Abstract
Purpose
Patients with relapsed metastatic germ cell tumor (GCT) can be cured with second-line and even third-line regimens. We report survival outcomes of patients treated with high-dose chemotherapy (HDCT) and peripheral-blood stem-cell transplantation (PBSCT) at Indiana University between 2004 and 2014.
Patients and Methods
We conducted a retrospective analysis of 364 consecutive patients with GCT who progressed after cisplatin-based combination chemotherapy and were subsequently treated with HDCT and PBSCT. Three hundred forty-one patients received two consecutive courses of HDCT consisting of 700 mg/m2 carboplatin and 750 mg/m2 etoposide, each for 3 consecutive days, and each followed by PBSCT. Twenty-three patients received only a single course of HDCT because of progressive disease or toxicity. Cox proportional hazards models were used to test predictors of disease progression.
Results
The median age was 32 years (range, 17 to 70 years). With a median follow-up of 3.3 years, the 2-year progression-free survival (PFS) was 60% (95% CI, 55% to 65%) and the 2-year overall survival was 66% (95% CI, 60% to 70%). Three hundred three patients received HDCT as second-line therapy with a 2-year PFS of 63% (95% CI, 57% to 68%), and 61 patients received HDCT as third-line or later therapy with a 2-year PFS of 49% (95% CI, 36% to 61%). In a multivariable analysis, factors associated with disease progression included use of HDCT as third-line or later therapy, platinum-refractory disease, mediastinal primary tumor site, nonseminoma histology, intermediate- or poor-risk disease at the time of GCT diagnosis, and human chorionic gonadotropin ≥ 1,000 mIU/mL at initiation of HDCT. There were nine treatment-related deaths. Secondary leukemia developed in five patients.
Conclusion
This large single-institution study demonstrates that patients with relapsed metastatic GCT are curable by HDCT plus PBSCT even when used in third-line or later therapy.
INTRODUCTION
Germ-cell tumors (GCT) are the most common cancer in men between 15 and 35 years of age and the incidence has risen during the past several decades.1 Patients with metastatic GCT are curable even in the presence of metastatic disease.2 The International Germ Cell Cancer Collaborative Group (IGCCCG) classified patients with metastatic GCT into good, intermediate, and poor risk disease on the basis of specified prognostic criteria.3 Per the IGCCCG, the good risk category represents 60%, intermediate risk represents 26%, and poor risk represents 14% of patients with metastatic GCT. Using contemporary cisplatin-based front-line combination chemotherapy, cure rates at our institution for good, intermediate, and poor risk disease are 90%, 84%, and 51%, respectively.4 Patients who relapse after initial chemotherapy, with anatomically confined disease, can still be cured by salvage surgery.5 The vast majority of patients, however, will be treated with salvage chemotherapy; the most effective regimen for these patients remains unsettled. Second-line standard-dose chemotherapy options include etoposide plus ifosfamide plus cisplatin (VIP), vinblastine plus ifosfamide plus cisplatin, or paclitaxel plus ifosfamide plus cisplatin.6-8
High-dose chemotherapy (HDCT) followed by bone marrow transplant was first investigated at Indiana University in 1986.9 Bone marrow transplantation was replaced by peripheral-blood stem cells in 1996. This allowed for more rapid engraftment and hence fewer delays in delivering a second course of HDCT. In our previous series of 184 consecutive patients with relapsed metastatic GCT treated with HDCT and peripheral-blood stem-cell transplant (PBSCT) between 1996 and 2004, long-term disease-free survival was achieved in 70% of patients in the second-line setting, and in 45% of patients who received a third-line or subsequent regimen.10
Investigators at Memorial Sloan Kettering Cancer Center (MSKCC) pioneered another widely used HDCT regimen, which incorporates paclitaxel and ifosfamide as induction chemotherapy and stem-cell mobilization followed by high-dose carboplatin and etoposide with PBSCT for three cycles (TI-CE regimen).11 In a phase I/II trial that enrolled 107 patients, the reported 5-year disease-free survival was 47% and overall survival (OS) was 52%. Given the lack of definitive phase III data for salvage therapy and limited outcome data reported from the past 10 years, we now report survival outcomes and prognostic criteria in 364 consecutive patients with relapsed metastatic GCT treated at Indiana University between 2004 and 2014 with HDCT followed by PBSCT for a planned two successive courses.
PATIENTS AND METHODS
Patients
Eligible patients had metastatic GCT that progressed after one or more standard cisplatin-etoposide–based combination chemotherapy regimens.2,12 After institutional review board approval was obtained, we conducted a retrospective analysis of 364 patients with relapsed metastatic GCT who received HDCT and PBSCT at Indiana University between December 2004 and December 2014. Clinical data were collected from electronic medical records, physical charts, and patient interviews. Abstracted data included patient and disease characteristics, prior surgical and chemotherapy treatment, date of HDCT, and survival outcomes. This study used the secure Web-based, Title 21 Code of Federal Regulations Part 11–compliant Research Electronic Data Capture system for data input. Patients with primary mediastinal nonseminomatous germ cell tumor (PMNSGCT) were included in this study. Patients with late relapse of GCT, defined as > 2 years after previous therapy, were excluded.
Patients who had platinum-refractory disease, defined as tumor progression within 4 weeks of cisplatin-based chemotherapy, proceeded directly to HDCT. Patients who had platinum-sensitive disease received one or two cycles of standard-dose chemotherapy, most commonly vinblastine plus ifosfamide plus cisplatin, before proceeding to HDCT. This was done in an attempt to reduce the bulk of tumor while preventing further disease progression before initiating HDCT.
Peripheral-blood stem cells were harvested after the patient’s bone marrow was stimulated using granulocyte colony-stimulating factor. These cells were then purified using a technique that has been previously described.13 Two courses of HDCT were planned for all patients. Each course consisted of carboplatin 700 mg/m2 body-surface area plus etoposide 750 mg/m2 intravenously on days 5, 4, and 3. Stem-cell infusion was performed on day 0.
Patients received bacterial, viral, and fungal prophylaxis according to the following regimen: ciprofloxacin 500 mg orally twice per day, acyclovir 400 mg orally twice per day, fluconazole 400 mg orally once per day, and vancomycin intravenously. Infectious prophylaxis was initiated on the day before stem-cell infusion and continued until neutrophil count recovery. Granulocyte colony-stimulating factor was delivered once per day after stem-cell infusion until neutrophil count recovery. After engraftment and end-organ recovery, the second cycle of HDCT was initiated. Some patients who achieved a complete or partial remission after HDCT were treated with maintenance oral etoposide delivered at a dose of 50 mg/m2 once per day for 21 days of 28-day cycles for a total of three cycles.14,15 This was given to patients with nonseminoma who achieved a serological complete remission and had hematologic recovery after HDCT.
The primary end point of this study was to estimate the 2-year PFS of patients with relapsed GCT treated with HDCT. Secondary end points were to estimate 2-year OS, to identify clinical factors prognostic for disease progression after HDCT, and to assess safety and toxicity.
Statistical Analysis
Analyses were performed using SAS software, version 9.4. Patient and disease characteristics at the beginning of HDCT were tabulated. PFS was calculated from the date of HDCT initiation to the date of relapse or date of death. Otherwise, the patient was censored at the date of last follow-up. PFS was estimated using Kaplan-Meier methods.
Univariable analysis was performed to identify prognostic factors for disease progression using the Wald χ2 test. Using a significance level of P < .05, factors that were significant in the univariable analysis for PFS were incorporated in a multivariable Cox proportional hazards model. This model was used to identify important clinical factors in predicting PFS and to estimate the hazard ratio for each variable while adjusting for other covariates.
Variables that were tested in the univariable analysis were use of HDCT as third-line or later regimen versus second-line therapy, platinum-refractory versus platinum-sensitive disease, primary tumor site (mediastinal v testes/retroperitoneal), tumor histology, risk per IGCCCG criteria at the time of GCT diagnosis,3 serum tumor marker level at initiation of HDCT including alpha-fetoprotein and human chorionic gonadotropin (hCG), and age at the time of HDCT initiation. Age was used as a continuous variable in this analysis.
RESULTS
Patient and Disease Characteristics
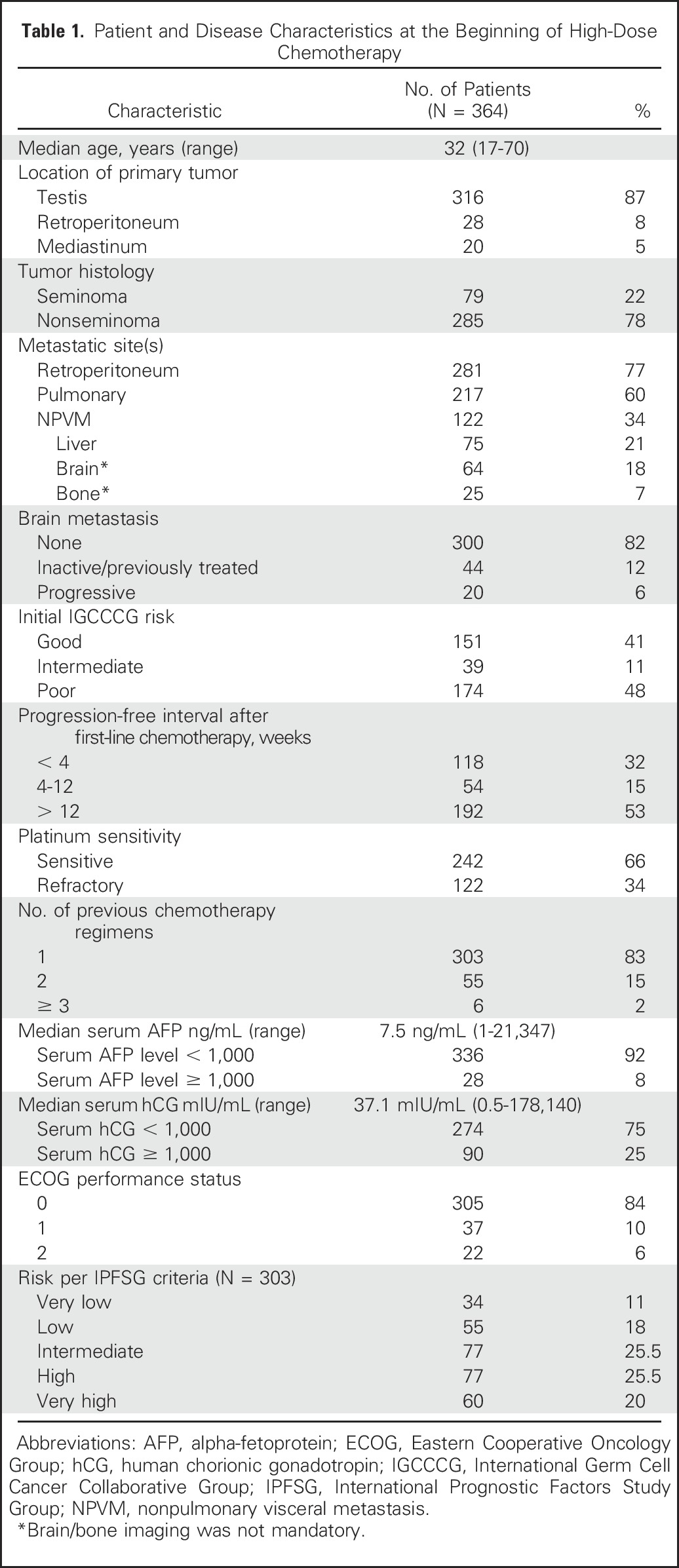
Three hundred sixty-four consecutive patients with relapsed metastatic GCT received HDCT and PBSCT at Indiana University between 2004 and 2014. The median age was 32 years (range, 17 to 70 years). Three hundred forty-two patients had an Eastern Cooperative Oncology Group performance status of 0 or 1 at initiation of HDCT. The primary tumor site was testis in 316 patients, retroperitoneum in 28 patients, and mediastinum in 20 patients. All patients with primary mediastinal primary tumor site had nonseminoma histology. Seventy-nine patients had pure seminoma and 285 had nonseminomatous GCT. Two hundred forty-two patients had platinum-sensitive disease and 122 patients had platinum-refractory disease, defined as tumor progression within 4 weeks of cisplatin-based chemotherapy. Three hundred three patients received HDCT in the second-line setting and 61 received HDCT in the third-line or later setting. Table 1 lists patient and disease characteristics at the time of starting the first course of HDCT.
Table 1.
Patient and Disease Characteristics at the Beginning of High-Dose Chemotherapy
Treatment Administration and Survival Outcomes
Two hundred forty-two patients received a single course of standard-dose chemotherapy before proceeding with HDCT. Three hundred forty-one patients (94%) received the planned two courses of HDCT. The median time between HDCT courses was 28 days (range, 18 to 58 days). Twenty-three patients received only a single course of HDCT because of progressive disease in 12 patients and toxicity in 11 patients. One hundred thirty-four patients received maintenance etoposide after HDCT. Among these patients, 15 received one cycle, 14 received two cycles, and 105 received three cycles of oral maintenance etoposide.
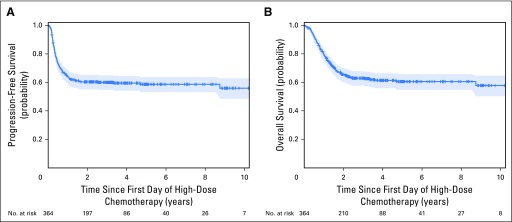
With a total of 364 patients and a median follow-up of 3.3 years, the estimated 2-year PFS was 60% (95% CI, 55% to 65%; Fig 1A). The estimated 2-year OS was 66% (95% CI, 60% to 70%; Fig 1B). Among the 143 patients who had disease recurrence after HDCT, the median time to relapse was 4.3 months (range, 1 to 30 months).
Fig 1.
Kaplan-Meier estimates of (A) progression-free survival and (B) overall survival for 364 patients.
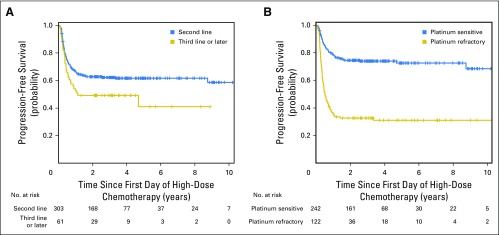
In breaking down the PFS by line of therapy, patients who received HDCT as second-line treatment had a 2-year PFS of 63% (95% CI, 57% to 68%) and patients who received HDCT as third-line or later therapy had a 2-year PFS of 49% (95% CI, 36% to 61%; P = .03). The 2-year OS rates for these subsets were 67% (95% CI, 61% to 72%) and 60% (95% CI, 46% to 71%), respectively (P = .05). Patients with platinum-refractory disease had 2-year PFS rates of 33% (95% CI, 24% to 41%) v 75% (95% CI, 69% to 80%) for platinum-sensitive patients (P < .001). Two-year OS rates for these subsets were 37% (95% CI, 30% to 45%) and 80% (95% CI, 75% to 85%), respectively (P < .001). Figure 2 depicts Kaplan-Meier estimates of PFS for patients who received HDCT according to the line of treatment and according to platinum sensitivity.
Fig 2.
Kaplan-Meier estimates of (A) progression-free survival according to line of therapy and (B) platinum sensitivity.
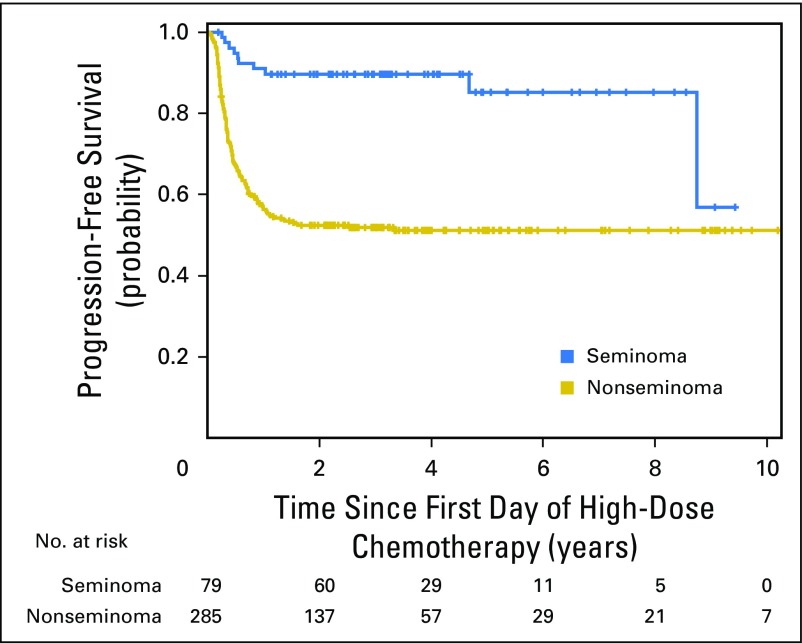
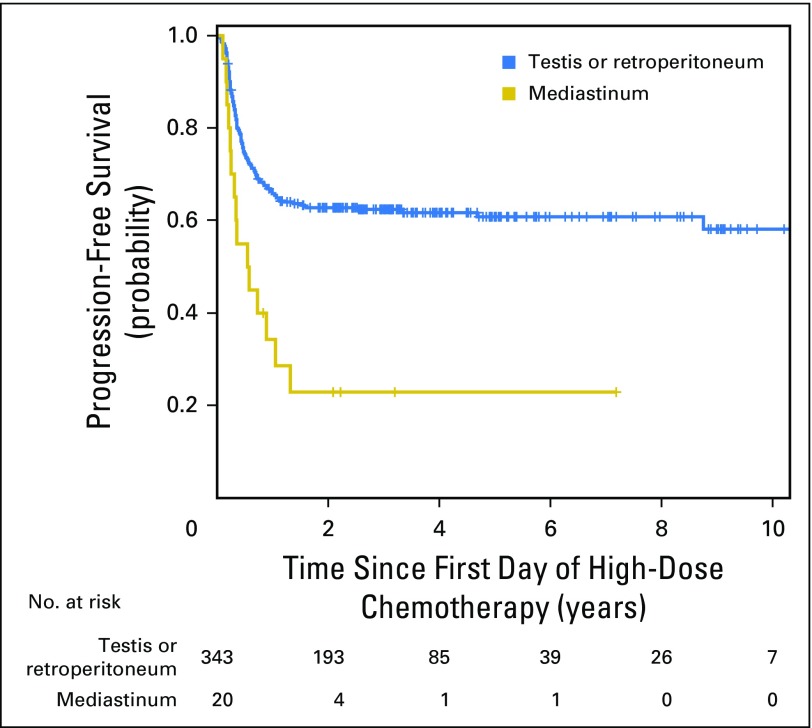
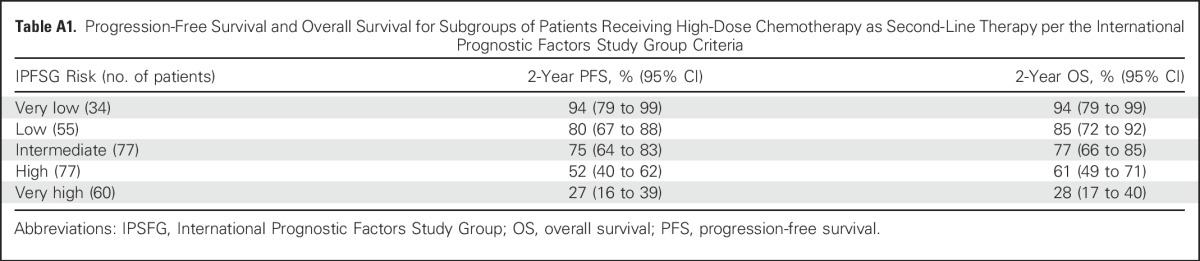
Patients with pure seminoma had 2-year PFS of 90% (95% CI, 81% to 95%) compared with 52% (95% CI, 46% to 58%) for nonseminomatous GCT (P < .001; Appendix Fig A1, online only). Two-year PFS rates for patients with PMNSGCT versus testicular/retroperitoneal primary tumor sites were 23% (95% CI, 7% to 43%) versus 63% (95% CI, 57% to 68%; P < .001), respectively (Appendix Fig A2, online only). Two-year PFS and 2-year OS for patients receiving HDCT in the second-line setting stratified per the International Prognostic Factors Study Group criteria are shown in Appendix Table A1 (online only).
Some patients with brain metastasis in this study were treated with radiation therapy before or after HDCT. Among the 64 patients with brain metastasis, 20 had progressive brain metastasis at initiation of HDCT. Eight of these 20 patients achieved remission and were disease-free after HDCT without additional surgery or radiation therapy. In addition, five of 20 patients with PMNSGCT were continuously disease-free after HDCT.
Univariable and Multivariable Analyses
Univariable and multivariable analyses were performed to evaluate the impact among the predictors on PFS while adjusting for other covariates. The results of the Cox regression analysis of all 364 patients are presented in Tables 2 and 3. The variables that were significant in the univariable analysis and included in the multivariable analysis were line of therapy (third-line or later regimen v second-line therapy), platinum-refractory versus platinum-sensitive disease, serum tumor marker level at initiation of HDCT including alpha-fetoprotein and hCG, histologic type (nonseminoma v seminoma), risk per IGCCCG criteria at the time of GCT diagnosis, and primary tumor site (mediastinal v testis/retroperitoneal).
Table 2.
Univariable Analysis for Prognostic Variables in 364 Patients
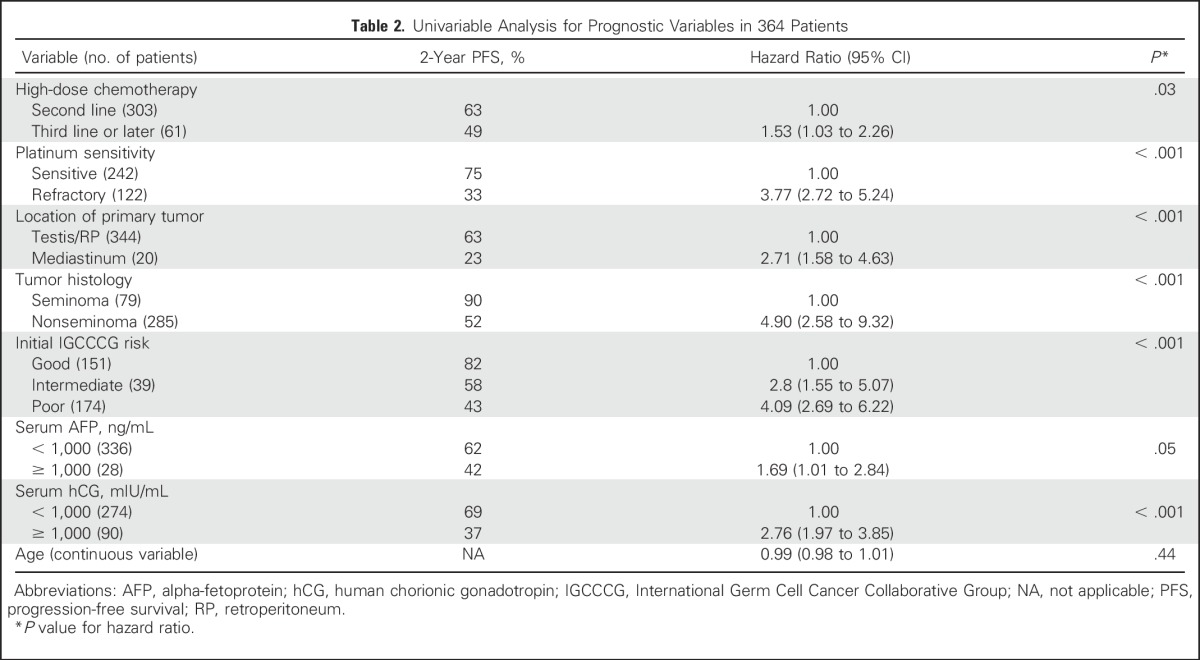
Table 3.
Multivariable Analysis for Prognostic Variables in 364 Patients
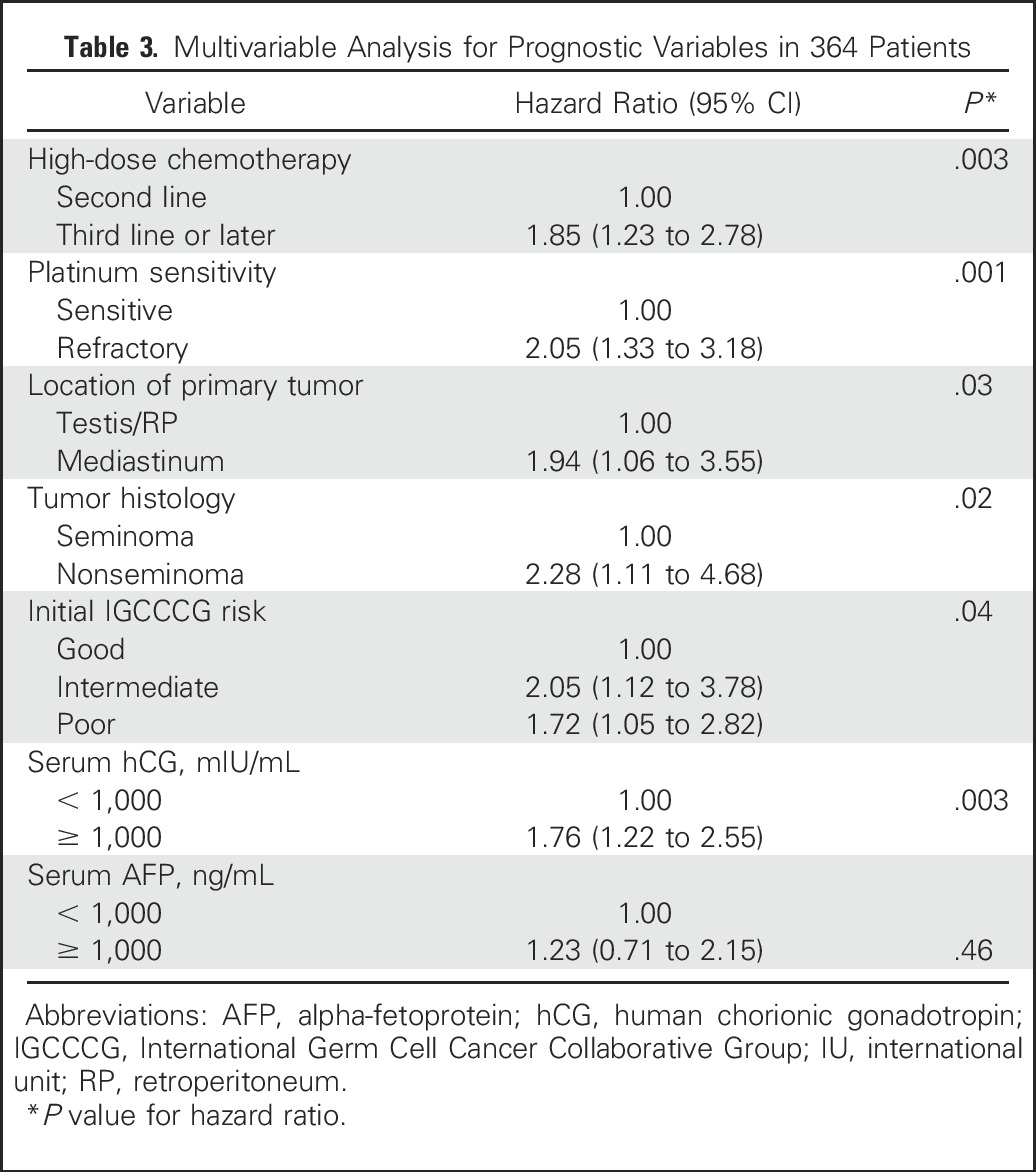
Significant variables that retained statistical significance in the multivariable analysis and had an adverse effect on PFS were use of HDCT as third-line or later therapy, platinum-refractory disease, mediastinal primary tumor site, nonseminoma histology, intermediate or poor risk disease per IGCCCG criteria,3 and hCG ≥ 1,000 mIU/mL at initiation of HDCT.
Toxicity
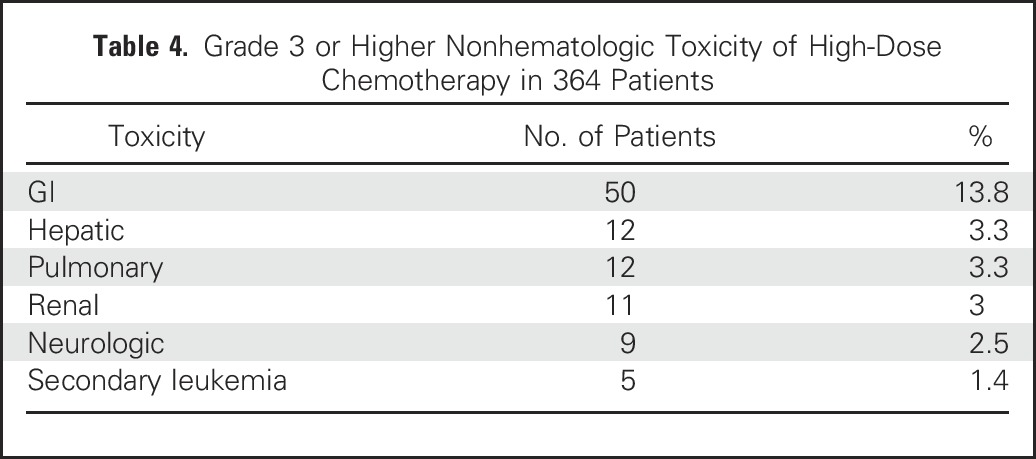
Grade 3 or higher nonhematologic toxic effects of HDCT are listed in Table 4. Renal toxicity was defined as a serum creatinine greater than three times the upper limit of normal range, GI defined as severe pain interfering with oral intake, neurologic defined as severe symptoms limiting self-care, pulmonary defined as dyspnea or hypoxia interfering with daily activities, and hepatic toxic effects included a value for liver-function tests that was greater than three times the upper limit of normal range. The most frequent adverse events were mucositis, nausea, and vomiting. Long-term adverse effects included peripheral neuropathy and ototoxicity.
Table 4.
Grade 3 or Higher Nonhematologic Toxicity of High-Dose Chemotherapy in 364 Patients
There were nine treatment-related deaths. Six deaths occurred during or within 30 days of completion of HDCT: four as a result of infectious complications of HDCT and two as a result of hepatic failure. Three deaths occurred > 30 days after completion of HDCT and were as a result of complications from secondary leukemia.
Secondary leukemia developed in five patients and the intervals from HDCT to diagnosis of leukemia were 17, 27, 36, 51, and 120 months. Among these five patients, four received HDCT as second-line and only one received HDCT as third-line therapy. Three patients who developed secondary leukemia had received maintenance oral etoposide after HDCT. Three of these five patients died as a result of complications related to leukemia and two are currently experiencing complete remission for their relapsed GCT and secondary leukemia. One patient developed a solid malignancy 6 months after HDCT in the form of metastatic mucinous adenocarcinoma of the GI tract. He died as a result of complications related to his solid malignancy.
DISCUSSION
In the current era, cisplatin-based combination chemotherapy will cure 83% of patients with metastatic GCT.16 Ninety percent of patients with IGCCCG good risk disease will achieve cure with front-line chemotherapy. Patients with intermediate and poor risk disease have less favorable outcomes, and a significant proportion will relapse and require salvage therapy.4 This study evaluates 364 consecutive patients with relapsed metastatic GCT treated with HDCT at our center between 2004 and 2014. To our knowledge, this is the largest single-institution study evaluating HDCT in patients with relapsed GCT.
With a median follow-up of 3.3 years, the 2-year PFS was 60% (95% CI, 55% to 65%) and 2-year OS was 66% (95% CI, 60% to 70%). These outcomes are similar to results reported from MSKCC using the TI-CE regimen.11 Although this study included a high proportion of patients with poor prognostic factors at initiation of HDCT, survival outcomes seem superior compared with previous studies evaluating standard-dose salvage chemotherapy.6,7,17
The multivariable analysis reported in this study identifies key prognostic factors in patients with relapsed GCT undergoing HDCT. Receiving HDCT in the third-line or subsequent setting, platinum-refractory disease, nonseminoma histology, mediastinal primary tumor site, IGCCCG intermediate or poor risk disease at the time of GCT diagnosis, and hCG ≥ 1,000 mIU/mL at start of HDCT were all independent predictors of progression after HDCT. These prognostic criteria are consistent with previous studies that reported prognostic factors in patients with relapsed GCT treated with HDCT.10,18,19 Importantly, a significant proportion of patients who had poor prognostic features achieved remission and were disease-free after HDCT.
Long-term remissions were achieved in patients receiving HDCT as third-line or subsequent therapy (2-year PFS, 49%) and in patients with platinum-refractory disease (2-year PFS, 33%). Switching from bone marrow transplant to PBSCT allowed for more rapid delivery of the second course of HDCT and improved outcomes in patients with PMNSGCT. This study included 20 patients with PMNSGCT. Five of these 20 patients (25%) were disease-free after HDCT. These results seem similar to those of patients with PMNSGCT in the MSKCC series, where five of 21 patients (24%) were disease-free after HDCT.11 In addition, this study included 64 patients with brain metastasis; among these, 20 patients had progressive brain metastasis at initiation of HDCT. Eight of these 20 patients achieved remission and were disease-free after HDCT.
The choice of initial salvage chemotherapy for relapsed metastatic GCT remains controversial. One of the challenges is determining which patients should be treated with salvage standard-dose chemotherapy versus HDCT. A randomized phase III study comparing sequential with a single course of HDCT showed superior OS in the arm receiving sequential HDCT.20 A prospective phase III trial did not show a difference in survival when comparing VIP for four cycles versus VIP for three cycles followed by HDCT with carboplatin and etoposide plus cyclophosphamide for one cycle.21
In 2011, Lorch et al17 reported outcomes from a large multi-institutional database evaluating 1,594 patients with relapsed GCT. This retrospective study included a diverse patient population stratified to prognostic subgroups according to the International Prognostic Factors Study Group.19 Patients were treated with heterogeneous salvage chemotherapy regimens between 1990 and 2008. In this study, HDCT achieved superior outcomes compared with standard-dose chemotherapy and there was an overall 56% decrease in the risk of progression after first salvage treatment, favoring HDCT. This translated into statistically significant improvement in OS with HDCT in all prognostic subgroups except the low-risk group. The superior outcomes with HDCT were more pronounced in patients with intermediate, high, or very high risk disease.
In the current study, patients with seminoma had the highest cure rate, with a 2-year PFS of 90% (95% CI, 81% to 95%). Even more importantly, cures were achieved in patients with platinum-refractory disease, PMNSGCT, progressive brain metastases, and in patients receiving HDCT as third-line or subsequent therapy. These patients have low chance of cure with standard-dose chemotherapy.17
Although this study had a large sample size of consecutive patients and long follow-up, there are limitations to report. Patients in this study were treated at an experienced high-volume referral center for metastatic GCT; hence survival outcomes for patients treated at other institutions might vary. In our opinion, these patients require multidisciplinary expertise and thus should be cared for at centers of testicular cancer excellence. Other studies have reported improved outcomes when patients with testicular cancer were treated at tertiary care centers.22,23 Patients who were treated with salvage HDCT had adequate performance status and end-organ function, and these factors might have contributed to selection bias. In addition, some prognostic groups had a small number of patients, prohibiting statistical significance in the multivariable analysis.
In conclusion, HDCT followed by PBSCT is effective salvage therapy for patients with relapsed GCT, delineating remarkable chemosensitivity of this disease. Patients with platinum-refractory disease and patients with poor prognostic features are prime candidates for HDCT. Treatment with high-dose carboplatin and etoposide was associated with low treatment-related mortality. Although potentially curative, HDCT has both short- and long-term quality-of-life issues, reflecting a burden of the patients who are cured. Optimal patient selection for HDCT versus standard-dose chemotherapy as initial salvage is currently being studied in a randomized phase III trial as part of an international collaboration (ClinicalTrials.gov identifier, NCT02375204).
Appendix
Fig A1.
Kaplan-Meier estimates of progression-free survival for seminoma versus nonseminoma.
Fig A2.
Kaplan-Meier estimates of progression-free survival for primary tumor site: testis/retroperitoneum versus mediastinum.
Table A1.
Progression-Free Survival and Overall Survival for Subgroups of Patients Receiving High-Dose Chemotherapy as Second-Line Therapy per the International Prognostic Factors Study Group Criteria
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: Nabil Adra, Rafat Abonour, Costantine Albany, Nasser H. Hanna, Lawrence H. Einhorn
Financial support: Lawrence H. Einhorn
Collection and assembly of data: Nabil Adra, Costantine Albany
Data analysis and interpretation: Nabil Adra, Sandra K. Althouse, Costantine Albany, Nasser H. Hanna, Lawrence H. Einhorn
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
High-Dose Chemotherapy and Autologous Peripheral-Blood Stem-Cell Transplantation for Relapsed Metastatic Germ Cell Tumors: The Indiana University Experience
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Nabil Adra
No relationship to disclose
Rafat Abonour
Research Funding: Amgen (Inst)
Sandra K. Althouse
No relationship to disclose
Costantine Albany
No relationship to disclose
Nasser H. Hanna
Research Funding: Merck (Inst), Bristol-Myers Squibb (Inst)
Lawrence H. Einhorn
Stock or Other Ownership: Amgen, Biogen Idec
Consulting or Advisory Role: Celgene
REFERENCES
- 1.Nigam M, Aschebrook-Kilfoy B, Shikanov S, et al. Increasing incidence of testicular cancer in the United States and Europe between 1992 and 2009. World J Urol. 2015;33:623–631. doi: 10.1007/s00345-014-1361-y. [DOI] [PubMed] [Google Scholar]
- 2.Hanna NH, Einhorn LH. Testicular cancer—Discoveries and updates. N Engl J Med. 2014;371:2005–2016. doi: 10.1056/NEJMra1407550. [DOI] [PubMed] [Google Scholar]
- 3.International Germ Cell Cancer Collaborative Group International Germ Cell Consensus Classification: A prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 4.Adra N, Ku K, Kalra M, et al: Survival outcomes of patients with metastatic germ cell tumor (mGCT) treated from 1998 to 2012: The Indiana University (IU) experience. J Clin Oncol 34, 2016 (suppl 2S; abstr 491)
- 5.Murphy BR, Breeden ES, Donohue JP, et al. Surgical salvage of chemorefractory germ cell tumors. J Clin Oncol. 1993;11:324–329. doi: 10.1200/JCO.1993.11.2.324. [DOI] [PubMed] [Google Scholar]
- 6.Loehrer PJ, Sr, Einhorn LH, Williams SD. VP-16 plus ifosfamide plus cisplatin as salvage therapy in refractory germ cell cancer. J Clin Oncol. 1986;4:528–536. doi: 10.1200/JCO.1986.4.4.528. [DOI] [PubMed] [Google Scholar]
- 7.Loehrer PJ, Sr, Gonin R, Nichols CR, et al. Vinblastine plus ifosfamide plus cisplatin as initial salvage therapy in recurrent germ cell tumor. J Clin Oncol. 1998;16:2500–2504. doi: 10.1200/JCO.1998.16.7.2500. [DOI] [PubMed] [Google Scholar]
- 8.Kondagunta GV, Bacik J, Donadio A, et al. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol. 2005;23:6549–6555. doi: 10.1200/JCO.2005.19.638. [DOI] [PubMed] [Google Scholar]
- 9.Nichols CR, Tricot G, Williams SD, et al. Dose-intensive chemotherapy in refractory germ cell cancer—A phase I/II trial of high-dose carboplatin and etoposide with autologous bone marrow transplantation. J Clin Oncol. 1989;7:932–939. doi: 10.1200/JCO.1989.7.7.932. [DOI] [PubMed] [Google Scholar]
- 10.Einhorn LH, Williams SD, Chamness A, et al. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med. 2007;357:340–348. doi: 10.1056/NEJMoa067749. [DOI] [PubMed] [Google Scholar]
- 11.Feldman DR, Sheinfeld J, Bajorin DF, et al. TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: Results and prognostic factor analysis. J Clin Oncol. 2010;28:1706–1713. doi: 10.1200/JCO.2009.25.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman DR, Bosl GJ, Sheinfeld J, et al. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672–684. doi: 10.1001/jama.299.6.672. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia S, Abonour R, Porcu P, et al. High-dose chemotherapy as initial salvage chemotherapy in patients with relapsed testicular cancer. J Clin Oncol. 2000;18:3346–3351. doi: 10.1200/JCO.2000.18.19.3346. [DOI] [PubMed] [Google Scholar]
- 14.Cooper MA, Einhorn LH. Maintenance chemotherapy with daily oral etoposide following salvage therapy in patients with germ cell tumors. J Clin Oncol. 1995;13:1167–1169. doi: 10.1200/JCO.1995.13.5.1167. [DOI] [PubMed] [Google Scholar]
- 15.Miller JC, Einhorn LH. Phase II study of daily oral etoposide in refractory germ cell tumors. Semin Oncol. 1990;17(1, suppl 2):36–39. [PubMed] [Google Scholar]
- 16.Ko JJ, Bernard B, Tran B, et al. Conditional survival of patients with metastatic testicular germ cell tumors treated with first-line curative therapy. J Clin Oncol. 2016;34:714–720. doi: 10.1200/JCO.2015.64.7909. [DOI] [PubMed] [Google Scholar]
- 17.Lorch A, Bascoul-Mollevi C, Kramar A, et al. Conventional-dose versus high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: Evidence from a large international database. J Clin Oncol. 2011;29:2178–2184. doi: 10.1200/JCO.2010.32.6678. [DOI] [PubMed] [Google Scholar]
- 18.Beyer J, Kramar A, Mandanas R, et al. High-dose chemotherapy as salvage treatment in germ cell tumors: A multivariate analysis of prognostic variables. J Clin Oncol. 1996;14:2638–2645. doi: 10.1200/JCO.1996.14.10.2638. [DOI] [PubMed] [Google Scholar]
- 19.International Prognostic Factors Study Group Prognostic factors in patients with metastatic germ cell tumors who experienced treatment failure with cisplatin-based first-line chemotherapy. J Clin Oncol. 2010;28:4906–4911. doi: 10.1200/JCO.2009.26.8128. [DOI] [PubMed] [Google Scholar]
- 20.Lorch A, Kleinhans A, Kramar A, et al. Sequential versus single high-dose chemotherapy in patients with relapsed or refractory germ cell tumors: Long-term results of a prospective randomized trial. J Clin Oncol. 2012;30:800–805. doi: 10.1200/JCO.2011.38.6391. [DOI] [PubMed] [Google Scholar]
- 21.Pico JL, Rosti G, Kramar A, et al. A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumours. Ann Oncol. 2005;16:1152–1159. doi: 10.1093/annonc/mdi228. [DOI] [PubMed] [Google Scholar]
- 22.Collette L, Sylvester RJ, Stenning SP, et al. Impact of the treating institution on survival of patients with “poor-prognosis” metastatic nonseminoma. J Natl Cancer Inst. 1999;91:839–846. doi: 10.1093/jnci/91.10.839. [DOI] [PubMed] [Google Scholar]
- 23.Stiller CA. Non-specialist units, clinical trials and survival from testicular cancer. Eur J Cancer. 1995;31A:289–291. doi: 10.1016/0959-8049(94)00486-o. [DOI] [PubMed] [Google Scholar]