Abstract
Obesity-related disease is a significant source of premature death and economic burden globally. It is also a common comorbidity in patients suffering from lung disease, affecting both severity and treatment success. However, this complex association between obesity and the lung is poorly understood. Autophagy is a self-recycling homeostatic process that has been linked to beneficial or deleterious effects, depending on the specific lung disease. Obesity affects autophagy in a tissue-specific manner, activating autophagy in adipocytes and impairing autophagy in hepatocytes, immune cells, and pancreatic β-cells, among others. Obesity is also characterized by chronic low-grade inflammation that can be modulated by the pro- and antiinflammatory effects of the autophagic machinery. Scant evidence exists regarding the impact of autophagy in obesity-related lung diseases, but there are communal pathways that could be related to disease pathogenesis. Important signaling molecules in obesity, including IL-17, leptin, adiponectin, NLRP3 inflammasome, and TLR-4, have been implicated in the pathogenesis of lung disease. These mediators are known to be modulated by autophagy activity. In this perspective, we highlight the recent advances in the understanding of autophagy in obesity-related conditions, as well as the potential mechanisms that can link autophagy and obesity in the pathogenesis of lung disease.
Keywords: autophagy, obesity, lung disease, inflammation, lipid metabolism
Clinical Relevance
Autophagy is a well-known important factor in lung disease pathogenesis, but its relationship with obesity in lung diseases has not been studied. With this perspective, we review what is known to date and propose future directions in this field.
Obesity is the result of an imbalance between energy intake and energy output and has reached pandemic level in developed countries. According to the World Health Organization, the prevalence of obesity has doubled since 1980, with 13% of the adult population being obese (1). Obesity accounts for 5.5–6.8% of the health budget in the United States, and obesity-related disease and organ dysfunction is a significant source of premature death and life years lost relative to life expectancy (2). In addition to well-known associated disorders such as type 2 diabetes mellitus, hepatic steatosis, and atherosclerosis, obesity has been implicated as a modulating factor in a variety of other human diseases. Obesity has been linked to increased incidence of colon, prostate, and hematologic malignancies (3), and worsening autoimmune conditions (4), but it may be protective in the critically ill and in vascular disorders such as postmyocardial infarction (5) or stroke (6).
Obesity-associated lung disease presents unique phenotypes among different lung pathologies, affecting both the severity of disease and its response to treatment. Obstructive sleep apnea (OSA) and obesity hypoventilation syndrome are the two diseases most intimately associated with obesity (7, 8). However, obesity has also been linked to several other lung pathologies, such as asthma, chronic obstructive pulmonary disease (COPD), and pulmonary fibrosis. Obese patients have a 50% higher incidence of asthma compared with nonobese patients (9). These patients tend to have a neutrophilic and Th17-driven inflammatory response that is less responsive to treatment with glucocorticoids (10–12). Patients with idiopathic pulmonary fibrosis (IPF) and obesity have higher waitlist mortality and 90-day post-transplant mortality (13, 14). Obese patients with acute respiratory distress syndrome have longer duration of mechanical ventilation and intensive care unit stays but have mortality rates similar to those of nonobese patients (15). In contrast, patients with COPD and obesity have lower in-hospital, in-hospital mortality, and readmission rates (16, 17).
This complex association between adipose tissue and the lung is poorly understood. Recently, autophagy, the highly conserved degradation of intracellular organelles and proteins, has been shown to play an important role in both obesity and pulmonary disease pathogenesis. This perspective review focuses on the functional role of autophagy-obesity–related pulmonary disease states.
Autophagy
Autophagy
Autophagy is a vital cellular process that degrades and recycles intracellular components through lysosomal degradation (18). Cytosolic material such as damaged organelles, lipid droplets (LDs), foreign pathogens, or unwanted cytosolic proteins are enveloped in double-membrane autophagosomes that fuse with lysosomes for degradation. This degradative process is coupled with conserving energy and key nutrients for cellular homeostasis and function, which confers a prosurvival effect. Autophagy can be regulated by numerous pathologic conditions such as infection, environmental stress, malignancy, as well as by metabolic derangement including starvation and obesity (19). There are unique autophagy pathways that involve receptors that confer selectivity to recognize ubiquitinated-tagged cargo through ubiquitin-binding domains and link them to double-membrane autophagosomes through light chain 3 (LC3) interacting regions (20).
Selective Autophagy
Selective autophagy denotes the removal or degradation of specific cellular organelles or components such as stressed endoplasmic reticulum (ER) (ER-phagy) (21–24), mitochondria (mitophagy) (25–28), LDs (lipophagy) (29–31), aggregated misfolded proteins (aggrephagy) (32–35), and microbes (xenophagy) (36–39). Furthermore, polyubiquitin chains are generated on the outer mitochondrial membrane during mitochondrial stress (40). Recruitment of additional proteins such as PINK1 and Parkin contribute to the generation of autophagosome-mediated degradation of mitochondria, leading to mitophagy (25). Impairment in mitophagy can lead to the accumulation of damaged mitochondria and to increased production of reactive oxygen species to propagate further cell damage (41). Lipid stores can also be used through autophagy to release free fatty acids (FFAs) for β-oxidation and energy production (42). Autophagy contributes to LD and triglyceride (TG) breakdown through either engulfing small LDs into autophagosomes or pinching a small portion of a bigger LD. In hepatocytes, autophagy blockade led to the accumulation of triglycerides and LDs that were colocalized with autophagic-associated proteins and compartments (29). Autophagy participates in the regulation of innate and adaptive immunity, playing a crucial role in the resistance to bacterial, viral, and parasitic infections (43). Autophagy can participate in regulating inflammatory signaling in immune cells. Autophagy-deficient macrophages have increased IL-1β production after endotoxin stimulation (44). Autophagy-associated antiinflammatory properties (43) have a broad range of influence, exerting regulation over inflammasome activation (44), IFN response (45), nuclear factor (NF)-κB signaling (46), lymphocyte development and function (47), and the production of IL-1α, IL-1β, and IL-18 (48–51).
Molecular Mechanism of Autophagy
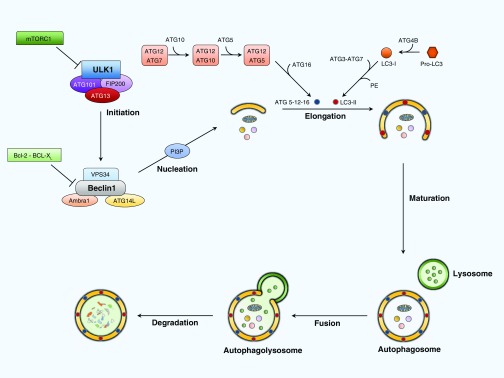
Numerous environmental factors regulate the activation or inhibition of autophagy. The mammalian target of rapamycin (mTOR) negatively regulates autophagy when there is an abundance of nutrients or growth factors (52). During starvation, AMP-activated protein kinase inhibits mTOR and activates uncoordinated-51–like protein kinase initiation complex (53), which enhances the activity of the Beclin 1 interacting complex that consists of Beclin 1 (BCL2 family proteins), VPS34 (a class III phosphatidylinositol-3 kinase), and ATG14L, leading to nucleation and formation of the autophagosome by increasing PI3P levels. The elongation of the autophagosome membrane requires two ubiquitin-like conjugation systems. The first is the ATG5–ATG12 complex, which is conjugated by ATG7 and ATG10 enzymes. The second requires ubiquitin-like protein microtubule–associated protein 1 LC3, also called ATG8, which is cleaved by ATG4B into LC3B-I and then converted to LC3B-II when conjugated with phosphatidylethanolamine by ATG3 and ATG7 (19). Conversion of LC3-I to LC3-II is a classic hallmark of autophagosome formation. Once the autophagosome is complete, it fuses with lysosomes to form autophagolysomes for content degradation (Figure 1) (53).
Figure 1.
Molecular mechanism of autophagy. Environmental signals modulate mammalian target of rapamycin (mTOR) complex 1 (mTORC1), negatively regulating autophagy by inhibiting the uncoordinated-51–like kinase 1 (ULK1) complex consisting of ULK1, ATG101, ATG13, and FIP200. Starvation and low ATP levels down-regulate mTOR and directly stimulate the ULK1 complex. The ULK1 complex positively regulates autophagy by activating the Beclin 1 interacting complex, which consists of Beclin 1 (BCL2 family proteins), VPS34 (a class III phosphatidylinositol-3 kinase), and ATG14L. This increases the levels of phosphatidylinositol 3-phosphate (PI3P), which promotes the nucleation of autophagosomal membrane. The elongation of the autophagosome membrane requires two ubiquitin-like conjugation systems. The first is the ATG5–ATG12 complex, which is conjugated by ATG7 and ATG10 enzymes. The second one requires the ubiquitin-like protein microtubule–associated protein 1 light chain 3 (LC3), also called ATG8, which is cleaved by ATG4B into LC3B-I. LC3B-I turns into the active LC3B-II after conjugation with phosphatidylethanolamine by ATG3 and ATG7. Once the double-membrane autophagosome is complete, it fuses with a lysosome to form the autophagolysosome to degrade the autophagosome contents. ATG, autophagy-related protein; FIP200, focal adhesion kinase family interacting protein of 200 kD; VPS34, vacuolar protein sorting 34.
Autophagy and Obesity
In the past decade, many advances have been made in the understanding of autophagy in the pathogenesis of human disease (19, 53–56). Obesity creates a chronic low-grade inflammatory state (57) that potentially can be modulated by autophagy-associated pathways. Regulation of tissue-specific autophagy has been shown to be critical in the development of obesity and obesity-associated metabolic disorders (58). Atg7+/− heterozygous mice are more prone to metabolic syndrome and inflammasome activation (59). Monoallelic loss of Beclin 2, which participates in autophagy, results in obesity, impaired glucose tolerance, and decreased insulin sensitivity (60).
Adipose tissue is a key regulator of lipid storage and is a major endocrine organ of the body. Obesity and high-fat diet (HFD) feeding up-regulates autophagy in adipocytes through induction of mitochondrial and ER stress (61). Aberrant autophagy activation leads to defective browning of the adipose tissue, diminishing its thermogenic capacity (61) and metabolic profile (62, 63). Autophagy also plays an important role in adipogenesis and differentiation. Stimulation of autophagy favors white adipocyte differentiation, whereas autophagy blockade favors brown adipocyte differentiation (64, 65). Adipocyte-specific Atg7−/− mice had lower baseline white adipose tissue (WAT) and body weight and improved metabolic profiles and were more resistant to HFD-induced obesity (64, 65). In humans, autophagy is up-regulated in adipocytes of obese patients with type 2 diabetes mellitus or insulin resistance, as evidenced by increased autophagy markers, associated transcription factors, and increased colocalization of LDs with LC3 in autophagosomes (66–70). Interestingly though, autophagy markers have also been found to be low in obese patients before bariatric surgery (71). Pharmacologic blockade of autophagy by mineralcorticoid receptor antagonists prevents weight gain after HFD and increases brown adipocyte transcripts and adipocyte count. Treatment with lipoxins in obese mice down-regulates autophagy in WAT, attenuates hepatic steatosis, and reduces inflammation (72, 73). Thus, autophagic activity in adipocytes promotes obesity through white adipocyte differentiation and augments obesity-associated disorders such as insulin resistance, hepatic steatosis, and inflammation.
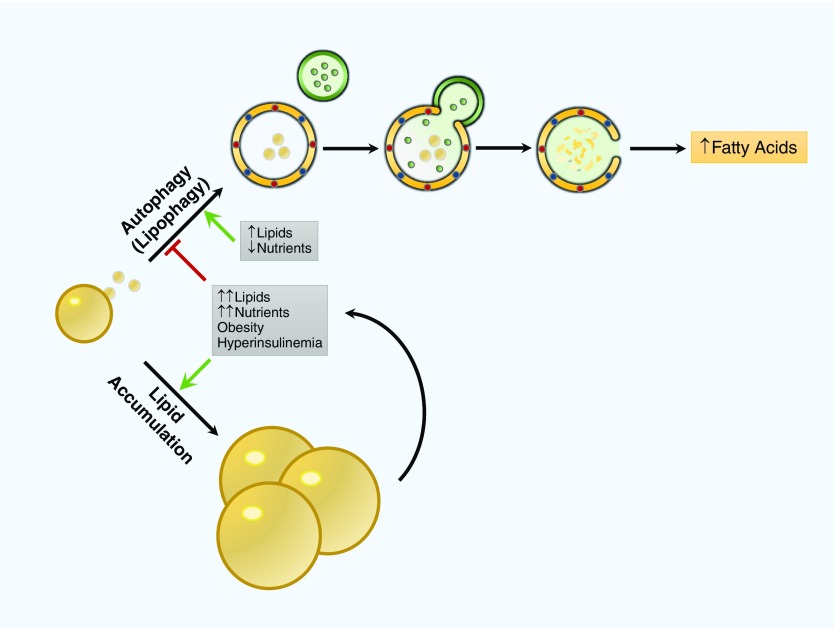
Hepatocytes convert FFAs into TGs for storage in LDs (74). Blockade of lipophagy leads to accumulation of TGs and LDs within autophagic compartments (29). Lipotoxicity, caused by either exogenous accumulation via HFD or endogenous accumulation via TG/LD, contributes to further inhibition of autophagy and worsens TG/LD accumulation (Figure 2) (29, 75). In obesity models, autophagy markers are down-regulated in hepatocytes compared with control subjects. Blockade of autophagy in hepatocytes leads to worsening of metabolic diseases such as insulin resistance, hepatic steatosis, and ER stress (76). Thus, autophagic activity in hepatocytes has a beneficial role and protects against obesity-associated metabolic disorders such as hepatic steatosis, insulin resistance, and impaired glucose metabolism.
Figure 2.
Role of autophagy in lipid metabolism in the liver. In hepatocytes, autophagy plays an important role in lipid turnover from lipid droplets. In starvation, autophagy degrades lipid droplets to increase free fatty acids and fuel β-oxidation. In obesity-related conditions such as hyperinsulinemia and lipid accumulation, autophagy is inhibited, which causes a predisposition toward more lipid accumulation and, in turn, further autophagy inhibition that, in organs such as the liver, can lead to hepatic steatosis.
FFAs can promote the generation of harmful reactive oxygen species in β-cells (77). Autophagy is up-regulated as a defense mechanism, protecting β-cells on FFA exposure (78). However, excessive FFAs can subsequently inhibit autophagy through impaired autophagosome maturation and turnover (79). Autophagy in skeletal muscle potentiates exercise-induced improvements in glucose homeostasis and insulin sensitivity in HFD mice (80). Controversy remains as to whether autophagy may be protective in non–exercise-related HFD models (81, 82). HFD impairs autophagy in the medial-basal hypothalamus, the central control for metabolic physiology and feeding behavior. Selective autophagy blockade of the hypothalamus leads to obesity and insulin resistance in mice. Starvation induces autophagy in agouti-related protein (AgRP) neurons, which produce AgRP to increase food intake (83). Autophagy blockade in these neurons during starvation attenuates AgRP release and confers a lean phenotype (84). Conversely, in pro-opiomelanocortin neurons that suppress food intake (83), autophagy blockade through ATG7 or ATG12 leads to increased obesity from impaired lipolysis and disrupted glucose homeostasis (85, 86).
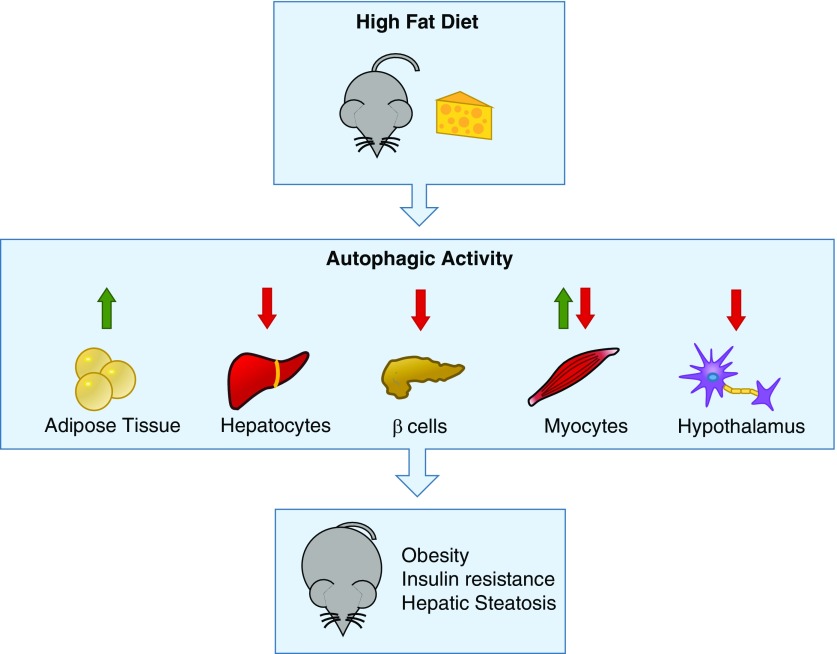
In summary, autophagy plays an important role in regulating obesity-related metabolic dysfunction. Lipid overload can affect autophagy, which can lead to decreased lipophagy, decreased mitochondrial turnover and increased ER stress, low-grade inflammation, and finally, insulin resistance, although differences in autophagy regulation can be cell specific (Figure 3). Currently, no studies have examined the effect of HFD on autophagy in the lung.
Figure 3.
Tissue-specific regulation of autophagy under high-fat diet conditions. Under high-fat diet conditions, mice have tissue-specific changes in autophagy. In adipose tissue, there is an increase in autophagic activity as a response to endoplasmic reticulum stress, leading to degradation of the antiinflammatory adipokine adiponectin. In hepatocytes, β cells, and hypothalamic neurons, there is decreased autophagy under a high-fat diet, leading to lipid accumulation, β-cell toxicity, and inflammation. In myocytes under exercise, there is an increase in autophagy, leading to decreased insulin resistance. Thus, aberrant autophagy contributes to obesity disease pathogenesis, leading to insulin resistance, hepatic steatosis, and inflammation.
Autophagy and Pulmonary Disease
Asthma
Significant advancements have been made in the understanding of autophagy in the pathogenesis of pulmonary disease, often through modulation of the inflammatory response. Autophagy has been studied in asthma, COPD, IPF, acute lung injury, OSA, and several other pulmonary diseases (87). Dendritic cell–specific ATG5–deficient mice exposed to house dust mites develop severe IL-17A–dependent, steroid-resistant asthma and unprovoked airway hyperresponsiveness (AHR) (88). However, during virally mediated asthma exacerbations, exuberant autophagy may decrease IFN-γ and increase viral load (89). In human subjects, single nucleotide polymorphisms in the ATG5 gene have been associated with asthma (90). Markers of autophagy were also elevated in the sputum granulocytes and eosinophils of subjects with asthma (91).
COPD
Markers of autophagy are elevated in patients with COPD from cigarette smoke or α-1 antitrypsin deficiency (92). Autophagy was also up-regulated in vitro (92–98) and in vivo in models of COPD and chronic bronchitis (95, 99). Inhibition of autophagy (93, 94, 100) and mitophagy (101) attenuate cigarette smoke–induced lung injury and chronic bronchitis. However, aggregophagy and xenophagy seem to be protective for disease pathogenesis (102, 103).
Pulmonary Fibrosis
Autophagy is associated with the degradation of collagen and is protective in in vivo models of pulmonary fibrosis (104–107). TGF-β, one of the hallmark cytokines of fibrosis, can inhibit autophagy through mTOR (108), favoring collagen deposition in fibroblasts (109). Autophagy marker levels are low in the lungs of patients with IPF (109, 110). Mitophagy can also have a potentially beneficial effect in the pathogenesis of pulmonary fibrosis. PINK1-deficient mice were susceptible to lung fibrosis induced by bleomycin (111, 112). The role PINK1 expression plays in the lungs of patients with IPF is controversial because there are reports on high (111) and low (112) levels related to ER stress (112).
Inhibition of autophagy and mitophagy decreases cell viability in acute lung injury (113, 114). Up-regulation of autophagy by low-dose cytoprotective carbon monoxide exposure can inhibit cell death in lung epithelial cells (115). In chronic/recurrent hypoxia animal models of OSA, autophagy is induced and may be protective for cardiac function (116). However, the activation of mitophagy can be detrimental in hypoxemic conditions (117).
Impact of Autophagy in the Pathogenesis of Obesity-related Lung Disease
Obesity and many pulmonary diseases share common signaling pathways related to inflammation. Several signaling molecules in obesity, including IL-17, leptin, adiponectin, NLRP3 inflammasome, and TLR-4, have been implicated in the pathogenesis of lung disease. Autophagy regulates inflammation through a variety of mechanisms (47). In obesity, autophagy impairment in the bone marrow derived macrophages and Kupfer cells of mice fed an HFD produces increased release of proinflammatory cytokines and macrophage proinflammatory polarization (118). Autophagy-deficient mice have increased inflammasome activation (59). In addition, autophagy deficiency in the hypothalamus induces IKKβ/NF-κB activation and inflammatory changes in the hypothalamus after HFD (119). Autophagy blockade in cultured human adipocytes leads to increased IL-1β, IL-6, and IL-8 secretion (69) and to the activation of ER stress–induced autophagy (120).
IL-17 is a known mediator of neutrophilic inflammation in the airways in various lung diseases (121, 122). IL-17 is also up-regulated in obesity associated with altered dendritic cell function (123). IL-1β is required for the production of IL-17A by CD4+ T cells. Autophagy decreases IL-1β (44, 124) by sequestering pro–IL-1 β, thus down-regulating IL-17A production (48). In obesity-associated asthma, IL-17A plays an essential role in disease severity and is required for AHR in a murine model because IL-17–deficient mice do not develop asthma under HFD (125). Increased IL-17 levels are correlated to worsening the exacerbating fibrosis in bleomycin-induced lung injury (126, 127). Loss of autophagy in dendritic cells leads to IL-17A–driven AHR in a murine asthma model (88). After infection with respiratory syncytial virus, lc3b−/− dendritic cells have altered innate cytokine production, leading to a Th17-skewed CD4+ T-cell response and lung injury (128). Similarly, airway epithelial cells deficient in LC3B had enhanced inflammasome activation and increased IL-1 and IL-17A production after respiratory syncytial virus infection (128). IL-17 is increased in the bronchial mucosa of patients with COPD (122, 129, 130) and asthma (130). Genetic deletion of IL-17 in mice was protective against cigarette smoke–induced lung inflammation and apoptosis of type II alveolar epithelial cells (131).
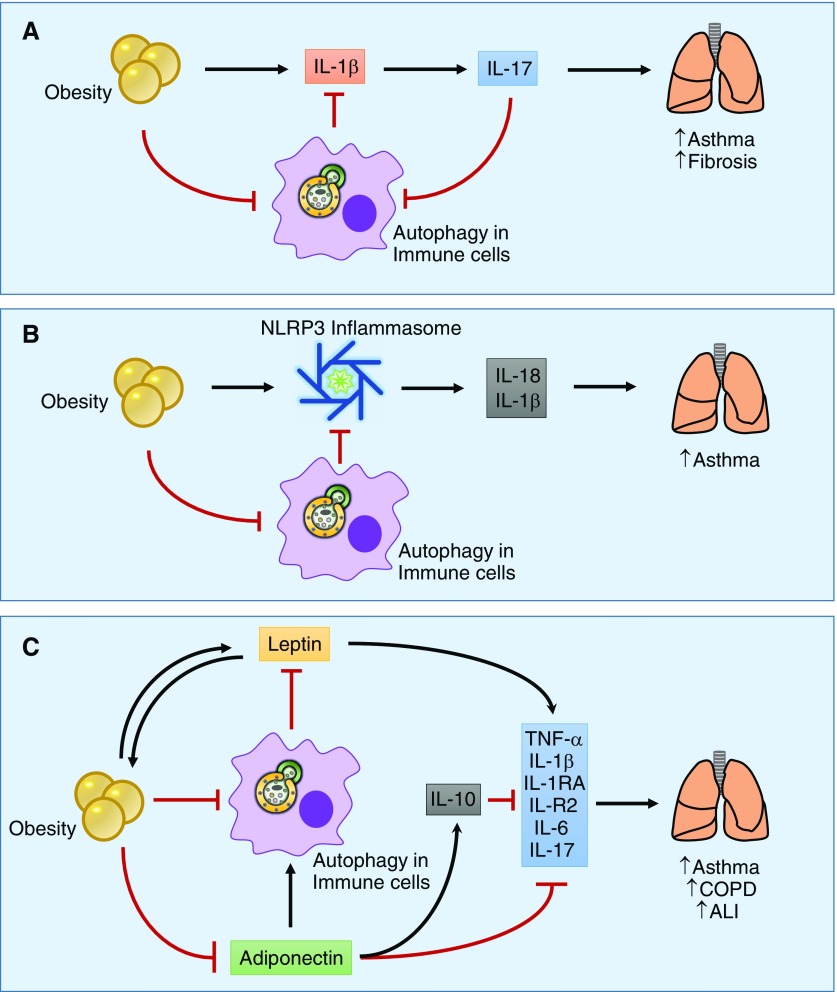
Innate lymphoid cells group 3 cells are lymphoid cells that lack B or T receptors and produce IL-17A as their signature cytokine (132). In mice under HFD conditions, innate lymphoid cells group 3 has been shown to be present in the lungs and can be stimulated by IL-1β produced by lung or adipose tissue macrophages to produce AHR (125). IL-17A has also been shown to play an important role in pulmonary fibrosis pathogenesis by stimulating collagen production. Autophagy is activated by IL-17 inhibition, promoting degradation of collagen in lung epithelial cells (133). IL-17 can also inhibit autophagy in lung epithelial cells by regulating phosphorylation of BCL2 (134). HFD conditions can cause interstitial disease similar to sarcoidosis and the progressive development of lung fibrosis (135). Thus, the regulation of IL-17 by autophagy could be altered in obesity and could lead to the pathogenesis of pulmonary diseases such as asthma and IPF (Figure 4A).
Figure 4.
Proposed mechanisms of obesity- and autophagy-related pathogenesis of lung disease. (A) Obesity induces the production of inflammatory cytokines such as IL-1β by macrophages in the adipose or lung tissue, leading to the production of IL-17. IL-17 has been correlated with worsening lung inflammation and injury in diseases such as asthma and fibrosis. Autophagy can sequester pro–IL-1β, decreasing IL-1β production and thus negatively regulating IL-17 levels. (B) Obesity is characterized by NLRP3 inflammasome activation that increases the production of inflammatory cytokines such as IL-1β and IL-18, which has been shown to contribute to lung disease pathogenesis. Autophagy can inhibit inflammasome activation, thereby decreasing IL-1β and IL-18 production. (C) Adipocytes are characterized by adipokine production such as leptin and adiponectin. Under obesity conditions, leptin levels are increased as a result of leptin resistance. Leptin has systemic effects and can increase the production of inflammatory cytokines. Adiponectin is decreased in obesity. Adiponectin has antiinflammatory properties through increasing production of IL-10 and inhibits the production of proinflammatory cytokines such as TNF-α, IL-1β, IL-1RA, IL-R2, IL-6, and IL-17. ALI, acute lung injury; COPD, chronic obstructive pulmonary disease; NLRP3, nod-like receptor protein-3.
The secretion of IL-1β and IL-18 is regulated by the NLRP3 inflammasome (136). In obesity, the NLRP3 inflammasome is activated by obesity-associated “danger signals” and participates in the regulation of T cells in the adipose tissue, contributing to a proinflammatory state (137). NLRP3 has been shown to be up-regulated in the adipocytes of obese patients with metabolic syndrome (138). Autophagy can regulate inflammatory responses related to NLRP3 inflammasome (50) and target ubiquitinated inflammasomes for degradation (139), limiting inflammation. Under HFD conditions, FFAs are able to induce inflammasome-dependent IL-1β and IL-18 production and inhibit the autophagosome formation that results in impaired insulin signaling (140). In a hyperoxia model, NLRP3-deficient mice are resistant to oxidative damage, and interestingly, this resistance is correlated to PINK1 expression (114). NLRP3 inflammasome (as well as IL-17) is required to develop AHR in obese mice (125), but the relation between autophagy and NLRP3 under obesity conditions in other lung diseases has not been studied. Thus, autophagy may act as a defense mechanism to limit obesity-associated inflammation and lung disease through the inhibition of the NLRP3 inflammasome–mediated IL-1β and IL-18 production (Figure 4B).
Obesity is correlated with higher levels of leptin, an adipokine that influences appetite. Leptin has also been shown to stimulate the production of inflammatory cytokines such as TNF-α, IL-1β, IL-1RA, IL-R2, and IL-6 in innate and adaptive immunity (141, 142). Airway epithelial cells express receptors for leptin and adiponectin, suggesting a potential ability to respond to this systemic mediator (143). Leptin levels have been correlated with the severity of COPD (143), asthma (143), and acute lung injury (ALI) (144). In patients with COPD, increases in leptin levels correlate with a proinflammatory state (145). Leptin polymorphisms have also been associated with COPD severity (146). Leptin has been shown to promote a Th17-mediated inflammatory response in lupus-prone mice (147) and to inhibit autophagy in CD4+ T cells (148). Inhibition of autophagy can increase leptin levels (85, 86), suggesting that leptin and autophagy regulate one another, contributing to both obesity and pulmonary disease (Figure 4C).
Adiponectin is another key adipokine in metabolism that is classically down-regulated in obesity. HFD/obesity-associated ER stress promotes the degradation of adiponectin through autophagy, and this has been associated with glucose intolerance or diabetes in human studies (149, 150). Adiponectin has also been shown to have antiinflammatory effects such as suppression of TNF-α, IL-6, and NF-κB and up-regulation of IL-10 (141). Mice deficient in adiponectin have increased IL-17A–mediated neutrophilic infiltration of the lung (151). Adiponectin has also been shown to regulate IL-17A release in other diseases such as psoriasis (152). Adiponectin is a known positive regulator of autophagy in myocytes (81), and adiponectin-induced autophagy has been found to have beneficial antiinflammatory effects in cardiovascular diseases (153, 154), but currently, no studies have examined its effects on autophagy in the lung. In macrophages stimulated with LPS, adiponectin can inhibit autophagy-mediated TNF-α production (155). Treatment with adiponectin can abolish AHR in asthma murine models (156) and decrease inflammation in ALI murine models (157, 158). Thus, adiponectin may play a role in augmenting autophagy-mediated immune modulating and attenuating obesity-associated inflammatory cytokine release and lung injury (Figure 4C).
TLR-4 signaling and ER stress are related to the proinflammatory response in obesity. HFD can stimulate TLR-4, which, in turn, increases the expression of proinflammatory cytokines that lead to mitochondrial and ER stress (61). ER stress is one of the most important stimulators of autophagy in WAT under obesity conditions (149, 150). However, TLR-4 has been shown to be protective for the maintenance of normal lung architecture because TLR-4–deficient mice have emphysema and increased autophagy levels after cigarette smoke exposure (95). In a murine model of hypercholesterolemia, mice were found to develop emphysema and TLR-4 signaling activation after feeding with HFD (159). Impairment of TLR-4–dependent autophagy activation in the bleomycin pulmonary fibrosis models exacerbates pulmonary fibrosis through the inhibition of autophagy-associated collagen degradation. This effect can then be reversed when autophagy is stimulated by rapamycin (160). All the above suggest that TLR-4 can exert different regulatory functions over autophagy, depending on the stimuli such as CS, FFAs, or profibrotic mediators.
Future studies should focus on the regulation of these obesity-related inflammatory mediators by stimulation and inhibition of autophagy under HFD conditions in animal models of asthma, IPF, ALI, OSA, and COPD. We hypothesize that the stimulation of autophagy in these models may attenuate HFD or obesity-associated lung injury.
Conclusions
Autophagy and obesity-related inflammation are involved in pulmonary disease pathogenesis. The growing interest in autophagy and its role in obesity-associated pulmonary disease is evolving. Obesity promotes a systemic proinflammatory environment that is exacerbated by obesity-associated suppression of autophagy. Defective autophagy leads to dysregulated IL-17, leptin, adiponectin, TLRs, and NLRP3 inflammasome activation, as well as to defective mitochondrial accumulation and persistent ER stress, potentially leading to worse lung injury. Currently the role of autophagy in obesity-related lung disease is still unclear, and efforts to identify these links, given the high prevalence of obesity among the world population, are needed.
Footnotes
Author Contributions: M.A.P. and A.M.K.C. contributed to the conception and design of the study; M.A.P. and K.C.M. contributed to the drafting of the manuscript; M.A.P., K.C.M., and A.M.K.C. contributed to the editing of the manuscript; K.C.M. contributed to the creation of the figures; A.M.K.C. contributed to the editing of the figures.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0045PS on February 22, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.World Health OrganizationObesity and overweight [Internet]. World Health Organization; 2015. [accessed 10 Jan 2016]Available from: http://www.who.int/mediacentre/factsheets/fs311/en/ [Google Scholar]
- 2.McNeil H, Segal L Quality of Life and Obesity. The Centre for Health Program Evaluation Monash University. 1999. Research report 17.
- 3.Strong AL, Burow ME, Gimble JM, Bunnell BA. Concise review: the obesity cancer paradigm: exploration of the interactions and crosstalk with adipose stem cells. Stem Cells. 2015;33:318–326. doi: 10.1002/stem.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y. Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev. 2014;13:981–1000. doi: 10.1016/j.autrev.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Karrowni W, Kennedy K, Jones P. Obesity paradox among survivors of acute myocardial infarction and its interaction with time. J Am Coll Cardiol. 2015;65(10S):A31. [Google Scholar]
- 6.Andersen KK, Olsen TS. The obesity paradox in stroke: lower mortality and lower risk of readmission for recurrent stroke in obese stroke patients. Int J Stroke. 2015;10:99–104. doi: 10.1111/ijs.12016. [DOI] [PubMed] [Google Scholar]
- 7.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291:2013–2016. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 8.Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc. 2008;5:218–225. doi: 10.1513/pats.200708-122MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beuther DA, Sutherland ER. Overweight, obesity, and incident asthma: a meta-analysis of prospective epidemiologic studies. Am J Respir Crit Care Med. 2007;175:661–666. doi: 10.1164/rccm.200611-1717OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 11.Scott HA, Gibson PG, Garg ML, Wood LG. Airway inflammation is augmented by obesity and fatty acids in asthma. Eur Respir J. 2011;38:594–602. doi: 10.1183/09031936.00139810. [DOI] [PubMed] [Google Scholar]
- 12.Telenga ED, Tideman SW, Kerstjens HA, Hacken NH, Timens W, Postma DS, van den Berge M. Obesity in asthma: more neutrophilic inflammation as a possible explanation for a reduced treatment response. Allergy. 2012;67:1060–1068. doi: 10.1111/j.1398-9995.2012.02855.x. [DOI] [PubMed] [Google Scholar]
- 13.Nathan SD, Shlobin OA, Ahmad S, Burton NA, Barnett SD, Edwards E. Comparison of wait times and mortality for idiopathic pulmonary fibrosis patients listed for single or bilateral lung transplantation. J Heart Lung Transplant. 2010;29:1165–1171. doi: 10.1016/j.healun.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Gries CJ, Bhadriraju S, Edelman JD, Goss CH, Raghu G, Mulligan MS. Obese patients with idiopathic pulmonary fibrosis have a higher 90-day mortality risk with bilateral lung transplantation. J Heart Lung Transplant. 2015;34:241–246. doi: 10.1016/j.healun.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Morris AE, Stapleton RD, Rubenfeld GD, Hudson LD, Caldwell E, Steinberg KP. The association between body mass index and clinical outcomes in acute lung injury. Chest. 2007;131:342–348. doi: 10.1378/chest.06-1709. [DOI] [PubMed] [Google Scholar]
- 16.Chailleux E, Laaban JP, Veale D. Prognostic value of nutritional depletion in patients with COPD treated by long-term oxygen therapy: data from the ANTADIR observatory. Chest. 2003;123:1460–1466. doi: 10.1378/chest.123.5.1460. [DOI] [PubMed] [Google Scholar]
- 17.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 18.Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:1845–1846. doi: 10.1056/NEJMc1303158. [DOI] [PubMed] [Google Scholar]
- 20.Stolz A, Ernst A, Dikic I. Cargo recognition and trafficking in selective autophagy. Nat Cell Biol. 2014;16:495–501. doi: 10.1038/ncb2979. [DOI] [PubMed] [Google Scholar]
- 21.Bernales S, Schuck S, Walter P. ER-phagy: selective autophagy of the endoplasmic reticulum. Autophagy. 2007;3:285–287. doi: 10.4161/auto.3930. [DOI] [PubMed] [Google Scholar]
- 22.Schuck S, Gallagher CM, Walter P. ER-phagy mediates selective degradation of endoplasmic reticulum independently of the core autophagy machinery. J Cell Sci. 2014;127:4078–4088. doi: 10.1242/jcs.154716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mochida K, Oikawa Y, Kimura Y, Kirisako H, Hirano H, Ohsumi Y, Nakatogawa H. Receptor-mediated selective autophagy degrades the endoplasmic reticulum and the nucleus. Nature. 2015;522:359–362. doi: 10.1038/nature14506. [DOI] [PubMed] [Google Scholar]
- 24.Lipatova Z, Shah AH, Kim JJ, Mulholland JW, Segev N. Regulation of ER-phagy by a Ypt/Rab GTPase module. Mol Biol Cell. 2013;24:3133–3144. doi: 10.1091/mbc.E13-05-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong YC, Holzbaur EL. Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci USA. 2014;111:E4439–E4448. doi: 10.1073/pnas.1405752111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 28.Geisler S, Holmström KM, Treis A, Skujat D, Weber SS, Fiesel FC, Kahle PJ, Springer W. The PINK1/Parkin-mediated mitophagy is compromised by PD-associated mutations. Autophagy. 2010;6:871–878. doi: 10.4161/auto.6.7.13286. [DOI] [PubMed] [Google Scholar]
- 29.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang CW, Miao YH, Chang YS. A sterol-enriched vacuolar microdomain mediates stationary phase lipophagy in budding yeast. J Cell Biol. 2014;206:357–366. doi: 10.1083/jcb.201404115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Zutphen T, Todde V, de Boer R, Kreim M, Hofbauer HF, Wolinski H, Veenhuis M, van der Klei IJ, Kohlwein SD. Lipid droplet autophagy in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 2014;25:290–301. doi: 10.1091/mbc.E13-08-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockenstein E, Schwach G, Ingolic E, Adame A, Crews L, Mante M, Pfragner R, Schreiner E, Windisch M, Masliah E. Lysosomal pathology associated with α-synuclein accumulation in transgenic models using an eGFP fusion protein. J Neurosci Res. 2005;80:247–259. doi: 10.1002/jnr.20446. [DOI] [PubMed] [Google Scholar]
- 33.Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D. Deficiency of ATP13A2 leads to lysosomal dysfunction, α-synuclein accumulation, and neurotoxicity. J Neurosci. 2012;32:4240–4246. doi: 10.1523/JNEUROSCI.5575-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nassif M, Valenzuela V, Rojas-Rivera D, Vidal R, Matus S, Castillo K, Fuentealba Y, Kroemer G, Levine B, Hetz C. Pathogenic role of BECN1/Beclin 1 in the development of amyotrophic lateral sclerosis. Autophagy. 2014;10:1256–1271. doi: 10.4161/auto.28784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crippa V, Sau D, Rusmini P, Boncoraglio A, Onesto E, Bolzoni E, Galbiati M, Fontana E, Marino M, Carra S, et al. The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS) Hum Mol Genet. 2010;19:3440–3456. doi: 10.1093/hmg/ddq257. [DOI] [PubMed] [Google Scholar]
- 36.Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120:159–162. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Li X, He S, Zhou X, Ye Y, Tan S, Zhang S, Li R, Yu M, Jundt MC, Hidebrand A, et al. Lyn delivers bacteria to lysosomes for eradication through TLR2-initiated autophagy related phagocytosis. PLoS Pathog. 2016;12:e1005363. doi: 10.1371/journal.ppat.1005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakurai A, Maruyama F, Funao J, Nozawa T, Aikawa C, Okahashi N, Shintani S, Hamada S, Ooshima T, Nakagawa I. Specific behavior of intracellular Streptococcus pyogenes that has undergone autophagic degradation is associated with bacterial streptolysin O and host small G proteins Rab5 and Rab7. J Biol Chem. 2010;285:22666–22675. doi: 10.1074/jbc.M109.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noda T, Yoshimori T. Between canonical and antibacterial autophagy: Rab7 is required for GAS-containing autophagosome-like vacuole formation. Autophagy. 2010;6:419–420. doi: 10.4161/auto.6.3.11419. [DOI] [PubMed] [Google Scholar]
- 40.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tal MC, Sasai M, Lee HK, Yordy B, Shadel GS, Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci USA. 2009;106:2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16:461–472. doi: 10.1038/nrm4024. [DOI] [PubMed] [Google Scholar]
- 43.Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011;469:323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 45.Jounai N, Takeshita F, Kobiyama K, Sawano A, Miyawaki A, Xin KQ, Ishii KJ, Kawai T, Akira S, Suzuki K, et al. The Atg5 Atg12 conjugate associates with innate antiviral immune responses. Proc Natl Acad Sci USA. 2007;104:14050–14055. doi: 10.1073/pnas.0704014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fliss PM, Jowers TP, Brinkmann MM, Holstermann B, Mack C, Dickinson P, Hohenberg H, Ghazal P, Brune W. Viral mediated redirection of NEMO/IKKγ to autophagosomes curtails the inflammatory cascade. PLoS Pathog. 2012;8:e1002517. doi: 10.1371/journal.ppat.1002517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deretic V, Kimura T, Timmins G, Moseley P, Chauhan S, Mandell M. Immunologic manifestations of autophagy. J Clin Invest. 2015;125:75–84. doi: 10.1172/JCI73945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harris J, Hartman M, Roche C, Zeng SG, O’Shea A, Sharp FA, Lambe EM, Creagh EM, Golenbock DT, Tschopp J, et al. Autophagy controls IL-1β secretion by targeting pro-IL-1β for degradation. J Biol Chem. 2011;286:9587–9597. doi: 10.1074/jbc.M110.202911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crişan TO, Plantinga TS, van de Veerdonk FL, Farcaş MF, Stoffels M, Kullberg BJ, van der Meer JW, Joosten LA, Netea MG. Inflammasome-independent modulation of cytokine response by autophagy in human cells. PLoS One. 2011;6:e18666. doi: 10.1371/journal.pone.0018666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 52.Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 54.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng Y, Ren X, Hait WN, Yang JM. Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol Rev. 2013;65:1162–1197. doi: 10.1124/pr.112.007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wensveen FM, Valentić S, Šestan M, Turk Wensveen T, Polić B. The “Big Bang” in obese fat: events initiating obesity-induced adipose tissue inflammation. Eur J Immunol. 2015;45:2446–2456. doi: 10.1002/eji.201545502. [DOI] [PubMed] [Google Scholar]
- 58.Stienstra R, Haim Y, Riahi Y, Netea M, Rudich A, Leibowitz G. Autophagy in adipose tissue and the β cell: implications for obesity and diabetes. Diabetologia. 2014;57:1505–1516. doi: 10.1007/s00125-014-3255-3. [DOI] [PubMed] [Google Scholar]
- 59.Lim YM, Lim H, Hur KY, Quan W, Lee HY, Cheon H, Ryu D, Koo SH, Kim HL, Kim J, et al. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nat Commun. 2014;5:4934. doi: 10.1038/ncomms5934. [DOI] [PubMed] [Google Scholar]
- 60.He C, Wei Y, Sun K, Li B, Dong X, Zou Z, Liu Y, Kinch LN, Khan S, Sinha S, et al. Beclin 2 functions in autophagy, degradation of G protein-coupled receptors, and metabolism. Cell. 2013;154:1085–1099. doi: 10.1016/j.cell.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okla M, Wang W, Kang I, Pashaj A, Carr T, Chung S. Activation of Toll-like receptor 4 (TLR4) attenuates adaptive thermogenesis via endoplasmic reticulum stress. J Biol Chem. 2015;290:26476–26490. doi: 10.1074/jbc.M115.677724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giralt M, Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology. 2013;154:2992–3000. doi: 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]
- 63.Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci USA. 2009;106:19860–19865. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009;119:3329–3339. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ost A, Svensson K, Ruishalme I, Brännmark C, Franck N, Krook H, Sandström P, Kjolhede P, Strålfors P. Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol Med. 2010;16:235–246. doi: 10.2119/molmed.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kovsan J, Blüher M, Tarnovscki T, Klöting N, Kirshtein B, Madar L, Shai I, Golan R, Harman-Boehm I, Schön MR, et al. Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab. 2011;96:E268–E277. doi: 10.1210/jc.2010-1681. [DOI] [PubMed] [Google Scholar]
- 68.Rodríguez A, Gómez-Ambrosi J, Catalán V, Rotellar F, Valentí V, Silva C, Mugueta C, Pulido MR, Vázquez R, Salvador J, et al. The ghrelin O-acyltransferase-ghrelin system reduces TNF-α-induced apoptosis and autophagy in human visceral adipocytes. Diabetologia. 2012;55:3038–3050. doi: 10.1007/s00125-012-2671-5. [DOI] [PubMed] [Google Scholar]
- 69.Jansen HJ, van Essen P, Koenen T, Joosten LA, Netea MG, Tack CJ, Stienstra R. Autophagy activity is up-regulated in adipose tissue of obese individuals and modulates proinflammatory cytokine expression. Endocrinology. 2012;153:5866–5874. doi: 10.1210/en.2012-1625. [DOI] [PubMed] [Google Scholar]
- 70.Nuñez CE, Rodrigues VS, Gomes FS, Moura RF, Victorio SC, Bombassaro B, Chaim EA, Pareja JC, Geloneze B, Velloso LA, et al. Defective regulation of adipose tissue autophagy in obesity. Int J Obes. 2013;37:1473–1480. doi: 10.1038/ijo.2013.27. [DOI] [PubMed] [Google Scholar]
- 71.Soussi H, Reggio S, Alili R, Prado C, Mutel S, Pini M, Rouault C, Clément K, Dugail I. DAPK2 downregulation associates with attenuated adipocyte autophagic clearance in human obesity. Diabetes. 2015;64:3452–3463. doi: 10.2337/db14-1933. [DOI] [PubMed] [Google Scholar]
- 72.Armani A, Cinti F, Marzolla V, Morgan J, Cranston GA, Antelmi A, Carpinelli G, Canese R, Pagotto U, Quarta C, et al. Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high-fat-diet-fed mice. FASEB J. 2014;28:3745–3757. doi: 10.1096/fj.13-245415. [DOI] [PubMed] [Google Scholar]
- 73.Börgeson E, Johnson AM, Lee YS, Till A, Syed GH, Ali-Shah ST, Guiry PJ, Dalli J, Colas RA, Serhan CN, et al. Lipoxin A4 attenuates obesity-induced adipose inflammation and associated liver and kidney disease. Cell Metab. 2015;22:125–137. doi: 10.1016/j.cmet.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin S, Parton RG. Lipid droplets: a unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 75.Park HW, Park H, Semple IA, Jang I, Ro SH, Kim M, Cazares VA, Stuenkel EL, Kim JJ, Kim JS, et al. Pharmacological correction of obesity-induced autophagy arrest using calcium channel blockers. Nat Commun. 2014;5:4834. doi: 10.1038/ncomms5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu HY, Han J, Cao SY, Hong T, Zhuo D, Shi J, Liu Z, Cao W. Hepatic autophagy is suppressed in the presence of insulin resistance and hyperinsulinemia: inhibition of FoxO1-dependent expression of key autophagy genes by insulin. J Biol Chem. 2009;284:31484–31492. doi: 10.1074/jbc.M109.033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DeFronzo RA. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract Suppl. 2004:9–21. doi: 10.1111/j.1368-504x.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 78.Ebato C, Uchida T, Arakawa M, Komatsu M, Ueno T, Komiya K, Azuma K, Hirose T, Tanaka K, Kominami E, et al. Autophagy is important in islet homeostasis and compensatory increase of β cell mass in response to high-fat diet. Cell Metab. 2008;8:325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 79.Las G, Serada SB, Wikstrom JD, Twig G, Shirihai OS. Fatty acids suppress autophagic turnover in β-cells. J Biol Chem. 2011;286:42534–42544. doi: 10.1074/jbc.M111.242412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, Xu A, Sweeney G. Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high-fat diet feeding in mice. Diabetes. 2015;64:36–48. doi: 10.2337/db14-0267. [DOI] [PubMed] [Google Scholar]
- 82.Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim H, Hur KY, Kim HK, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med. 2013;19:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 83.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 84.Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, Cuervo AM, Singh R. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell Metab. 2011;14:173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coupé B, Ishii Y, Dietrich MO, Komatsu M, Horvath TL, Bouret SG. Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metab. 2012;15:247–255. doi: 10.1016/j.cmet.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Quan W, Kim HK, Moon EY, Kim SS, Choi CS, Komatsu M, Jeong YT, Lee MK, Kim KW, Kim MS, et al. Role of hypothalamic proopiomelanocortin neuron autophagy in the control of appetite and leptin response. Endocrinology. 2012;153:1817–1826. doi: 10.1210/en.2011-1882. [DOI] [PubMed] [Google Scholar]
- 87.Nakahira K, Cloonan SM, Mizumura K, Choi AM, Ryter SW. Autophagy: a crucial moderator of redox balance, inflammation, and apoptosis in lung disease. Antioxid Redox Signal. 2014;20:474–494. doi: 10.1089/ars.2013.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suzuki Y, Maazi H, Sankaranarayanan I, Lam J, Khoo B, Soroosh P, Barbers RG, James Ou JH, Jung JU, Akbari O.Lack of autophagy induces steroid-resistant airway inflammation J Allergy Clin Immunol[online ahead of print] 14 Nov 2015. DOI: 10.1016/j.jaci.2015.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu Q, Jiang D, Huang C, van Dyk LF, Li L, Chu HW. Trehalose-mediated autophagy impairs the anti-viral function of human primary airway epithelial cells. PLoS One. 2015;10:e0124524. doi: 10.1371/journal.pone.0124524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Poon AH, Chouiali F, Tse SM, Litonjua AA, Hussain SN, Baglole CJ, Eidelman DH, Olivenstein R, Martin JG, Weiss ST, et al. Genetic and histologic evidence for autophagy in asthma pathogenesis. J Allergy Clin Immunol. 2012;129:569–571. doi: 10.1016/j.jaci.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ban GY, Pham DL, Trinh TH, Lee SI, Suh DH, Yang EM, Ye YM, Shin YS, Chwae YJ, Park HS. Autophagy mechanisms in sputum and peripheral blood cells of patients with severe asthma: a new therapeutic target. Clin Exp Allergy. 2016;46:48–59. doi: 10.1111/cea.12585. [DOI] [PubMed] [Google Scholar]
- 92.Chen ZH, Kim HP, Sciurba FC, Lee SJ, Feghali-Bostwick C, Stolz DB, Dhir R, Landreneau RJ, Schuchert MJ, Yousem SA, et al. Egr-1 regulates autophagy in cigarette smoke-induced chronic obstructive pulmonary disease. PLoS One. 2008;3:e3316. doi: 10.1371/journal.pone.0003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lam HC, Cloonan SM, Bhashyam AR, Haspel JA, Singh A, Sathirapongsasuti JF, Cervo M, Yao H, Chung AL, Mizumura K, et al. Histone deacetylase 6-mediated selective autophagy regulates COPD-associated cilia dysfunction. J Clin Invest. 2013;123:5212–5230. doi: 10.1172/JCI69636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen ZH, Lam HC, Jin Y, Kim HP, Cao J, Lee SJ, Ifedigbo E, Parameswaran H, Ryter SW, Choi AM. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc Natl Acad Sci USA. 2010;107:18880–18885. doi: 10.1073/pnas.1005574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.An CH, Wang XM, Lam HC, Ifedigbo E, Washko GR, Ryter SW, Choi AM. TLR4 deficiency promotes autophagy during cigarette smoke-induced pulmonary emphysema. Am J Physiol Lung Cell Mol Physiol. 2012;303:L748–L757. doi: 10.1152/ajplung.00102.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hwang JW, Chung S, Sundar IK, Yao H, Arunachalam G, McBurney MW, Rahman I. Cigarette smoke-induced autophagy is regulated by SIRT1-PARP-1-dependent mechanism: implication in pathogenesis of COPD. Arch Biochem Biophys. 2010;500:203–209. doi: 10.1016/j.abb.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim HP, Wang X, Chen ZH, Lee SJ, Huang MH, Wang Y, Ryter SW, Choi AM. Autophagic proteins regulate cigarette smoke-induced apoptosis: protective role of heme oxygenase-1. Autophagy. 2008;4:887–895. doi: 10.4161/auto.6767. [DOI] [PubMed] [Google Scholar]
- 98.Fujii S, Hara H, Araya J, Takasaka N, Kojima J, Ito S, Minagawa S, Yumino Y, Ishikawa T, Numata T, et al. Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. OncoImmunology. 2012;1:630–641. doi: 10.4161/onci.20297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hidvegi T, Stolz DB, Alcorn JF, Yousem SA, Wang J, Leme AS, Houghton AM, Hale P, Ewing M, Cai H, et al. Enhancing autophagy with drugs or lung-directed gene therapy reverses the pathological effects of respiratory epithelial cell proteinopathy. J Biol Chem. 2015;290:29742–29757. doi: 10.1074/jbc.M115.691253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cloonan SM, Lam HC, Ryter SW, Choi AM. “Ciliophagy”: the consumption of cilia components by autophagy. Autophagy. 2014;10:532–534. doi: 10.4161/auto.27641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, Glass K, Owen CA, Mahmood A, Washko GR, et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest. 2014;124:3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tran I, Ji C, Ni I, Min T, Tang D, Vij N. Role of cigarette smoke-induced aggresome formation in chronic obstructive pulmonary disease-emphysema pathogenesis. Am J Respir Cell Mol Biol. 2015;53:159–173. doi: 10.1165/rcmb.2014-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Monick MM, Powers LS, Walters K, Lovan N, Zhang M, Gerke A, Hansdottir S, Hunninghake GW. Identification of an autophagy defect in smokers’ alveolar macrophages. J Immunol. 2010;185:5425–5435. doi: 10.4049/jimmunol.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Korfhagen TR, Le Cras TD, Davidson CR, Schmidt SM, Ikegami M, Whitsett JA, Hardie WD. Rapamycin prevents transforming growth factor-α–induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2009;41:562–572. doi: 10.1165/rcmb.2008-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simler NR, Howell DC, Marshall RP, Goldsack NR, Hasleton PS, Laurent GJ, Chambers RC, Egan JJ. The rapamycin analogue SDZ RAD attenuates bleomycin-induced pulmonary fibrosis in rats. Eur Respir J. 2002;19:1124–1127. doi: 10.1183/09031936.02.00281602. [DOI] [PubMed] [Google Scholar]
- 106.Tulek B, Kiyan E, Toy H, Kiyici A, Narin C, Suerdem M. Anti-inflammatory and anti-fibrotic effects of sirolimus on bleomycin-induced pulmonary fibrosis in rats. Clin Invest Med. 2011;34:E341. doi: 10.25011/cim.v34i6.15894. [DOI] [PubMed] [Google Scholar]
- 107.Yoshizaki A, Yanaba K, Yoshizaki A, Iwata Y, Komura K, Ogawa F, Takenaka M, Shimizu K, Asano Y, Hasegawa M, et al. Treatment with rapamycin prevents fibrosis in tight-skin and bleomycin-induced mouse models of systemic sclerosis. Arthritis Rheum. 2010;62:2476–2487. doi: 10.1002/art.27498. [DOI] [PubMed] [Google Scholar]
- 108.Gui YS, Wang L, Tian X, Li X, Ma A, Zhou W, Zeng N, Zhang J, Cai B, Zhang H, et al. mTOR overactivation and compromised autophagy in the pathogenesis of pulmonary fibrosis. PLoS One. 2015;10:e0138625. doi: 10.1371/journal.pone.0138625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patel AS, Lin L, Geyer A, Haspel JA, An CH, Cao J, Rosas IO, Morse D. Autophagy in idiopathic pulmonary fibrosis. PLoS One. 2012;7:e41394. doi: 10.1371/journal.pone.0041394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nho RS, Hergert P. IPF fibroblasts are desensitized to type I collagen matrix-induced cell death by suppressing low autophagy via aberrant Akt/mTOR kinases. PLoS One. 2014;9:e94616. doi: 10.1371/journal.pone.0094616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Patel AS, Song JW, Chu SG, Mizumura K, Osorio JC, Shi Y, El-Chemaly S, Lee CG, Rosas IO, Elias JA, et al. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-β1 in pulmonary fibrosis. PLoS One. 2015;10:e0121246. doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125:521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tanaka A, Jin Y, Lee SJ, Zhang M, Kim HP, Stolz DB, Ryter SW, Choi AM. Hyperoxia-induced LC3B interacts with the Fas apoptotic pathway in epithelial cell death. Am J Respir Cell Mol Biol. 2012;46:507–514. doi: 10.1165/rcmb.2009-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang Y, Sauler M, Shinn AS, Gong H, Haslip M, Shan P, Mannam P, Lee PJ. Endothelial PINK1 mediates the protective effects of NLRP3 deficiency during lethal oxidant injury. J Immunol. 2014;192:5296–5304. doi: 10.4049/jimmunol.1400653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee SJ, Ryter SW, Xu JF, Nakahira K, Kim HP, Choi AM, Kim YS. Carbon monoxide activates autophagy via mitochondrial reactive oxygen species formation. Am J Respir Cell Mol Biol. 2011;45:867–873. doi: 10.1165/rcmb.2010-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maeda H, Nagai H, Takemura G, Shintani-Ishida K, Komatsu M, Ogura S, Aki T, Shirai M, Kuwahira I, Yoshida K. Intermittent-hypoxia induced autophagy attenuates contractile dysfunction and myocardial injury in rat heart. Biochim Biophys Acta. 2013;1832:1159–1166. doi: 10.1016/j.bbadis.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 117.Haslip M, Dostanic I, Huang Y, Zhang Y, Russell KS, Jurczak MJ, Mannam P, Giordano F, Erzurum SC, Lee PJ. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler Thromb Vasc Biol. 2015;35:1166–1178. doi: 10.1161/ATVBAHA.114.304865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu K, Zhao E, Ilyas G, Lalazar G, Lin Y, Haseeb M, Tanaka KE, Czaja MJ. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy. 2015;11:271–284. doi: 10.1080/15548627.2015.1009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meng Q, Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IκB kinase β (IKKβ)/NF-κB pathway. J Biol Chem. 2011;286:32324–32332. doi: 10.1074/jbc.M111.254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yin J, Wang Y, Gu L, Fan N, Ma Y, Peng Y. Palmitate induces endoplasmic reticulum stress and autophagy in mature adipocytes: implications for apoptosis and inflammation. Int J Mol Med. 2015;35:932–940. doi: 10.3892/ijmm.2015.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ivanov S, Bozinovski S, Bossios A, Valadi H, Vlahos R, Malmhäll C, Sjöstrand M, Kolls JK, Anderson GP, Lindén A. Functional relevance of the IL-23-IL-17 axis in lungs in vivo. Am J Respir Cell Mol Biol. 2007;36:442–451. doi: 10.1165/rcmb.2006-0020OC. [DOI] [PubMed] [Google Scholar]
- 122.Roos AB, Sandén C, Mori M, Bjermer L, Stampfli MR, Erjefält JS. IL-17A is elevated in end-stage chronic obstructive pulmonary disease and contributes to cigarette smoke-induced lymphoid neogenesis. Am J Respir Crit Care Med. 2015;191:1232–1241. doi: 10.1164/rccm.201410-1861OC. [DOI] [PubMed] [Google Scholar]
- 123.Carolan E, Tobin LM, Mangan BA, Corrigan M, Gaoatswe G, Byrne G, Geoghegan J, Cody D, O’Connell J, Winter DC, et al. Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. J Immunol. 2015;194:5775–5780. doi: 10.4049/jimmunol.1402945. [DOI] [PubMed] [Google Scholar]
- 124.Fujishima Y, Nishiumi S, Masuda A, Inoue J, Nguyen NM, Irino Y, Komatsu M, Tanaka K, Kutsumi H, Azuma T, et al. Autophagy in the intestinal epithelium reduces endotoxin-induced inflammatory responses by inhibiting NF-κB activation. Arch Biochem Biophys. 2011;506:223–235. doi: 10.1016/j.abb.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 125.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, Iwakura Y, Israel E, Bolger K, Faul J, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, Wynn TA. Bleomycin and IL-1β-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207:535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.François A, Gombault A, Villeret B, Alsaleh G, Fanny M, Gasse P, Adam SM, Crestani B, Sibilia J, Schneider P, et al. B cell activating factor is central to bleomycin- and IL-17-mediated experimental pulmonary fibrosis. J Autoimmun. 2015;56:1–11. doi: 10.1016/j.jaut.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 128.Reed M, Morris SH, Owczarczyk AB, Lukacs NW. Deficiency of autophagy protein Map1-LC3b mediates IL-17-dependent lung pathology during respiratory viral infection via ER stress-associated IL-1. Mucosal Immunol. 2015;8:1118–1130. doi: 10.1038/mi.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Di Stefano A, Caramori G, Gnemmi I, Contoli M, Vicari C, Capelli A, Magno F, D’Anna SE, Zanini A, Brun P, et al. T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol. 2009;157:316–324. doi: 10.1111/j.1365-2249.2009.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Doe C, Bafadhel M, Siddiqui S, Desai D, Mistry V, Rugman P, McCormick M, Woods J, May R, Sleeman MA, et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. 2010;138:1140–1147. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chang Y, Al-Alwan L, Audusseau S, Chouiali F, Carlevaro-Fita J, Iwakura Y, Baglole CJ, Eidelman DH, Hamid Q. Genetic deletion of IL-17A reduces cigarette smoke-induced inflammation and alveolar type II cell apoptosis. Am J Physiol Lung Cell Mol Physiol. 2014;306:L132–L143. doi: 10.1152/ajplung.00111.2013. [DOI] [PubMed] [Google Scholar]
- 132.Montaldo E, Juelke K, Romagnani C. Group 3 innate lymphoid cells (ILC3s): origin, differentiation, and plasticity in humans and mice. Eur J Immunol. 2015;45:2171–2182. doi: 10.1002/eji.201545598. [DOI] [PubMed] [Google Scholar]
- 133.Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG, Wang XX, Liu HZ, Sun W, Hu ZW. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-β1-dependent and -independent mechanisms. J Immunol. 2011;187:3003–3014. doi: 10.4049/jimmunol.1004081. [DOI] [PubMed] [Google Scholar]
- 134.Liu H, Mi S, Li Z, Hua F, Hu ZW. Interleukin 17A inhibits autophagy through activation of PIK3CA to interrupt the GSK3B-mediated degradation of BCL2 in lung epithelial cells. Autophagy. 2013;9:730–742. doi: 10.4161/auto.24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Samokhin AO, Bühling F, Theissig F, Brömme D. ApoE-deficient mice on cholate-containing high-fat diet reveal a pathology similar to lung sarcoidosis. Am J Pathol. 2010;176:1148–1156. doi: 10.2353/ajpath.2010.090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Esser N, L’homme L, De Roover A, Kohnen L, Scheen AJ, Moutschen M, Piette J, Legrand-Poels S, Paquot N. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia. 2013;56:2487–2497. doi: 10.1007/s00125-013-3023-9. [DOI] [PubMed] [Google Scholar]
- 139.Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McCallister JW, Adkins EJ, O’Brien JM., Jr Obesity and acute lung injury. Clin Chest Med. 2009;30:495–508, viii. doi: 10.1016/j.ccm.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- 143.Sideleva O, Suratt BT, Black KE, Tharp WG, Pratley RE, Forgione P, Dienz O, Irvin CG, Dixon AE. Obesity and asthma: an inflammatory disease of adipose tissue not the airway. Am J Respir Crit Care Med. 2012;186:598–605. doi: 10.1164/rccm.201203-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Barazzone-Argiroffo C, Muzzin P, Donati YR, Kan CD, Aubert ML, Piguet PF. Hyperoxia increases leptin production: a mechanism mediated through endogenous elevation of corticosterone. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1150–L1156. doi: 10.1152/ajplung.2001.281.5.L1150. [DOI] [PubMed] [Google Scholar]
- 145.Schols AM, Creutzberg EC, Buurman WA, Campfield LA, Saris WH, Wouters EF. Plasma leptin is related to proinflammatory status and dietary intake in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1220–1226. doi: 10.1164/ajrccm.160.4.9811033. [DOI] [PubMed] [Google Scholar]
- 146.Ye XW, Xiao M, Ye J, Zhang XY, Xiao J, Feng YL, Wen FQ. The polymorphism -2548G/A in leptin and severity of chronic obstructive pulmonary disease. Int J Immunogenet. 2011;38:45–50. doi: 10.1111/j.1744-313X.2010.00968.x. [DOI] [PubMed] [Google Scholar]
- 147.Yu Y, Liu Y, Shi FD, Zou H, Matarese G, La Cava A. Cutting edge: leptin-induced RORγt expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J Immunol. 2013;190:3054–3058. doi: 10.4049/jimmunol.1203275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cassano S, Pucino V, La Rocca C, Procaccini C, De Rosa V, Marone G, Matarese G. Leptin modulates autophagy in human CD4+CD25- conventional T cells. Metabolism. 2014;63:1272–1279. doi: 10.1016/j.metabol.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhou L, Liu F. Autophagy: roles in obesity-induced ER stress and adiponectin downregulation in adipocytes. Autophagy. 2010;6:1196–1197. doi: 10.4161/auto.6.8.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhou L, Liu M, Zhang J, Chen H, Dong LQ, Liu F. DsbA-L alleviates endoplasmic reticulum stress-induced adiponectin downregulation. Diabetes. 2010;59:2809–2816. doi: 10.2337/db10-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kasahara DI, Kim HY, Williams AS, Verbout NG, Tran J, Si H, Wurmbrand AP, Jastrab J, Hug C, Umetsu DT, et al. Pulmonary inflammation induced by subacute ozone is augmented in adiponectin-deficient mice: role of IL-17A. J Immunol. 2012;188:4558–4567. doi: 10.4049/jimmunol.1102363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shibata S, Tada Y, Hau CS, Mitsui A, Kamata M, Asano Y, Sugaya M, Kadono T, Masamoto Y, Kurokawa M, et al. Adiponectin regulates psoriasiform skin inflammation by suppressing IL-17 production from γδ-T cells. Nat Commun. 2015;6:7687. doi: 10.1038/ncomms8687. [DOI] [PubMed] [Google Scholar]
- 153.Li C, Wang Z, Wang C, Ma Q, Zhao Y. Perivascular adipose tissue-derived adiponectin inhibits collar-induced carotid atherosclerosis by promoting macrophage autophagy. PLoS One. 2015;10:e0124031. doi: 10.1371/journal.pone.0124031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Qi GM, Jia LX, Li YL, Li HH, Du J. Adiponectin suppresses angiotensin II-induced inflammation and cardiac fibrosis through activation of macrophage autophagy. Endocrinology. 2014;155:2254–2265. doi: 10.1210/en.2013-2011. [DOI] [PubMed] [Google Scholar]
- 155.Pun NT, Subedi A, Kim MJ, Park PH PLoS One Staff. Correction: globular adiponectin causes tolerance to LPS-induced TNF-α expression via autophagy induction in RAW 264.7 macrophages: involvement of SIRT1/FoxO3A axis. PLoS One. 2015;10:e0130370. doi: 10.1371/journal.pone.0124636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118:389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 157.Konter JM, Parker JL, Baez E, Li SZ, Ranscht B, Denzel M, Little FF, Nakamura K, Ouchi N, Fine A, et al. Adiponectin attenuates lipopolysaccharide-induced acute lung injury through suppression of endothelial cell activation. J Immunol. 2012;188:854–863. doi: 10.4049/jimmunol.1100426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shah D, Romero F, Duong M, Wang N, Paudyal B, Suratt BT, Kallen CB, Sun J, Zhu Y, Walsh K, et al. Obesity-induced adipokine imbalance impairs mouse pulmonary vascular endothelial function and primes the lung for injury. Sci Rep. 2015;5:11362. doi: 10.1038/srep11362. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 159.Goldklang M, Golovatch P, Zelonina T, Trischler J, Rabinowitz D, Lemaître V, D’Armiento J. Activation of the TLR4 signaling pathway and abnormal cholesterol efflux lead to emphysema in ApoE-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1200–L1208. doi: 10.1152/ajplung.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang HZ, Wang JP, Mi S, Liu HZ, Cui B, Yan HM, Yan J, Li Z, Liu H, Hua F, et al. TLR4 activity is required in the resolution of pulmonary inflammation and fibrosis after acute and chronic lung injury. Am J Pathol. 2012;180:275–292. doi: 10.1016/j.ajpath.2011.09.019. [DOI] [PubMed] [Google Scholar]