Abstract
Purpose
To determine the pathologic complete response (pCR) rate in estrogen receptor (ER) –positive primary breast cancer triaged to chemotherapy when the protein encoded by the MKI67 gene (Ki67) level was > 10% after 2 to 4 weeks of neoadjuvant aromatase inhibitor (AI) therapy. A second objective was to examine risk of relapse using the Ki67-based Preoperative Endocrine Prognostic Index (PEPI).
Methods
The American College of Surgeons Oncology Group (ACOSOG) Z1031A trial enrolled postmenopausal women with stage II or III ER-positive (Allred score, 6 to 8) breast cancer whose treatment was randomly assigned to neoadjuvant AI therapy with anastrozole, exemestane, or letrozole. For the trial ACOSOG Z1031B, the protocol was amended to include a tumor Ki67 determination after 2 to 4 weeks of AI. If the Ki67 was > 10%, patients were switched to neoadjuvant chemotherapy. A pCR rate of > 20% was the predefined efficacy threshold. In patients who completed neoadjuvant AI, stratified Cox modeling was used to assess whether time to recurrence differed by PEPI = 0 score (T1 or T2, N0, Ki67 < 2.7%, ER Allred > 2) versus PEPI > 0 disease.
Results
Only two of the 35 patients in ACOSOG Z1031B who were switched to neoadjuvant chemotherapy experienced a pCR (5.7%; 95% CI, 0.7% to 19.1%). After 5.5 years of median follow-up, four (3.7%) of the 109 patients with a PEPI = 0 score relapsed versus 49 (14.4%) of 341 of patients with PEPI > 0 (recurrence hazard ratio [PEPI = 0 v PEPI > 0], 0.27; P = .014; 95% CI, 0.092 to 0.764).
Conclusion
Chemotherapy efficacy was lower than expected in ER-positive tumors exhibiting AI-resistant proliferation. The optimal therapy for these patients should be further investigated. For patients with PEPI = 0 disease, the relapse risk over 5 years was only 3.6% without chemotherapy, supporting the study of adjuvant endocrine monotherapy in this group. These Ki67 and PEPI triage approaches are being definitively studied in the ALTERNATE trial (Alternate Approaches for Clinical Stage II or III Estrogen Receptor Positive Breast Cancer Neoadjuvant Treatment in Postmenopausal Women: A Phase III Study; clinical trial information: NCT01953588).
INTRODUCTION
For postmenopausal women with clinical stage II or III estrogen receptor (ER) –positive breast cancer, neoadjuvant aromatase inhibition (AI) is an underused and low-toxicity alternative to chemotherapy for increasing breast conservation rates.1 A barrier to greater adoption of neoadjuvant AI is high response variability. We therefore postulated that an early switch from AI to neoadjuvant chemotherapy could produce better clinical outcomes for tumors that responded poorly to AI. Conversely, adjuvant AI alone may be sufficient to prevent relapse in tumors highly responsive to neoadjuvant AI.
Pathologic complete response (pCR) after systemic chemotherapy remains a controversial clinical trial end point,2,3 and alternatives are needed, particularly in ER-positive, human epidermal growth factor receptor 2–negative disease (HER2-negative), where pCR rates are low. Data from a neoadjuvant study comparing letrozole and tamoxifen in postmenopausal women with ER-positive breast cancer—P024 (Preoperative Treatment of Postmenopausal Breast Cancer Patients with Letrozole: A Randomized Double-Blind Multicenter Study)4-6—was previously used to generate the Preoperative Endocrine Prognostic Index (PEPI).7 PEPI requires pathologic stage (tumor size and nodal status), in addition to levels of the protein encoded by the MKI67 gene (Ki67), and Allred ER score measured on the surgical specimen (with surgery conducted during uninterrupted endocrine therapy). Patients with a PEPI score of 0 (pT1 or pT2, pN0, Ki67 ≤ 2.7%, Allred score > 2) from the P024 trial were found to have a low risk of relapse. Similar findings were observed in the neoadjuvant IMPACT trial (Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen)2, but no subsequent validation efforts have been reported.
The American College of Surgeons Oncology Group (ACOSOG) Z1031A randomized phase II clinical trial was designed to determine which AI (anastrozole, letrozole, or exemestane) should be recommended for future testing against chemotherapy in the neoadjuvant setting (ACOSOG is now a part of the Alliance for Clinical Trials in Oncology). The major initial finding from ACOSOG Z1031A was that half of the patients who were considered candidates for mastectomy or inoperable before neoadjuvant AI therapy had successful breast-conserving surgery.8 When the enrollment in ACOSOG Z1031A was complete, an amendment was introduced (ACOSOG Z1031B) that triaged patients with tumors exhibiting a Ki67 > 10% in a tumor biopsy 2 to 4 weeks after starting AI to standard chemotherapy. The hypothesis being tested was that the pCR rate would be at least 20% in this AI-resistant population. Herein, we report the pCR results from ACOSOG Z1031B as well as the time to recurrence by PEPI status among all ACOSOG Z1031 patients who completed neoadjuvant AI treatment.
METHODS
Establishment of an Early Ki67 Cut Point for Triage to Chemotherapy
An on-treatment Ki67 threshold for switching from neoadjuvant AI therapy to neoadjuvant chemotherapy was established using data from the Preoperative Letrozole study (POL)9 and the IMPACT trial.10 A Ki67 value of > 10% at 1 month in the POL study was associated with a higher PEPI score (P = .01), a smaller number of patients in the PEPI-0 group (P = .08), and worse relapse-free survival (P = .0016). Similarly, the IMPACT data confirmed that a 2-week Ki67 > 10% predicted a higher PEPI score (P = .001), smaller numbers of patients in the PEPI-0 group (P = .004), and worse relapse-free survival (P = .008; Data Supplement; Appendix Table A1 and Figure A1A, online only). Combining these studies revealed only one PEPI-0 case among 51 patients with a 2- to 4-week Ki67 value of > 10%. Thus, according to the PEPI model, patients with a Ki67 value of 10% at 2 to 4 weeks had a < 2% chance of a favorable PEPI score that would allow them to safely avoid chemotherapy under current guidelines.
Patient Eligibility
Eligible patients were postmenopausal with invasive breast cancer (clinical stage T2 to T4c, N0 to N3, M0). Additional criteria have been previously described.8 This study was supported by the Clinical Trials Support Unit and approved by the institutional review boards of all participating institutions. Each participant signed an institutional review board–approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Treatment Schema
Patients enrolled in ACOSOG Z1031B underwent a core breast biopsy for Ki67 determination after 2 weeks of AI therapy. If the Ki67 was ≤ 10%, the patient continued AI therapy for another 12 to 14 weeks and then proceeded to surgery. Women whose 2-week Ki67 level was > 10% were offered either a National Comprehensive Cancer Network–approved neoadjuvant chemotherapy regimen or surgery at the discretion of their providers and/or the patients. If the biopsy core contained insufficient tumor to perform the Ki67 assay, patients could elect to undergo a repeat biopsy at 4 weeks or continue on AI therapy. If severe treatment-related toxicity was reported or the patient refused further AI therapy, surgery was recommended. Within 14 days of registration, patients underwent a complete physical examination with tumor assessment. Every 4 weeks, patients underwent a physical examination, toxicity assessment, and tumor assessment using February 2000 WHO criteria. If tumor progression was suspected, ultrasound or mammogram was required, and neoadjuvant AI was discontinued if progression was confirmed. Blood and biopsy specimens for correlative studies were collected at baseline, 2 to 4 weeks after the start of neoadjuvant AI treatment, on discontinuation of neoadjuvant treatment, and at surgery. For patients with PEPI = 0 disease, management without chemotherapy was recommended but not mandatory to determine the acceptability of this recommendation.
AI Treatment
Before the release of the ACOSOG Z1031A results,8 eligible patients were randomly assigned to 16 to 18 weeks of neoadjuvant AI with exemestane 25 mg once daily, letrozole 2.5 mg once daily, or anastrozole 1 mg once daily. After the release of the data, patients could choose either letrozole or anastrozole treatment.
Statistical Considerations
The primary end point for ACOSOG Z1031B was the pCR rate among the women who after 2 weeks of neoadjuvant AI therapy had a tumor Ki67 level of > 10% and switched to neoadjuvant chemotherapy. pCR was defined as histologic evidence of no invasive tumor cells in the surgical breast specimen or ipsilateral lymph nodes. A one-stage phase II clinical trial design was chosen to assess the pCR rate. With a sample size of 35 and a one-sided α = 0.10, a one-sample binomial test of proportions would have a 90% chance of declaring success with a pCR rate of at least 20% compared with the null hypothesis that the pCR was 5% (at least four pCRs were needed to conclude pCR rate ≥ 20%). A 90% binomial confidence interval for the true pCR was also constructed. On the basis of the IMPACT study, it was estimated that 235 eligible women would need to enroll to obtain 35 women with a 2-week Ki67 > 10% willing to switch to neoadjuvant chemotherapy. The long-term PEPI outcome study cohort excluded women from both ACOSOG Z1031A and Z1031B who withdrew consent not having started neoadjuvant AI, had metastases or bilateral invasive breast cancer at registration, failed to undergo surgery, had alternative therapy before surgery, had radiographic confirmed disease progression or new primary disease during neoadjuvant AI, failed to have sentinel lymph node or axillary lymph node dissection, or lacked sufficient tissue to obtain a Ki67 or Allred score. In addition, ACOSOG Z1031B patients with 2- or 4-week Ki67 > 10% who remained on AI were excluded. Time to breast cancer recurrence (TBCR) was defined as the time from surgery to first local, regional, or distant disease recurrence. Patients without documented disease recurrence were censored at the date of their last disease evaluation. TBCR was estimated using the Kaplan-Meier method11 Stratified Cox modeling (with cohort and adjuvant chemotherapy use as strata) was used to assess whether TBCR differed with respect to PEPI 0 status.12 The Alliance Statistics and Data Center conducted data collection and statistical analyses. Data were locked on January 11, 2016.
Ki-67 CLIA Assay for ACOSOG Z1031A and Z1031B
For both ACOSOG Z1031 cohorts, a Ki67 clinical trial assay was performed at the College of American Pathologists– and the Clinical Laboratory Improvement Amendments–certified Washington University anatomic and molecular pathology laboratory using the CONFIRM anti-Ki67 (30-9) rabbit monoclonal primary antibody as a prediluted reagent on a Benchmark XT platform according to the manufacturer instructions (Ventana, Tucson, AZ). Tonsil was used as the assay positive control.
Ki-67 Quantification Approaches
A single experienced pathologist (D.C.A.) conducted the real-time Ki67 scoring for ACOSOG Z1031B. If the estimated rate was low (< 2.7%), or high (> 10%), a whole slide estimate was conducted. If the score was between 2.7% and 10%, point counting was conducted using an ocular grid, at least three high-power fields with a minimum of 100 cells scored. Retrospective analysis of ACOSOG Z1031A used the iScan Coreo scanner (Ventana) with the Companion Algorithm Ki-67 (30-9) software. The imaging approach required three to 10 areas of interest be selected at 4× magnification excluding ductal carcinoma in situ, vessels, and lymphocytes and avoiding perinecrotic or necrotic areas. The image analysis result was reviewed to ensure the software was correctly differentiating between benign and malignant cells, and, if not, the case was triaged to visual point counting. The visual point counting approach required color photomicrographs with a background grid taken at 40× of at least three fields selected based on invasive tumor content and the quality of the histology, not on Ki67 staining pattern, to obtain tumor cell count of at least 200. The scorer counted the total number of tumor cells and the number of Ki67-positive cells that intersected with first grid line and every third gridline thereafter.
Gene Expression Analysis to Study Cell Cycle–Regulated Genes
RNA preparation and Agilent 44K gene array analysis(Agilent Technologies, Santa Clara, CA) approaches were carried out as previously described.2 The microarray contained probes for 720 of the 874 genes previously identified as having periodic expression in the cell division cycle of HeLa cells (Data Supplement).13 Gene expression levels in each tumor were normalized to the number of standard deviations from the median expression value across all the tumors. A multigene proliferation score (MGPS) for a tumor was the average normalized expression of the 772 genes for that tumor analysis.14 The microarray data sets have been submitted to Gene Expression Omnibus (GSE87411).
RESULTS
ACOSOG Z1031B Patient Cohort
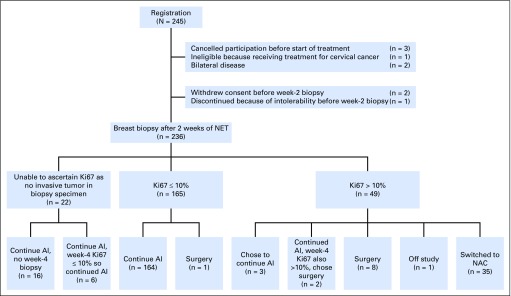
From October 1, 2009 to November 15, 2011, 245 patients were enrolled into ACOSOG Z1031B. At week 2, 165 women (69.9%) had a Ki67 value of ≤ 10%; 49 women (20.8%) had a Ki67 value > 10%, and 22 women (9.3%) had insufficient tumor to make a Ki67 determination. Patient and disease characteristics of these 236 women are presented in Table 1 and the CONSORT diagram in Figure 1.
Table 1.
Patient and Disease Characteristics for the ACOSOG Z1031B Cohort Sorted by Early on-Treatment Ki67 Categories
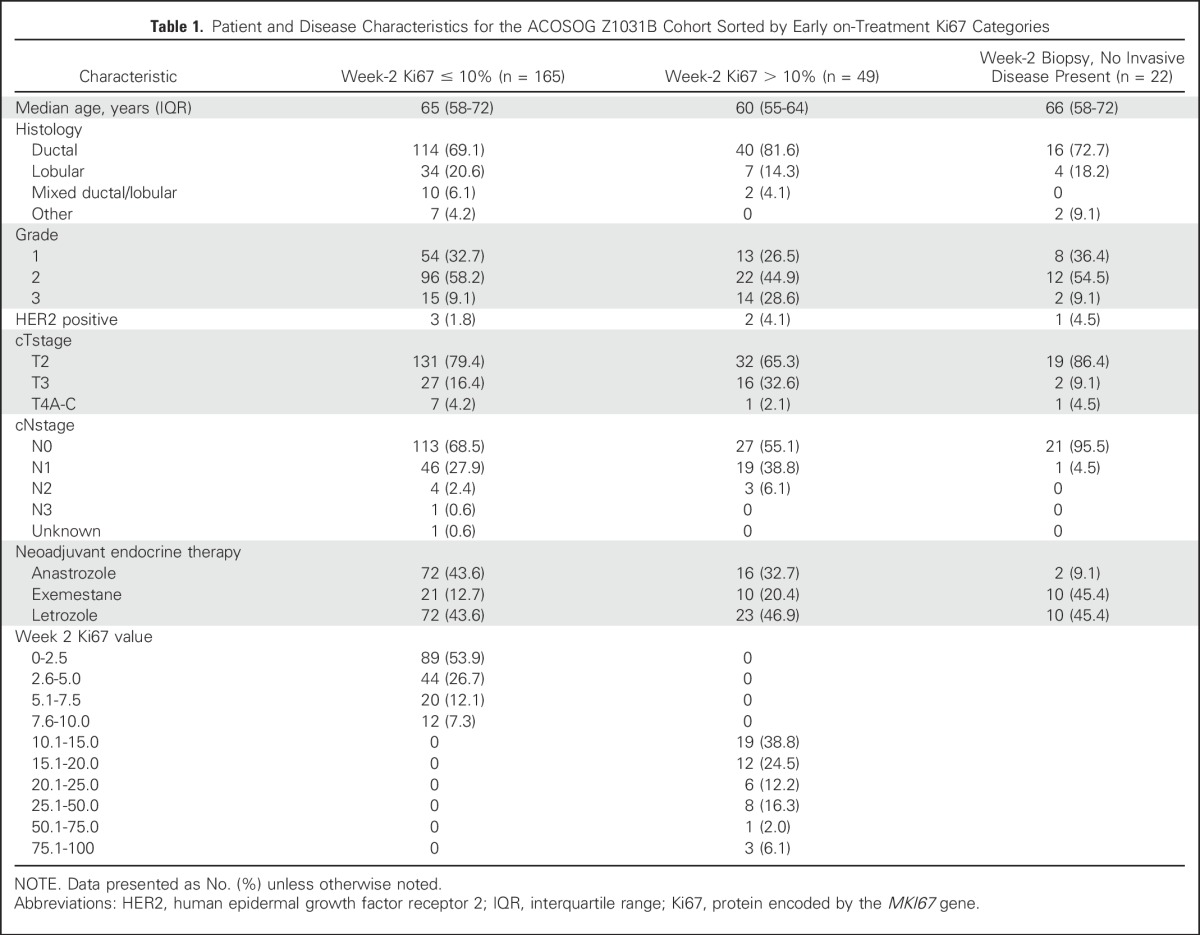
Fig 1.
CONSORT diagram for the ACOSOG Z1031B patients. AI, aromatase inhibitor; Ki67, protein encoded by the MKI67 gene; NAC, neoadjuvant chemotherapy; NET, neoadjuvant endocrine therapy.
Neoadjuvant Chemotherapy Efficacy for ER-Positive Tumors Exhibiting a 2- to 4-Week Ki67 Value > 10% After Starting Aromatase Inhibition
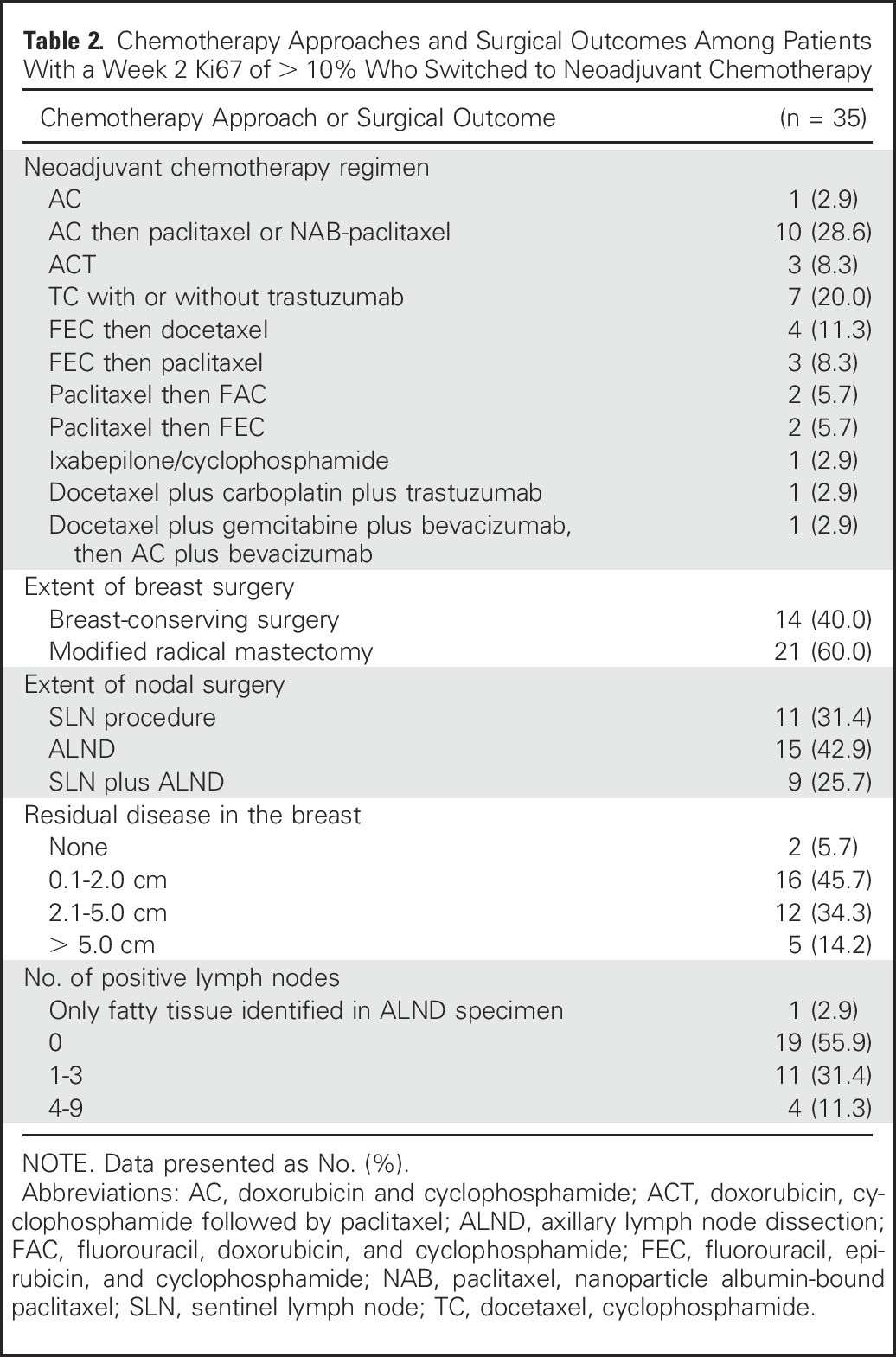
Among the 49 patients whose week 2–Ki67 was > 10%, 35 patients switched to neoadjuvant chemotherapy; three patients continued with AI, eight patients went directly to surgery, two patients went to surgery after a rebiopsy at week 4 and again found their Ki67 to be > 10%; one patient pursued treatment outside of this study. Twenty-five (71.4%) of the 35 had an anthracycline-containing regimen (Table 2). Six patients (17.1%) failed to complete their planned course of chemotherapy because of intolerability. There were two (5.7%; 95% CI, 0.7% to 19.1%) pCRs among these 35 patients. A pCR occurred in a 55-year-old woman with cT2N0 ductal breast cancer with a 2-week Ki67 of 80.0% treated with doxorubicin and cyclophosphamide (AC) plus docetaxel. The second pCR was in a 59-year-old woman with cT3N1 ductal breast cancer with a 2-week Ki67 of 31.7% subsequently treated with docetaxel plus gemcitabine plus bevacizumab followed by AC plus bevacizumab.
Table 2.
Chemotherapy Approaches and Surgical Outcomes Among Patients With a Week 2 Ki67 of > 10% Who Switched to Neoadjuvant Chemotherapy
Neoadjuvant Outcomes for ACOSOG Z1031B Patients Whose Week 2 to 4 Ki67 Was ≤ 10%
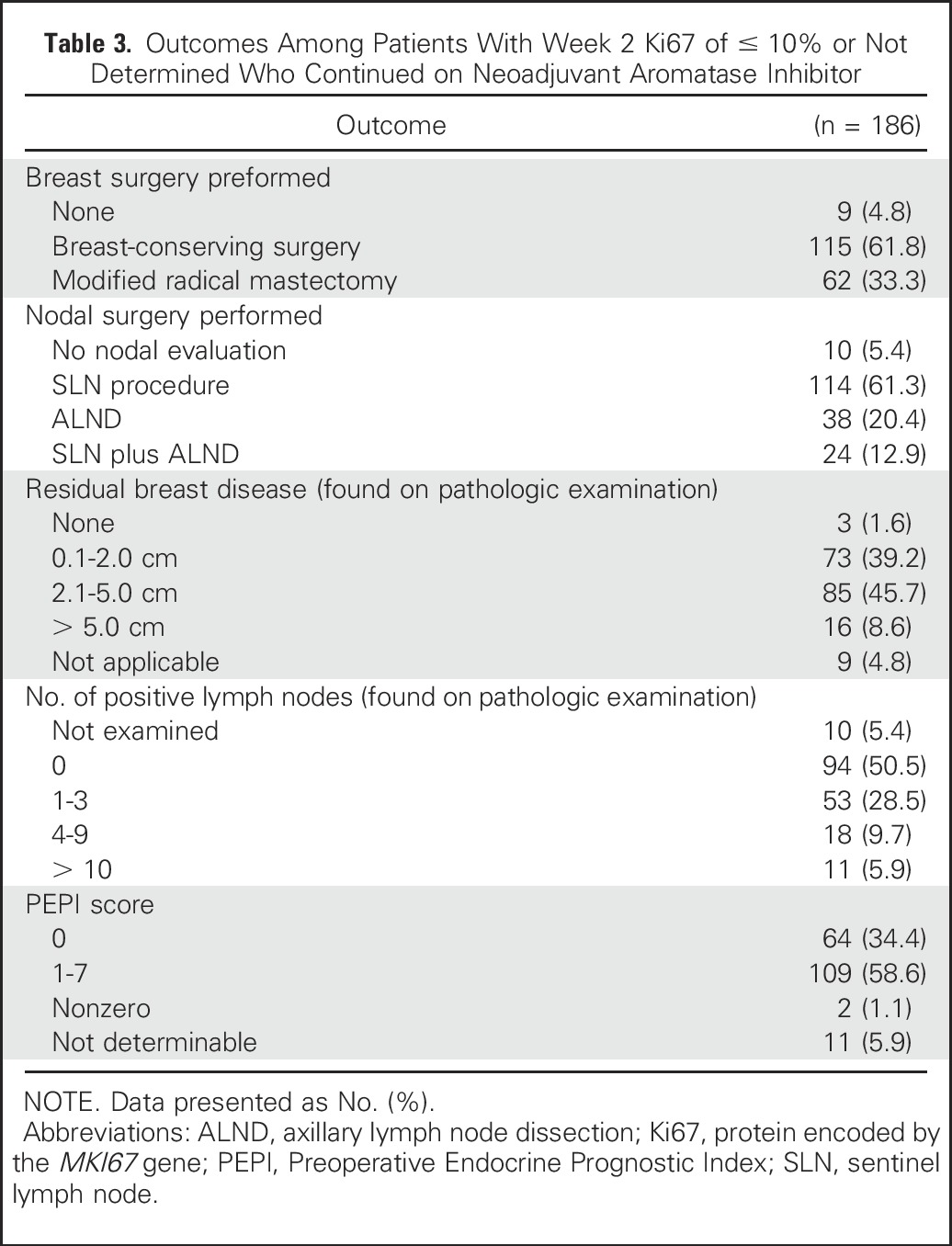
There were 187 patients whose 2-week Ki67 value was either ≤ 10% (165) or not determined because of insufficient tumor in the biopsy specimen (22). One of 187 patients whose week 2–Ki67 value was ≤ 10% chose to go directly to surgery rather than continue on AI. Of 22 patients whose 2-week Ki67 value was not obtained, all chose to remain on AI either after a rebiopsy at week 4 Ki67 of ≤ 10% (six patients) or after forgoing a rebiopsy at 4 weeks (16 patients; see Fig 1 for exact disposition of all patients). Among the remaining 177 patients, pathologic evaluation revealed no residual disease in the breast or lymph nodes in three patients, in only the breast in 92 patients, and in both the breast and lymph nodes in 82 patients (Table 3). The pCR rate among the 186 women who completed AI was therefore 1.6% (95% CI, 0.3% to 4.6%). PEPI scores were: 0 in 64 patients; 1 to 7 in 109 patients; and not determined in 13 patients because of progression during neoadjuvant AI therapy (two patients), lack of Allred score or Ki67 from surgical specimen (four patients), or failure to undergo surgery for reasons other than progression (seven patients). Thus, the PEPI-0 rate was 34.4% (95% CI, 27.6% to 41.7%).
Table 3.
Outcomes Among Patients With Week 2 Ki67 of ≤ 10% or Not Determined Who Continued on Neoadjuvant Aromatase Inhibitor
Adjuvant Chemotherapy Decisions for Patients With a PEPI = 0
Management without adjuvant chemotherapy was the preferred protocol approach for patients in the PEPI = 0 category. This occurred in 57 of 64 PEPI = 0 patients (89%). In contrast, 45 (41.2%) of 109 patients with a PEPI > 0 received adjuvant chemotherapy.
Long-Term Outcomes for ACOSOG Z1031 Patients According to PEPI Status
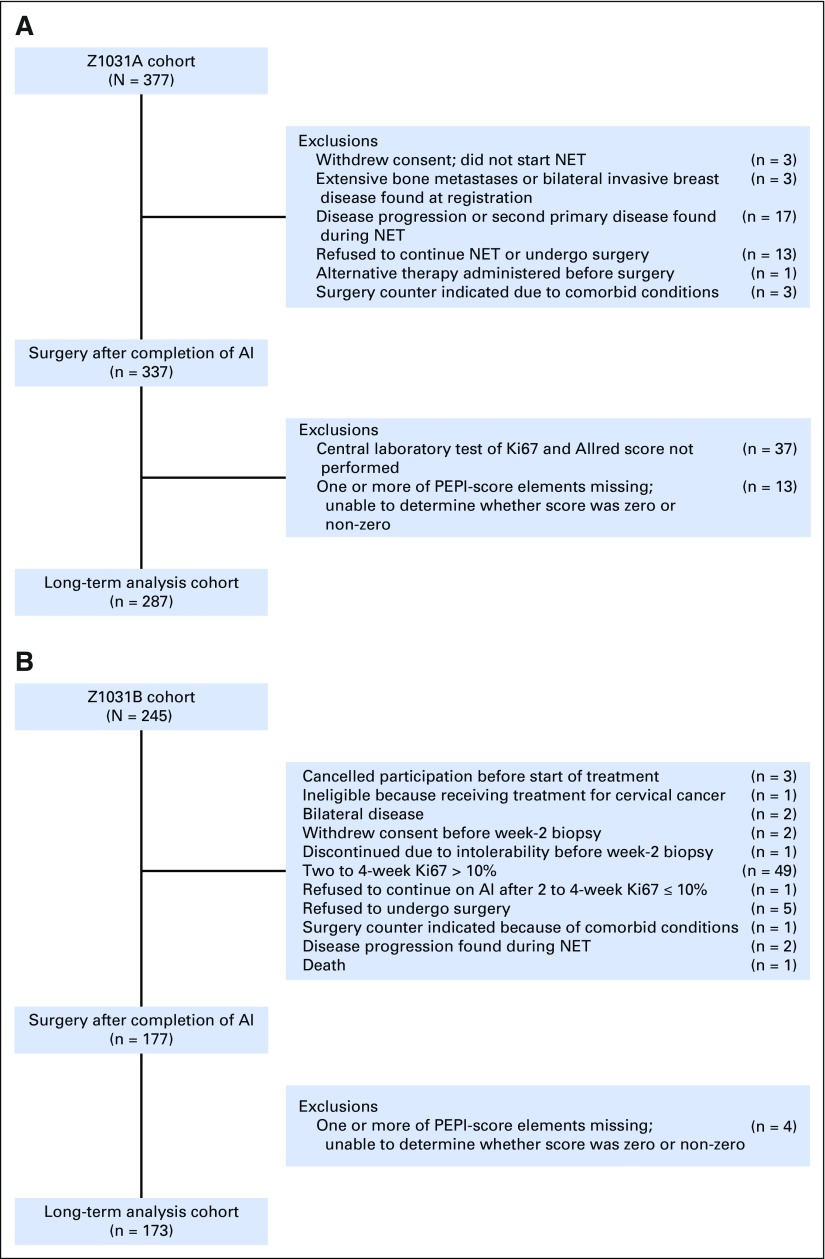
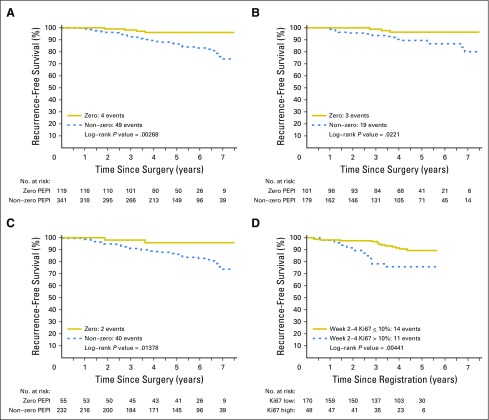
Clinical outcomes after surgery were examined in 287 ACOSOG Z1031A patients and 173 ACOSOG Z1031B patients who completed neoadjuvant AI (REMARK, Fig 2). Overall, 119 (25.9%) tumors were categorized as PEPI = 0. Adjuvant chemotherapy was administered in 18 (15.1%) of 119 PEPI = 0 cases and 162 (47.5%) of 341 PEPI > 0 patients. Adjuvant radiation was administered in 80 (67.2%) of 119 PEPI = 0 cases and 242 (71.0%) of 341 PEPI > 0 cases. At data lock, all patients have been followed until death or a median of 5.5 years postsurgery, with 365 patients alive without a disease progression, 21 alive with disease recurrence, 32 dead after disease recurrences, and 42 patients dead as a result of a second cancer (six patients), noncancer causes (28 patients), or unknown causes (eight patients). A total of 49 patients with PEPI > 0 disease experienced recurrence: local (seven), regional (two), distant (39), and locoregional/distant (one); and four patients with PEPI = 0 disease experienced recurrence (all distant). The hazard of breast cancer recurrence for PEPI = 0 cases relative to the PEPI > 0 cases was 0.27 (P = .014; 95% CI, 0.092 to 0.764) when stratifying by cohort and known adjuvant chemotherapy use. Kaplan-Meier plots of the time to breast cancer recurrence by PEPI = 0 versus PEPI > 0 status are presented in Figure 3A for the combined cohort and in Figure 3B for those patients who did not receive adjuvant chemotherapy. Because the Ki67 assay approach in ACOSOG Z1031A is being tested prospectively in the ALTERNATE (Alternate Approaches for Clinical Stage II or III Estrogen Receptor Positive Breast Cancer Neoadjuvant Treatment in Postmenopausal Women: A Phase III Study) trial, the PEPI-0 outcome for the ACOSOG Z1031A cohort is shown separately (Fig 3C).
Fig 2.
REMARK diagrams for the long-term outcome analysis by Preoperative Endocrine Therapy Prognostic Index (PEPI) score for (A) ACOSOG Z1031A patients, and (B) ACOSOG Z1031B patients. AI, aromatase inhibitor; Ki67, protein encoded by the MKI67 gene; NET, neoadjuvant endocrine therapy.
Fig 3.
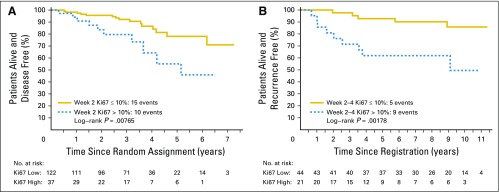
Kaplan-Meier analysis by Preoperative Endocrine Prognostic Index (PEPI) = 0 (pathologic stage II or IIA, surgical specimen protein encoded by the MKI67 gene (Ki67) < 2.7%, and estrogen receptor Allred score > 2) versus PEPI > 0 (A) for all patients, (B) for patients who did not receive adjuvant chemotherapy, and (C) for patients in the ACOSOG Z1031A cohort alone. (D) Outcomes for patients according to the 10% Ki67 cut point on ACOSOG Z1031B.
Prognosis for ACOSOG Z1031B Patients According to Ki67 at 2 to 4 Weeks
The 35 patients who were triaged to chemotherapy have been followed at least 1 year postsurgery or to death. At the time of the data lock, there were 24 patients alive without a disease progression, six were alive with disease progression, two had died of breast cancer, and three had died as a result of noncancer causes. With a median follow-up of 4.4 years post registration, the risk of relapse was increased for those with 2- to 4-week Ki67 > 10% (log rank P = .004; Fig 3D).
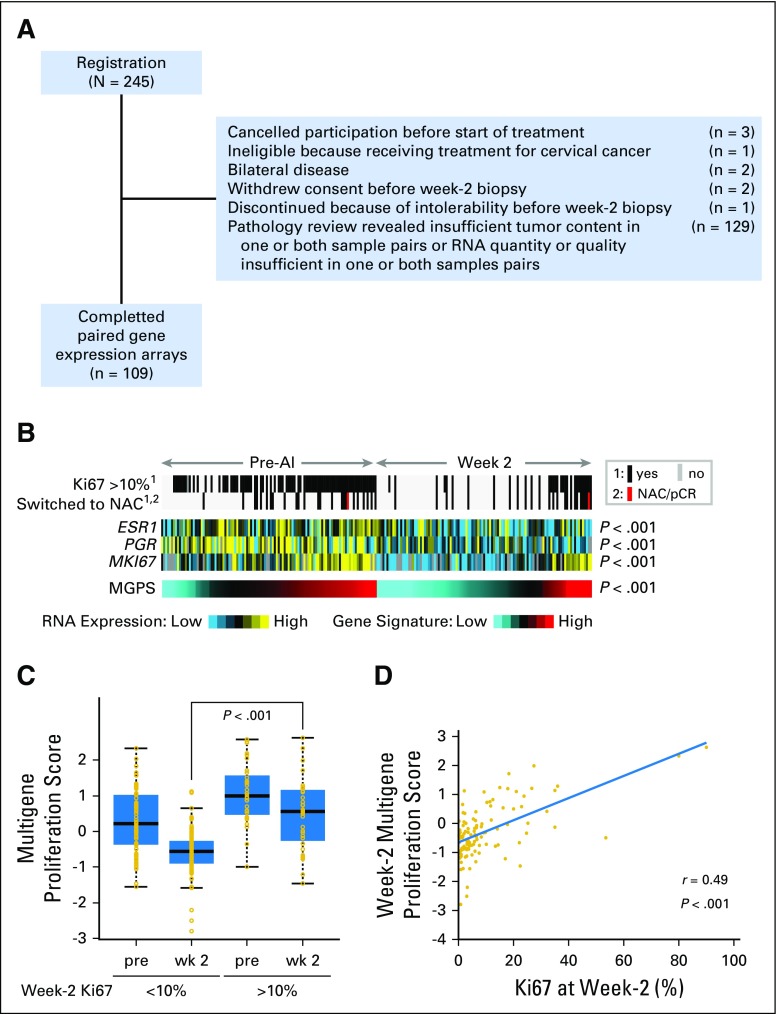
Gene Expression Analysis and Treatment-Induced Tumor Proliferation Status
The low pCR rate to chemotherapy in ACOSOG Z1031B prompted a further investigation of tumor proliferation status. Paired high tumor content frozen tumor samples before and after 2 or 4 weeks of AI were available in 109 of the 245 patients and were used for mRNA expression profiling (REMARK, Fig 4A). Proliferation status was determined using a noncommercial MGPS.14 Pairwise analysis demonstrated mRNA levels for ER, progesterone receptor, and Ki67, and MGPS values were markedly suppressed with treatment (Wilcoxon signed rank P > .001). Box plots comparing the MGPS scores at baseline and 2 weeks are illustrated in Figure 4C. The MGPS scores were higher at both baseline and after 2 weeks in the cohort, with 2-week Ki67 values of > 10% compared with ≤ 10% (Fig 4C; P < .001). The Spearman correlation coefficient at 2 to 4 weeks between Ki67 values and the MGPS was 0.49 (Fig 4D).
Fig 4.
Gene expression–based analysis of proliferation in 109 patients at baseline and 2-week paired samples (including a small number of patients for whom the data were derived from a 4-week sample) from the ACOSOG Z1031B cohort only (A) REMARK diagram. (B) The effect of aromatase inhibitor (AI) treatment on mRNA levels for estrogen receptor, progesterone receptor, and protein encoded by the MKI67 gene (Ki67) as a heat map showing marked suppression with treatment. The gray and black bars indicate samples associated with Ki67 values above or below 10% and patients who received chemotherapy. The red bar indicates the one patient in this analysis who experienced a pathologic complete response (pCR) to chemotherapy. The lowest bar provides a heat map from the multigene proliferation score (MGPS, cell cycle), also showing marked suppression of treatment, but also identifying patients who have presently high levels of gene expression from cell cycle–related genes that overlap with patients with Ki67 levels of > 10% and received chemotherapy. (C) Box plots comparing the MGPS scores at baseline and 2 weeks in samples associated with 2-week Ki67 < 10% or > 10% showing higher scores at both baseline and at 2 weeks for patients with Ki67 scores > 10% (Wilcoxon signed rank test P ≤ .001 for both comparisons). (D) Correlation between Ki67 values and MGPS values at 2 weeks with the Pearson’s correlation coefficient. MGPS, multigene proliferation; NAC, neoadjuvant chemotherapy.
DISCUSSION
The PEPI is a distinct prognostic approach for ER-positive breast cancer that depends on tumor features after neoadjuvant endocrine therapy. PEPI integrates residual disease burden and the cell cycle (Ki67) response providing a simple approach to deescalating adjuvant treatment after neoadjuvant AI for patients in the PEPI = 0 category. These patients, who had a risk of relapse of 3% with a median follow-up of approximately 5 years, are therefore unlikely to benefit from adjuvant chemotherapy. A weakness of the study is that the relapse risk estimate is based on only 119 cases; thus, PEPI validation efforts should continue.
Concerns regarding the variability in Ki67 analysis have been discussed extensively.15 The imaging analysis approach to Ki67 estimation used for the ACOSOG Z1031A cohort is promising (Fig 3C), but conclusive data using this methodology await the results of the ALTERNATE trial.
The low pCR rate for ACOSOG Z1031B patients who switched to neoadjuvant chemotherapy contradicts the hypothesis that AI-resistant proliferation in ER-rich tumors is associated with enhanced chemotherapy response. The muted chemotherapy response was unlikely because of the Ki67 assay failure to capture highly proliferative tumors, because an independent examination of tumor cell cycle activity with a multigene proliferation score indicated significantly higher expression of cell cycle–dependent genes when the Ki67 was > 10% at 2 to 4 weeks (Figs 4C and 4D). Low chemotherapy responsiveness could reflect the postmenopausal status of the patient cohort,16 the high ER content of tumors eligible for neoadjuvant endocrine therapy,17,18 or the use of endocrine therapy before neoadjuvant chemotherapy. However, the latter hypothesis seems unlikely because, unlike prior studies that raised this concern,19 endocrine therapy was not administered concurrently with chemotherapy.
Our data provide further support for an assessment of prognosis in ER-positive breast cancer on the basis of post–neoadjuvant endocrine therapy tumor characteristics. Triaging patients to neoadjuvant chemotherapy on the basis of failure to suppress Ki67 is feasible but highlights the relative chemotherapy-resistant nature of strongly ER-positive yet AI-resistant disease in postmenopausal women and the early relapse risk faced by patients in this category in all three studies where this question can be examined (Fig 3D; Data Supplement). The development of new treatment options for intrinsically AI-resistant disease will likely depend on new insights into the molecular basis for primary endocrine therapy resistance.20,21
ACKNOWLEDGMENT
We thank Dr Jo Anne Zujewski for her long-term support and guidance for the neoadjuvant endocrine therapy studies we have conducted with the Cancer Treatment and Evaluation Program of the National Cancer Institute. We also thank the clinical trial participants and their families.
Appendix
Table A1.
Early Ki67 Assessments and Outcome in IMPACT and POL Trials
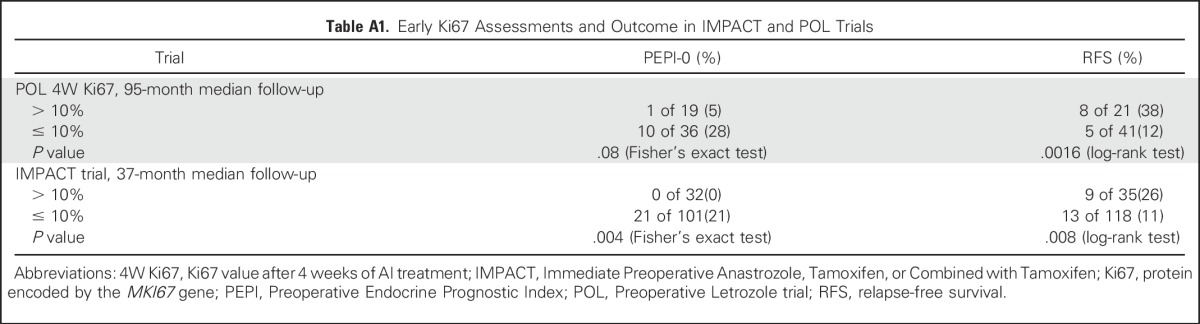
Fig A1.
Relapse patterns in the two independent training sets used to justify the 10% early on treatment cut point for triage to chemotherapy in Z1031B. (A) is based on data from the IMPACT trial10 and (B) from data derived from the Preoperative Letrozole (POL) Trial9. In both studies, on-treatment Ki67 values of >10% identified in a biopsy taken at 2 weeks (IMPACT) or 4 weeks (POL) are associated with a significantly higher risk of subsequent relapse over time than patients with values of ≤10% at the same time point.
Footnotes
Supported by the National Cancer Institute of the National Institutes of Health Grants No. U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA180857, U10CA180858, U10CA180870, U10CA180833, and legacy grants CA31946, CA33601, CA105409, U10CA031983, U10CA037347, U10CA047577, U10CA077440 with grants R01-CA095614 to M.J.E., U24-CA114736, U24CA196171, U10-CA076001, and U01-CA114722 from the National Cancer Institute; the Breast Cancer Research Foundation; a Komen St Louis Affiliate Clinical Trials Grant; and support for Z1031 from Pfizer and Novartis and image analysis support for the iScan Coreo scanner from Roche Diagnostics. M.J.E. is a McNair Medical Foundation Scholar and the recipient of a Cancer Prevention Research Institute of Texas established investigator award.
M.J.E. and V.J.S. are joint first authors.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the companies involved.
Clinical trial information: NCT00265759.
See accompanying Editorial on page 1031
AUTHOR CONTRIBUTIONS
Conception and design: Matthew J. Ellis, Vera J. Suman, Yu Tao, Michael Barnes, Mitchell Dowsett, Gary Unzeitig, Marilyn Leitch, John A. Olson Jr, D. Craig Allred, Kelly Hunt
Administrative support: Matthew J. Ellis, Marilyn Leitch
Provision of study materials or patients: Matthew J. Ellis, Mark Watson, Gary Unzeitig, Timothy Pluard Pat Whitworth, Gildy Babiera, J. Michael Guenther, Zoneddy Dayao, David Ota, John A. Olson Jr, Kelly Hunt
Collection and assembly of data: Matthew J. Ellis, Jeremy Hoog, Rodrigo Goncalves, Souzan Sanati, Katherine DeSchryver, Erika Crouch, Amy Brink, Mark Watson, Michael Barnes, G. Thomas Budd, Eric Winer, Paula Silverman, Laura Esserman, Lisa Carey, Gary Unzeitig, Timothy Pluard, Pat Whitworth, Gildy Babiera, J. Michael Guenther, Zoneddy Dayao, David Ota, John A. Olson Jr, D. Craig Allred, Kelly Hunt
Data analysis and interpretation: Matthew J. Ellis, Vera J. Suman, Rodrigo Goncalves, Chad J. Creighton, Jingqin Luo, Yu Tao, Cynthia X. Ma, Gary Unzeitig, Kelly Hunt
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ki67 Proliferation Index as a Tool for Chemotherapy Decisions During and After Neoadjuvant Aromatase Inhibitor Treatment of Breast Cancer: Results From the American College of Surgeons Oncology Group Z1031 Trial (Alliance)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Matthew J. Ellis
Employment: Bioclassifier
Leadership: Bioclassifier
Stock or Other Ownership: Bioclassifier
Consulting or Advisory Role: Novartis, Pfizer, Celgene, AstraZeneca, NanoString Technologies
Patents, Royalties, Other Intellectual Property: Patent on PAM50, which generates royalties on the license to Bioclassifier, Prosigna, and Nanostring
Travel, Accommodations, Expenses: Pfizer, AstraZeneca
Vera J. Suman
No relationship to disclose
Jeremy Hoog
No relationship to disclose
Rodrigo Goncalves
No relationship to disclose
Souzan Sanati
No relationship to disclose
Chad J. Creighton
No relationship to disclose
Katherine DeSchryver
No relationship to disclose
Erika Crouch
No relationship to disclose
Amy Brink
No relationship to disclose
Mark Watson
Patents, Royalties, Other Intellectual Property: Holder of several patents on mammaglobin gene as a biomarker; unrelated to current work
Jingqin Luo
No relationship to disclose
Yu Tao
No relationship to disclose
Michael Barnes
Employment: Roche/Ventana Medical Systems
Leadership: Roche/Ventana Medical Systems
Stock or Other Ownership: Roche
Research Funding: Roche/Ventana Medical Systems
Patents, Royalties, Other Intellectual Property: Roche/Ventana Medical Systems
Travel, Accommodations, Expenses: Roche/Ventana Medical Systems
Mitchell Dowsett
Honoraria: Novartis, Pfizer, Myriad Genetics
Consulting or Advisory Role: Genoptix, Genentech, Janssen Oncology, GTx, Radius Health
Research Funding: Roche (Inst), AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Radius Health (Inst)
Other Relationship: Institute of Cancer Research
G. Thomas Budd
No relationship to disclose
Eric Winer
Consulting or Advisory Role: Leap Therapeutics
Paula Silverman
No relationship to disclose
Laura Esserman
No relationship to disclose
Lisa Carey
Research Funding: GlaxoSmithKline, Genentech
Cynthia X. Ma
Research Funding: Puma Biotechnology (Inst), Pfizer (Inst), Eisai (Inst)
Gary Unzeitig
No relationship to disclose
Timothy Pluard
Speakers' Bureau: Genentech, Novartis, Pfizer
Pat Whitworth
Consulting or Advisory Role: Cianna Medical, Intact Medical, Prelude Medical, Genomic Health, Agendia NV, Myriad Genetics, Prosigna
Research Funding: Intact Medical, Cianna Medical, Agendia NV, Prelude Medical, InVitae, Prosigna
Gildy Babiera
Consulting or Advisory Role: MD Anderson Physician’s Network
J. Michael Guenther
No relationship to disclose
Zoneddy Dayao
No relationship to disclose
David Ota
No relationship to disclose
Marilyn Leitch
Consulting or Advisory Role: Celgene, Genomic Health, Castle Biosciences
John A. Olson Jr
Stock or Other Ownership: Core Prognostex
D. Craig Allred
No relationship to disclose
Kelly Hunt
Consulting or Advisory Role: Armada Health Care
REFERENCES
- 1.Goncalves R, Ma C, Luo J, et al. : Use of neoadjuvant data to design adjuvant endocrine therapy trials for breast cancer. Nat Rev Clin Oncol 9:223-229, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cortazar P, Zhang L, Untch M, et al. : Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384:164-172, 2014 [DOI] [PubMed] [Google Scholar]
- 3.DeMichele A, Yee D, Berry DA, et al. : The neoadjuvant model is still the future for drug development in breast cancer. Clin Cancer Res 21:2911-2915, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eiermann W, Paepke S, Appfelstaedt J, et al. : Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann Oncol 12:1527-1532, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Ellis MJ, Coop A, Singh B, et al. : Letrozole is more effective neoadjuvant endocrine therapy than tamoxifen for ErbB-1- and/or ErbB-2-positive, estrogen receptor-positive primary breast cancer: Evidence from a phase III randomized trial. J Clin Oncol 19:3808-3816, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Ellis MJ, Coop A, Singh B, et al. : Letrozole inhibits tumor proliferation more effectively than tamoxifen independent of HER1/2 expression status. Cancer Res 63:6523-6531, 2003 [PubMed] [Google Scholar]
- 7.Ellis MJ, Tao Y, Luo J, et al. : Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J Natl Cancer Inst 100:1380-1388, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis MJ, Suman VJ, Hoog J, et al. : Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. J Clin Oncol 29:2342-2349, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. doi: 10.1016/j.jamcollsurg.2009.01.035. Olson JA Jr, Budd GT, Carey LA, et al: Improved surgical outcomes for breast cancer patients receiving neoadjuvant aromatase inhibitor therapy: Results from a multicenter phase II trial. J Am Coll Surg 208:906-914, 2009; discussion 915-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowsett M, Smith IE, Ebbs SR, et al. : Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst 99:167-170, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 12.Peto R, Peto J: Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A 135:185-207, 1972 [Google Scholar]
- 13.Whitfield ML, Sherlock G, Saldanha AJ, et al. : Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell 13:1977-2000, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creighton CJ: Multiple oncogenic pathway signatures show coordinate expression patterns in human prostate tumors. PLoS One 3:e1816, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dowsett M, Nielsen TO, A’Hern R, et al. : Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 103:1656-1664, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher B, Jeong JH, Bryant J, et al. : Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet 364:858-868, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Loibl S, Jackisch C, Lederer B, et al. : Outcome after neoadjuvant chemotherapy in young breast cancer patients: A pooled analysis of individual patient data from eight prospectively randomized controlled trials. Breast Cancer Res Treat 152:377-387, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Huober J, von Minckwitz G, Denkert C, et al. : Effect of neoadjuvant anthracycline-taxane-based chemotherapy in different biological breast cancer phenotypes: Overall results from the GeparTrio study. Breast Cancer Res Treat 124:133-140, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Albain KS, Barlow WE, Ravdin PM, et al. : Adjuvant chemotherapy and timing of tamoxifen in postmenopausal patients with endocrine-responsive, node-positive breast cancer: A phase 3, open-label, randomised controlled trial. Lancet 374:2055-2063, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis MJ, Ding L, Shen D, et al. : Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature 486:353-360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma CX, Reinert T, Chmielewska I, et al. : Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer 15:261-275, 2015 [DOI] [PubMed] [Google Scholar]