Abstract
Both prenatal organophosphate (OP) and pyrethroid (PYRE) insecticide exposures have been inconsistently linked with poorer neurodevelopmental outcomes. However, given that neither exposure occurs in isolation, and both classes are neurotoxic, it is important to consider both classes when evaluating these outcomes. Employing biomarkers of pesticide exposure, this research describes the effects of prenatal urinary metabolite levels of PYRE and OP insecticides, measured in both the second and third trimesters of pregnancy and postnatal urinary metabolite levels measured at 2 months of age, on child neurodevelopment at three months of age. Neurodevelopmental data were obtained by administration of the Bayley Scales of Infant Development-II (BSID-II). Generalized linear models using maximum likelihood estimation were used to evaluate the relationship between the indicators of motor and mental neurobehavioral development obtained for 118 infants and prenatal insecticide exposure, accounting for the concurrent infant insecticide exposure. Urinary measures of the PYRE metabolites 3-phenoxybenzoic acid (3PBA) and trans-3-(2,2-dichlorovinyl)-2,2 dimethylcyclopropane-1-carboxylic acid (trans-DCCA) in the third trimester of pregnancy had significant, albeit opposite, effects on mental functioning at three months of age. We observed no significant (p < 0.05) effects on motor development. These results were robust to second month infant urine measures of 3,5,6-trichloro-2-pyridinol (metabolite of OP chlorpyrifos), which independently had a significant and negative influence on mental functioning. Prenatal PYRE exposures exert heterogeneous effects by class on mental, but not motor, functioning at three months of age.
Keywords: pyrethroids, organophosphates, neurodevelopment, infant, BSID-II
1. Introduction
Pyrethroids (PYREs) constitute the majority of commercial household insecticides. PYREs have been replacing organophosphate insecticides (OPs), including chlorpyrifos and diazinon, for residential applications following consent decrees reached between manufacturers and the U.S. Environmental Protection Agency [1,2]. Residential sales were eliminated as of December 31, 2001 and December 31, 2004 for chlorpyrifos and diazinon, respectively. While OPs still have approved agricultural uses, there have been increases in the uses of pyrethroids over time.
Prenatal OP pesticide exposure has been associated with neonatal neurodevelopmental deficits [3-5]. Using the Brazelton Neonatal Behavioral Assessment Scale, Young et al. found a detrimental impact of in utero OP exposure on reflex functioning among infants assessed after three days of life [3]. Engel et al. (2007) and colleagues replicated Young et al.'s finding generally, however found the deleterious effect to be strongest for infants in the first day of life [4]. Furthermore, Engel et al. (2007) demonstrated that the effect of OP exposures, as indicated by the urinary dimethylphosphate metabolites, depended on hepatic paraoxonase-1 expression, which is, compared to adults, much lower in children [4,6].
Rauh et al. demonstrated the effects of prenatal OP exposure on the human brain [7]. In a study of 40 children aged 6 and 11 years of age, they surmised that chlorpyrifos exposure resulted in enlargement of the brains, thought to be due to enlargement of underlying white matter. These authors stated that either dose-related thinning or reduced morphological brain measures from the OP exposure resulted in impaired cognitive functioning. In a previous study, these authors found prenatal OP exposures to have an inverse dose-response effect on cognition among 264 7-year-old children living in an urban environment [8]. That work followed previous findings by the same group that as three-year-olds, the children with higher prenatal levels of chlorpyrifos in cord blood plasma exhibited significantly more delays in psychomotor and mental development using the Bayley Scales of Infant Development; these were delays that had not yet developed by two years of age [9]. These inquiries suggest a latency aspect to the deleterious effects of prenatal OP exposures. Other research has arrived at similar conclusions. Fortenberry et al. noted marginally increased Attention Deficit Hyperactivity Disorder (ADHD)-related behavior in children 6-11 years of age who were exposed in utero to chlorpyrifos [10].
Shorter-term effects of prenatal exposures have produced inconsistent findings. In the agricultural setting of Salinas Valley, California, Eskenazi et al. investigated the relationship between prenatal dialkylphosphate (DAP) metabolites in urine and DDE in cord blood with neurodevelopment measured at three time points after birth [11]. These authors reported that by twelve months of age, the DAP metabolites measured in the children were positively and significantly associated with motor and mental development. The authors speculated this non-intuitive finding could be attributable to a healthier diet of fruits and vegetables contaminated with these pesticides, or that children with higher cognitive functioning may be more likely to explore their environments and thus be exposed via non-dietary ingestion of contaminated dust and surface residues.
Pyrethroids are purportedly safer insecticides for humans; their lower volatility compared with the OPs diazinon and chlorpyrifos limits inhalation exposures when used in the residential environment; their rapid UV hydrolysis limits residues in food commodities. However, studies have suggested that there are indeed adverse effects in infants associated with pyrethroid exposures. Sinha et al. documented the potential for pyrethroids, specifically allethrin, to cross the blood-brain barrier during both the prenatal and postnatal period [12]. This finding is significant in that the blood-brain barrier is not fully formed until about six months of age and, thus, is not able to offer full protection to the infant during early life [13]. This finding suggests that the effects of postnatal insecticide exposures may vary by time since birth.
Williams et al. demonstrated that prenatal exposures to piperonyl butoxide (PBO), a pyrethroid synergist, resulted in significantly delayed mental development using the Bayley Scales of Infant Development (BSID-II) at 36-months of age, although the researchers did not find any effects due to cis- or trans-permethrin exposures [14]. Following the work of Williams et al., Horton et al. hypothesized that neurocognitive scores in children aged 36 months would be significantly delayed as a consequence of prenatal exposure to permethrin and PBO. Therefore they collected maternal personal air in the 3rd trimester and neonatal cord blood in order to measure potential exposures to permethrin and PBO. The authors found no significant associations between cis- or trans-permethrin and 36-month indicators of mental and motor development [15]. However, they did find an inverse relationship between mental scores and PBO exposure (1.2 point decrement per log-unit increase in PBO exposure). Ostrea Jr. et al. reported no significant effect between exposures to the PYREs bioallethrin, transfluthrin, cyfluthrin and/or cypermethrin and child development at two years of age [16]. However, Xue et al. found a negative and significant relationship of prenatal pyrethroid exposure and neural and mental development of Chinese infants at one year of age [17]. Thus, the literature on the effects of pyrethroid exposures on child development is contradictory, perhaps due to the timing of either the exposures, the exposure assessments, or the dose itself.
This study evaluated the neurodevelopmental effects in infants associated with both prenatal and postnatal OP and PYRE exposures, as assessed by urinary metabolites in overnight urine; prenatal exposures were assessed using maternal urine collected once in both the second and third trimesters of pregnancy; postnatal exposures were assessed using overnight urine collected at 2 months of age. Infant neurodevelopment was evaluated via the Bayley Scales of Infant Development (BSID-II) at three months of age. Because several of the metabolites had detection frequencies on the order of 2-30%, we concentrated the initial analyses on those metabolites for which the median value of the measure was above the limit of detection (LOD).
2. Material and Methods
2.1 Study Design
A cohort of 174 pregnant women was recruited from central Ohio obstetric clinics and offices from 2002 to 2005, and the infants from 140 of them were followed for up to 2 years, with urine collection at 2, 9, 16 and 23 months of age. We report here on the 140 maternal-infant dyads for which maternal urine was collected in both 2nd and 3rd trimesters, infant urine was collected at 2 months of age and BSID-II testing was completed at 3 months of age. The prenatal questionnaire covered standard demographic information as well as pre-pregnancy weight. Final pregnancy weight gain was recovered from the infant medical record as well as from questionnaire data. We used multiple imputation to derive maternal weight at each time point in the pregnancy when a urine sample was collected. This imputation method is described in detail below. Other collected data included potentially confounding family-based factors (e.g., race, socioeconomic status, maternal education), and relevant clinical information pertaining to the pregnancy and birth event (e.g., birth weight, gestational maturity, smoking status, etc.).
For maternal urine collection, women were provided a large polyethylene container and instructed to provide all urine voided over the longest period of the day associated with nighttime sleep, even if this included multiple voids. The women were asked to provide an estimate of the hours covered by the urine void(s). Similarly, for infant urine collection parents were provided a list of available diaper sizes and once they selected the most appropriate size, they were provided with a pre-weighed diaper and asked to use that for the longest period of time covered by overnight sleep. Parents were asked to record the duration of sleep time (as a proxy for the length of time covered by the urine voids) and the infant's weight. For those parents who did not know the precise infant weight, we used the mean weight of the diaper size selected by the parent. All urine samples were collected on the day generated, and delivered to the analysis laboratory on gel ice packs. Urine samples were analyzed for the suite of OP and PYRE metabolites listed in Table 1. The analytical method is detailed in the Appendix. At 3 months of age, neurodevelopmental data were obtained by administration of the Bayley Scales of Infant Development-II (BSID-II).
Table 1. Urinary Metabolites.
| Metabolite class | Acronym | Parent Pesticide |
|---|---|---|
| Phenoxybenzoic PYRE metabolites | ||
| 3-phenoxybenzoic acid | 3PBA | Cypermethrin, λ-Cyhalothrin, Deltamethrin, Fenpropathrin, Permethrin,Tralomethrin, Esfenvalerate,Sumithrin |
| 4-fluoro-3-phenoxybenzoic acid | 4F-3PBA | Cyfluthrin |
| Divinylcyclopropyl Carboxylic Acid PYRE metabolites | ||
| cis- and trans-3-(2,2-dimethylvinylcyclopropane-1-carboxylic acid) | DMCA1, DMCA2 | Pyrethrin I (most abundant component of pyrethrin mix), Tetramethrin, Resmethrin, Sumithrin |
| cis- and trans-3-(2,2-dichlorovinyl)-2,2 dimethylcyclopropane-1-carboxylic acid | cis-DCCA, trans-DCCA | Permethrin, Cypermethrin, Cyfluthrin |
| 4-chlorophenyl-2-isopropylacetic acid | CIAA | Esfenvalerate |
| cis- and trans-3-(2,2-dicbromovinyl)-2,2 dimethylcyclopropane-1-carboxylic acid | DBCA | Deltamethrin |
| Phenolic OP metabolites | ||
| 3,5,6-trichloro-2-pyridinol | TCPy | Chlorpyrifos |
| 2-isopropyl-4-methyl-6-hydroxypyrimidine | IMPy | Diazinon |
2.2 Imputation
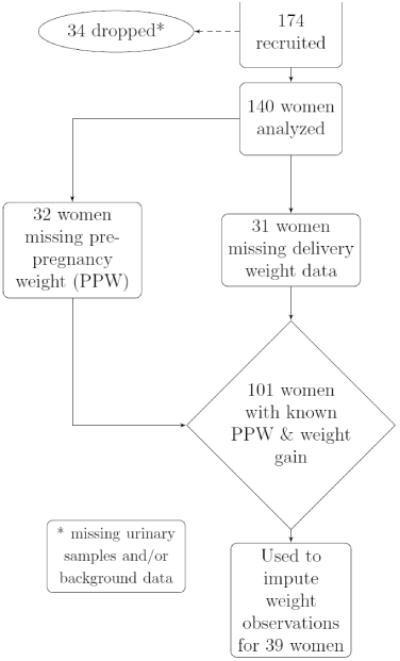
The preliminary data analyses included imputation as an alternative to case-deletion [18], in order to preserve the otherwise complete records of women for whom pre–pregnancy weight and/or weight gain was unknown. Linear interpolation was used to estimate second and/or third trimester weights once pre-pregnancy weight and pregnancy weight gain were determined. Figure 1, describing the sample of women that comprised the known sample to use in the imputation, shows that there were complete data for 101 women and these were used to impute missing values for 39 women.
Figure 1. Flowchart describing the missingness of pre–pregnancy weight and weight gain data.
We pursued imputation using a multivariate normal model (MVN) [19] and imputation by chained equations (ICE) using cross imputation (all variables to be included in a model were used to predict all other variables in the model, known as a fully conditional specification (FCS)) [20], for each imputed weight variable. We specifically used these multiple imputation (MI) procedures as simulation techniques to replace each missing data point with a set of m>1 plausible values (based on known relevant predictors). These imputations were created through a Bayesian process. A parametric model for the complete data was specified. We used the missing-at-random assumption, with the underlying assumption that missing pre-pregnancy weight or weight gain could be imputed using other variables that predict weight (i.e. age, smoking status, etc.).
A prior distribution was applied to the unknown model parameters (parameters of variables that predict weight among the missing observations); m independent draws were simulated from the conditional distribution of the missing data given the observed data by Bayes' Theorem. We used m=10 draws to achieve precision of the imputation procedure and to reduce variability when averaging. The m versions of the complete data were analyzed by standard complete-data methods, and the results were combined to yield estimates, standard errors, and p-values that formally incorporated uncertainty imposed by missing data. High correlations of imputations across both strategies indicated a successful and reliable imputation approach.
2.3 Regression Analyses
Generalized linear models using maximum likelihood estimation (MLE) were used to test the association of the retained metabolites with motor and mental neurodevelopment outcomes at three months of age. Separate models analyzed the effects of (1) the prenatal levels in the absence of concurrent measures and (2) the prenatal and postnatal concentrations along with other maternal and pregnancy characteristics. We defined the complete models to be the following:
| (1) |
where represents the Bayley's neurodevelopmental score outcome for B =1 (motor) and B =2 (mental), miv,T denotes V metabolites measured during the second or third trimester, miu,P reflects U metabolites measured after birth and dig includes maternal and pregnancy-related variables that might impact neurodevelopment. Metabolites are included if their median values are greater than the LOD. Maternal and pregnancy-related variables are included in a forward selection approach. Only variables that significantly (p < 0.05 as assessed by t-test) improve model fit will be retained and presented.
Institutional review board approval was granted by The Ohio State University IRB. All analyses were completed with Stata 11.1 [21].
3. Results and Discussion
Relevant sample descriptive statistics are given in Table 2. Mothers had a mean age of approximately 30 years, and the sample consisted of 15% non-white mothers. The mean gestational age was 9.07 months and the mean birth weight was 120 ounces (3402 grams). Five children were born low birth weight (LBW). The mean, non-imputed weight gain was almost 33 pounds. The majority of the women in the sample had completed at least high school (0.90) and had a previous child (maternal prior parity = 0.96). The mean category for household income was 7.42, which reflects a middle class sample of women (household income of between $40,000 and $60,000).
Table 2. Sample descriptive statistics (no imputations).
| Variable | N | Mean | Std. Dev. | Minimum | Median | Maximum |
|---|---|---|---|---|---|---|
| Child birth weight (ounces) | 109 | 119.39 | 18.41 | 79.4 | 121.4 | 176 |
| Low birth weight status | 110 | 0.045 | 0.21 | 0 | 0 | 1 |
| Gestational age (in days) | 110 | 272.08 | 12.24 | 212.1 | 273.7 | 290.5 |
| No. of cigarettes smoked/day | 139 | 2.60 | 6.53 | 0 | 0 | 30 |
| Maternal race (nonwhite=1) | 137 | 0.15 | 0.36 | 0 | 0 | 1 |
| Maternal age in years | 139 | 29.7 | 5.41 | 18.8 | 30.7 | 40.7 |
| Bayley's mental score - 3 mo | 119 | 102.9 | 3.9 | 93 | 103 | 125 |
| Bayley's motor score - 3 mo | 119 | 101 | 4.49 | 85 | 101 | 108 |
| Pre-pregnancy weight (in lbs) | 108 | 182.4 | 84.7 | 126 | 176 | 306 |
| Weight gain (in lbs) | 101 | 32.9 | 18.9 | 7 | 31 | 91.9 |
| Education > high school | 137 | 0.9 | 0.3 | 0 | 1 | 1 |
| Maternal prior parity | 140 | 0.96 | 1.10 | 0 | 1 | 6 |
| Household income | 137 | 7.4 | 1.9 | 1 | 8 | 9 |
| Mother had diabetes | 140 | 0.05 | 0.22 | 0 | 0 | 1 |
| Child female sex | 91 | 0.49 | 0.50 | 0 | 0 | 1 |
| Pregnancy interval | 140 | 3914.1 | 4514.4 | 15 | 1024 | 13533 |
| First primary care visit | 136 | 63.1 | 23.7 | 12 | 61 | 135 |
Note: To have a fully observed weight gain outcome for each woman, it is necessary to know both the pre-pregnancy and delivery weights. Thus, although delivery weights were missing for only 31 women, 39 women had missing weight gain outcomes.
For the mental BSID 3-month scores, the mean was 102.92 with a SD=3.88 (n=119). Clinically relevant delays, defined as mental BDI scores two standard deviations or more below the mean (i.e., 95 or lower) [22], were associated with six children. For the motor BDI 3-month scores, the mean was 101 with a SD=4.49 (n=119); the clinical delay based on a motor BDI score of 92 or lower was associated with six children. None of the LBW children experienced developmental delays at three months according to these definitions.
The correlation between the imputations using the MVN method and the ICE cross method was 0.88 for each metabolite analyzed. We report metabolite levels in ng/kg/day using the ICE imputation strategy. Further details on the imputation and interpolation techniques are available by request.
Tables 3 and 4 give the descriptive statistics for the OP and PYRE metabolites in maternal and infant samples, respectively. All metabolites with a median value above the LOD were retained for analyses. Metabolites and their respective period of observance are bolded. Four metabolites from the second trimester were included (i.e., TCPy, trans-DCCA, cis-DCCA and 3PBA), three in the third trimester (i.e., TCPy, trans-DCCA and 3PBA) and three from the post-partum measure (i.e., TCPy, 3PBA and IMPy).
Table 3. Maternal excretion of in ng/kg/day, by trimester of pregnancy (with ICE imputations).
| Metabolite | Trimester | N | N(%) <LOD | AM | SE | Min | 25% | Median | 75% | Max |
|---|---|---|---|---|---|---|---|---|---|---|
| TCPy | T=2 | 140 | 3(2.1) | 24.07 | 1.59 | 0.00056 | 11.41 | 19.62 | 31.45 | 131.52 |
| T=3 | 138 | 8(5.8) | 29.34 | 3.16 | 0.0005 | 12.68 | 20.45 | 34.39 | 334.72 | |
| Overall | 278 | 11(4) | 26.69 | 1.77 | 0.0005 | 11.80 | 19.76 | 33.97 | 334.72 | |
| IMPy | T=2 | 140 | 118(84) | 3.13 | 1.05 | 0.00024 | 0.00037 | 0.00041 | 0.00049 | 116.51 |
| T=3 | 138 | 118(86) | 4.79 | 1.85 | 0.00021 | 0.00035 | 0.00038 | 0.00046 | 202.14 | |
| Overall | 278 | 236(85) | 3.96 | 1.06 | 0.00021 | 0.00036 | 0.0004 | 0.00048 | 202.14 | |
| 3PBA | T=2 | 140 | 7(5) | 18.73 | 4.44 | 0.00072 | 3.91 | 7.28 | 16.18 | 568.99 |
| T=3 | 138 | 15(11) | 12.05 | 1.33 | 0.00065 | 3.88 | 8.36 | 13.16 | 100.56 | |
| Overall | 278 | 22(8) | 15.41 | 2.33 | 0.00065 | 3.90 | 8.05 | 14.31 | 568.99 | |
| 4F-3PBA | T=2 | 140 | 115(82) | 1.32 | 0.60 | 0.00048 | 0.00074 | 0.00082 | 0.00101 | 81.30 |
| T=3 | 138 | 119(86) | 0.61 | 0.18 | 0.00043 | 0.00068 | 0.00075 | 0.00089 | 15.23 | |
| Overall | 278 | 234(84) | 0.96 | 0.31 | 0.00043 | 0.00071 | 0.00080 | 0.00096 | 81.3 | |
| DMCA1 | T=2 | 140 | 137(98) | 0.35 | 0.22 | 0.00096 | 0.0014 | 0.0016 | 0.0018 | 21.22 |
| T=3 | 138 | 135(98) | 0.59 | 0.35 | 0.00086 | 0.0013 | 0.0015 | 0.0017 | 33.55 | |
| Overall | 278 | 272(98) | 0.47 | 0.20 | 0.00086 | 0.0014 | 0.0016 | 0.0018 | 33.55 | |
| DMCA2 | T=2 | 140 | 137(98) | 0.69 | 0.44 | 0.00096 | 0.0014 | 0.0016 | 0.0018 | 44.91 |
| T=3 | 138 | 137(98) | 0.10 | 0.10 | 0.00086 | 0.0013 | 0.0015 | 0.0017 | 14.28 | |
| Overall | 278 | 274(99) | 0.40 | 0.23 | 0.00086 | 0.0014 | 0.0016 | 0.0018 | 44.91 | |
| Cis-DCCA | T=2 | 140 | 65(46) | 7.31 | 1.32 | 0.0011 | 0.0016 | 2.56 | 10.32 | 133.29 |
| T=3 | 138 | 75(54) | 4.54 | 0.55 | 0.001 | 0.0015 | 0.0019 | 8.26 | 28.70 | |
| Overall | 278 | 140(50) | 5.94 | 0.72 | 0.001 | 0.0016 | 0.015 | 9.34 | 133.29 | |
| Trans-DCCA | T=2 | 140 | 55(39) | 14.88 | 2.98 | 0.0011 | 0.0017 | 5.97 | 16.80 | 262.44 |
| T=3 | 138 | 61(44) | 9.85 | 1.27 | 0.001 | 0.0015 | 5.10 | 14.30 | 92.59 | |
| Overall | 278 | 116(42) | 12.38 | 1.63 | 0.001 | 0.0016 | 5.51 | 14.82 | 262.44 | |
| CIAA | T=2 | 140 | 140(100) | 0.0008 | <0.01 | 0.00048 | 0.00069 | 0.0008 | 0.00089 | 0.0011 |
| T=3 | 138 | 135(98) | 0.48 | 0.43 | 0.0004 | 0.00065 | 0.00075 | 0.00084 | 59.01 | |
| Overall | 278 | 275(99) | 0.24 | 0.21 | 0.00043 | 0.00068 | 0.00078 | 0.00088 | 59.01 | |
| DBCA | T=2 | 140 | 140(100) | 0.0008 | 0 | 0.00048 | 0.00069 | 0.0008 | 0.00089 | 0.0011 |
| T=3 | 138 | 138(100) | 0.00075 | <0.01 | 0.0004 | 0.00065 | 0.00075 | 0.00083 | 0.0011 | |
| Overall | 278 | 278(100) | 0.00078 | <0.01 | 0.00043 | 0.00068 | 0.00078 | 0.00087 | 0.0011 |
Metabolites and their respective period of observance are bolded.
Table 4. Infant excretion in ng/kg/day at three months after birth (non-imputed).
| Metabolite | N | N(%) <LOD | AM | SE of AM | Min | 25% | Median | 75% | Max |
|---|---|---|---|---|---|---|---|---|---|
| TCPy | 124 | 35(28) | 14.67 | 3.42 | 0.0092 | 0.0157 | 7.6 | 14.45 | 399 |
| IMPy | 124 | 46(37) | 17.89 | 3.98 | 0.0035 | 0.00708 | 5.6 | 17.45 | 409.8 |
| 3PBA | 124 | 33(27) | 23.54 | 3.61 | 0.0092 | 0.0168 | 13.9 | 26 | 315.2 |
| 4F-3PBA | 124 | 117(94) | 0.54 | 0.24 | 0.0071 | 0.0119 | 0.0129 | 0.0157 | 20.4 |
| DMCA1 | 124 | 116(94) | 1.31 | 0.59 | 0.0141 | 0.0240 | 0.0257 | 0.0314 | 63.4 |
| DMCA2 | 124 | 117(94) | 2.50 | 0.99 | 0.0141 | 0.0240 | 0.0257 | 0.0314 | 75.1 |
| Cis-DCCA | 124 | 104(84) | 3.54 | 1.01 | 0.0141 | 0.0240 | 0.0277 | 0.0314 | 81.9 |
| Trans-DCCA | 124 | 94(76) | 9.23 | 2.62 | 0.0141 | 0.0240 | 0.0283 | 0.0345 | 261.3 |
| CIAA | 140 | 140(100) | 0.0008 | <0.01 | 0.00048 | 0.00069 | 0.0008 | 0.00089 | 0.0011 |
| DBCA | 140 | 140(100) | 0.0008 | 0 | 0.00048 | 0.00069 | 0.0008 | 0.00089 | 0.0011 |
Metabolites and their respective period of observance are bolded.
Using ArcGIS (ESRI 2011), we mapped the location of exposures for the metabolites with a median value above the LOD. Figure 2 gives the tertile urinary metabolite concentrations at each trimester (second trimester represented on the left and the third trimester given on the right) among the sample of women tested in Central Ohio. The yellow dots denote metabolite concentrations at the limit of detection (LOD), with increasing concentrations represented by darker shades. Cumulatively assessed, these figures show that the dispersion of higher concentrations of the urinary metabolite concentrations are fairly random.
Figure 2. Metabolite concentrations ng/kg/day by location in Central Ohio, using imputed data (N=140).
Table 5 presents the main results of our analyses. Four models are presented. The first model gives the associations between prenatal metabolites meeting our inclusion criterion and the motor development score. Model 2 repeats the first model and adds the infant metabolite levels at two months and pregnancy and maternal characteristics that met the significance criterion using the forward selection approach with the variables listed in Table 2. Including these variables (birth weight, gestational age and maternal education status) produced a significant and negative impact of the chlorpyrifos metabolite in the third trimester on motor functioning: a one nanogram per kilogram of body weight per day increase in exposure reduced the Bayley's motor functioning score by -0.031. Maternal education greater than high school (β3 = 4.73, p < 0.01) and a longer gestational age (β3 = 0.164, p < 0.01) each enhanced motor activity.
Table 5. Generalized linear model using maximum likelihood estimation results.
| Outcome | Motor Assessments at 3 months | Mental Assessments at 3 months | ||
|---|---|---|---|---|
| Model | 1 | 2 | 3 | 4 |
| Metabolite | N=118 | N=105 | N=118 | N=112 |
|
| ||||
| PANEL A: Prenatal (Trimester) | ||||
|
| ||||
| TCPy – (2) | -0.020 (0.023) | 0.016 (0.034) | -0.004 (0.016) | -0.025 (0.026) |
| TCPy – (3) | -0.023 (0.019) | -0.031** (0.015) | 0.003 (0.021) | 0.008 (0.024) |
| 3PBA – (2) | -0.003 (0.012) | -0.013 (0.015) | 0.008 (0.011) | 0.008 (0.011) |
| 3PBA – (3) | -0.035 (0.041) | -0.025 (0.036) | -0.097*** (0.037) | -0.119*** (0.038) |
| DCCA-t – (2) | 0.006 (0.028) | 0.033 (0.024) | 0.025 (0.026) | 0.033 (0.025) |
| DCCA-t – (3) | 0.004 (0.035) | 0.009 (0.037) | 0.093** (0.041) | 0.101** (0.040) |
| DCCA-c – (2) | -0.029 (0.083) | -0.043 (0.092) | -0.104 (0.070) | -0.109 (0.068) |
|
| ||||
| PANEL B: Postnatal (months) | ||||
|
| ||||
| TCPy – (3) | 0.003 (0.005) | -0.015*** (0.005) | ||
| IMPy – (3) | 0.002 (0.005) | -0.002 (0.004) | ||
| 3PBA – (3) | 0.013 (0.007) | 0.014 (0.009) | ||
|
| ||||
| PANEL C: Pregnancy and Maternal Characteristics | ||||
|
| ||||
| Birth weight | -0.015 (0.026) | |||
| Gestation (in days) | 0.164*** (0.057) | |||
| Maternal education | 4.73*** (1.70) | |||
| Maternal race nonwhite | -1.91** (0.839) | |||
|
| ||||
| Constant | 100.37*** (2.62) | 93.83*** (3.01) | 101.94*** (2.10) | 104.44*** (2.41) |
|
| ||||
| BIC | 1710.93 | 1210.12 | 1021.27 | 912.62 |
Panel C also included number of days since birth when development was assessed. Robust standard errors in parentheses. Birth weight and gestation were mean-centered. Maternal education is binary indicator denoting whether a mother graduated from high school (1=yes, 0=no). BIC refers to the Bayesian information criterion.
p<0.10,
p<0.05,
p<0.01
Model 3 gives the associations between prenatal metabolites and mental assessments, and Model 4 repeats Model 3 and adds the infant metabolite levels at two months of age and non-white ethnicity. For Models 3 and 4 we found several metabolites that significantly predict cognitive functioning. In particular, higher urinary levels of 3-phenoxybenzoic acid (3PBA) in the third trimester of pregnancy were associated with negative effects on the mental development score: each one nanogram per kilogram of body weight per day increase in the 3PBA metabolite produced an approximately 0.10 to 0.12 decline in the mental score. Trans-DCCA produced a significantly positive influence on mental development, an influence that was comparable to the effect of 3PBA: each one nanogram per kilogram of body weight per day increase in the trans-DCCA metabolite in the third trimester produced a 0.10 increase in the mental score.
When controlling for concurrent infant exposures at three months of age, these metabolites retain their significant effects. Furthermore, higher infant urinary levels of TCPy (metabolite of the OP chlorpyrifos) (TCPy) were associated with a negative influence on mental score: each one nanogram per kilogram of body weight per day increase in the TCPy metabolite produced a 0.02 decline in mental score. The TCPy effect on mental development was about a tenth of the magnitude of the 3PBA metabolite. Non-white maternal race also had significant decrements on mental development, with a reduction of almost two points (β3 = -1.91, p < 0.05) on the Bayley's index for children born to non-white mothers.
All regression analyses were repeated with the insertion of a binary variable indicating whether the mother's weight (either pre-pregnancy weight or delivery weight) was imputed (results not shown). None of the significant results changed, demonstrating that our results are not sensitive to the imputation procedure. To further inspect the contradictory result for the PYREs, we re-analyzed Models 3 through 4 in Table 5 by removing one of the two third trimester PYRE metabolite (3PBA or trans-DCCA). When removing the trans-DCCA metabolite, the 3PBA metabolite remained negative and significant (β1,3PBA = -0.059, p = 0.038). However, when removing the 3PBA metabolite, the trans-DCCA metabolite no longer remained significant (β1,DCCT−t = 0.021, p = 0.623).
In this paper, we found that the concentrations of two pesticide metabolites in the third trimester of pregnancy were associated with infant mental development at three months of age: 3-phenoxybenzoic acid (3PBA) and trans-3-(2,2-dichlorovinyl)-2,2 dimethylcyclopropane-1- carboxylic acid (trans-DCCA).
These metabolites had opposite effects, although their magnitudes were comparable. This is perplexing because permethrin, the most commonly used PYRE, devolves to both 3PBA and a mixture of cis/trans-DCCA. These effects were robust to concurrent infant exposures, namely exposure to chlorpyrifos (TCPy) and well as other maternal and pregnancy characteristics. We also noted an association between exposure to the TCPy metabolite in the third trimester and motor functioning at three months of age.
The epidemiological work here substantiates the neurological assessment of the consequences of OP and PYRE exposures. Torres-Altoro et al. discussed the detrimental effects of chlorpyrifos on dopamine (DA) and glutamatergic neurotransmission effectors in corticostriatalmotor circuitry [23]. Aldridge et al. cited toxic effects of chlorpyrifos on hippocampal DA content as well as the influence of nontoxic levels of chlorpyrifos on serotonin-related behavioral abnormalities [24]. In the rat model the PYRE insecticide permethrin and OP chlorpyrifos increased striatal levels of glial fibrillary acidic protein (GFAP), which is used as a marker to distinguish astrocytes from other glial cells during development [25,26]. However, increases may not occur in the presence of combined exposures [27], demonstrating the utility of our inquiry. Nevertheless, this inflammatory activation of astrocytes may impact the glutamate release and uptake mechanisms in the central nervous system [28, 29]. These insecticides may enhance astrocyte presence and/or functioning, which then facilitates the uptake of glutamate and, consequently, deleteriously affect neuronal functionality.
Relatedly, the striatum might play a more important role than the cerebellum in the ability to adjust to a novel movement in the context of a series of previously practiced movements [30]. Cerebellar neuropathology in the form of Purkinje cell loss results in dysregulation of cerebellar neural circuitry modulating prefrontal cortex DA release, which may negatively impact the ability to engage in practiced or repetitive behaviors. Purkinje cells are susceptible to apoptosis by glutamate release [31], although they may not be directly susceptible to all permethrin exposures or in the absence of contextual effects [32, 33]. Alternatively, the lack of a strong effect on motor development could be a consequence of time. Changes in motor functioning may not occur for some time after birth, in which case post-natal observations at three months would miss such previously documented influences [34].
With respect to the PYRE pesticides, Malaviya et al. found evidence of dopamine surges among neonatal rats exposed to fenvalerate and cypermethrin during the gestational period [35]. Such exposures may even lead to a cascade of escalating effects. Wojcikowski and colleagues noted that stimulation or inhibition of the brain dopaminergic system may cause changes in cytochrome P450 activity of physiological, pharmacological and toxicological significance [36, 37]. In their investigation of the effects of prenatal exposure to deltamethrin on behavioral activity in rat offspring, Johri et al. (2006) discussed how an increase in cytochrome P450 activity may lead to the accumulation of deltamethrin and/or a short-lived reactive metabolite to levels that may be sufficient to alter the behavioral activity of the offspring [38].
The contradictory association of 3PBA and trans-DCCA metabolites with mental development at three months of age is unexpected. However, it suggests that differential effects on neurodevelopment, particularly cognitive functioning, from prenatal exposure to parent pesticides, including cypermethrin and permethrin, deserve further scrutiny. For example, Nasuti et al. (2003) suggested a different effect of type I (i.e., permethrin) and type II (i.e., cypermethrin) pyrethroids on sodium channels [39], particularly the opening duration of such channels [40], with type II pyrethroids inducing a much longer opening unless also in the presence of a type I pyrethroid, which displaces the former resulting in shorter openings. A state of prolonged activation among sodium channels can induce significant changes in neuronal excitability and function [41]. Alternatively, potassium channels that are activated by an increase in cytoplasmic levels of sodium ions [42] (known as KNa channels) may contribute to learning deficits that we identified in our sample [43]. Importantly, this literature suggests that our negative 3PBA metabolite finding on mental development may be a consequence of exposure to a type II pyrethroid, whereas the positive DCCA-t metabolite finding may reflect exposure to a type I pyrethroid.
4. Conclusion
The current study adds to the existing literature in several important ways. First, we considered OP and PYRE insecticide exposures at two time points during pregnancy to assess the potential for windows of vulnerability. This is a useful advancement, as OPs and PYREs have very short half-lives in humans and metabolite urine concentrations show considerable intra-individual variation across time [44, 45]. Second, we investigated the importance of prenatal exposures while controlling for current infant exposures. We considered infant exposures at 2-months of age as potentially confounding the relationship between prenatal exposures and infant development. Other studies have collected data on pesticide exposures for both mother and child [9], but omitted controlling for infant exposures when assessing the relationship between prenatal exposures and neurodevelopment, thus potentially underestimating the true effect of the prenatal exposures. In addition, we calculated maternal and infant exposures in terms of ng of metabolite in urine/kg/d, thus normalizing for weight and urine volume and using a metric that is more compatible with a risk framework than can be achieved using the conventional ng/mL metric. Finally, we included numerous OP and PYRE metabolites for several important reasons: (1) people are exposed to both insecticide classes from dietary sources and from product use in and around the home, (2) exposures rarely occur in isolation and (3) cumulative and/or synergistic effects may need to be considered. Other studies have investigated the effects of childhood OP and PYRE exposures on behavioral problems, but no current research is available to isolate prenatal effects [46].
This study is the first to examine the concomitant effects of prenatal PYRE and OP pesticide exposure on infant development outcomes at three months after birth. Furthermore, these results control for concurrent infant exposures. Given these study dynamics, this investigation presents the most rigorous analysis of the effects of these pesticides on infant neurodevelopment. However, a number of limitations apply to the results. First, these results may not have clinical significance for two reasons. First, few children experienced clinically significant development delays. Second, the contrasting results between pyrethrin metabolites (3PBA and DCCA-trans, specifically) for mental development may obscure any clinically meaningful outcomes. The effects of the levels of these two metabolites effectively cancel one another out (if concomitantly exposed) such that any remaining effect on mental functioning may essentially be white noise or attributable to another pesticide exposure at a different time period (pre- or post–natally to TCPy, for example). Second, a large number of the metabolites did not meet our inclusion criterion. Not including these metabolites in our regressions should not be used to imply we are stating the parent pesticides associated with these metabolites have no effect on infant neurodevelopment at three months of age. Rather, given the lack of substantial data, we exercised caution and decided to refrain from currently incorporating into our analyses these other metabolites as is, including DMCA1, DMCA2, CIAA, DBCA and 4F-3PBA. Future work should attempt to expand upon our present capabilities to further assess whether the associations we observed remain true in a more exhaustive model.
Acknowledgments
Although the research described in the article has been funded wholly or in part by the U.S. Environmental Protection Agency's Science to Achieve Results (STAR) program through grant R-82861101 (PI: J.R. Wilkins III), it has not been subjected to any EPA review and therefore does not necessarily reflect the views of the Agency, and no official endorsement should be inferred.
Appendix
Analytes
The insecticide metabolites analyzed here, their abbreviations, the isotopically-labelled analogues (surrogate recovery standard; SRS) added for quantification, as well as insecticides that devolve to each metabolite are listed in Table A1.
Table A1. Organophosphate (OP) and Pyrethroid insecticide metabolites analyzed.
| Metabolite class/metabolite | Acronym | Labeled analoguea | Parent pesticide(s) |
|---|---|---|---|
| Phenolic OP | |||
| 3,5,6-trichloro-2-pyridinol | TCPy | 13C2-15N1-TCPy | Chlorpyrifos |
| 2-isopropyl-6-methyl-4-pyrimidinol | IMPy | 13C4-IMPy | Diazinon |
| Phenoxybenzoic Pyrethroid | |||
| 3-Phenoxybenzoic acid | 3PBA | 13C6-3PBA | Permethrin, cypermethrin, deltamethrin, esfenvalerate, sumithrin, tralomethrin |
| 4-Fluoro-3-phenoxybenzoic acid | 4F-3PBA | 13C6-3PBA | Cyfluthrin |
| Vinylcyclopropyl and other Pyrethroid | |||
| Cis-& trans-3-(2,2-dimethylvinyl)-2,2-dimethylcycplopropane-1-carboxylic acid | DMCA-1b DMCA-2b |
Pyrethrin I, tetramethrin, resmethrin, sumithrin | |
| Cis-& trans-3-(2,2-dichlorovinyl)-2,2-dimethylcycplopropane-1-carboxylic acid | DCCA-cis DCCA-trans |
Permethrin, cypermethrin, cyfluthrin | |
| Cis-3-(2,2-dibromovinyl)-2,2-dimethylcycplopropane-1-carboxylic acid | DBCA-cis | 3,5-Dibromobenzoic acidc | Deltamethrin |
| 4-chlorophenyl-2-isopropylacetic acid | CIAA | Esfenvalerate |
Added to urine extract for isotope dilution analysis
DMCA-1 is probably cis-DMCA; DMCA-2 is probably trans-DMCA.
3,5-DBBA is not isotopically labeled, but used due to similarity of substituent groups and unlikely presence in urine
Table A2. Analytical Method parameters in urine samples.
| Metabolite | Derivatization method for metabolitea | Ions monitored, m/z | Limit of Detection in urine, ng/mLb | Recovery, % mean ± sd (n=20)c,d |
|---|---|---|---|---|
| TCPy | silylation | 254, 256 | 0.1 | 99 ± 7 |
| IMPy | silylation | 209, 210 | 0.03 | 91 ± 23 |
| 3PBA | methylation | 228, 197 | 0.1 | 88 ± 11 |
| 4F-3PBA | methylation | 246, 215 | 0.1 | 84 ± 12 |
| DCCA-cis | silylation | 265, 267 | 0.2 | 60 ± 30 |
| DCCA-trans | silylation | 265, 267 | 0.2 | 45 ± 17 |
| DMCA-1 | silylation | 225, 73 | 0.2 | 45 ± 23 |
| DMCA-2 | silylation | 225, 73 | 0.2 | 46 ± 18 |
| CIAA | silylation | 269, 271 | 0.1 | 66 ± 22 |
| DBCA-cis | methylation | 253, 255 | 0.1 | 98 ± 13 |
All metabolites except TCPy and IMPy can be derivatized by either method; method listed is the one providing greater sensitivity, and hence the one used here for detection and quantification
3X S/N from standards
Concentration of metabolites in control urine,ng/mL used for spike samples: TCPy: 1.07±0.24; 3PBA: 0.51±0.09; DCCA-cis: 0.29 ±0.20; DCCA-trans: 0.23±0.20 IMPy: 0.37±0.57
Fortification level for spiked urine was 5 ng/mL for each metabolite or sum of isomers (DCCA isomers were each 2.5 ng/mL; DMCA isomers were 1 and 4 ng/mL for isomers 1 and 2 respectively)
Reference standards of TCPy, IMPy, 3PBA, 4F-3PBA, and DCCA-cis/trans, as well as labeled IMPy and labeled 3PBA, were obtained from Cambridge Isotopes Laboratory; CIAA, DMCA-cis/trans and DBCA-cis were obtained from Dr. EhrenstorferGmBH; labeled TCPy was obtained as a gift from Dow AgroSciences; other chemicals such as internal standards were obtained from Sigma-Aldrich; solvents were pesticide grade; control urine was obtained from BioReclamation, Inc.
Extraction and GC/MS Analysis
Table A2 provides information about the analytical method including the derivatization method used for each metabolite, the ions monitored, the limit of detection, and the recovery of metabolites from fortified control urine. The concentrations of metabolites in the unfortified urine and the fortification levels of metabolites in these QC samples are provided as footnotes.
The metabolite conjugates in a 5 mL aliquot of the urine were hydrolyzed at 80 ± 5°C for 1 h after addition of 50 ng of each SRS, 0.5 mL of concentrated HCl and 11.5 mL of methanol. The urine:methanol extract was diluted with 100 mL of 20% NaCl in MilliQ water, and applied to a C18 SPE cartridge (Phenomenex Strata 500 mg) that had been conditioned in sequence with10 mL methanol, 10 mL MilliQ water, and 6 mL of 1:10 acetonitrile:0.025 M phosphoric acid. The eluent was collected for subsequent recovery of IMPy. The SPE cartridge was dried under full vacuum for 1 h, and then eluted with 1.4 mL of dry acetonitrile. This resulting eluent, called the primary fraction, contained all metabolites except IMPy and its labeled analogue. The Internal Standard bromobiphenyl and methylation derivatization check standard 3,4-D (3,4-dichlorophenoxy acetic acid) were added to the primary fraction eluent. The pH of the IMPy fraction was adjusted to pH=5 with conc NaOH. This aqueous extract was partitioned with 2 × 50 mL dichloromethane (DCM). The DCM extract was drained through 10 g of muffled sodium sulfate, concentrated to 1 mL and solvent exchanged into 1 mL of dry acetonitrile; the Internal Standard dibromobiphenyl was added.
The primary fraction was divided into two aliquots of 500 uL each. For methylation of one portion, 25 uL of methanol was added and the solution was derivatized with ethereal diazomethane generated in situ from diazald and KOH. The solution was treated for a minimum of 30 sec or until the solution turned a distinct bright yellow color. The solution was allowed to sit for 30 min at room temperature and was then purged with dry nitrogen to remove residual diazomethane. For silylation of the second aliquot of the primary metabolite fraction, as well as the IMPy fraction, 75 uL of MTBSTFA was added and the extract was allowed to react in a 60 ± 5°C oven for 1 h. The samples and standards were analyzed using GC/MS in the multiple ion detection (MID) mode (HP/Agilent 6890 GC and 5973 MS). The GC column was the same for all analyses: DB-1701 column (30 m, 0.25 mm ID, 0.15 um film thickness, J&W), with helium as carrier gas. Methylated samples were analyzed with the following GC temperature program: 90-130 C @25C/min, 130-200 C/min, 200-280 C @ 15 C/min. Silylated samples were analyzed with the following temperature program: 100- 130 C @ 25 C/min, 130- 200 C @ 2 C/min, 200-280 C @ 20 C/min.
Quantification of the gas chromatography/mass spectrometry (GC/MS) data was performed using the internal standard method and a linear least squares regression-derived 6-point calibration curve that was generated and analyzed concurrently with each sample set. Each sample set consisted of 18 samples, one sample duplicate, one control urine sample, and one spiked control urine. A two-step approach to isotope dilution quantification was used, with the recovery of each labeled analogue determined first and then applied to the analyte. This was done so as to track general method performance through labeled analogue recoveries. Concentrations of those metabolites without a labeled analogue were adjusted by that analyte's recovery from the concurrently analyzed spiked sample.
Footnotes
Author Contributions: Kyle R. Fluegge performed the statistical analysis, managed the literature searches and wrote the first draft of the manuscript. Marcia Nishioka and J.R.Wilkins III wrote the protocol, managed the analyses of the study and edited the manuscript. All authors read and approved the final manuscript.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.U.S. EPA. Chlorpyrifos Revised Risk Assessment and Agreement with Registrants. Washington, DC: U.S. Environmental Protection Agency; 2000. [Google Scholar]
- 2.U.S. EPA. Diazinon Revised Risk Assessment and Agreement with Registrants. Washington, DC: U.S. Environmental Protection Agency; 2001. [Google Scholar]
- 3.Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, Barr DB, Furlong CE, Holland NT. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26(2):199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, Wetmur JG, Wolff MS. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. American Journal of Epidemiology. 2007;165(12):1397–404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- 5.Jurewicz J, Hanke W. Prenatal and childhood exposure to pesticides and neurobehavioral development: review of epidemiological studies. International Journal of Occupational Medicine and Environmental Health. 2008;21(2):121–32. doi: 10.2478/v10001-008-0014-z. [DOI] [PubMed] [Google Scholar]
- 6.Furlong CE, Holland N, Richter RJ, Bradman A, Ho A, Eskenazi B. PON1 status of farmworker mothers and children as a predictor of organophosphate sensitivity. Pharmacogenetics and Genomics. 2006;16(3):183–90. doi: 10.1097/01.fpc.0000189796.21770.d3. [DOI] [PubMed] [Google Scholar]
- 7.Rauh VA, Perera FP, Horton MK, Whyatt RM, Bansal R, Hao X, Liu J, Barr DB, Slotkin TA, Peterson BS. Brain anomalies in children exposed prenatally to a common organophosphate pesticide. Proceedings of the National Academy of Sciences. 2012 May 15;109(20):7871–7876. doi: 10.1073/pnas.1203396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. Seven year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environmental Health Perspectives. 2011;119(8):1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, Whitehead R, Tang D, Whyatt RW. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6):e1845–59. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortenberry GZ, Meeker JD, Sánchez BN, Barr DB, Panuwet P, Bellinger D, Schnaas L, Solano-González M, Ettinger AS, Hernandez-Avila M, Hu H. Urinary 3, 5, 6-trichloro-2-pyridinol (TCPY) in pregnant women from Mexico City: Distribution, temporal variability, and relationship with child attention and hyperactivity. International Journal of Hygiene and Environmental Health. 2014;217(2):405–12. doi: 10.1016/j.ijheh.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskenazi B, Marks AR, Bradman A, Harley K, Bart DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environmental Health Perspectives. 2007;115(5):792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha C, Agrawal AK, Islam F, Seth K, Chaturvedi RK, Shukla S, Seth PK. Mosquito repellent (pyrethroid-based) induced dysfunction of blood–brain barrier permeability in developing brain. International Journal of Developmental Neuroscience. 2004;22(1):31–37. doi: 10.1016/j.ijdevneu.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Adinolfi M. The development of the human blood-CSF-brain barrier. Developmental Medicine & Child Neurology. 1985;27(4):532–7. doi: 10.1111/j.1469-8749.1985.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 14.Williams MK, Rundle A, Holmes D, Reyes M, Hoepner LA, Barr DB, Camann DE, Perera FP, Whyatt RM. Changes in pest infestation levels, self-reported pesticide use, and permethrin exposure during pregnancy after the 2000-2001 US Environmental Protection Agency restriction of organophosphates. Environmental Health Perspectives. 2008;116(12):1681–1688. doi: 10.1289/ehp.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horton MK, Rundle A, Camann DE, Barr DB, Rauh VA, Whyatt RM. Impact of prenatal exposure to piperonyl butoxide and permethrin on 36-month neurodevelopment. Pediatrics. 2011;127(3):e699–706. doi: 10.1542/peds.2010-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ostrea EM, Jr, Reyes A, Villanueva-Uy E, Pacifico R, Benitez B, Ramos E, Bernardo RC, Bielawski DM, Delaney-Black V, Chiodo L, Janisse JJ. Fetal exposure to propoxur and abnormal child neurodevelopment at 2 years of age. Neurotoxicology. 2012;33(4):669–75. doi: 10.1016/j.neuro.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue Z, Li X, Su Q, Xu L, Zhang P, Kong Z, Xu J, Teng J. Effect of synthetic pyrethroid pesticide exposure during pregnancy on the growth and development of infants. Asia-Pacific Journal of Public Health. 2013;25(4 suppl):72S–9S. doi: 10.1177/1010539513496267. [DOI] [PubMed] [Google Scholar]
- 18.Manca A, Palmer S. Handling missing data in patient-level cost-effectiveness analysis alongside randomised clinical trials. Applied Health Economics and Health Policy. 2005;4(2):65–75. doi: 10.2165/00148365-200504020-00001. [DOI] [PubMed] [Google Scholar]
- 19.Rubin DB, Schafer JL. Efficiently creating multiple imputations for incomplete multivariate normal data. Proceedings of the Statistical Computing Section of the American Statistical Association. 1990;83:88. [Google Scholar]
- 20.Van Buren S. Multiple imputation of discrete and continuous data by fully conditional specification. Statistical Methods in Medical Research. 2007;16(3):219–42. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 21.StataCorp. Stata statistical software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 22.Bright Futures Steering Committee, Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: An algorithm for developmental surveillance and screening. Pediatrics. 2006;118(1):405–20. doi: 10.1542/peds.2006-1231. [DOI] [PubMed] [Google Scholar]
- 23.Torres-Altoro MI, Mathur BN, Drerup JM, Thomas R, Lovinger DM, O'Callaghan JP, Bibb JA. Organophosphates dysregulate dopamine signaling, glutamatergic neurotransmission, and induce neuronal injury markers in striatum. Journal of Neurochemistry. 2011;119(2):303–313. doi: 10.1111/j.1471-4159.2011.07428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environmental Health Perspectives. 2005;113(8):1027–31. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittman JT, Dodd CA, Klein BG. Immunohistochemical changes in the mouse striatum induced by the pyrethroid insecticide permethrin. International Journal of Toxicology. 2003;22(5):359–70. doi: 10.1177/109158180302200504. [DOI] [PubMed] [Google Scholar]
- 26.Garcia SJ, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos targets developing glia: effects on glial fibrillary acidic protein. Developmental Brain Research. 2002;133(2):151–61. doi: 10.1016/s0165-3806(02)00283-3. [DOI] [PubMed] [Google Scholar]
- 27.Dodd CA, Klein BG. Pyrethroid and organophosphate insecticide exposure in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine mouse model of Parkinson's disease: an immunohistochemical analysis of tyrosine hydroxylase and glial fibrillary acidic protein in dorsolateral striatum. Toxicology and Industrial Health. 2009;25(1):25–39. doi: 10.1177/0748233709102752. [DOI] [PubMed] [Google Scholar]
- 28.Mense SM, Sengupta A, Lan C, Zhou M, Bentsman G, Volsky DJ, Whyatt RM, Perera FP, Zhang L. The common insecticides cyfluthrin and chlorpyrifos alter the expression of a subset of genes with diverse functions in primary human astrocytes. Toxicological Sciences. 2006;93(1):125–35. doi: 10.1093/toxsci/kfl046. [DOI] [PubMed] [Google Scholar]
- 29.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 32(1):1–4. [PubMed] [Google Scholar]
- 30.Laforce R, Doyon J. Differential role for the striatum and cerebellum in response to novel movements using a motor learning paradigm. Neuropsychologia. 2002;40(5):512–7. doi: 10.1016/s0028-3932(01)00128-2. [DOI] [PubMed] [Google Scholar]
- 31.Balchen T, Diemer NH. The AMPA antagonist, NBQX, protects against ischemia-induced loss of cerebellar Purkinje cells. Neuroreport. 1992;3(9):785–788. doi: 10.1097/00001756-199209000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Rahman A, Abou-Donia SM, El-Masry EM, Shetty AK, Abou-Donia MB. Stress and combined exposure to low doses of pyridostigmine bromide, DEET, and permethrin produce neurochemical and neuropathological alterations in cerebral cortex, hippocampus, and cerebellum. Journal of Toxicology and Environmental Health, Part A. 2004;67(2):163–92. doi: 10.1080/15287390490264802. [DOI] [PubMed] [Google Scholar]
- 33.Asari MA, Abdullah MS, Abdullah S. Effect of early neonatal exposure to deltamethrin on the purkinje cell number in rat cerebellum. The Malaysian Journal of Medical Sciences. 2008;15(3):14–21. [PMC free article] [PubMed] [Google Scholar]
- 34.Abou-Donia MB, Khan WA, Dechkovskaia AM, Goldstein LB, Bullman SL, Abdel-Rahman A. In utero exposure to nicotine and chlorpyrifos alone, and in combination produces persistent sensorimotor deficits and Purkinje neuron loss in the cerebellum of adult offspring rats. Archives of Toxicology. 2006;80(9):620–31. doi: 10.1007/s00204-006-0077-1. [DOI] [PubMed] [Google Scholar]
- 35.Malaviya MA, Husain R, Seth PK. Perinatal effects of two pyrethroid insecticides on brain neurotransmitter function in the neonatal rat. Veterinary and Human Toxicology. 1993;35(2):119–22. [PubMed] [Google Scholar]
- 36.Wójcikowski J, Golembiowska K, Daniel WA. The regulation of liver cytochrome P450 by the brain dopaminergic system. Current Drug Metabolism. 2007;8(6):631–638. doi: 10.2174/138920007781368872. [DOI] [PubMed] [Google Scholar]
- 37.Wójcikowski J, Gołembiowska K, Daniel WA. Regulation of liver cytochrome P450 by activation of brain dopaminergic system: physiological and pharmacological implications. Biochemical Pharmacology. 2008;76(2):258–267. doi: 10.1016/j.bcp.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 38.Johri A, Yadav S, Singh RL, Dhawan A, Ali M, Parmar D. Long lasting effects of prenatal exposure to deltamethrin on cerebral and hepatic cytochrome P450s and behavioral activity in rat offspring. European Journal of Pharmacology. 2006;544(1):58–68. doi: 10.1016/j.ejphar.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 39.Nasuti C, Cantalamessa F, Falcioni G, Gabbianelli R. Different effects of Type I and Type II pyrethroids on erythrocyte plasma membrane properties and enzymatic activity in rats. Toxicology. 2003;191(2):233–44. doi: 10.1016/s0300-483x(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 40.Motomura H, Narahashi T. Interaction of tetramethrin and deltamethrin at the single sodium channel in rat hippocampal neurons. Neurotoxicology. 2001;22(3):329–39. doi: 10.1016/s0161-813x(01)00023-7. [DOI] [PubMed] [Google Scholar]
- 41.Jarecki BW, Piekarz AD, Jackson JO, Cummins TR. Human voltage-gated sodium channel mutations that cause inherited neuronal and muscle channelopathies increase resurgent sodium currents. The Journal of Clinical Investigation. 2010;120(1):369–78. doi: 10.1172/JCI40801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaczmarek LK, Slack Slick. sodium-activated potassium channels. ISRN Neuroscience. 2013;2013 doi: 10.1155/2013/354262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bausch AE, Dieter R, Nann Y, Hausmann M, Meyerdierks N, Kaczmarek LK, Ruth P, Lukowski R. The sodium-activated potassium channel Slack is required for optimal cognitive flexibility in mice. Learning & Memory. 2015;22(7):323–35. doi: 10.1101/lm.037820.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaneko H, Miyamoto J. Pyrethroid chemistry and metabolism. Handbook of Pesticide Toxicology. 2001;2:1263–88. [Google Scholar]
- 45.Wessels D, Barr DB, Mendola P. Use of biomarkers to indicate exposure of children to organophosphate pesticides: implications for a longitudinal study of children's environmental health. Environmental Health Perspectives. 2003;111(16):1939–1946. doi: 10.1289/ehp.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oulhote Y, Bouchard MF. Urinary metabolites of organophosphate and pyrethroid pesticides and behavioral problems in Canadian children. Environmental Health Perspectives (Online) 2013;121(11-12):1378–1384. doi: 10.1289/ehp.1306667. [DOI] [PMC free article] [PubMed] [Google Scholar]


