a β-barrel fold. α-helical membrane proteins contain one or more transmembrane-spanning domains (TMDs) that consist of hydrophobic α-helices, whereas monomeric β-barrel proteins contain between 8–26 TMDs consisting of amphipathic β-strands. The β-strands are arranged in a linear antiparallel β-sheet, with the first strand and last strand interacting at a junction to form the classic barrel-like shape from which their name is derived (Fig. 1a, b). Whereas α-helical transmembrane proteins can be found in all cellular membranes, β-barrel transmembrane proteins are only found within the outer membranes of Gram-negative bacteria and the endosymbionts apicoplasts, chloroplasts, and mitochondria; and more generally referred to as β-barrel outer membrane proteins (OMPs)1, 2. OMPs are essential for many cellular processes, including nutrient import, outer membrane biogenesis and motility3. Additionally, for pathogenic bacteria, OMPs can function as virulence factors that mediate pathogenesis and infection4, 5. Despite their essential roles, the mechanism for the biogenesis of OMPs remains poorly understood.
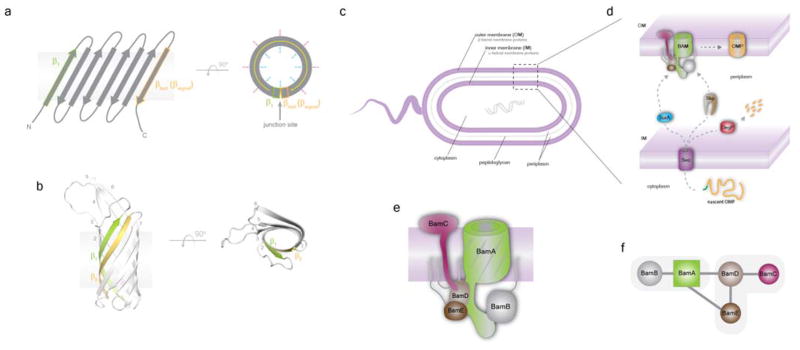
Figure 1. A journey toward the biogenesis of a β-barrel outer membrane protein.
a. Schematic of the architecture of a generic β-barrel outer membrane proteins (OMP), which consists of a linear arrangement of an even number of antiparallel β-strands (ranging from 8–26) where the first strand and last strand (also referred to as the β-signal) interact at the junction site to form a barrel shape within the membrane. Residues oriented inside the barrel domain (indicated by cyan arrows) are primarily hydrophilic, interacting with solvent, other domains, and/or other proteins, while the residues oriented outside the barrel domain (indicated by red arrows) are almost exclusively hydrophobic, mediating interactions with the hydrophobic core of the membrane. b. An example of an OMP, the structure of Ail (PBD ID 3QRA) from Y. pestis containing eight strands. The first strand is shown in green and the last strand is shown in yellow. c. Gram-negative bacteria contain both an inner membrane and an outer membrane, where the inner membrane partitions the cytoplasm and periplasm and peptidoglycan. The inner membrane contains exclusively α-helical membrane proteins, whereas the outer membrane contains almost exclusively β-barrel outer membrane proteins; only a few examples of the outer membrane containing α-helical membrane proteins are known. d. The biogenesis of an OMP begins within the cytoplasm where it is translated with an N-terminal leader sequence (green). It is then translocated across the inner membrane into the periplasm by the Sec machinery. The periplasmic chaperones SurA (primary pathway) and Skp (rescue pathway) then stabilize and further escort the nascent OMP to the BAM complex for final insertion into the outer membrane. If OMPs become misfolded and cannot be rescued by Skp, they will be degraded by DegP to prevent toxicity to the cell. e. In E. coli, the BAM complex consists of five components: the essential core component BamA, which is an OMP itself, and four accessory lipoproteins termed BamB, BamC, BamD and BamE, each containing an N-terminal post-translational lipid modification that anchors them to the inner leaflet of the outer membrane. Based on microscopy studies, the majority of the soluble domain of BamC is found at the surface of the bacteria, as depicted. However, recent structural studies show close association with BamD, and further studies are required to determine whether the proposed presence of BamC at the surface serves as part of its role within the BAM complex. f. The interaction network within the BAM complex, based on experimental studies, including results from recent structural studies. The BAM complex has been purified as two separate modules containing BamAB and BamCDE.
The unique properties of OMPs offer some clues about the machinery that is responsible for placing them into the outer membrane. OMPs have a much different fold than α-helical membrane proteins; they consist of a barrel-shaped β-sheet containing an intricate hydrogen bonding network that creates an extremely stable tertiary structure which is often resistant to denaturants such as sodium dodecyl sulfate6, 7. Each amphipathic strand contains hydrophobic residues that point towards the outside of the barrel and mediate interactions with the membrane bilayer, whereas the residues that point towards the inside or the lumen of the barrel are typically hydrophilic residues that interact with solvent, protein substrates and/or accessory proteins (Fig. 1a). These properties of OMPs make their biogenesis a unique and complex folding problem that requires complex machinery to accomplish.
Our current understanding of OMP biogenesis has come primarily from studies in Gram-negative bacteria such as Escherichia coli, Neisseria meningitidis, and Neisseria gonorrhoeae. Unlike α-helical membrane proteins, OMPs must be transported across the inner membrane, periplasm and peptidoglycan before they are inserted into the outer membrane 8–11 (Fig. 1c). This journey begins in the cytoplasm, where nascent OMPs are fully translated with an N-terminal leader sequence that directs them to the Sec export machinery, which transports the nascent OMPs across the inner membrane into the periplasm8–10, 12 (Fig. 1d). Chaperones in the periplasm then stabilize the nascent OMPs and escort them across the periplasm to the outer membrane13. The chaperone SurA has been implicated to be the key chaperone during targeting of OMPs ; however, Skp has also been shown to have a role during chaperone-mediated biogenesis for some OMPs, even rescuing ‘off-pathway’ OMPs that are usually chaperoned by SurA13. However, recent studies indicate a more complicated pathway where OMPs may experience complex chaperone networks that are driven by energy landscapes within the periplasm14–16. Misfolded OMPs that cannot be rescued are degraded by the periplasmic protease DegP to prevent their accumulation and thus cellular toxicity13, 17–19. Once at the outer membrane, the nascent OMPs are delivered to the β-barrel assembly machinery (BAM) complex, which mediates their biogenesis into the outer membrane8–10, 20. The pathway to the BAM complex and hence OMP biogenesis is complicated by a number of factors, including the peptidoglycan, which is part of the cell wall that forms a rigid mesh within the periplasm, separating the inner membrane and the outer membrane (Fig. 1c). The chaperone-stabilized nascent OMPs must pass through the peptidoglycan to reach the BAM complex within the outer membrane. Another barrier for OMP biogenesis is the absence of a known energy source, such as nucleotide hydrolysis in the periplasm or at the outer membrane21. Without a novel mechanism, the energy required to insert OMPs into the inner membrane or outer membrane would be too great to overcome in vivo. However, once nascent OMPs arrive at the outer membrane, it has been postulated that the BAM complex acts as a catalyst on the membrane itself to facilitate insertion of OMPs22–24. No analogous catalyst has yet been identified within the inner membrane, which possibly explains why no OMPs have been found at that membrane.
The BAM complex consists of multiple components which can vary by species23, 25–28. In E. coli, the BAM complex consists of five components: the essential core component BamA, which is an OMP itself, and four accessory lipoproteins termed BamB, BamC, BamD and BamE, each containing an N-terminal post-translational lipid modification that anchors them to the inner leaflet of the outer membrane9, 10, 29, 30 (Fig. 1e). BamB and BamD have been shown to interact directly with the periplasmic domain of BamA while BamC and BamE interact with BamD, leading to the isolation of primarily BamAB and BamCDE modules in vivo9, 10, 29, 31–35 (Fig. 1f). Both BamA and BamD are essential for cell viability; however, all components are required for efficient OMP biogenesis in vivo31–33, 36, 37. In vitro studies have further demonstrated the vital role of BamB for optimal folding of the two OMPs OmpT and EspP, possibly mediating the delivery of substrate from SurA to the BAM complex, a process that is still not well understood36, 37.
In this Progress article, we discuss recent structural studies that have advanced our understanding of the structure of the fully assembled BAM complex and the interactions between the individual components. We also review the mechanistic insights that have been gained from structural data, mutagenesis studies, molecular dynamics simulations, and explore two emerging models for BAM-mediated OMP biogenesis in bacteria.
The structure of the BAM complex
The structures of the Bam proteins
In the past decade, the structures of all of the periplasmic domains of the Bam accessory proteins have been reported29, 35 (Fig. 2a). BamB adopts an eight-bladed β-propeller fold containing WD40-like motifs, suggesting that it may play a role as a scaffold within the BAM complex38–41. The structure of BamC contains an unstructured N-terminal domain followed by two helix-grip domains, each of which was solved individually42–44. Interestingly, in vivo studies have shown the helix-grip domains of BamC to be surface-exposed in E. coli45. Here, fluorescently labeled antibodies, which were raised against fully folded BamC, were used to specifically label BamC located outside of E. coli. While labeling was still observed when the C-terminal helix-grip domain was removed, no labeling was observed with a construct lacking both helix-grip domains. Exactly how surface exposure of BamC occurs or what role this may have within the BAM complex remains to be determined. BamD consists of five tetratricopeptide repeat (TPR) domains, and it has been suggested that BamD has a role in activating BamA and/or interacts with substrates43, 46, 47. BamE contains an ααβββ fold and has been shown to enhance the interaction of BamD with BamA33, 43, 48, 49. Although the structures of these Bam accessory components have been solved, they offered few clues about how the BAM complex interacts with nascent OMPs or how it mediates their biogenesis.
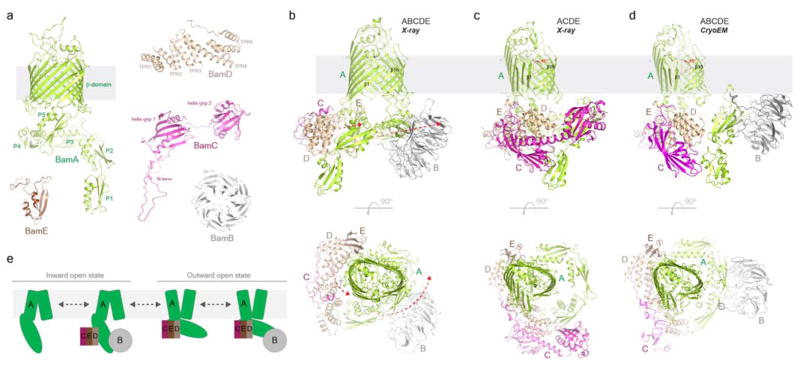
Figure 2. The structures of the β-barrel assembly machinery complex.
a. The individual structures of all the Bam components. BamA (green) contains an N-terminal periplasmic domain consisting of five polypeptide transport-associated (POTRA) repeat domains (P1 – P5) and a C-terminal 16-stranded β-barrel domain. BamB is an eight bladed β-propeller structure containing multiple WD40-like repeats and may serve as a scaffolding protein. BamC, which has been shown to be presented at the surface, contains multiple domains, including an unstructured N-terminal domain followed by two helix-grip domains. BamD contains five tetratricopeptide repeat (TPR) domains and forms the primary interaction with BamA. BamE contains an ααβββ globular fold and interacts with both BamA and BamD, helping to stabilize the intact complex. b,c. The structure of the BAM complex was recently reported with (PDB ID 5D0O and 5AYW) (b) and without BamB (PDB ID 5D0Q and 5EKQ) (c) by X-ray crystallography, revealing an unprecedented 45° shift of the first half of the barrel domain of BamA. The direction of the conformational changes observed within the POTRA domains are indicated. d. More recently, the structure of the BAM complex with BamB was reported using cryoEM (PDB ID 5LJO), revealing a similar conformation as observed in the X-ray crystal structure lacking BamB (part c). e. These structures suggest that the role of the Bam accessory proteins may be to regulate the conformation of BamA during OMP biogenesis. In all structures reported to date, two major conformations have now been observed for BamA. In the first, the barrel domain of BamA shows an ‘inward open’ conformation where the exit pore is occluded, with POTRA5 being located away from the barrel domain to enable access to the lumen from the periplasm. In the second, the barrel domain of BamA shows an ‘outward open’ conformation where the exit pore is now fully open, as POTRA5 shifts to occlude access to the barrel lumen from the periplasm. These conformational states were initially thought to be modulated by BamB, however, both have now been observed in the presence and absence of BamB. Exactly what role the conformational changes serve or how they are regulated remains unknown. The structure of BamA alone (PDB ID 4C4V and 4N75) has also been reported and was found in an ‘inward open’ state; however, BamA alone would presumably be rare in vivo.
The crystal structure of BamA offered the first hint to how the BAM complex may function in OMP biogenesis23, 24, 50–54. BamA consists of an N-terminal periplasmic domain that contains five polypeptide transport associated (POTRA) domains and a C-terminal 16-stranded β-barrel domain20, 23, 35 (Fig. 2a). Shortly after the BAM complex was first discovered, it was shown that both BamB and BamD interact with BamA via its POTRA domains31, 32, 34. Other studies suggested that the POTRA domains may also interact with the nascent OMP substrate prior to insertion into the outer membrane53, 54. More recently, several reports revealed that the atomic structure of the β-barrel domain of BamA is novel and has unique properties that were not previously seen in other OMPs with known structure20, 23, 24, 35. Whereas BamA contains sixteen β-strands as predicted, strands β1 and β16, which are located at the junction site that closes the barrel, were found to be substantially shorter, resulting in a drastically thinned hydrophobic belt (phenylalanine, tryptophan, and tyrosine residues found along the outside of the barrel domain that assist in maintaining buoyancy within the membrane)20, 23, 24, 51, 52. Furthermore, in two of the reported crystal structures, strand β16 was found partially unzipped from β1, sitting in a bent conformation pointing toward the lumen of the barrel domain, leaving only a few hydrogen bonds at the junction23, 51, 52. These studies offered the first evidence that the barrel domain of BamA contains a lateral gate into the membrane, which would create a direct portal for the insertion of nascent OMPs directly into the outer membrane during biogenesis23, 24. Moreover, an exit pore was identified that has been proposed to mediate the formation of extracellular loops of nascent OMPs24, 52.
The structure of the fully assembled complex
The first complex crystal structure of Bam components was that of BamD bound to a BamC construct that lacks the C-terminal helix-grip domain. The structure revealed that the unstructured N-terminal domain of BamC almost exclusively mediates the interaction with BamD55. The next complex reported was that of a fusion between POTRA3, POTRA4 and POTRA5 of BamA with BamB56, 57. This crystal structure further confirmed previous studies that indicated that BamB binds along POTRA3 in proximity to the linker with POTRA2. This interaction may have a role in regulating the conformational changes within the POTRA domains that were previously observed along this hinge point between POTRA2 and POTRA350, 53, 54. Later, the crystal structure of a fusion between POTRA4 and POTRA5 with BamD was reported, showing that TPR3 and TPR4 of BamD mediate the primary interactions with only POTRA5 of BamA, agreeing well with previous studies31, 58. The exact role of BamD binding to BamA remains unclear; however, studies have suggested that BamD may activate BamA59.
Four crystal structures of the assembled BAM complex were recently reported, providing the molecular details for how the full complex is assembled, the interactions between the individual Bam components, and associated conformational changes60–62 (Fig. 2b, c). Two of the reported structures lack BamB, whereas two of the structures contain BamB, yet are the same structures with identical space groups and cell parameters61, 62. More recently, a fifth structure of the BAM complex with BamB was reported using cryoEM63. BamC was found interacting with BamD as observed previously, yet the helix-grip domains were found in various states and conformations along the periplasmic side of the complex with one or both disordered, indicating the lack of a stable interaction with other Bam components. While this seemingly conflicts with the previous in vivo studies indicating the helix-grip domains are surface exposed45, the X-ray and cryoEM structures of the BAM complex were determined in non-native environments and in the absence of substrates, which may be required to observe the native topology of BamC. Proper controls were demonstrated in the in vivo studies, with a construct lacking both helix-grip domains showing no surface labeling, providing the most convincing evidence that BamC truly is surface exposed. And since no stable interaction of BamC with the surface of BamA has been described, it is not surprising that BamC was observed to be dynamic in the reported structures. Aside from BamD, where flexing of the molecule was observed within the complex, the individual structures of BamB, BamC and BamE remained mostly unchanged with only slight localized conformational changes. In all structures of the BAM complex, the binding interactions of BamC with BamD, BamD with BamA, and BamB with BamA closely mirror those observed in the previously described complex crystal structures55–58. In addition, BamE was found to interact with TPR4 and TPR5 of BamD and unexpectedly also with POTRA5 of BamA, which may explain previous studies reporting that BamE enhances the interaction of BamD with BamA33. Further, TPR1 and TPR2 of BamD were found to interact with POTRA1 and POTRA2 of BamA, which may stabilize a ring-like quaternary structure along the periplasmic portion of the BAM complex60–62. Although this ring-like structure has been suggested to be important for OMP biogenesis, no mechanism has been postulated describing exactly how it contributes to the function of the BAM complex. While the recent structures show BamD interacting with POTRA1, POTRA2 and POTRA5, only the interaction with POTRA5 was consistently observed and supported by in vivo studies31, suggesting the other interactions may be artefacts.
Conformational cycling of BamA
The most important observations taken from the recent structures of the BAM complex are the drastic conformational changes observed in BamA60–63 (Fig. 2b, c, d). In total four conformational states are observed for the POTRA domains. Two states were observed in the crystal structures that lack BamB, in which POTRA1-4 undergo ~30 Å shift from one another, whereas POTRA5 remains mostly unchanged, being located away from the barrel domain, thus enabling access to the lumen of the barrel domain60, 61 (Fig. 2c). The third conformational state was observed in the cryoEM structure which closely resembles one of the crystal structures that lack BamB; however only slight shifts were detected, primarily in POTRA1 and POTRA263 (Fig. 2d). The fourth conformational state was observed in the crystal structure with BamB, in which POTRA1-5 undergo ~40 Å shift with POTRA5 now being located directly under the barrel domain fully occluding access the barrel lumen61, 62 (Fig. 2b). The POTRA domains of BamA were previously shown to be dynamic, and it is expected that the BAM complex itself is also dynamic. Therefore, it remains to be determined whether the observed conformations of BamA are truly due to binding of the Bam accessory proteins or just an artifact of crystallization and/or in vitro sample preparation23, 50, 53, 54.
Based on the X-ray crystal structures, two conformations were observed for the β-barrel domain of BamA, and the different states first seemed to be dependent on whether BamB was present or not60–62. In the presence of BamB, BamA contains a classic barrel domain with a fully formed junction site (inward open conformation)61, 62 (Fig. 2b). By contrast, in the absence of BamB, an unprecedented conformational change was observed, where the first half of the barrel domain undergoes a ~45° rotation, starting with strand β1 and diminishing up to strand β9, with extracellular loops 1–3 fully opening along the previously identified exit pore (outward open conformation)60, 61 (Fig. 2c). However, in the recent cryoEM structure which contains BamB63, the barrel domain of BamA was also found in an outward open conformation (Fig. 2d). Exactly why different conformations are observed between the X-ray crystal structure and the cryoEM structure of the fully assembled BAM complexes in the absence of BamB is not known; however, one possible explanation may be that different detergents were used during structure determination.
One consequence of the conformational change from an inward open to an outward open conformation of the barrel domain is that the junction gets completely disrupted with no hydrogen bonds being formed between strands β1 and β1660, 61. Despite the dynamic conformational changes observed for BamA, the Bam accessory proteins accommodate these changes in BamA primarily as rigid bodies. With the existing structural data, it is tempting to speculate that the roles of BamB and BamCDE may be to regulate the cycling of BamA between ‘inward open’ and ‘outward open’ conformations (Fig. 2e). Crosslinking studies have provided further evidence for the observed structural changes24, 61, which may explain previous observations from genetic and functional studies that indicated that conformational cycling of BamA during OMP biogenesis is regulated by Bam components 64.
These conformational changes in BamA could possibly help drive the biogenesis of OMPs by directly threading nascent OMPs into the outer membrane and/or by further priming the membrane for OMPs insertion. However, additional experimental verification is certainly required to fully test this hypothesis.
Mechanistic insights into OMP biogenesis
Molecular dynamics simulations reveal clues towards mechanism
Molecular dynamics simulations have had a key role in unraveling the mechanisms of OMP biogenesis since the first BamA structures were solved23, 24. Although these structures revealed a closed barrel, molecular dynamics simulations on the 1–2 μs time scale showed the junction between strands β1 and β16 of BamA to be dynamic23, 24. The simulations also showed a distinct thinning of the membrane near the proposed lateral gate23, 24. Notably, at the time the simulations used a simplified lipid membrane and/or were performed at an increased temperature, which leaves in question the biological relevance of the observed dynamics. However, biochemical experiments have confirmed the importance of lateral gating; disulfide cross-linking the gate at various points rendered BamA non-functional, leading to a loss of viability, whereas reducing the cross-link restored function24, 61. Similarly, a hypothetical exit pore between the extracellular loops of BamA that was observed in simulations was confirmed to be relevant to BamA function in cross-linking studies24, 61.
More recent molecular dynamics simulations of BamA have focused on the dynamics of the five POTRA domains of BamA65. It was found that in addition to a high degree of flexibility between the domains, the POTRA domains can also bind to the membrane surface. Only certain membrane-bound conformations were observed to be compatible with BamB and/or BamD binding65. Finally, simulations of the entire BAM complex, namely the 3.9-Å BamACDE structure (PDB 5D0Q) and the 2.9-Å BamABCDE structure (PDB 5D0O), have also been carried out61. These simulations showed that the distinct conformations of each complex are stable on the 100-ns time scale. Removal of some or all Bam accessory proteins from the complexes showed an increase in dynamics for the remaining components; for example, POTRA3 and POTRA4 move away from the membrane when BamB is removed61. Simulations of over 2.8 μs together with functional studies led to the conclusion that the POTRA domains of BamA (which are arranged in a ring-like architecture) and accessory proteins that are located below the BamA barrel shift between distinct conformations to modulate the conformation of the β-barrel, thereby alternating exposure to the periplasm or to the membrane61.
Mechanistic models for BAM-mediated biogenesis
Although several hypotheses have been proposed for how the BAM complex mediates OMP biogenesis, two popular mechanistic models are summarized here. Neither of the models has been conclusively demonstrated experimentally to apply to all OMPs and it may be that different types of OMPs interact with the BAM complex in a distinct way, depending on their complexity. The first model proposes that, based on their sequence, OMPs fold themselves and just need a locally disturbed membrane to organically fold into. In this model, the role of the BAM complex is to function as a catalyst by locally destabilizing the membrane bilayer and to traffic the nascent OMP into close proximity to the primed membrane for insertion into the outer membrane20, 22–24, 66 (Fig. 3a).
Figure 3. Mechanistic models for the role of the BAM complex in OMP biogenesis.
a. The first model proposes that β-barrel outer membrane proteins (OMPs) fold intrinsically and just need a locally disturbed membrane to organically fold into. In this model, nascent OMPs are first synthesized in the cytoplasm with an N-terminal leader sequence (removed by proteases at the inner membrane) which directs them to the Sec translocon for transport across in the inner membrane into the periplasm (step 1). Next, the periplasmic chaperones SurA or Skp (not shown) binds the nascent OMPs and delivers them to the BAM complex (step 2), which serves as a catalyst to locally destabilize the membrane bilayer as indicated) and to traffic the nascent OMP into close proximity to the primed membrane for insertion into the outer membrane (step 3). The current hypothesis is that trafficking to the BAM complex may be regulated by the β-signal of the nascent OMP. b. The second model proposes a more systematic mechanism whereby the SurA- and/or Skp-stabilized nascent OMP is delivered to the BAM complex, which then systematically folds it into the membrane. This model shares the initial two steps (step 1 and step 2) with the first model (part a), where nascent OMPs are imported into the periplasm and stabilized by chaperones such as SurA and Skp (not shown). Next, the nascent OMPs are delivered to components of the BAM complex (possibly BamB, BamD, and/or the POTRA domains of BamA) and folding and insertion are initiated by the β-signal of the nascent OMP (step 3)). Upon opening of the lateral gate of BamA, a β-hairpin has been hypothesized to bind the exposed N-terminal strand of BamA by β-augmentation. The barrel of the nascent OMP integrates directly into the barrel of BamA, thus satisfying the requirement that hydrophilic residues point inside the barrel, while hydrophobic residues point outside and mediate interaction with the membrane. The nascent OMP continues to grow either strand by strand or by a β-hairpin at a time (steps 4–6)). To prevent the formation of a super-pore in the outer membrane, the nascent OMP eventually ‘buds’ away from the barrel domain of BamA. We hypothesize that maturation occurs when the first strand of the nascent OMP comes into proximity of the junction site, whereby the last strand of the nascent OMP unpairs from BamA and then pairs with its own first strand (step 7)). This results in the termination of the folding process, forming an independent barrel which then diffuses into the outer membrane, while the BAM complex is left primed for another round of OMP folding.
The first model can be rationalized by the fact that some OMPs can readily and efficiently fold into perturbed membranes; however, this is not true for larger, more complex OMPs, including those that are multimeric. To address this, the second model proposes a more systematic mechanism whereby nascent OMPS that are stabilized by SurA and/or Skp are delivered to BamA and/or other Bam proteins and then systematically threaded into the membrane, possibly strand by strand or by one β-hairpin at a time29, 67, 68 (Fig. 3b). To satisfy the amphipathic properties of the new strands, each is formed by β-augmentation, a process in which existing β-strands serve as templates for the formation of new strands, using the exposed edges of the BamA barrel following lateral opening or following the large conformational change observed in the most recent structures of the assembled BAM complex lacking BamB. Strands would be added and an enlarged barrel is formed, which would expand only to a certain point until it begins to bud away from the BamA barrel, forming its own barrel within the membrane. It has been hypothesized that the C-terminal strand (also referred to as the β-signal) of the nascent OMPs would interact with the first strand of the BamA barrel as an initiating step for biogenesis; however, this notion remains to be shown experimentally. While the lateral gate formed by BamA enables the formation of strands directly into the membrane, the exit pore would mediate the formation of extracellular loops, some of which can be as large as 100 residues or more4. Barrel growth of the nascent OMP would continue until the termination step, which is triggered when the first strand of the new OMP forms and comes into close proximity with the transient junction. We hypothesize that the C-terminal strand of the new OMP has an inherently higher affinity for its native first strand than it does for that of BamA. Therefore, strand exchange occurs, which completes the biogenesis of the new OMP where it can freely diffuse into the outer membrane. This concept can be rationalized by the fact that the junctions of most OMPs share many more hydrogen bonds than the five or six that the first strand of BamA can offer.
Outlook
In Gram-negative bacteria, it is generally accepted that the BAM complex is responsible for the biogenesis of most, if not all, OMPs. Given the amphipathic nature of the β-strand building blocks, the diversity in the number of strands, and the fact that there is no energy source such as nucleotide hydrolysis at the outer membrane, the mechanism for the biogenesis of OMPs is arguably more complex than that for α-helical membrane proteins. Collectively, studies over the past decade have greatly advanced our understanding of the journey OMPs must take to reach their final destination within the outer membrane. Furthermore, the recently solved structures of the BAM complex, which reveal unprecedented conformational changes in the β-barrel domain of BamA, cycling between ‘inward open’ and ‘outward open’, it is clear that the gap between what is known about the biogenesis of α-helical versus β-barrel membrane proteins is rapidly closing.
It was shown that the BAM complex can also partner with the translocation and assembly module (TAM) complex to mediate the biogenesis of some autotransporters and the usher protein FimD69–72. More recently, in Borrelia burgdorferi, the TamB component of TAM was shown to interact directly with BamA and to be an essential component of the BAM complex73. Exactly how the BAM and TAM systems communicate with one another, how they decide their substrates, and what mechanistic role each may have in OMP biogenesis remains to be determined.
With the new structures of the BAM complex hot off the press60–63, we now have the molecular blueprints that are necessary to design experiments to precisely resolve the molecular and mechanistic details responsible for the biogenesis of OMPs. Moving forward, there are many unanswered questions that require further investigation. For example, what role does BamC have and does its proposed presentation at the cell surface contribute to OMP biogenesis? If so, how? Where does the energy come from that drives the BAM complex? Could the destabilized membrane and folding energy be all that are required? Do nascent OMPs really interact directly with the POTRA domains of BamA and if so, how? This idea has been around for many years but without any convincing evidence. Furthermore, if nascent OMPs do in fact interact directly with BamA or even another Bam protein, is the N-terminus or the C-terminus recognized first? How important are the sequences of the first and last strands of nascent OMPs? It can be rationalized that they must be complementary and this may even dictate the number of strands in an OMP. With the recent structures of the BAM complex, it is tempting to propose that the role of the accessory proteins is to regulate the conformational cycling of BamA; however, additional experiments are certainly required to support this hypothesis. Still, if true, this would align with the fact that BamA is the only evolutionarily conserved component of the BAM complex present also in mitochondria and in plastids such as apicoplasts and chloroplasts. Finally, the mechanism for how the BAM complex mediates OMP biogenesis has been a topic of much debate. Therefore, which proposed mechanism is correct, if any? Are there other, more plausible mechanisms that have been overlooked? Could the BAM complex mediate unique folding pathways tailored to the substrate OMP itself? With the structure of the fully assembled BAM complex now known, it is anticipated that many of these missing pieces of the mechanistic puzzle will soon be revealed.
Acknowledgments
NN is supported by the Department of Biological Sciences at Purdue University, a Showalter Trust Award, and by the National Institute of Allergy and Infectious Diseases (1K22AI113078-01); JCG is supported by a CAREER award from the National Science Foundation (MCB-1452464); SKB is supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Biographies
Nicholas Noinaj is an Assistant Professor in the Department of Biological Sciences at Purdue University. He received dual B.A. degrees in chemistry and mathematics at Berea College (Berea, Kentucky) and his Ph.D. in biochemistry at the University of Kentucky (Lexington, Kentucky). He did his postdoc within the Intramural Research Program at NIH/NIDDK. He joined the structural biology group at Purdue University in 2014 and his research focuses on structure determination of membrane protein folding machineries in pathogenic bacteria and chloroplasts.
James C. Gumbart is an Assistant Professor of Physics at the Georgia Institute of Technology (Atlanta, Georgia, USA). He received his B.S. from Western Illinois University (Macomb, Illinois, USA) and his Ph.D. from the University of Illinois (Urbana, Illinois, USA). His lab’s research is focused on the structure, mechanics, and function of the bacterial cell envelope.
Susan Buchanan is Chief of the Section on Structural Biology of Membrane Proteins, in the National Institute for Diabetes & Digestive & Kidney Diseases, at the National Institutes of Health. She joined the NIDDK as a Tenure Track Investigator in 2001 and is currently a Senior Investigator and Chief of the Laboratory of Molecular Biology, NIDDK. Research in Dr. Buchanan’s laboratory focuses on structure determination of integral membrane proteins using X-ray crystallography and functional analysis of these proteins using biophysical, biochemical, and cell biological techniques.
Footnotes
Competing interests statements
The authors declare no competing interests.
Subject terms:
Biological sciences / Structural biology / X-ray crystallography [URI /631/535/1266]
Biological sciences / Microbiology / Bacteria / Bacterial development [URI /631/326/41/2528]
Biological sciences / Microbiology / Bacteria / Bacterial structural biology [URI /631/326/41/2536]
Biological sciences / Biochemistry / Proteins / Membrane proteins [URI /631/45/612/1237]
Biological sciences / Microbiology / Bacteria / Cellular microbiology [URI /631/326/41/88]
References
- 1.Walther DM, Rapaport D, Tommassen J. Biogenesis of beta-barrel membrane proteins in bacteria and eukaryotes: evolutionary conservation and divergence. Cell Mol Life Sci. 2009;66:2789–804. doi: 10.1007/s00018-009-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Misra R. Assembly of the beta-Barrel Outer Membrane Proteins in Gram-Negative Bacteria, Mitochondria, and Chloroplasts. ISRN Mol Biol. 2012;2012:708203. doi: 10.5402/2012/708203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulz GE. beta-Barrel membrane proteins. Curr Opin Struct Biol. 2000;10:443–7. doi: 10.1016/s0959-440x(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 4.Noinaj N, et al. Structural basis for iron piracy by pathogenic Neisseria. Nature. 2012;483:53–8. doi: 10.1038/nature10823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang JH, Tong J, Tan KS, Gabriel K. From evolution to pathogenesis: the link between beta-barrel assembly machineries in the outer membrane of mitochondria and gram-negative bacteria. Int J Mol Sci. 2012;13:8038–50. doi: 10.3390/ijms13078038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heller KB. Apparent molecular weights of a heat-modifiable protein from the outer membrane of Escherichia coli in gels with different acrylamide concentrations. J Bacteriol. 1978;134:1181–3. doi: 10.1128/jb.134.3.1181-1183.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noinaj N, Kuszak AJ, Buchanan SK. Heat Modifiability of Outer Membrane Proteins from Gram-Negative Bacteria. Methods Mol Biol. 2015;1329:51–6. doi: 10.1007/978-1-4939-2871-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricci DP, Silhavy TJ. The Bam machine: A molecular cooper. Biochim Biophys Acta. 2012;1818:1067–84. doi: 10.1016/j.bbamem.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knowles TJ, Scott-Tucker A, Overduin M, Henderson IR. Membrane protein architects: the role of the BAM complex in outer membrane protein assembly. Nat Rev Microbiol. 2009;7:206–14. doi: 10.1038/nrmicro2069. [DOI] [PubMed] [Google Scholar]
- 10.Hagan CL, Silhavy TJ, Kahne D. beta-Barrel membrane protein assembly by the Bam complex. Annu Rev Biochem. 2011;80:189–210. doi: 10.1146/annurev-biochem-061408-144611. [DOI] [PubMed] [Google Scholar]
- 11.Rollauer SE, Sooreshjani MA, Noinaj N, Buchanan SK. Outer membrane protein biogenesis in Gram-negative bacteria. Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2015.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Driessen AJ, Nouwen N. Protein translocation across the bacterial cytoplasmic membrane. Annu Rev Biochem. 2008;77:643–67. doi: 10.1146/annurev.biochem.77.061606.160747. [DOI] [PubMed] [Google Scholar]
- 13.Sklar JG, Wu T, Kahne D, Silhavy TJ. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes & Development. 2007;21:2473–2484. doi: 10.1101/gad.1581007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon CP, Zaccai NR, Fleming PJ, Gessmann D, Fleming KG. Membrane protein thermodynamic stability may serve as the energy sink for sorting in the periplasm. Proc Natl Acad Sci U S A. 2013;110:4285–90. doi: 10.1073/pnas.1212527110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costello SM, Plummer AM, Fleming PJ, Fleming KG. Dynamic periplasmic chaperone reservoir facilitates biogenesis of outer membrane proteins. Proc Natl Acad Sci U S A. 2016;113:E4794–800. doi: 10.1073/pnas.1601002113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Geyter J, et al. Protein folding in the cell envelope of Escherichia coli. Nat Microbiol. 2016;1:16107. doi: 10.1038/nmicrobiol.2016.107. [DOI] [PubMed] [Google Scholar]
- 17.Krojer T, Garrido-Franco M, Huber R, Ehrmann M, Clausen T. Crystal structure of DegP (HtrA) reveals a new protease-chaperone machine. Nature. 2002;416:455–459. doi: 10.1038/416455a. [DOI] [PubMed] [Google Scholar]
- 18.Krojer T, et al. Structural basis for the regulated protease and chaperone function of DegP. Nature. 2008;453:885–890. doi: 10.1038/nature07004. [DOI] [PubMed] [Google Scholar]
- 19.Ge X, et al. DegP primarily functions as a protease for the biogenesis of beta-barrel outer membrane proteins in the Gram-negative bacterium Escherichia coli. FEBS J. 2014;281:1226–40. doi: 10.1111/febs.12701. [DOI] [PubMed] [Google Scholar]
- 20.Noinaj N, Rollauer SE, Buchanan SK. The beta-barrel membrane protein insertase machinery from Gram-negative bacteria. Curr Opin Struct Biol. 2015;31:35–42. doi: 10.1016/j.sbi.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun V. Energy-coupled transport and signal transduction through the gram-negative outer membrane via TonB-ExbB-ExbD-dependent receptor proteins. FEMS Microbiol Rev. 1995;16:295–307. doi: 10.1111/j.1574-6976.1995.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 22.Gessmann D, et al. Outer membrane beta-barrel protein folding is physically controlled by periplasmic lipid head groups and BamA. Proc Natl Acad Sci U S A. 2014;111:5878–83. doi: 10.1073/pnas.1322473111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noinaj N, et al. Structural insight into the biogenesis of beta-barrel membrane proteins. Nature. 2013;501:385–390. doi: 10.1038/nature12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noinaj N, Kuszak Adam J, Balusek C, Gumbart James C, Buchanan Susan K. Lateral Opening and Exit Pore Formation Are Required for BamA Function. Structure. 2014;22:1055–1062. doi: 10.1016/j.str.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paramasivam N, Habeck M, Linke D. Is the C-terminal insertional signal in Gram-negative bacterial outer membrane proteins species-specific or not? BMC Genomics. 2012;13:510. doi: 10.1186/1471-2164-13-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb CT, Heinz E, Lithgow T. Evolution of the beta-barrel assembly machinery. Trends Microbiol. 2012;20:612–20. doi: 10.1016/j.tim.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Volokhina EB, Beckers F, Tommassen J, Bos MP. The beta-barrel outer membrane protein assembly complex of Neisseria meningitidis. J Bacteriol. 2009;191:7074–85. doi: 10.1128/JB.00737-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anwari K, et al. A modular BAM complex in the outer membrane of the alpha-proteobacterium Caulobacter crescentus. PLoS One. 2010;5:e8619. doi: 10.1371/journal.pone.0008619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim KH, Aulakh S, Paetzel M. The bacterial outer membrane beta-barrel assembly machinery. Protein Sci. 2012;21:751–68. doi: 10.1002/pro.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onufryk C, Crouch ML, Fang FC, Gross CA. Characterization of Six Lipoproteins in the σE Regulon. Journal of Bacteriology. 2005;187:4552–4561. doi: 10.1128/JB.187.13.4552-4561.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu T, et al. Identification of a Multicomponent Complex Required for Outer Membrane Biogenesis in Escherichia coli. Cell. 2005;121:235–245. doi: 10.1016/j.cell.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Malinverni JC, et al. YfiO stabilizes the YaeT complex and is essential for outer membrane protein assembly in Escherichia coli. Molecular microbiology. 2006;61:151–64. doi: 10.1111/j.1365-2958.2006.05211.x. [DOI] [PubMed] [Google Scholar]
- 33.Sklar JG, et al. Lipoprotein SmpA is a component of the YaeT complex that assembles outer membrane proteins in Escherichia coli. Proceedings of the National Academy of Sciences. 2007;104:6400–6405. doi: 10.1073/pnas.0701579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vuong P, Bennion D, Mantei J, Frost D, Misra R. Analysis of YfgL and YaeT interactions through bioinformatics, mutagenesis, and biochemistry. J Bacteriol. 2008;190:1507–17. doi: 10.1128/JB.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Neil PK, Rollauer SE, Noinaj N, Buchanan SK. Fitting the Pieces of the beta-Barrel Assembly Machinery Complex. Biochemistry. 2015;54:6303–11. doi: 10.1021/acs.biochem.5b00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roman-Hernandez G, Peterson JH, Bernstein HD. Reconstitution of bacterial autotransporter assembly using purified components. Elife. 2014;3:e04234. doi: 10.7554/eLife.04234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hagan CL, Kim S, Kahne D. Reconstitution of Outer Membrane Protein Assembly from Purified Components. Science. 2010;328:890–892. doi: 10.1126/science.1188919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noinaj N, Fairman JW, Buchanan SK. The Crystal Structure of BamB Suggests Interactions with BamA and Its Role within the BAM Complex. J Mol Biol. 2011;407:248–60. doi: 10.1016/j.jmb.2011.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heuck A, Schleiffer A, Clausen T. Augmenting beta-Augmentation: Structural Basis of How BamB Binds BamA and May Support Folding of Outer Membrane Proteins. J Mol Biol. 2011;406:659–66. doi: 10.1016/j.jmb.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Kim KH, Paetzel M. Crystal Structure of Escherichia coli BamB, a Lipoprotein Component of the beta-Barrel Assembly Machinery Complex. J Mol Biol. 2011;406:667–78. doi: 10.1016/j.jmb.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 41.Jansen KB, Baker SL, Sousa MC. Crystal structure of BamB from Pseudomonas aeruginosa and functional evaluation of its conserved structural features. PLoS One. 2012;7:e49749. doi: 10.1371/journal.pone.0049749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim KH, Aulakh S, Tan W, Paetzel M. Crystallographic analysis of the C-terminal domain of the Escherichia coli lipoprotein BamC. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011;67:1350–8. doi: 10.1107/S174430911103363X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albrecht R, Zeth K. Structural basis of outer membrane protein biogenesis in bacteria. J Biol Chem. 2011;286:27792–803. doi: 10.1074/jbc.M111.238931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warner LR, et al. Structure of the BamC two-domain protein obtained by Rosetta with a limited NMR data set. J Mol Biol. 2011;411:83–95. doi: 10.1016/j.jmb.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webb CT, et al. Dynamic association of BAM complex modules includes surface exposure of the lipoprotein BamC. J Mol Biol. 2012;422:545–55. doi: 10.1016/j.jmb.2012.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandoval CM, Baker SL, Jansen K, Metzner SI, Sousa MC. Crystal structure of BamD: an essential component of the beta-Barrel assembly machinery of gram-negative bacteria. J Mol Biol. 2011;409:348–57. doi: 10.1016/j.jmb.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong C, Hou HF, Yang X, Shen YQ, Dong YH. Structure of Escherichia coli BamD and its functional implications in outer membrane protein assembly. Acta Crystallogr D Biol Crystallogr. 2012;68:95–101. doi: 10.1107/S0907444911051031. [DOI] [PubMed] [Google Scholar]
- 48.Kim KH, et al. Structural characterization of Escherichia coli BamE, a lipoprotein component of the beta-barrel assembly machinery complex. Biochemistry. 2011;50:1081–90. doi: 10.1021/bi101659u. [DOI] [PubMed] [Google Scholar]
- 49.Knowles TJ, et al. Structure and function of BamE within the outer membrane and the beta-barrel assembly machine. EMBO Rep. 2011;12:123–8. doi: 10.1038/embor.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gatzeva-Topalova PZ, Warner LR, Pardi A, Sousa MC. Structure and flexibility of the complete periplasmic domain of BamA: the protein insertion machine of the outer membrane. Structure. 2010;18:1492–501. doi: 10.1016/j.str.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albrecht R, et al. Structure of BamA, an essential factor in outer membrane protein biogenesis. Acta Crystallographica Section D. 2014;70:1779–1789. doi: 10.1107/S1399004714007482. [DOI] [PubMed] [Google Scholar]
- 52.Ni D, et al. Structural and functional analysis of the β-barrel domain of BamA from Escherichia coli. The FASEB Journal. 2014;28:2677–2685. doi: 10.1096/fj.13-248450. [DOI] [PubMed] [Google Scholar]
- 53.Gatzeva-Topalova PZ, Walton TA, Sousa MC. Crystal Structure of YaeT: Conformational Flexibility and Substrate Recognition. Structure. 2008;16:1873–1881. doi: 10.1016/j.str.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim S, et al. Structure and Function of an Essential Component of the Outer Membrane Protein Assembly Machine. Science. 2007;317:961–964. doi: 10.1126/science.1143993. [DOI] [PubMed] [Google Scholar]
- 55.Kim KH, Aulakh S, Paetzel M. Crystal structure of beta-barrel assembly machinery BamCD protein complex. J Biol Chem. 2011;286:39116–21. doi: 10.1074/jbc.M111.298166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jansen KB, Baker SL, Sousa MC. Crystal structure of BamB bound to a periplasmic domain fragment of BamA, the central component of the beta-barrel assembly machine. J Biol Chem. 2015;290:2126–36. doi: 10.1074/jbc.M114.584524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Z, et al. Structural basis for the interaction of BamB with the POTRA3-4 domains of BamA. Acta Crystallogr D Struct Biol. 2016;72:236–44. doi: 10.1107/S2059798315024729. [DOI] [PubMed] [Google Scholar]
- 58.Bergal HT, Hopkins AH, Metzner SI, Sousa MC. The Structure of a BamA-BamD Fusion Illuminates the Architecture of the beta-Barrel Assembly Machine Core. Structure. 2016;24:243–51. doi: 10.1016/j.str.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ricci DP, Hagan CL, Kahne D, Silhavy TJ. Activation of the Escherichia coli beta-barrel assembly machine (Bam) is required for essential components to interact properly with substrate. Proc Natl Acad Sci U S A. 2012;109:3487–91. doi: 10.1073/pnas.1201362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bakelar J, Buchanan SK, Noinaj N. The structure of the beta-barrel assembly machinery complex. Science. 2016;351:180–6. doi: 10.1126/science.aad3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gu Y, et al. Structural basis of outer membrane protein insertion by the BAM complex. Nature. 2016;531:64–9. doi: 10.1038/nature17199. [DOI] [PubMed] [Google Scholar]
- 62.Han L, et al. Structure of the BAM complex and its implications for biogenesis of outer-membrane proteins. Nat Struct Mol Biol. 2016;23:192–6. doi: 10.1038/nsmb.3181. [DOI] [PubMed] [Google Scholar]
- 63.Iadanza MG, et al. Lateral opening in the intact beta-barrel assembly machinery captured by cryo-EM. Nat Commun. 2016;7:12865. doi: 10.1038/ncomms12865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rigel NW, Ricci DP, Silhavy TJ. Conformation-specific labeling of BamA and suppressor analysis suggest a cyclic mechanism for beta-barrel assembly in Escherichia coli. Proc Natl Acad Sci U S A. 2013;110:5151–6. doi: 10.1073/pnas.1302662110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fleming PJ, et al. BamA POTRA Domain Interacts with a Native Lipid Membrane Surface. Biophys J. 2016;110:2698–709. doi: 10.1016/j.bpj.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burgess NK, Dao TP, Stanley AM, Fleming KG. Beta-barrel proteins that reside in the Escherichia coli outer membrane in vivo demonstrate varied folding behavior in vitro. J Biol Chem. 2008;283:26748–58. doi: 10.1074/jbc.M802754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Estrada Mallarino L, et al. TtOmp85, a beta-barrel assembly protein, functions by barrel augmentation. Biochemistry. 2015;54:844–52. doi: 10.1021/bi5011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stegmeier JF, Andersen C. Characterization of pores formed by YaeT (Omp85) from Escherichia coli. J Biochem. 2006;140:275–83. doi: 10.1093/jb/mvj147. [DOI] [PubMed] [Google Scholar]
- 69.Gruss F, et al. The structural basis of autotransporter translocation by TamA. Nat Struct Mol Biol. 2013;20:1318–20. doi: 10.1038/nsmb.2689. [DOI] [PubMed] [Google Scholar]
- 70.Heinz E, Selkrig J, Belousoff MJ, Lithgow T. Evolution of the Translocation and Assembly Module (TAM) Genome Biol Evol. 2015;7:1628–43. doi: 10.1093/gbe/evv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Selkrig J, et al. Discovery of an archetypal protein transport system in bacterial outer membranes. Nat Struct Mol Biol. 2012;19:506–10. S1. doi: 10.1038/nsmb.2261. [DOI] [PubMed] [Google Scholar]
- 72.Selkrig J, Leyton DL, Webb CT, Lithgow T. Assembly of beta-barrel proteins into bacterial outer membranes. Biochim Biophys Acta. 2014;1843:1542–50. doi: 10.1016/j.bbamcr.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 73.Iqbal H, Kenedy MR, Lybecker M, Akins DR. The TamB ortholog of Borrelia burgdorferi interacts with the beta-barrel assembly machine (BAM) complex protein BamA. Mol Microbiol. 2016 doi: 10.1111/mmi.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]