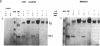Abstract
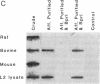
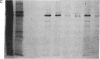
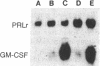
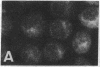
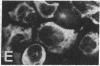
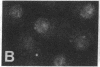
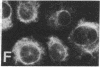
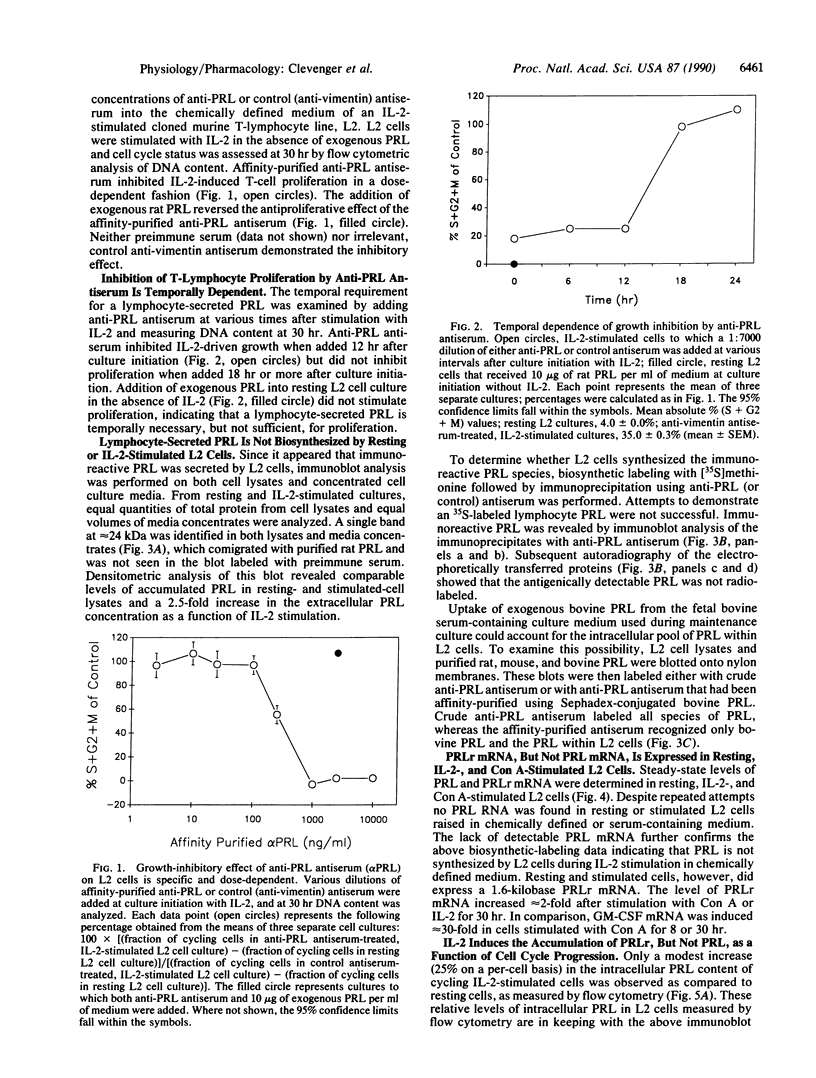
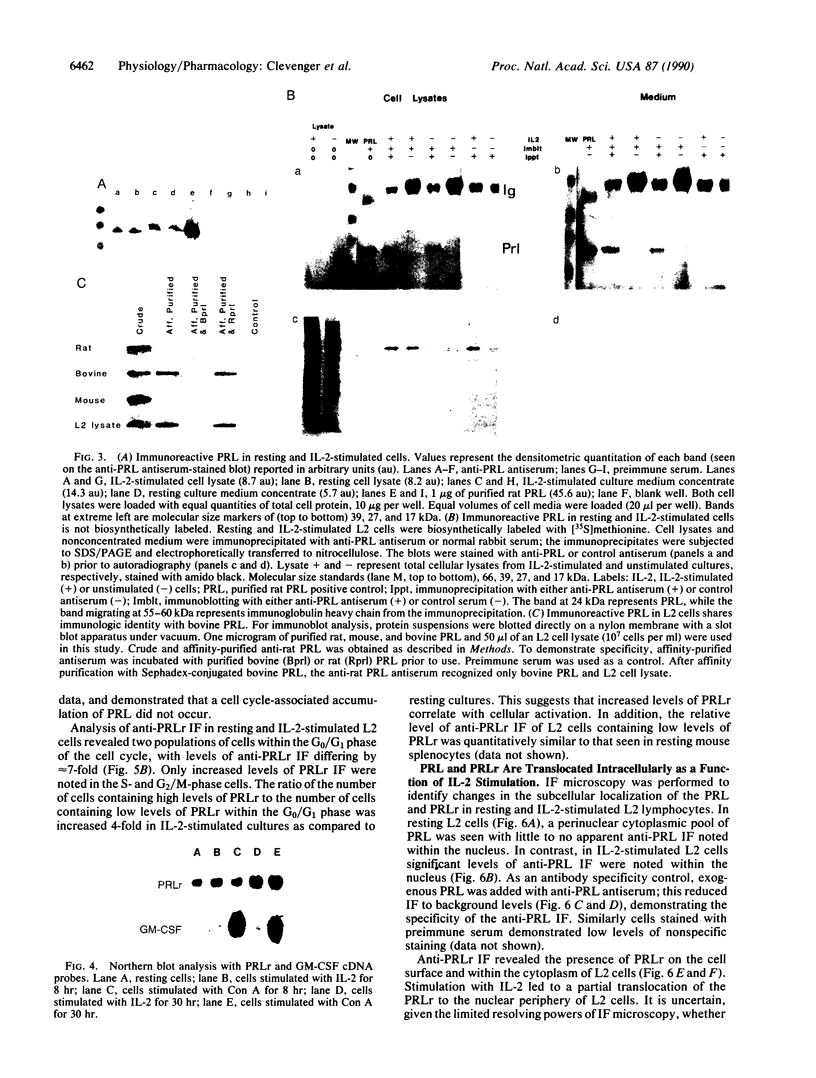
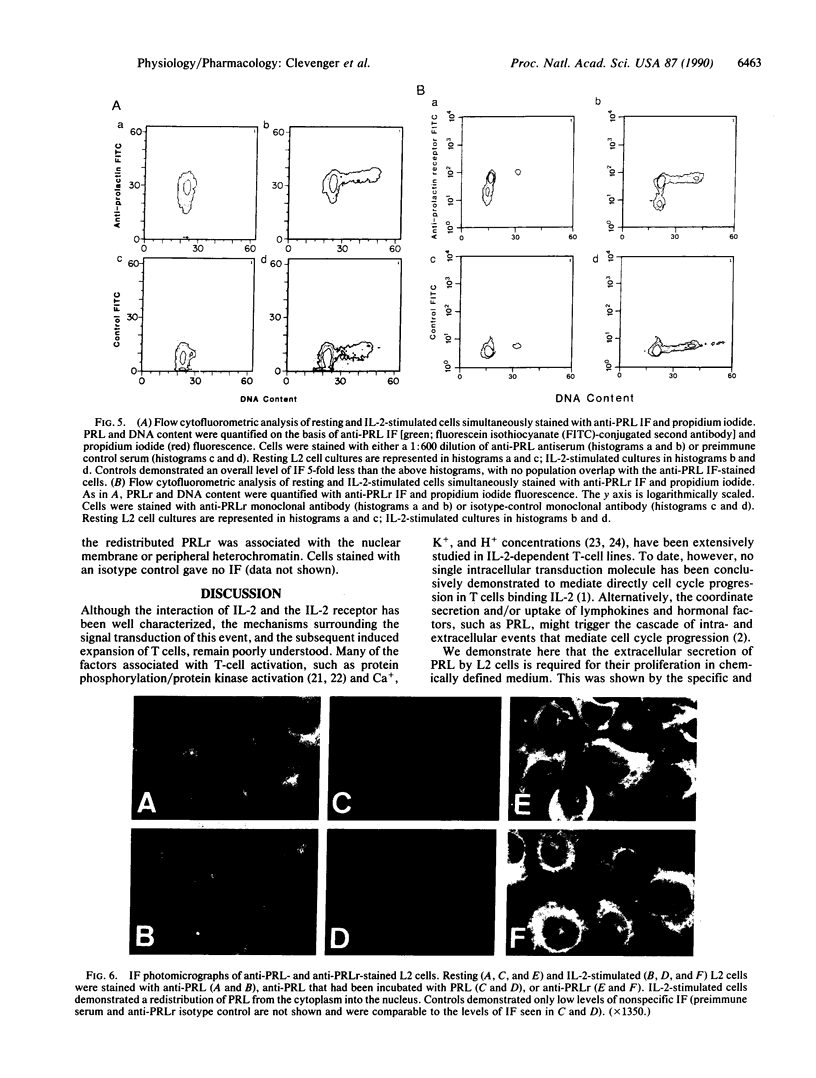
The requirement for prolactin in interleukin 2-driven T-cell proliferation was evaluated. Addition of an anti-prolactin antiserum resulted in the specific inhibition of T-cell proliferation in a time- and dose-dependent manner. Synthesis of prolactin and its mRNA, however, did not occur during interleukin 2 stimulation. Instead, previously internalized prolactin, presumably from fetal bovine serum, appears to serve as the source of prolactin under serum-free conditions. A 7-fold increase in a prolactin receptor occurred as a function of cell cycle progression; accumulation of a 1.6-kilobase prolactin receptor mRNA increased approximately 2-fold. Interleukin 2 stimulation induced the translocation of prolactin into the nucleus and prolactin receptor to the nuclear periphery. These data indicate that extracellular prolactin is requisite for T-cell proliferation and suggest that the effects of prolactin are exerted in the nucleus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berczi I., Nagy E., Kovacs K., Horvath E. Regulation of humoral immunity in rats by pituitary hormones. Acta Endocrinol (Copenh) 1981 Dec;98(4):506–513. doi: 10.1530/acta.0.0980506. [DOI] [PubMed] [Google Scholar]
- Bernton E. W., Meltzer M. S., Holaday J. W. Suppression of macrophage activation and T-lymphocyte function in hypoprolactinemic mice. Science. 1988 Jan 22;239(4838):401–404. doi: 10.1126/science.3122324. [DOI] [PubMed] [Google Scholar]
- Carrier M., Emery R. W., Wild-Mobley J., Perrotta N. J., Russell D. H., Copeland J. G. Prolactin as a marker of rejection in human heart transplantation. Transplant Proc. 1987 Aug;19(4):3442–3443. [PubMed] [Google Scholar]
- Clevenger C. V., Bauer K. D., Epstein A. L. A method for simultaneous nuclear immunofluorescence and DNA content quantitation using monoclonal antibodies and flow cytometry. Cytometry. 1985 May;6(3):208–214. doi: 10.1002/cyto.990060306. [DOI] [PubMed] [Google Scholar]
- Clevenger C. V., Epstein A. L., Bauer K. D. Modulation of the nuclear antigen p105 as a function of cell-cycle progression. J Cell Physiol. 1987 Mar;130(3):336–343. doi: 10.1002/jcp.1041300305. [DOI] [PubMed] [Google Scholar]
- Davis J. A., Linzer D. I. Autocrine stimulation of Nb2 cell proliferation by secreted, but not intracellular, prolactin. Mol Endocrinol. 1988 Aug;2(8):740–746. doi: 10.1210/mend-2-8-740. [DOI] [PubMed] [Google Scholar]
- Davis J. A., Linzer D. I. Expression of multiple forms of the prolactin receptor in mouse liver. Mol Endocrinol. 1989 Apr;3(4):674–680. doi: 10.1210/mend-3-4-674. [DOI] [PubMed] [Google Scholar]
- DiMattia G. E., Gellersen B., Bohnet H. G., Friesen H. G. A human B-lymphoblastoid cell line produces prolactin. Endocrinology. 1988 Jun;122(6):2508–2517. doi: 10.1210/endo-122-6-2508. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Anderson W. B. Interleukin-2 stimulates association of protein kinase C with plasma membrane. Nature. 1985 May 16;315(6016):233–235. doi: 10.1038/315233a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gelfand E. W., Mills G. B., Cheung R. K., Lee J. W., Grinstein S. Transmembrane ion fluxes during activation of human T lymphocytes: role of Ca2+, Na+/H+ exchange and phospholipid turnover. Immunol Rev. 1987 Feb;95:59–87. doi: 10.1111/j.1600-065x.1987.tb00500.x. [DOI] [PubMed] [Google Scholar]
- Giss B. J., Walker A. M. Mammotroph autoregulation: intracellular fate of internalized prolactin. Mol Cell Endocrinol. 1985 Oct;42(3):259–267. doi: 10.1016/0303-7207(85)90057-7. [DOI] [PubMed] [Google Scholar]
- Glick B. Interrelation of the avian immune and neuroendocrine systems. J Exp Zool. 1984 Dec;232(3):671–682. doi: 10.1002/jez.1402320336. [DOI] [PubMed] [Google Scholar]
- Hartmann D. P., Holaday J. W., Bernton E. W. Inhibition of lymphocyte proliferation by antibodies to prolactin. FASEB J. 1989 Aug;3(10):2194–2202. doi: 10.1096/fasebj.3.10.2787766. [DOI] [PubMed] [Google Scholar]
- Imboden J. B., Weiss A. The T-cell antigen receptor regulates sustained increases in cytoplasmic free Ca2+ through extracellular Ca2+ influx and ongoing intracellular Ca2+ mobilization. Biochem J. 1987 Nov 1;247(3):695–700. doi: 10.1042/bj2470695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe B. D., Sabath D. E., Johnson G. D., Moscinski L. C., Johnson K. R., Rovera G., Nauseef W. M., Prystowsky M. B. Myeloperoxidase and oncogene expression in GM-CSF induced bone marrow differentiation. Oncogene. 1988 Feb;2(2):167–174. [PubMed] [Google Scholar]
- Katoh M., Raguet S., Zachwieja J., Djiane J., Kelly P. A. Hepatic prolactin receptors in the rat: characterization using monoclonal antireceptor antibodies. Endocrinology. 1987 Feb;120(2):739–749. doi: 10.1210/endo-120-2-739. [DOI] [PubMed] [Google Scholar]
- Lee S. C., Sabath D. E., Deutsch C., Prystowsky M. B. Increased voltage-gated potassium conductance during interleukin 2-stimulated proliferation of a mouse helper T lymphocyte clone. J Cell Biol. 1986 Apr;102(4):1200–1208. doi: 10.1083/jcb.102.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum J. B., Infante A. J., Makker D. M., Yang F., Bowman B. H. Transferrin synthesis by inducer T lymphocytes. J Clin Invest. 1986 Mar;77(3):841–849. doi: 10.1172/JCI112381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger B., Weiss A., Imboden J., Laing T., Stobo J. D. The role of protein kinase C in transmembrane signaling by the T cell antigen receptor complex. Effects of stimulation with soluble or immobilized CD3 antibodies. J Immunol. 1987 Oct 15;139(8):2755–2760. [PubMed] [Google Scholar]
- Montgomery D. W., Zukoski C. F., Shah G. N., Buckley A. R., Pacholczyk T., Russell D. H. Concanavalin A-stimulated murine splenocytes produce a factor with prolactin-like bioactivity and immunoreactivity. Biochem Biophys Res Commun. 1987 Jun 15;145(2):692–698. doi: 10.1016/0006-291x(87)91020-5. [DOI] [PubMed] [Google Scholar]
- Neckers L. M., Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolin J. M. Incorporation of regulatory peptide hormones by individual cells of the adrenal cortex: prolactin-adrenocorticotrophin differences. Peptides. 1980 Fall;1(3):249–255. doi: 10.1016/0196-9781(80)90062-5. [DOI] [PubMed] [Google Scholar]
- Nolin J. M. Intracellular prolactin in rat corpus luteum and adrenal cortex. Endocrinology. 1978 Feb;102(2):402–406. doi: 10.1210/endo-102-2-402. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Kibler R., Matrisian L., Larson D. F., Poulos B., Magun B. E. Prolactin receptors on human T and B lymphocytes: antagonism of prolactin binding by cyclosporine. J Immunol. 1985 May;134(5):3027–3031. [PubMed] [Google Scholar]
- Sissom J. F., Eigenbrodt M. L., Porter J. C. Anti-growth action on mouse mammary and prostate glands of a monoclonal antibody to prolactin receptor. Am J Pathol. 1988 Dec;133(3):589–595. [PMC free article] [PubMed] [Google Scholar]