Abstract
Purpose
The purpose of this study was to examine outcomes associated with hormonal maintenance therapy (HMT) compared with routine observation (OBS) after primary cytoreductive surgery and platinum-based chemotherapy in women with stage II to IV low-grade serous carcinoma of the ovary or peritoneum.
Patients and Methods
Eligibility criteria for patients from our database were: treatment with primary surgery followed by platinum-based chemotherapy, stage II to IV disease, at least 2 years of follow-up for patients who had not experienced recurrence, and adequate clinical information. The two groups were compared for progression-free survival (PFS) and overall survival, and a multivariable Cox regression analysis was performed. Subset analyses for patients who were disease free or had persistent disease were also performed.
Results
Between 1981 and 2013, 203 eligible patients—133 who underwent OBS and 70 who received HMT—were seen at our institution. Median PFS for patients who underwent OBS was 26.4 months, compared with 64.9 months for those who received HMT (P < .001). No statistically significant difference in overall survival was observed between the two groups (102.7 v 115.7 months, respectively). For subgroups of women who were disease free or had persistent disease, median PFS was superior for those who received HMT (81.1 v 30.0 months; P < .001 and 38.1 v 15.2 months; P < .001, respectively). Women who received HMT had a significantly lower risk of disease progression compared with those who underwent OBS (hazard ratio, 0.44; 95% CI, 0.31 to 0.64; P < .001).
Conclusion
Women with stage II to IV low-grade serous carcinoma who received HMT after primary treatment had significantly longer PFS compared with women who underwent OBS. These findings warrant further investigation using a prospective trial design.
INTRODUCTION
Low-grade serous carcinoma (LGSC) of the ovary or peritoneum is a rare histologic subtype that seems to originate de novo or after diagnosis of a serous tumor of low malignant potential (LMP). Several publications from our group have highlighted the relative resistance of this subtype to conventional chemotherapy.1-5 A recent report from the Arbeitsgemeinschaft Gynaekologische Onkologie confirmed the observation that LGSC is not as responsive to chemotherapy as high-grade serous carcinoma (HGSC).6 These findings have accelerated the search for alternative effective therapies, including hormonal therapy and targeted agents.7-9
For several years, similarities between luminal breast cancer and LGSC have been recognized. A high proportion of LGSCs have estrogen receptor (ER) and progesterone receptor (PR) expression, and hormonal therapy seems to provide clinical benefit in > 70% of relapses.7,10,11 In addition, in both tumor types, women ≤ 35 years of age have worse outcomes compared with older women.5
On the basis of our clinical experience, LGSC does not seem to be completely resistant to platinum-based chemotherapy. Furthermore, in the absence of prospective data indicating otherwise, we continue to recommend platinum plus taxane chemotherapy to women with newly diagnosed stage II to IV LGSC after primary cytoreductive surgery. Others have begun to abandon postoperative chemotherapy in favor of hormonal therapy despite lack of data from prospective clinical trials. Since the early 1980s, several patients seen at our institution have received hormonal therapy after completion of primary platinum-based chemotherapy. The purpose of this study is to examine outcomes associated with hormonal maintenance therapy (HMT) compared with routine observation (OBS) after primary cytoreductive surgery and platinum-based chemotherapy in women with stage II to IV LGSC.
PATIENTS AND METHODS
Our institutional review board approved the Low-Grade Serous Tumor Database, a longitudinal database established in 2007. Data are collected both retrospectively and prospectively. A waiver of informed consent was granted for patients not seen at our institution for ≥ 1 year. All others provided written informed consent. Eligibility criteria for this study were: treatment with primary cytoreductive surgery followed by platinum-based chemotherapy, pathologically confirmed stage II to IV LGSC of the ovary or peritoneum, OBS or HMT within 3 months after completion of postoperative chemotherapy, ≥ 2 years of follow-up for patients who had not experienced recurrence, and adequate clinical information. The choice of OBS versus HMT was determined by attending physician discretion and patient consent.
We identified 544 potentially eligible patients. We excluded 341 because of: incomplete clinical information (no confirmation of pathologic diagnosis of LGSC and/or lack of clinical information regarding date of disease progression; n = 81), disease progression during primary chemotherapy or change in chemotherapy (n = 76), neoadjuvant chemotherapy (n = 51), treatment in a clinical trial (n = 26), non–platinum-based or no chemotherapy (n = 23), wrong histology or stage (n = 39), hormonal therapy started > 3 months after completion of primary chemotherapy (n = 16), insufficient follow-up time (n = 10), or miscellaneous reasons (eg, synchronous primary cancer, radiotherapy; n = 19). Therefore, 203 patients constituted the study cohort.
Pathology slides were reviewed by MD Anderson gynecologic pathologists and documented as LGSC of the ovary or peritoneum. Criteria for diagnosis of LGSC have been previously reported.4,12 Information on ER and PR expression by immunohistochemical staining of tumor tissue was captured whenever available from pathology documents.
Database elements included: demographics, primary surgery, International Federation of Gynecology and Obstetrics stage, perioperative studies, systemic therapies, disease status at completion of primary treatment, OBS versus HMT after primary chemotherapy, date of initial disease progression, disease status at last contact, and date of last contact or death.
Statistical analyses were performed using IBM SPSS Statistics (version 21; Armonk, NY). Progression-free survival (PFS) was calculated from date of primary surgery to date of disease progression or death, whichever occurred first. Disease progression was determined by serial imaging examinations and/or doubling of nadir serum cancer antigen 125. Overall survival (OS) was calculated from date of primary surgery to date of last contact or death resulting from any cause. Cumulative distributions of OS and PFS were estimated using the Kaplan-Meier method.13 The log-rank test was used to compare differences between survival curves. Cox proportional hazards regression was used to model the association between key variables and PFS and OS. The proportional hazards assumption was checked for all models. Variables with P values < .25 on univariable analysis were included in the multivariable models. All P values were two sided. P values < .05 were considered statistically significant.
RESULTS
Between 1981 and 2013, 203 patients met eligibility criteria. After primary cytoreductive surgery and platinum-based chemotherapy, 133 patients underwent OBS, and 70 received HMT. Demographic and clinical characteristics are listed in Table 1. Most patients were white, had ovarian LGSC, and had stage III disease. No significant differences between groups were found for most categories. A significantly higher proportion of women in the HMT group had LGSC after original diagnosis of serous LMP tumor (17.1% v 6%) and persistent tumor at completion of primary chemotherapy (60.0% v 8.3%).
Table 1.
Patient Demographic and Clinical Characteristics (N = 203)
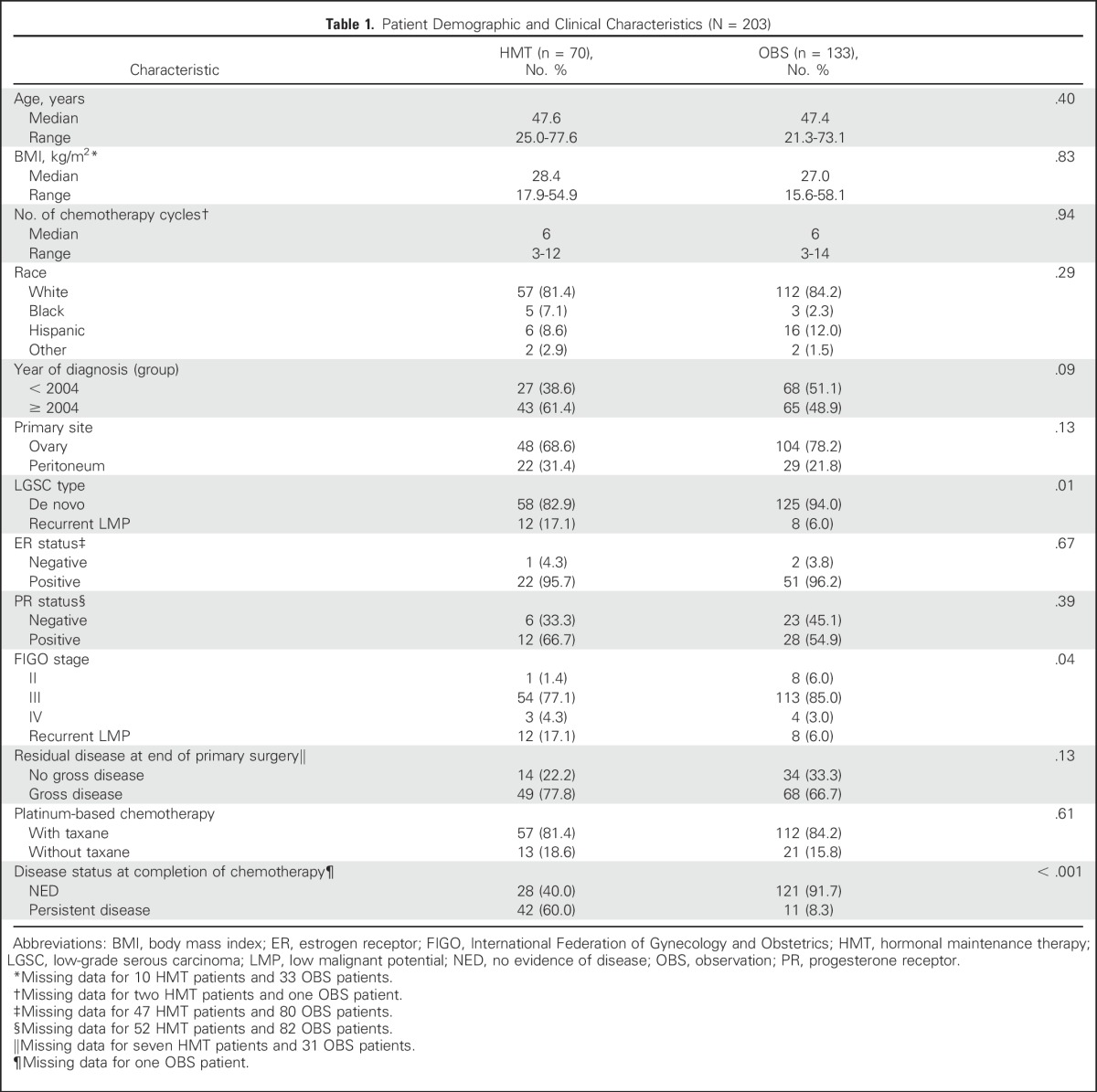
Of the 70 patients who received HMT, 38 (54.3%) received letrozole, two (2.9%) anastrozole, 20 (28.6%) tamoxifen, five (7.1%) leuprolide acetate, two each leuprolide acetate with either letrozole (2.9%) or tamoxifen (2.9%), and one (1.4%) depot medroxyprogesterone acetate. Nine women—five who received letrozole and four who received tamoxifen—required a change in therapy because of adverse effects. Median duration of HMT was 33.3 months (range, 1 to 223.2 months).
Median follow-up time for the entire group was 70.8 months, with a median of 80.3 months for patients undergoing OBS and 54.9 months for those receiving HMT. Of the 160 patients who had experienced recurrence at the time of analysis, imaging studies before the date of disease progression were available in 112 (70%). Median interval between these two time points was 5.5 months for the OBS group and 5.9 months for the HMT group (P = .85).
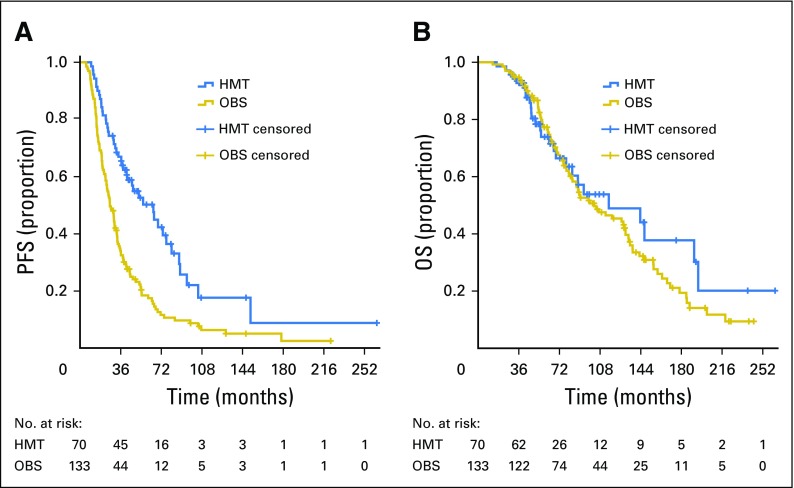
Median PFS for the entire group was 32.6 months (95% CI, 28.4 to 36.9). Median PFS for the OBS group was 26.4 months (95% CI, 21.8 to 31.0), compared with 64.9 months (95% CI, 43.5 to 86.3) for the HMT group (P < .001; Fig 1A). Median OS for the entire group was 104.7 months (95% CI, 75.1 to 134.3). No statistically significant difference in OS was observed between the OBS and HMT groups (102.7 v 115.7 months; P = .42; Fig 1B). Within the OBS group, there was no significant difference in median OS between women who eventually received hormonal therapy for progression or recurrence and those who never received hormonal therapy (106.8 v 102.7 months; P = .37).
Fig 1.
(A) Progression-free survival (PFS; P < .001) and (B) overall survival (OS; P = .42) for the overall study population. HMT, hormonal maintenance therapy; OBS, observation.
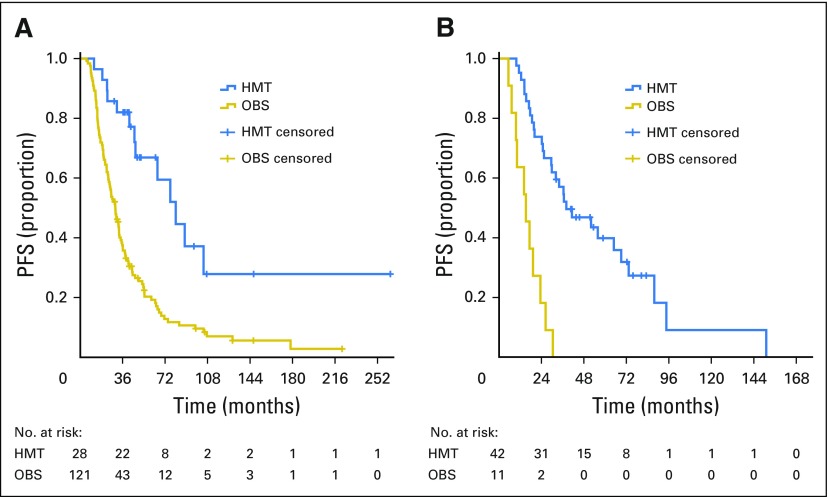
In women who were clinically disease free at the completion of platinum-based chemotherapy, median PFS was 30.0 months (95% CI, 24.9 to 35.1) for the OBS group and 81.1 months (95% CI, 54.9 to 107.2) for the HMT group (Fig 2A). In women with persistent disease at completion of chemotherapy, median PFS was 15.2 months (95% CI, 7.6 to 22.9) for the OBS group and 38.1 months (95% CI, 17.8 to 58.4) for the HMT group (Fig 2B). A stratified log-rank test comparing HMT and OBS groups adjusted for disease status resulted in P < .001.
Fig 2.
Progression-free survival (PFS) for patients who had (A) no evidence of disease and (B) persistent disease at completion of primary chemotherapy; stratified log-rank test by disease status, P < .001. HMT, hormonal maintenance therapy; OBS, observation.
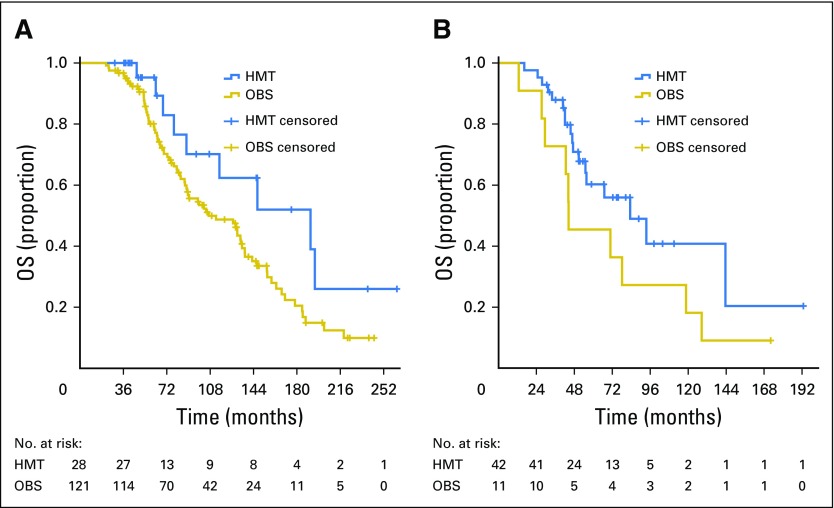
In women who were clinically disease free at the end of primary chemotherapy, median OS was 191.3 months (95% CI, 93.5 to 289.0) for the HMT group and 106.8 months (95% CI, 70.2 to 143.4) for the OBS group (Fig 3A). In women who had persistent disease, median OS was 83.3 months (95% CI, 48.2 to 118.4) for the HMT group and 44.4 months (95% CI, 13.9 to 74.8) for the OBS group (Fig 3B). A stratified log-rank test adjusted for disease status resulted in P = .014.
Fig 3.
Overall survival (OS) for patients who had (A) no evidence of disease and (B) persistent disease at completion of primary chemotherapy; stratified log-rank test by disease status, P = .014. HMT, hormonal maintenance therapy; OBS, observation.
Information on ER and/or PR expression was available in 76 and 69 patients, respectively (Table 1). ER and PR expression was present in 96% and 58% of patients, respectively. When analyzed by ER or PR expression status, patients receiving HMT had better outcomes. Among ER-positive patients, those receiving HMT had longer median PFS than those undergoing OBS (73.3 months; 95% CI, 61.5 to 85.2 v 29.9 months; 95% CI, 22.6 to 37.1; P = .001). Median OS for patients receiving HMT was longer than that in patients undergoing OBS, but this did not reach statistical significance (191.3 months; 95% CI, 107.4 to 275.1 v 127.0 months; 95% CI, 77.2 to 176.8, respectively; P = .056). For PR-positive patients, median PFS for those receiving HMT was longer than that for patients undergoing OBS (73.3 months; 95% CI, 59.0 to 87.7 v 22.6 months; 95% CI, 9.0 to 36.1, respectively; P = .001). For PR-negative patients, those receiving HMT had better PFS than those undergoing OBS (69.1 months; 95% CI, 5.1 to 133.1 v 29.9 months; 95% CI, 25.5 to 34.2, respectively; P = .04). There were no differences in median OS for PR-positive patients. When ER-positive/PR-positive patients were compared with ER-positive/PR-negative patients, there were no significant differences in median PFS or OS.
Tables 2 and 3 list the univariable and multivariable Cox proportional hazards regression results for PFS and OS, respectively. Variables with P < .25 on univariable analysis for PFS included year of diagnosis, age, group, stage, and site. After adjusting for these in a multivariable setting, age, group, stage, and site remained significantly associated with disease progression. Compared with women age ≤ 35 years of age, older women had a significantly lower likelihood of experiencing progression (hazard ratio [HR], 0.66; 95% CI, 0.44 to 0.98; P = .04). Women who received HMT had a significantly lower risk of progression compared with women who underwent OBS (HR, 0.44; 95% CI, 0.31 to 0.64; P < .001). Women with stage II or III disease or those with recurrent LMP tumors had a significantly lower risk of progression compared with women with stage IV disease. Women with a primary peritoneal tumor had a significantly lower risk of progression compared with those with an ovarian primary tumor (HR, 0.57; 95% CI, 0.38 to 0.86; P = .007). ER and PR status were excluded from the Cox regression analysis because of the large number of patient cases with missing data.
Table 2.
Univariable and Multivariable Cox Proportional Hazards for PFS

Table 3.
Univariable and Multivariable Cox Proportional Hazards for OS
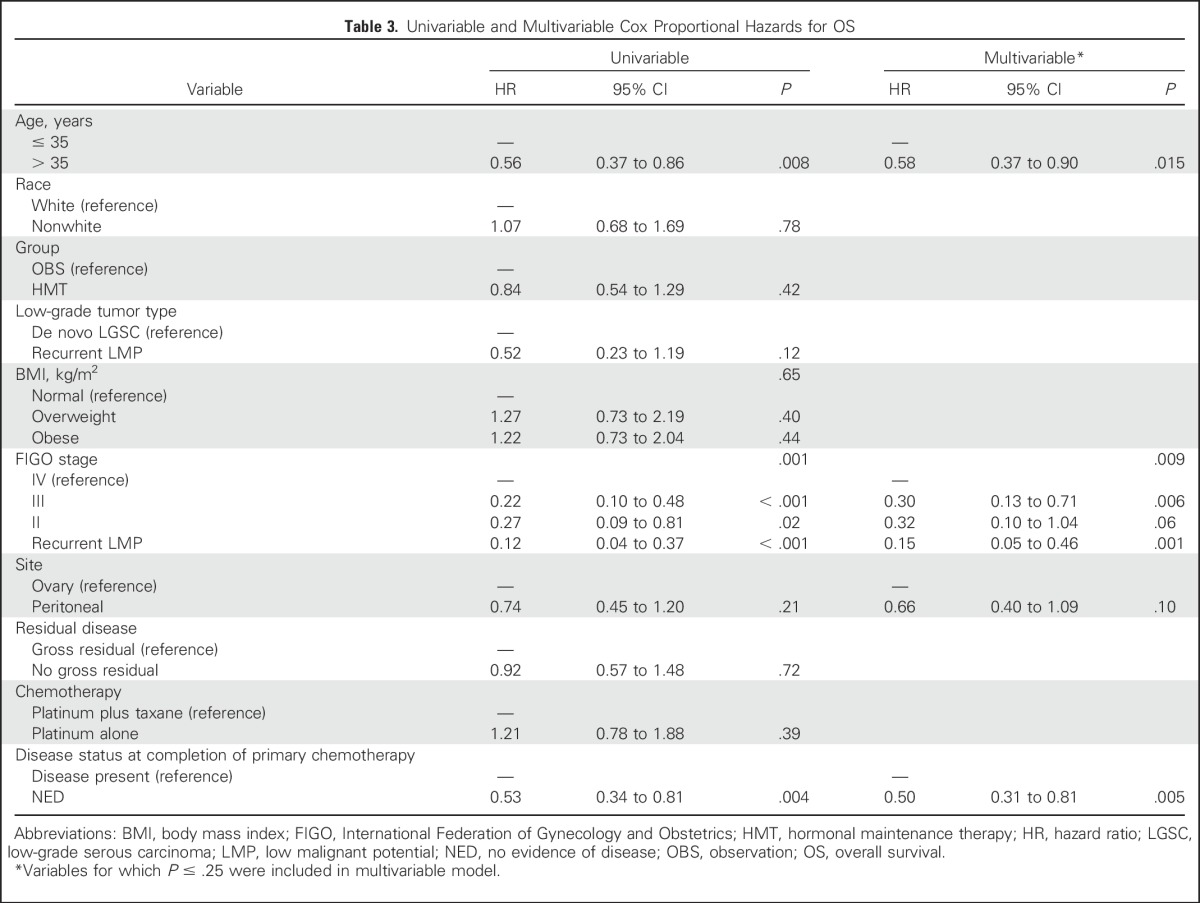
After including age, stage, site, and disease status at completion of primary chemotherapy in the multivariable Cox proportional hazards regression for OS, a lower risk of dying was associated with age > 35 years (HR, 0.58; 95% CI, 0.37 to 0.90; P = .015), stage II or III disease or LGSC resulting from a recurrent LMP tumor, and being disease free at completion of primary chemotherapy (HR, 0.50; 95% CI, 0.31 to 0.81; P = .005).
DISCUSSION
The principal findings of our study indicate that women with stage II to IV LGSC who received HMT after primary treatment had significantly longer PFS than women who underwent OBS. Median PFS was superior for the HMT group compared with the OBS group not only in the entire study cohort but also in the two disease status subgroups: women who were clinically disease free at completion of primary chemotherapy and women with persistent disease. Although the survival outcomes between the HMT and OBS groups were not statistically significant in the overall study population (P = .42), after adjusting for disease status, median OS for the HMT group was significantly longer than that for the OBS group (P = .014).
By multivariable analysis, a lower likelihood of disease progression was significantly associated with age > 35 years, HMT, stage, and primary peritoneal tumor. Factors significantly associated with lower risk of dying included age > 35 years, stage, and being disease free at completion of primary chemotherapy.
Over the past decade, a series of publications from our group has indicated that LGSC is less responsive to conventional chemotherapy than HGSC.1-6 These reports documented > 40% frequency of persistent disease at completion of primary platinum-based chemotherapy in women with stage II to IV LGSC of the ovary or peritoneum1,4,5 and response rates of < 5% in both the neoadjuvant2 and salvage chemotherapy settings.3 Recently, Grabowski et al,6 reviewing more than 5,000 patients accrued to four phase III trials in the Arbeitsgemeinschaft Gynaekologische Onkologie metadatabase, found that the response rate to chemotherapy in suboptimally debulked LGSC was significantly lower than that in suboptimally debulked HGSC (23% v 90%, respectively).
Some have interpreted the reports of the last decade to conclude that platinum-based chemotherapy is of no benefit in first-line treatment of LGSC and should be abandoned. In our view, that perspective is premature based on available data. Although LGSC is indolent and not as chemotherapy sensitive as HGSC, it is not entirely chemotherapy resistant. Responses are observed in some women, and although response may not be documented based on standard imaging criteria, serum cancer antigen 125 declines significantly in a sizable proportion of patients.2,3,14 Furthermore, a high proportion of women have stable disease for a period of time.2,3 The dilemma associated with interpretation of stable disease is determining whether it is a result of the indolent nature of LGSC or reflects a beneficial effect of therapy. Given the absence of contradictory evidence from prospective trials, platinum plus taxane chemotherapy remains the standard postoperative treatment for women with newly diagnosed stage II to IV LGSC. However, the reality that LGSC is relatively chemotherapy resistant has clearly stimulated the search for alternative therapies.
One such alternative therapeutic strategy is hormonal therapy. Since the 1980s, we have known that a variety of hormonal therapies may have modest efficacy in epithelial ovarian cancer.15-27 However, epithelial ovarian cancer is not a single disease but rather several different cancers. For the past several years, our clinical observations indicate that well-differentiated ovarian cancers seem to respond to hormonal therapies more commonly than poorly differentiated ovarian cancers, especially for grade 1 (now classified as low grade) serous and grade 1 endometrioid carcinomas.
Although current evidence from clinical practice provides clues in predicting which ovarian cancers are more likely to respond to hormonal therapy, there are no definitive answers based on systematic clinical investigations. Factors that have been studied to varying degrees include ER status, histologic subtype, and histologic grade. ER expression varies depending on histologic subtype. Lindgren et al28 observed ERα expression in serous and endometrioid carcinomas but not in mucinous carcinomas. Fujimura et al29 reported the frequency of ERα and ERβ in different histologic subtypes: clear cell (0% and 39.3%, respectively), serous (97.2% and 41.7%, respectively), mucinous (70% and 30%, respectively), and endometrioid (100% and 75%, respectively). In addition, an Ovarian Tumor Tissue Analysis Consortium study found higher frequencies of ER expression in LGSC (87% of 104), HGSC (81% of 1,691), and endometrioid carcinoma (77% of 475), compared with clear cell (20% of 381) and mucinous carcinomas (21% of 197).30
In studies that directly compared ER expression based on histologic grade of serous carcinoma, Wong et al10 observed a significantly higher rate of ER expression in LGSC (58% of 43) than in HGSC (27% of 48; P = .003). Using different immunohistochemical platforms and cutoff scores, Escobar et al11 reported higher frequencies of ER expression than Wong et al in LGSC (96%) and HGSC (83%). Of several methods used to measure ER expression, only the Dako platform (Agilent, Santa Clara, CA) revealed a significant difference between LGSC and HGSC.
Several studies have demonstrated a correlation between the presence of ER and response to hormonal therapy in ovarian cancer of all subtypes, whereas others have suggested the absence of ER is associated with negligible response rates.22,24,27,31,32 However, to determine the true effectiveness of hormonal therapy, subtype-specific trials for women with LGSC are clearly needed. In our report of hormonal therapy in recurrent LGSC, we detailed the clinical course of 64 women who received 89 separate regimens.7 Overall response rate was 9%, with an additional 62% of patient regimens resulting in stable disease, for an overall clinical benefit rate of 71%. Additionally, median PFS was 7.4 months. Of 50 patients with available ER and PR expression data, patients with ER-positive/PR-positive tumors had a longer median time to progression than those with ER-positive/PR-negative tumors. The difference approached but did not reach statistical significance. In the current study, information on ER expression was available in 76 (37%) of 203 patients, and only three patients were ER negative. In all subgroup analyses (ER-positive, PR-positive, and PR-negative patients), the HMT group had better outcomes than the OBS group. None of the findings suggested which patients experienced greater benefit from HMT. Because only three of 76 patients with available data were ER negative, meaningful comparisons were not possible.
Increasingly, LGSC seems to bear a striking resemblance to ER-positive breast cancer. In addition to ER expression, similar characteristics include response to anti-estrogen therapy and the observation that women age ≤ 35 years have significantly worse prognosis than older women.5,7 Another common trait is that ER-positive breast cancer seems to be less chemotherapy sensitive than ER-negative breast cancer.33,34
The strategy of administering HMT after completion of primary platinum-based chemotherapy evolved at our institution over several years and was primarily based on two observations: the relative chemotherapy resistance of LGSC and the clinical benefit of anti-estrogen therapy in some women with recurrent LGSC. Interestingly, this development has essentially mirrored the standard treatment of high-risk, ER-positive breast cancer.35
Although a majority of patients in our HMT cohort received aromatase inhibitors, our study does not provide major clues regarding specific optimal endocrine therapies. Even in ER-positive breast cancer, no single drug is considered to be the best option.
A study such as ours has several known limitations, including its retrospective nature, long study period, incomplete data, potential referral bias, heterogeneous therapies, and varying follow-up practice patterns. Nevertheless, observational studies often yield valid results.36,37 In addition, we found no significant differences in the interval between prior imaging and diagnosis of disease progression between the two groups.
As in any retrospective study, selection bias was a concern. We sought to analyze a homogeneous cohort of women who underwent primary treatment for this rare ovarian cancer subtype, after which their clinical management diverged to HMT or OBS. We wanted to explore whether HMT had any significant impact on clinical outcome. Although women who received neoadjuvant chemotherapy were identified in our database, this group was excluded from our study because these patients seem to have a different clinical course. In our prior study of 25 women with LGSC of the ovary or peritoneum treated with neoadjuvant chemotherapy, median OS was 56.1 months, which is approximately half that of women who undergo primary cytoreductive surgery followed by platinum-based chemotherapy.2 For women who received neoadjuvant chemotherapy and were excluded from our study, median PFS was 28.9 months, and for the subgroup who received HMT, median PFS was 28.9 months, compared with 24.3 months for the OBS subgroup (P = .48). In addition, their median OS was only 59.8 months. The contrast in PFS and OS between patients who received neoadjuvant chemotherapy and those who underwent primary surgery followed by chemotherapy is notable and provides a rationale for not combining these clinically distinct subgroups.
We also excluded patients who experienced disease progression during primary chemotherapy because, by definition, they never had the opportunity to receive HMT or undergo OBS before progression. Median OS for this group was 42.5 months, which was to be expected in this poor-prognosis group. In addition, median OS for those patients within this cohort who subsequently received hormonal therapy for progression or recurrence was 48.6 months, compared with 26.0 months for those who never received hormonal therapy for progression or recurrence.
In summary, women with stage II to IV LGSC of the ovary or peritoneum who received HMT after primary chemotherapy had significantly longer PFS than women who underwent OBS. The findings of this hypothesis-generating study are potentially practice changing and warrant further investigation using a prospective trial design. A phase III randomized trial is currently under development to compare platinum-based chemotherapy followed by placebo versus platinum-based chemotherapy followed by HMT versus hormonal therapy alone for women with stage II to IV LGSC of the ovary or peritoneum after primary cytoreductive surgery. On the basis of our previous experience, women who undergo neoadjuvant chemotherapy will be ineligible for this trial. However, patients who develop progressive disease during primary treatment will be included in an intent-to-treat analysis.
Footnotes
Supported in part by the Sara Brown Musselman Fund for Serous Ovarian Cancer Research and the MD Anderson Cancer Center Support Grant from the National Cancer Institute, Grant No. P30 CA016672.
Presented at the 52nd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 3-7, 2016.
AUTHOR CONTRIBUTIONS
Conception and design: David M. Gershenson, Charlotte C. Sun
Collection and assembly of data: David M. Gershenson, Anais Malpica, Charlotte C. Sun
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Hormonal Maintenance Therapy for Women With Low-Grade Serous Cancer of the Ovary or Peritoneum
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
David M. Gershenson
Stock or Other Ownership: Johnson & Johnson, Pfizer, Biogen Idec, Celgene, AbbVie, GlaxoSmithKline, Merck
Consulting or Advisory Role: Clovis Oncology
Patents, Royalties, Other Intellectual Property: Royalties from Elsevier; royalties from UpToDate
Diane C. Bodurka
No relationship to disclose
Robert L. Coleman
Honoraria: National Comprehensive Cancer Network
Consulting or Advisory Role: Clovis Oncology, Genentech, Esperance Pharmaceuticals, National Comprehensive Cancer Network, Department of Defense Congressionally Directed Medical Research Program
Research Funding: AstraZeneca, Esperance Pharmaceuticals, OncoMed, Array BioPharma, Clovis Oncology, Amgen, Johnson & Johnson, Merck
Travel, Accommodations, Expenses: Millennium Pharmaceuticals, Merck, Amgen, AstraZeneca, Array BioPharma, Gradalis, Bayer HealthCare Pharmaceuticals, Clovis Oncology, Genentech, Research to Practice, University of California Irvine, New Mexico Cancer Center, University of Miami, University of Cincinnati Cancer Center
Karen H. Lu
No relationship to disclose
Anais Malpica
Honoraria: Oakstone Publishing, Array BioPharma, Wolters Kluwer
Charlotte C. Sun
Research Funding: AstraZeneca
REFERENCES
- 1.Gershenson DM, Sun CC, Lu KH, et al. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet Gynecol. 2006;108:361–368. doi: 10.1097/01.AOG.0000227787.24587.d1. [DOI] [PubMed] [Google Scholar]
- 2.Schmeler KM, Sun CC, Bodurka DC, et al. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2008;108:510–514. doi: 10.1016/j.ygyno.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Gershenson DM, Sun CC, Bodurka D, et al. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol Oncol. 2009;114:48–52. doi: 10.1016/j.ygyno.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Schmeler KM, Sun CC, Malpica A, et al. Low-grade serous primary peritoneal carcinoma. Gynecol Oncol. 2011;121:482–486. doi: 10.1016/j.ygyno.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 5.Gershenson DM, Bodurka DC, Lu KH, et al. Impact of age and primary disease site on outcome in women with low-grade serous carcinoma of the ovary or peritoneum: Results of a large single-institution registry of a rare tumor. J Clin Oncol. 2015;33:2675–2682. doi: 10.1200/JCO.2015.61.0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grabowski JP, Harter P, Heitz F, et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer: An analysis of the AGO Study Group metadatabase. Gynecol Oncol. 2016;140:457–462. doi: 10.1016/j.ygyno.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Gershenson DM, Sun CC, Iyer RB, et al. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2012;125:661–666. doi: 10.1016/j.ygyno.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grisham RN, Iyer G, Sala E, et al. Bevacizumab shows activity in patients with low-grade serous ovarian and primary peritoneal cancer. Int J Gynecol Cancer. 2014;24:1010–1014. doi: 10.1097/IGC.0000000000000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farley J, Brady WE, Vathipadiekal V, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: An open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14:134–140. doi: 10.1016/S1470-2045(12)70572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong KK, Lu KH, Malpica A, et al. Significantly greater expression of ER, PR, and ECAD in advanced-stage low-grade ovarian serous carcinoma as revealed by immunohistochemical analysis. Int J Gynecol Pathol. 2007;26:404–409. doi: 10.1097/pgp.0b013e31803025cd. [DOI] [PubMed] [Google Scholar]
- 11.Escobar J, Klimowicz AC, Dean M, et al. Quantification of ER/PR expression in ovarian low-grade serous carcinoma. Gynecol Oncol. 2013;128:371–376. doi: 10.1016/j.ygyno.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Fader AN, Java J, Krivak TC, et al. The prognostic significance of pre- and post-treatment CA-125 in grade 1 serous ovarian carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2014;132:560–565. doi: 10.1016/j.ygyno.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangioni C, Franceschi S, La Vecchia C, et al. High-dose medroxyprogesterone acetate (MPA) in advanced epithelial ovarian cancer resistant to first- or second-line chemotherapy. Gynecol Oncol. 1981;12:314–318. doi: 10.1016/0090-8258(81)90131-1. [DOI] [PubMed] [Google Scholar]
- 16.Tropé C, Johnsson JE, Sigurdsson K, et al. High-dose medroxyprogesterone acetate for the treatment of advanced ovarian carcinoma. Cancer Treat Rep. 1982;66:1441–1443. [PubMed] [Google Scholar]
- 17.Shirey DR, Kavanagh JJ, Jr., Gershenson DM, et al. Tamoxifen therapy of epithelial ovarian cancer. Obstet Gynecol. 1985;66:575–578. [PubMed] [Google Scholar]
- 18.Hatch KD, Beecham JB, Blessing JA, et al. Responsiveness of patients with advanced ovarian carcinoma to tamoxifen: A Gynecologic Oncology Group study of second-line therapy in 105 patients. Cancer. 1991;68:269–271. doi: 10.1002/1097-0142(19910715)68:2<269::aid-cncr2820680209>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Kavanagh JJ, Roberts W, Townsend P, et al. Leuprolide acetate in the treatment of refractory or persistent epithelial ovarian cancer. J Clin Oncol. 1989;7:115–118. doi: 10.1200/JCO.1989.7.1.115. [DOI] [PubMed] [Google Scholar]
- 20.Fromm GL, Freedman RS, Fritsche HA, et al. Sequentially administered ethinyl estradiol and medroxyprogesterone acetate in the treatment of refractory epithelial ovarian carcinoma in patients with positive estrogen receptors. Cancer. 1991;68:1885–1889. doi: 10.1002/1097-0142(19911101)68:9<1885::aid-cncr2820680906>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 21.Williams CJ. Tamoxifen for relapse of ovarian cancer. Cochrane Database Syst Rev. 2001;(1):CD001034. doi: 10.1002/14651858.CD001034. [DOI] [PubMed] [Google Scholar]
- 22.Bowman A, Gabra H, Langdon SP, et al. CA125 response is associated with estrogen receptor expression in a phase II trial of letrozole in ovarian cancer: Identification of an endocrine-sensitive subgroup. Clin Cancer Res. 2002;8:2233–2239. [PubMed] [Google Scholar]
- 23.Papadimitriou CA, Markaki S, Siapkaras J, et al. Hormonal therapy with letrozole for relapsed epithelial ovarian cancer: Long-term results of a phase II study. Oncology. 2004;66:112–117. doi: 10.1159/000077436. [DOI] [PubMed] [Google Scholar]
- 24.Smyth JF, Gourley C, Walker G, et al. Antiestrogen therapy is active in selected ovarian cancer cases: The use of letrozole in estrogen receptor-positive patients. Clin Cancer Res. 2007;13:3617–3622. doi: 10.1158/1078-0432.CCR-06-2878. [DOI] [PubMed] [Google Scholar]
- 25.Argenta PA, Thomas SG, Judson PL, et al. A phase II study of fulvestrant in the treatment of multiply-recurrent epithelial ovarian cancer. Gynecol Oncol. 2009;113:205–209. doi: 10.1016/j.ygyno.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Zheng H, Kavanagh JJ, Hu W, et al. Hormonal therapy in ovarian cancer. Int J Gynecol Cancer. 2007;17:325–338. doi: 10.1111/j.1525-1438.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- 27.Argenta PA, Um I, Kay C, et al. Predicting response to the anti-estrogen fulvestrant in recurrent ovarian cancer. Gynecol Oncol. 2013;131:368–373. doi: 10.1016/j.ygyno.2013.07.099. [DOI] [PubMed] [Google Scholar]
- 28.Lindgren PR, Cajander S, Bäckström T, et al. Estrogen and progesterone receptors in ovarian epithelial tumors. Mol Cell Endocrinol. 2004;221:97–104. doi: 10.1016/j.mce.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Fujimura M, Hidaka T, Kataoka K, et al. Absence of estrogen receptor-alpha expression in human ovarian clear cell adenocarcinoma compared with ovarian serous, endometrioid, and mucinous adenocarcinoma. Am J Surg Pathol. 2001;25:667–672. doi: 10.1097/00000478-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Sieh W, Köbel M, Longacre TA, et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis Consortium study. Lancet Oncol. 2013;14:853–862. doi: 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langdon SP, Hirst GL, Miller EP, et al. The regulation of growth and protein expression by estrogen in vitro: A study of 8 human ovarian carcinoma cell lines. J Steroid Biochem Mol Biol. 1994;50:131–135. doi: 10.1016/0960-0760(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 32.Langdon SP, Hawkes MM, Lawrie SS, et al. Oestrogen receptor expression and the effects of oestrogen and tamoxifen on the growth of human ovarian carcinoma cell lines. Br J Cancer. 1990;62:213–216. doi: 10.1038/bjc.1990.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17:460–469. doi: 10.1200/JCO.1999.17.2.460. [DOI] [PubMed] [Google Scholar]
- 34.Earl H, Provenzano E, Abraham J, et al. Neoadjuvant trials in early breast cancer: Pathological response at surgery and correlation to longer term outcomes—What does it all mean? BMC Med. 2015;13:234–244. doi: 10.1186/s12916-015-0472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stockler M, Wilcken NR, Ghersi D, et al. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer. Cancer Treat Rev. 2000;26:151–168. doi: 10.1053/ctrv.1999.0161. [DOI] [PubMed] [Google Scholar]
- 36.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878–1886. doi: 10.1056/NEJM200006223422506. [DOI] [PubMed] [Google Scholar]
- 37.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]