Abstract
Neonatal chronic lung disease, also known as bronchopulmonary dysplasia (BPD), is the most common complication of premature birth, affecting up to 30% of very low birth weight infants. Improved medical care has allowed for the survival of the most premature infants and has significantly changed the pathology of BPD from a disease marked by severe lung injury to the “new” form characterized by alveolar hypoplasia and impaired vascular development. However, increased patient survival has led to a paucity of pathologic specimens available from infants with BPD. This, combined with the lack of a system to model alveolarization in vitro, has resulted in a great need for animal models that mimic key features of the disease. To this end, a number of animal models have been created by exposing the immature lung to injuries induced by hyperoxia, mechanical stretch, and inflammation and most recently by the genetic modification of mice. These animal studies have 1) allowed insight into the mechanisms that determine alveolar growth, 2) delineated factors central to the pathogenesis of neonatal chronic lung disease, and 3) informed the development of new therapies. In this review, we summarize the key findings and limitations of the most common animal models of BPD and discuss how knowledge obtained from these studies has informed clinical care. Future studies should aim to provide a more complete understanding of the pathways that preserve and repair alveolar growth during injury, which might be translated into novel strategies to treat lung diseases in infants and adults.
Keywords: bronchopulmonary dysplasia, BPD, animal models, preterm neonates, lung development
Clinical Relevance
Bronchopulmonary dysplasia is a neonatal chronic lung disease characterized by alveolar hypoplasia and impaired pulmonary vascular development, and remains the most common complication of premature birth. This review describes the most extensively used animal models of chronic lung disease and summarizes how information obtained from these models has identified key molecular pathways that direct alveolarization; has elucidated how inflammation, mechanical ventilation, and oxygen treatment adversely affect these processes; and has been translated into novel treatments to care for premature infants.
Infants born prematurely frequently develop respiratory failure secondary to biochemical and structural immaturity of their lungs and insufficient respiratory drive. Although mechanical ventilation (MV) and O2–rich gas are life-saving treatments, these therapies also promote lung injury. Thus, more than 30% of preterm babies born before 30 weeks gestational age develop neonatal chronic lung disease, also known as bronchopulmonary dysplasia (BPD), a disease with significant morbidity and mortality. Infants with BPD are at increased risk for long-term hospitalization, recurrent respiratory ailments in early infancy, and life-long consequences from impaired pulmonary and neurologic development (1–8). Affected infants may remain O2 dependent for months, and although few remain O2 dependent beyond 2 years of age (4, 9), respiratory symptoms reflecting disturbed lung growth are often evident for many years (5, 9–14). Extreme prematurity even in the absence of BPD appears to have long-term effects on lung function, with the majority of very low birth weight (VLBW) infants without a history of BPD, demonstrating evidence of airway obstruction and diffusion defects years later (15). How BPD or prematurity alters pulmonary aging and whether this results in early lung function decline or increases the risk for adult lung diseases such as COPD is an ongoing discussion (8).
During the past two decades, advances in medical therapy have greatly improved the survival of premature infants. The use of antenatal steroids to accelerate lung maturation, the development of surfactant replacement therapy for acute respiratory failure, the institution of lung protective strategies of ventilation, and an optimization of nutritional support have contributed to an overall decrease in the mortality of VLBW infants. Accompanying this increase in survival, the clinical, radiographic, and pathological features of BPD have changed significantly. The lung pathology of infants with the “old form” of BPD originally described by Northway and colleagues (16) was characterized by evidence of severe lung injury, including inflammation, protein-rich lung edema, extensive airway epithelial metaplasia, peribronchial fibrosis, and marked airway and pulmonary vascular smooth muscle hypertrophy (17–19). In contrast, birth of VLBW infants during the canalicular and saccular stages of lung development appears to disrupt the normal program of alveolar and vascular development, resulting in the “new BPD,” characterized by alveolar simplification, dysmorphic capillaries, and increased in vascular and airway smooth muscle cells (20–23). Abnormal deposition of the extracellular matrix (ECM) components (e.g., elastin and collagen) and interstitial fluid accumulation are also observed (24–26).
Although of great clinical relevance, elucidating the pathophysiology of neonatal chronic lung disease in the postsurfactant era has become increasingly challenging. With the reduction in BPD-associated mortality, the availability of pathologic specimens has decreased. Furthermore, there is a paucity of in vitro systems that effectively model the complex 3-dimensional processes of alveolar formation and vascularization. Thus, defining the pathophysiology of BPD has relied, to a large extent, on the detailed observations made in animal models that mimic many features of this condition. Knowledge gained from these animal models has contributed great insight into the pathophysiology of the old and new forms of BPD and has led to changes in the clinical treatment regimen.
This review details some of the most extensively used animal models of BPD and summarizes the information learned from these models regarding the key molecular pathways (e.g., growth factor signaling) that direct alveolarization, vascular development, and ECM composition and how inflammation, MV, and O2 treatment adversely affect these processes. This is followed by a discussion of how data obtained from animal studies have been translated into current treatment strategies for the care of premature infants.
Overview of Animal Models of BPD
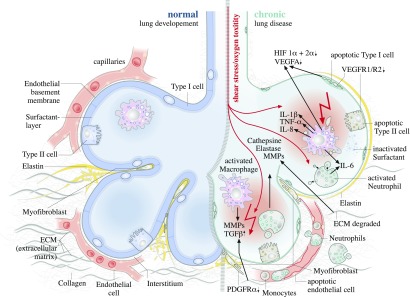
Numerous animal models have been developed to study the impairments in lung development that characterize BPD. Many of the commonly used models induce lung injury by 1) altering the amount of inspired O2, 2) applying stretch through MV, or 3) inducing pre- or postnatal inflammation. The recent development of genetically modified murine models has allowed the identification of key molecular pathways that direct the individual components of alveolarization. Figure 1 schematically summarizes key findings from animal studies, relating the effects of hyperoxia and mechanical stretch to the characteristic inflammatory changes reproduced by different models of BPD. The resulting remodeling of the ECM and alteration in growth factor signaling alters epithelial, endothelial, and (myo)fibroblast survival and proliferation, culminating in disruptions in pulmonary capillary development and distorted alveolarization.
Figure 1.
Pathophysiologic changes to the developing lung after exposure to oxygen and shear stress. Normal lung development is depicted on the Ieft; abnormal processes are depicted on the right. ECM, extracellular matrix; HIF, hypoxia-inducible factor; MMP, matrix metalloproteinase; VEGF-A, vascular endothelial growth factor A; VEGF-R1, vascular endothelial growth factor receptor 1; VEGF-R2, vascular endothelial growth factor receptor 2.
Hyperoxia
The recognition that high levels of inspired O2 are detrimental to the lung was made by investigators over one half century ago. After the seminal publication by Northway and colleagues describing the radiographic and histologic features of BPD, these same authors demonstrated that exposure of newborn guinea pigs to hyperoxia resulted in radiographic abnormalities that resembled the early stage of BPD in premature infants (27, 28). The injurious effects of O2 can be replicated in many species; however, rodents are particularly well suited to model BPD given that the newborn rodent is born during the saccular stage of lung development. Exposure of newborn mice to 100% O2 for 7 days was shown to result in an initial phase of acute injury (including pulmonary edema and hemorrhage), followed by a chronic repair phase, characterized by fibroblast proliferation and collagen deposition (29). Further studies demonstrated that hyperoxia not only induces lung injury but also disrupts lung structure, affecting alveolarization and vascularization. Exposure of newborn rats to 100% O2 during the first week of life causes airspace enlargement that remains evident weeks later (30). Subsequent experiments demonstrated that even more moderate amounts of O2 (60–85% FiO2) cause durable impairments in lung structure (31), alter growth factor expression, and decrease lung cell proliferation (32) (Figure 1).
These detrimental effects of hyperoxia on lung cells have been replicated in vitro. High levels of O2 suppress alveolar epithelial type II proliferation via posttranscriptional mechanisms (33). Oxygen levels above 50% also impair endothelial proliferation, in part via the inactivation of fibroblast growth factors (34); reduce the numbers of endothelial progenitor cells (EPCs) in the blood, lung, and bone marrow in neonatal mice (35); and alter the function of a side population of resident lung cells believed to have endothelial potential (36).
Many of the pulmonary responses to hyperoxia are developmentally regulated. Chronic O2 exposure induces opposite apoptotic and proliferative responses in neonatal and adult lung cells in rats (37). In mice, hyperoxia reduces EPCs in the developing lung but not in adult animals (35), and in clinical studies, hyperoxia impairs the growth of endothelial colony-forming cells obtained from preterm but not from term infants (38). Furthermore, O2–induced pulmonary vascular disease is age-dependent in mice, and neonatal exposure to hyperoxia appears to influence adult cardiopulmonary function and life-span in adult mice (39) and to alter pulmonary immune and oxidative stress responses (40, 41). Some of these sustained effects may be the results of epigenetic changes, related to the ability of O2 to alter histone deacetylase activity in the neonatal mouse lung (42), with evidence suggesting that DNA methylation mediates neonatal programming of O2 sensitivity in adulthood (43).
In summary, models exposing the immature lung to hyperoxia are clinically relevant. However, the O2 concentrations used in most of the above-mentioned studies exceed the levels of O2 supplementation currently applied to the preterm infant. In addition, animal models of hyperoxia lack the fluctuations in O2 concentrations that are clinically observed in preterm infants, which could potentially induce differences in molecular signaling not reproduced in the experimental setting. Nonetheless, the ability to standardize these models, the good reproducibility, and the ability to perform studies addressing long-term follow-up after neonatal hyperoxic injury make the model attractive to many researchers in the field and have allowed for its significant contributions to our current understanding in BPD.
Mechanical Stretch of the Premature Lung
The short-term MV of premature lambs and baboons models were initially developed to explore hyaline membrane disease (44, 45). Subsequently, the application of chronic MV resulted in the creation of novel animal models of BPD. An early study reported the effects of mechanically ventilating a small group of premature baboons with levels of O2 ranging from 95 to 100% for 8 to 17 days, all of which developed histopathologic evidence of BPD (46, 47). Further studies in this preterm baboon model reinforced the notion that hyperoxia is damaging to the immature lung by demonstrating the universal appearance of BPD in premature baboons ventilated for up to 11 days with an FiO2 of 1.0, in contrast to the absence of BPD in animals ventilated with O2 supplemented only on an as-needed basis (48). This premature baboon model, originally developed in the presurfactant era, has undergone modifications including antenatal exposure to maternal glucocorticoids, postnatal surfactant treatment, and assisted ventilation with more modest inflating pressures and concentrations of inspired O2, allowing it to better mimic the “new BPD” (49). A similar strategy of MV with O2–rich gas also induces lung pathology consistent with BPD in premature lambs (49–60). These studies in larger animals were integral in allowing mechanistic insight into the pathology of BPD and permitting the testing of therapeutic strategies currently used in the care of premature infants, including surfactant replacement therapy (61), high-frequency oscillatory ventilation (62), and nitric oxide (NO) (55).
Although the clinical condition and postnatal management of these premature animals closely resembled infants with BPD (49, 59), the costs and structural demands of these experiments using large animal models are substantial. Therefore, additional models of BPD were developed by applying similar strategies to smaller animals. MV of preterm rabbits was used to study hyaline membrane disease (63) and to evaluate therapeutic interventions such as surfactant replacement and inhaled glucocorticoids. MV of newborn rats induces airspace enlargement and decreases alveolar numbers by 24 hours (64), and a similar effect occurs with the MV of newborn mice (65). This latter model, although technically challenging given the small size of the mouse pups, allows investigators to take advantage of genetically modified mice to more mechanistically explore key molecular targets (66–68). However, limitations of these murine models include the application of these injuries in pups born at term and the inability to maintain these smaller animals for chronic ventilation experiments.
Figure 1 summarizes the inflammatory changes and disruption of the ECM that result from mechanical stretch on the developing lung, leading to alterations in growth factor signaling that affect endothelial and epithelial cells.
Infection, Inflammation, and Their Role in BPD
Evidence suggests that pre- and postnatal inflammation contribute to the pathogenesis of BPD. Clinical studies demonstrate an imbalance in pro- and antiinflammatory cytokines in tracheal aspirates from infants who later acquire BPD, which has been reviewed in detail (69, 70). The association between chorioamnionitis with the subsequent development of BPD (71) gave further support to the notion that, in some cases, BPD may have a prenatal inflammatory origin or may be aggravated by early inflammation (20, 72, 73). The cellular inflammatory response observed in experimental and clinical BPD is dominated by the influx of neutrophils and macrophages into the lungs and heightened neutrophil-derived elastase activity BPD (68–70, 74, 75). Neutrophils are activated in response to MV (75), and neutrophils are persistently elevated in the bronchoalveolar lavage fluid obtained from premature infants that develop BPD (71). LPS disrupts lung branching in early saccular mouse lung explants, an effect that depends on NF-κB activation in macrophages (72). Additional inflammatory cells also appear to play a role in BPD. Connective tissue mast cells accumulate in the lungs of preterm infants with BPD (73), and data suggest that autoreactive T cells may contribute to the premature baboon model of BPD (76).
Given that clinical studies cannot discriminate between the specific impacts of individual contributors to the pathogenesis of BPD, animal models using antenatal infection, endotoxin exposure, and/or transgenic mice have been developed to explore the link between lung inflammation and BPD. Antenatal endotoxin administration in rats (77) arrests alveolarization, and in preterm lambs, this effect appears to be time- and dose dependent (78). Studies in the premature baboons suggest that the efficacy of the immune response plays an important role in determining the outcome of perinatal inflammation of lung development. Animals that remain colonized with Ureaplasma urealyticum require more O2 and ventilatory support and show more severe lung inflammation at necropsy (54). In contrast, lung function and O2 need was less in preterm baboons that eradicated U. urealyticum. Even inflammation in the absence of infection leads to structural remodeling of the lung, including altered organization of ECM components, and impaired alveolarization and angiogenesis. The combination of hyperoxia and MV, a stimulus that impairs alveolarization, induces airway inflammation and cytokine expression in premature baboons (48), rodents, and rabbits (79–83). These inflammatory processes have been shown to be completely independent from the presence of pulmonary infections (84, 85). Moreover, the combination of these stimuli potentiates lung injury in sheep and mice (85, 86).
Additional animal studies have begun to elucidate the signaling molecules mediating these pathologic processes. Overexpression of IL-1β in lung epithelial cells disrupts postnatal lung morphogenesis in the mouse (88, 89). In this model, matrix metalloproteinase (MMP)-9 plays a protective role, with MMP-9 deficiency further exaggerating the alveolar hypoplasia (90). In mice, cytokine production induced by ante- or perinatal endotoxin directly correlates with toll-like receptor 4 expression (91). Intraamniotic endotoxin increases the levels of IFN-γ–inducible protein-10 and monokine induced by IFN-γ in preterm lamb lungs, leukocyte chemoattractants that are angiostatic (92). In contrast, intraamniotic exposure of mice in the early saccular stage of development stimulates angiogenesis and increases expression of the angiogenic cytokines monocyte chemoattractant protein-1 and macrophage inflammatory protein-1α in fetal lung explants (93). Figure 1 underlines the critical roles of inflammatory cells such as activated macrophages and neutrophils in the production of cytokines, proteases, and other injurious factors that remodel the ECM and disrupt alveolar and vascular development.
Disruption of Molecular Pathways Controlling Alveolarization
The introduction of genetically modified mouse models has allowed the elucidation of numerous signaling pathways that are essential for lung development. As an example, the dependency of alveolarization on pulmonary vascular development was first revealed by studies examining vascular endothelial growth factor signaling in mice (94). A comprehensive discussion of the molecular pathways contributing to alveolarization can be found in a number of excellent recent reviews (95, 96). Here we focus on how animal models have contributed to our understanding of key components of alveolar development and its pathology: 1) the development of the pulmonary capillary bed, 2) remodeling of the ECM, and 3) the role of (altered) growth factor signaling. Here, the transforming growth factor (TGF)-β/bone morphogenic protein (BMP) family serves as an excellent example because it plays a central role in vascular development and in matrix remodeling and is altered by hyperoxia, stretch, and inflammation.
Pathways Directing Vascular Development
Pathologic examination of lung tissue from animal models and patients with BPD reveals fewer small arteries and an abnormal distribution of vessels within the distal lung (97–99). These and other data led to the formation of the vascular hypothesis of BPD, wherein disruption of pulmonary angiogenesis induces the impaired alveolarization characteristic of BPD (20). In contrast, some studies have suggested that the pulmonary vascular network may be increased in BPD. Analysis of postmortem lungs from preterm and near-term infants exposed to short-term or long-term ventilation found that MV was associated with an expansion of the distal pulmonary circulation. However, this expanded network was abnormally formed, retaining the primitive dual capillary network characteristic of the saccular stage of development, with simplified nonbranching vessels (100). Data from LPS-treated mouse lung explants suggest that inflammation may simulate abnormal angiogenesis in the developing lung via the up-regulation of angiogenic CC chemokines such as macrophage inflammatory protein-1α and monocyte chemoattractant protein-1 (93).
Extensive experimental evidence derived from animal models has provided support for the notion that vascular development is an essential component of normal alveolarization and that stimuli that block pulmonary angiogenesis disrupt secondary septation. Administration of an antibody against platelet endothelial cell (EC) adhesion molecule-1 in neonatal rats inhibits EC function and disrupts alveolarization (101). Chronic ventilation of preterm baboons prevents the physiologic increase in platelet EC adhesion molecule-1 protein and capillary density normally observed in utero, in keeping with human studies that demonstrate that the capillary network fails to expand in patients with BPD. Additional studies using a variety of models have begun to identify the specific signaling pathways that drive angiogenesis in the developing lung. Two of the predominant and best-studied pathways are discussed in detail below.
Hypoxia-inducible factor.
Members of the hypoxia-inducible factor (HIF) family of transcription factors are tightly regulated by O2 tension and are key mediators of angiogenesis during development and disease. Under conditions of low O2 tension, HIF is stabilized, translocates into the nucleus, and binds to hypoxia-responsive elements located within the promoters of target genes, thus activating genes that increase O2 delivery and allow for the metabolic adaptation to hypoxia. The transition of the pulmonary circulation from the hypoxic fetal environment to the relatively hyperoxic postnatal state likely results in rapid molecular changes mediated via O2–sensitive pathways. HIF molecules play a key role in mediating the initial pulmonary adaptation and further lung maturation in part by regulating the expression of the HIF downstream target vascular endothelial growth factor (VEGF).
A role for the HIF pathway in regulating developmental angiogenesis was demonstrated by the targeted deletion of HIF family members in mice. Loss of HIF-1α in mice results in embryonic lethality at E10.5, with null embryos demonstrating multiple cardiac malformations and abnormally dilated blood vessels, perhaps due to the death of supporting mesenchymal cells (102). Targeted deletion of aryl hydrocarbon receptor nuclear translocator, the dimerization partner for HIF-1α and HIF-2α, also results in embryonic lethality at E10.5, with affected embryos displaying defective angiogenesis of the yolk sac and branchial arteries (103). Although embryonic lethality resulting from the absence of HIF-1α appears to be independent of VEGF, loss of HIF-2α in mice decreases alveolar epithelial cell expression of VEGF and causes fatal respiratory distress syndrome (RDS) due to insufficient surfactant production by alveolar type (AT)II cells (94), and a similar phenotype is induced by deleting the hypoxia-responsive elements located within the VEGF promoter.
In keeping with these data, preterm lambs suffering from RDS have decreased levels of HIF-1α and HIF-2α in association with diminished VEGF mRNA expression and a corresponding increase in the expression of the HIF inhibitor prolyl hydroxylase domain (PHD)-2 (104). In newborn mice, Hosford and Olson reported that HIF-2α gene expression increased during early alveolarization, but hyperoxia suppressed this increase (105). However, in that study, HIF-2α levels did not correlate with VEGF levels in the hyperoxia-exposed group, suggesting that additional factors may regulate VEGF in hyperoxia and that some effects of disrupted HIF-2α signaling may be independent of VEGF. Overexpression of an O2–insensitive form of HIF-2α in ATII cells also disrupts alveolarization, resulting in an abnormal accumulation of type II cells, decreased numbers of type I cells, and abnormalities in the pathways directing surfactant synthesis (106). These data suggest that HIF-2α may play a role in alveolar maturation and that tight spatial and temporal control of HIF-2α is essential for normal lung development. Stabilizing active HIF with the pharmacologic PHD inhibitor FG-4095 enhances angiogenesis of human lung microvascular ECs in vitro (107). Furthermore, augmenting HIF signaling in vivo by PHD inhibition ameliorates the physiologic consequences of BPD in preterm baboons, increasing alveolar surface area and improving oxygenation and lung compliance (108).
VEGF.
VEGF, a key EC mitogen and down-stream target of HIF, is a regulator of angiogenesis and fetal lung maturation. Studies in embryonic mouse tissue demonstrated that VEGF expression is temporally and spatially restricted and suggest that VEGF links airway and blood vessel formation by stimulating neovascularization at the leading edge of branching airways (109). Dysregulation of this temporal and spatial control of VEGF is detrimental to lung development. Overexpression of VEGF in alveolar epithelial cells causes embryonic mortality, increasing the growth of the pulmonary blood vessels but disrupting branching morphogenesis and ATI cell differentiation (110). However, intrauterine administration of anti–VEGF receptor 2 (VEGF-R2) antibodies later in embryonic development induces RDS, perhaps by blunting ATII surfactant production (94). Administration of similar antibodies in the perinatal period disrupts early alveolar development in mice (111). Inhibiting constitutive activation of NF-κB, a direct regulator of VEGF-R2 during alveolarization, impairs pulmonary angiogenesis and disrupts alveolarization in neonatal mice (112). Moreover, VEGF signaling appears to be important in maintaining lung structure even outside of lung development: chronic treatment of adult rats with the VEGF receptor blocker SU5416 induces alveolar septal cell apoptosis and causes airspace enlargement consistent with emphysema (113).
Decreased VEGF signaling may be one mechanism leading to the reduced pulmonary capillary volume and impaired alveolarization observed in very preterm baboons (52), and numerous studies have demonstrated an essential role for VEGF in murine embryonic vasculo- and alveologenesis. The expression of VEGF and one of its receptors, VEGF-R1, is decreased in the preterm baboon model of BPD. MV of 2- to 4-day-old mice with 40% O2 reduces gene and protein expression of VEGF-A and VEGF-R2 in the lung and results in lung structural abnormalities consistent with evolving BPD (114). These studies suggest that enhancement of VEGF may have therapeutic potential for BPD. In contrast to the effect observed during embryonic development, overexpression of VEGF in newborn rats increases survival, promotes lung angiogenesis, and prevents hyperoxia-induced alveolar damage (115). Furthermore, VEGF preserves endothelium-dependent vasodilation in fetal sheep, potentially by up-regulating endothelial NO synthase (eNOS) expression. These findings may be particularly important given that a subgroup of infants with BPD develop pulmonary hypertension.
The Role of ECM in Secondary Septation
The process of secondary septation, whereby secondary crests outgrow into the surrounding mesenchyme and allow for the division of the alveolar ducts into alveolar sacs and formation of the capillary bed, depend on the formation of a scaffold by the ECM, comprised of collagen, glycoproteins, and elastin (116, 117).
Elastin fiber assembly.
The onset of septa formation is heralded by the deposition of elastin at specific sites in the walls of the developing saccules, a paradigm that was informed in large part from data obtained from animal models. The essential role for elastin in lung development was established by studies showing that deletion of the Eln gene in mice leads to neonatal death from cardiorespiratory failure, reducing terminal airway branching and impairing pulmonary vasculogenesis (118, 119). The role of elastin in BPD was also outlined in the premature lamb model, where impaired alveolar and microvascular development is associated with an accumulation of excessive and disordered elastin (56). A similar abnormal accumulation of elastin is noted on lung pathology from premature infants who have died from BPD (22–24). Cyclic stretch of the lung during postnatal growth induces tropoelastin gene expression, thus leading to increased elastin deposition (57, 120–123). The abnormal abundance and distribution of elastin is especially notable in blunted secondary crests, areas where focal deposits of distal elastin normally define loci of future alveoli. Ventilation of newborn mice disrupts elastin deposition, increases elastase activity, and reduces the expression of proteins critical for elastic fiber assembly (114). Moreover, in this model, the alterations in elastin breakdown and deposition are associated with increased lung cell apoptosis and defective septation, similar to that observed in infants suffering from BPD (66). These changes to the ECM appear to be primarily induced by cyclic stretch in the absence of hyperoxia because similar studies have demonstrated that ventilating mice with air (21%) induces the same pathologic effects (66).
In contrast, animal models of BPD using severe and prolonged hyperoxia to induce lung injury showed varying results regarding pulmonary elastin and collagen expression. Hyperoxia inhibits fetal rat lung fibroblast proliferation, type I collagen gene expression, and net collagen production in vitro (124). However, other studies in neonatal mice and rats showed that hyperoxia increases pulmonary type I collagen, tropoelastin, and connective tissue growth factor, a fibroblast mitogen that promotes collagen deposition (125, 126).
Data from clinical studies support the notion that increased degradation of elastin is associated with lung injury and the development of BPD. Pulmonary inflammation increases elastase activity in the tracheal aspirates of premature infants, which likely impairs elastin deposition and alveolar formation by enhancing elastin breakdown (71, 127, 128). This idea is reinforced by the observation that infants with BPD, and mice and lambs exposed to cyclic stretch with O2–rich gas, exhibit elevated levels of urine desmosine, a sensitive marker of elastin breakdown (57, 127, 129–130).
ECM remodeling.
Remodeling of the ECM is primarily driven by serine proteases (e.g., neutrophil proteases and trypsin), MMPs, and the papain family of proteases (cathepsin B, H, K, L, and S). MMP-1, MMP-2, and MMP-9 are strongly expressed during alveolarization in humans and mice (131, 132), and degradation of the ECM is necessary for angiogenesis. However, enhanced proteolytic activity appears to be detrimental to the developing lung. Increased lung levels of MMPs (133), cathepsins, and cathepsin activity in bronchoalveolar lavage fluid are found in baboon models of BPD (134, 135). Moreover, the balance of matrix proteases and their inhibitors may have a broader effect on lung function outside of influencing ECM remodeling. For example, the serine protease inhibitor B1, expressed by neutrophils and macrophages, protects against the degradation of surfactant proteins by neutrophil elastase in baboon models of BPD (135). Further studies are necessary to clarify how proteolytic activity in the lung is tightly regulated during normal development and the mechanisms that alter this regulation during BPD.
The TGF-β/BMP Superfamily in Normal and Abnormal Lung Development
The TGF-β superfamily, including the BMPs, plays an important role in lung development by influencing the cellular composition of the lung via the regulation of endothelial and epithelial cell survival (136) and regulating ECM production and remodeling.
The expression and localization of TGF-β/BMP family members change significantly during lung development in mice and humans (137, 138). TGF-β is activated in the vascular and airway smooth muscle and in the alveolar and airway epithelium throughout late lung development, and active TGF-β signaling is required for normal late lung development (95). Similarly, BMP signaling is increased during the saccular and alveolar stages of lung development, suggesting a role for BMP in septal and vascular development (138). BMP family members stimulate the proliferation and migration of ECs by increasing the expression of angiogenic factors such as VEGF-R2 (139) and stimulate pulmonary EC angiogenesis by activating the Wingless (Wnt) signaling pathway (140).
Studies in murine models where key components of TGF-β/BMP signaling were disrupted have highlighted the importance of these pathways on alveolar development. The absence of latent TGF-β binding protein-3, a modulator of TGF-β secretion and activation, transiently decreases TGF-β activity in the lung at postnatal Days 4 to 6, altering cell proliferation, inhibiting septation, and inducing emphysematous changes in the lung parenchyma (141). Epithelial cell–specific abrogation of Alk3-mediated BMP signaling in the prenatal lung disrupts distal airway formation in mice (142). The functions of TGF-β in the lung are cell specific, with disruption of TGF-β type II receptor in epithelial cells delaying postnatal lung alveolarization and with disruption in mesenchymal cells resulting in mildly abnormal lung branching and organ defects such as diaphragmatic hernia (143). Overexpression of TGF-β1 in the newborn rat lung induces pathologic changes consistent with BPD (144), which are prevented by adenoviral-mediated gene transfer of decorin, a natural inhibitor of TGF-β activity (145, 146). In contrast, null mutation of Smad3, a key downstream effector of the TGF-β pathway, initially results in a subtle lung phenotype characterized by failure of normal organization of the ECs. However, this early phenotype culminates in the development of pulmonary emphysema in association with excessive MMP activity (147, 148). Thus, the balance of TGF-β signaling appears to be critical to alveolar development, with both excessive and insufficient TGF-β activity being detrimental to distal lung growth.
TGF-β signaling is disrupted in animal models of BPD. Ventilation increases the expression of TGF-β in preterm lambs as early as 1 day after initiation of MV (149). The impaired alveolarization and decreased respiratory compliance observed in neonatal mice exposed to hyperoxia are associated with potentiated TGF-β, but diminished BMP signaling and a similar imbalance in TGF-β/BMP signaling have been observed in murine lung epithelial cells and primary lung fibroblasts exposed to 85% O2 in vitro. As a counter player to fibroblast growth factor-β and IL-1β, factors that lower elastin mRNA in human lung fibroblasts, TGF-β increases elastin by transcriptional and posttranscriptional mechanisms (150). Exposure of primary lung fibroblasts to 85% O2 enhances the TGF-β–stimulated production of tropoelastin as well as type I collagen-α, tissue inhibitor of metalloproteinase-1, and tenascin-C (138). Exposure of primary alveolar type (AT)II cells to 85% O2 increases the susceptibility to TGF-β–induced apoptosis, whereas primary pulmonary artery smooth muscle cells are unaffected (138). Combined with evidence showing that TGF-β impairs keratinocyte growth factor–stimulated proliferation of ATII cells, these data suggest that increases in TGF-β induced by hyperoxia may contribute to the alveolar hypoplasia characteristic of BPD by enhancing apoptosis and decreasing proliferation of ATII cells (151).
From Bench to Bedside: The Use of Animal Models to Discover and Implement New Treatment Strategies
Numerous therapeutic strategies currently in use or being investigated to reduce the incidence of BPD in premature infants have evolved, in large part, from data derived from animal studies. Important trials tested the feasibility and effectiveness of nasal continuous positive airway pressure in premature baboons and sheep as a means of minimizing the need for MV, decreasing lung inflammation, and reducing or preventing chronic lung injury (56, 152). Surfactant replacement therapy was first demonstrated to be efficacious in animal models of RDS (153), improving alveolar expansion and reducing hyaline membrane disease in premature primates (154), and improving oxygenation and ventilation in premature lambs (155). These and similar studies provided the rationale for clinical trials of surfactant replacement therapy, including reports of short-term increases in oxygenation in preterm infants after surfactant replacement (156, 157), a randomized controlled trial demonstrating significantly reductions in mortality (158), and a report about the development of moderate to severe BPD (159, 160). Similarly, animal models of RDS were integral in establishing the beneficial effects of antenatal glucocorticoids, including improvements in lung function, decreases in lung water content, increases in mean alveolar volumes, and maturation of the surfactant system (161–163). Conversely, experimental studies have highlighted the detrimental effects of postnatal glucocorticoid administration on cerebral development and myelination (164, 165), cardiac and renal function, and lifespan (166–168), thus corroborating results from clinical studies that demonstrated short-term (e.g., hyperglycemia, hypertension, growth failure) and long-term (e.g., cerebral palsy, developmental delay) adverse effects of postnatal steroids (169, 170). Data derived from animal studies also supported a key role for retinoids in lung development and repair after injury (171–178), in keeping with results from randomized, controlled trials that showed that retinol treatment in VLBW infants significantly decreases the incidence of BPD (179, 180).
The role of NO in the regulation of perinatal pulmonary vascular tone has been well established, and animal models have helped to inform the notion that decreased NO synthesis contributes to the development of BPD (181, 182). Prolonged MV of premature lambs and baboons diminishes NOS activity and eNOS expression (53, 60, 181), and deficiency of eNOS exaggerates the adverse effects of hypoxia on alveolarization (183). Furthermore, administration of inhaled NO (iNO) to mechanically ventilated preterm lambs and baboons improves lung function, increases alveolar counts (55, 184), and reduces EC apoptosis in neonatal rats after VEGF inhibition (185). However, although data from these experimental studies suggested that iNO may decrease the incidence of death and BPD in premature infants (184, 186), results from clinical trials failed to demonstrate a clear benefit of iNO on the development of BPD (187, 188).
Animal models remain essential for the investigation and testing of novel strategies to prevent or treat BPD. There is great interest in studying whether cell-based therapies could be an effective treatment for BPD, including mesenchymal stem cells, human amniotic fluid stem cells, and EPCs, comprehensively detailed in a recent review (189). Intravenous or intratracheal administration of bone marrow stromal cells (BMSCs) or the conditioned media from these cells blunts the adverse effects of hyperoxia on the immature mouse lung (190, 191). In both studies, engraftment was low, and the beneficial effects of the BMSC conditioned media were comparable to or better than that of the BMSCs, suggesting the importance of a paracrine-mediated mechanism. Further experimental studies are needed to delineate the appropriate dose and timing of administration and to provide long-term safety and efficacy data before translating these studies to the clinic.
Conclusions
What Have We Learned?
During the past quarter-century, advances in medical care of premature infants, including the widespread use of antenatal steroids and surfactant, has greatly improved the survival of VLBW infants. However, with this increase in survival, the main focus of perinatal care is now on the prevention of long-term complications of prematurity. nCLD, the result of lung injury induced by O2, mechanical stretch, and inflammation superimposed on functionally and structural immature lung, is one of the most important causes of impaired long-term outcome in the preterm infant. The increasing paucity of human tissue available from patients with BPD and the absence of models that can mimic alveolarization in vitro have resulted in the development of numerous animal models that have been used to investigate the pathogenesis of BPD. Information obtained using these models has permitted the identification of stimuli (e.g., O2, stretch, and inflammation), which are highly disruptive to the normal program of alveolar development. The development of genetically modified mice has permitted the elucidation of numerous molecular pathways that direct key components of secondary septation, including the expansion of the pulmonary vascular bed via angiogenesis and the remodeling of the ECM, and how alterations in growth factor pathways, such as TGF-β/BMP, influence alveolar development. These models have been integral in the development and/or implementation of therapies that are part of the standard of care for VLBW infants, including antenatal glucocorticoids, surfactant replacement, lung protective and noninvasive strategies of ventilation, and retinol therapy.
Data from animal studies have been integral in highlighting that secondary septation requires the complex interplay between multiple cell types within the lung, allowing for coordinated development of the pulmonary capillary bed and terminal airspaces, orchestrated in part by the deposition and remodeling of ECM. Distinct molecular pathways direct the individual components of secondary septation, and abrogation of a single pathway is often sufficient to disrupt the entire process of alveolarization. To this end, a number of injuries, including hyperoxia, mechanical stretch, and inflammation, impair alveolarization and contribute to BPD by altering one or more of these molecular pathways, resulting in impaired lung development by altering the differentiation, proliferation, and migration of epithelial cells, ECs, and myofibroblasts. These injuries alter central growth factor signaling cascades that not only affect cell survival and fate but also ECM composition.
Moving forward, the challenge will be not only to understand the mechanisms regulating normal lung growth but also to begin to clarify the pathways that mediate lung repair and regeneration after injury. Focus is needed on understanding how the separate molecular pathways regulating alveolarization interact in a cell-specific manner at each stage of development and during injury and repair given that the global augmentation or inhibition of these signaling cascades (e.g., HIF and TGF-β/BMP) may negatively affect physiologic developmental and regenerative processes. Here, the identification of biomarkers that might herald the onset of BPD at early stages of the disease will be critical for the design of personalized treatment regimens. The mechanisms by which clinical variables, such as intrauterine growth retardation (192–194) and gender (195), influence the risk for BPD need to be better addressed in animal studies. Similarly, a better understanding of the impact of the genetics on this heterogeneous disease, as suggested by twin (196) and genetic association studies (197, 198), is critical and may lead to developing individualized treatments to more successfully prevent or treat BPD.
Further refinement and modification of the current animal models to better mimic the human condition is important. This would include limiting the levels of O2 used to cause lung injury in animals to those currently used in preterm infants and developing models in which fluctuations of O2 saturation are experimentally induced. In addition, a comparison of the structural abnormalities induced observed in animal models (i.e., sheep or baboons) at different stages of gestation treated with therapies more similar to current standard of care (steroids, surfactant, etc.) might allow a better understanding of whether the “new” BPD is the result of the greater immaturity of the infant at birth or a reflection in the changes in medical care of premature infants that has occurred over the past 2 decades. Similar studies may also be helpful in understanding why late preterm infants (32–36 wk gestational age) present with more long-term pulmonary and neurologic complications than anticipated. Finally, because the earliest survivors of BPD are reaching adulthood, evidence is emerging that suggests that early injuries during alveolar development may represent the childhood antecedent of adult lung disease. Thus, the creation of “double-hit” animal models of disease could be integral in understanding whether mild injuries during lung development modulate physiologic aging of the lung or alter the susceptibility to additional injuries later in life.
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2013-0014TR on September 11, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 2.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 3.Doyle LW, Cheung MM, Ford GW, Olinsky A, Davis NM, Callanan C. Birth weight <1501 g and respiratory health at age 14. Arch Dis Child. 2001;84:40–44. doi: 10.1136/adc.84.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenough A, Alexander J, Burgess S, Chetcuti PA, Cox S, Lenney W, Turnbull F, Shaw NJ, Woods A, Boorman J, et al. Home oxygen status and rehospitalisation and primary care requirements of infants with chronic lung disease. Arch Dis Child. 2002;86:40–43. doi: 10.1136/adc.86.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gross SJ, Iannuzzi DM, Kveselis DA, Anbar RD. Effect of preterm birth on pulmonary function at school age: a prospective controlled study. J Pediatr. 1998;133:188–192. doi: 10.1016/s0022-3476(98)70219-7. [DOI] [PubMed] [Google Scholar]
- 6.Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. Impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk for chronic lung disease. Pediatrics. 2005;115:655–661. doi: 10.1542/peds.2004-1238. [DOI] [PubMed] [Google Scholar]
- 7.Skidmore MD, Rivers A, Hack M. Increased risk of cerebral palsy among very low-birthweight infants with chronic lung disease. Dev Med Child Neurol. 1990;32:325–332. doi: 10.1111/j.1469-8749.1990.tb16944.x. [DOI] [PubMed] [Google Scholar]
- 8.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 9.Greenough A, Alexander J, Burgess S, Bytham J, Chetcuti PA, Hagan J, Lenney W, Melville S, Shaw NJ, Boorman J, et al. Preschool healthcare utilisation related to home oxygen status. Arch Dis Child Fetal Neonatal Ed. 2006;91:F337–F341. doi: 10.1136/adc.2005.088823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Northway WH, Jr, Moss RB, Carlisle KB, Parker BR, Popp RL, Pitlick PT, Eichler I, Lamm RL, Brown BW., Jr Late pulmonary sequelae of bronchopulmonary dysplasia. N Engl J Med. 1990;323:1793–1799. doi: 10.1056/NEJM199012273232603. [DOI] [PubMed] [Google Scholar]
- 11.Greenough A, Dimitriou G, Bhat RY, Broughton S, Hannam S, Rafferty GF, Leipälä JA. Lung volumes in infants who had mild to moderate bronchopulmonary dysplasia. Eur J Pediatr. 2005;164:583–586. doi: 10.1007/s00431-005-1706-z. [DOI] [PubMed] [Google Scholar]
- 12.Hjalmarson O, Sandberg KL. Lung function at term reflects severity of bronchopulmonary dysplasia. J Pediatr. 2005;146:86–90. doi: 10.1016/j.jpeds.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 13.Welsh L, Kirkby J, Lum S, Odendaal D, Marlow N, Derrick G, Stocks J EPICure Study Group. The EPICure study: maximal exercise and physical activity in school children born extremely preterm. Thorax. 2010;65:165–172. doi: 10.1136/thx.2008.107474. [DOI] [PubMed] [Google Scholar]
- 14.Filippone M, Carraro S, Baraldi E.From BPD to COPD? The hypothesis is intriguing but we lack lung pathology data in humans Eur Respir J 2010351419–1420.author reply 1420 [DOI] [PubMed] [Google Scholar]
- 15.Cazzato S, Ridolfi L, Bernardi F, Faldella G, Bertelli L. Lung function outcome at school age in very low birth weight children. Pediatr Pulmonol. 2013;48:830–837. doi: 10.1002/ppul.22676. [DOI] [PubMed] [Google Scholar]
- 16.Northway WH, Jr,, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 17.Chambers HM, van Velzen D. Ventilator-related pathology in the extremely immature lung. Pathology. 1989;21:79–83. doi: 10.3109/00313028909059539. [DOI] [PubMed] [Google Scholar]
- 18.Hislop AA, Haworth SG. Pulmonary vascular damage and the development of cor pulmonale following hyaline membrane disease. Pediatr Pulmonol. 1990;9:152–161. doi: 10.1002/ppul.1950090306. [DOI] [PubMed] [Google Scholar]
- 19.Van Lierde S, Cornelis A, Devlieger H, Moerman P, Lauweryns J, Eggermont E. Different patterns of pulmonary sequelae after hyaline membrane disease: heterogeneity of bronchopulmonary dysplasia? A clinicopathologic study. Biol Neonate. 1991;60:152–162. doi: 10.1159/000243402. [DOI] [PubMed] [Google Scholar]
- 20.Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res. 1999;46:641–643. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol. 1998;29:710–717. doi: 10.1016/s0046-8177(98)90280-5. [DOI] [PubMed] [Google Scholar]
- 23.Margraf LR, Tomashefski JF, Jr, Bruce MC, Dahms BB. Morphometric analysis of the lung in bronchopulmonary dysplasia. Am Rev Respir Dis. 1991;143:391–400. doi: 10.1164/ajrccm/143.2.391. [DOI] [PubMed] [Google Scholar]
- 24.Thibeault DW, Mabry SM, Ekekezie II, Truog WE. Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics. 2000;106:1452–1459. doi: 10.1542/peds.106.6.1452. [DOI] [PubMed] [Google Scholar]
- 25.Thibeault DW, Mabry SM, Ekekezie II, Zhang X, Truog WE. Collagen scaffolding during development and its deformation with chronic lung disease. Pediatrics. 2003;111:766–776. doi: 10.1542/peds.111.4.766. [DOI] [PubMed] [Google Scholar]
- 26.Turner BS, Bradshaw W, Brandon D.Neonatal lung remodeling: structural, inflammatory, and ventilator-induced injury J Perinat Neonatal Nurs 200519362–376.quiz 377–368 [DOI] [PubMed] [Google Scholar]
- 27.Han RN, Buch S, Tseu I, Young J, Christie NA, Frndova H, Lye SJ, Post M, Tanswell AK. Changes in structure, mechanics, and insulin-like growth factor-related gene expression in the lungs of newborn rats exposed to air or 60% oxygen. Pediatr Res. 1996;39:921–929. doi: 10.1203/00006450-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Northway WH, Jr, Rosan RC, Shahinian L, Jr, Castellino RA, Gyepes MT, Durbridge T. Radiologic and histologic investigation of pulmonary oxygen toxicity in newborn guinea pigs. Invest Radiol. 1969;4:148–155. doi: 10.1097/00004424-196905000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Bonikos DS, Bensch KG, Ludwin SK, Northway WH., Jr Oxygen toxicity in the newborn: the effect of prolonged 100 per cent O2 exposure on the lungs of newborn mice. Lab Invest. 1975;32:619–635. [PubMed] [Google Scholar]
- 30.Randell SH, Mercer RR, Young SL. Neonatal hyperoxia alters the pulmonary alveolar and capillary structure of 40-day-old rats. Am J Pathol. 1990;136:1259–1266. [PMC free article] [PubMed] [Google Scholar]
- 31.Dauger S, Ferkdadji L, Saumon G, Vardon G, Peuchmaur M, Gaultier C, Gallego J. Neonatal exposure to 65% oxygen durably impairs lung architecture and breathing pattern in adult mice. Chest. 2003;123:530–538. doi: 10.1378/chest.123.2.530. [DOI] [PubMed] [Google Scholar]
- 32.Warner BB, Stuart LA, Papes RA, Wispé JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol. 1998;275:L110–L117. doi: 10.1152/ajplung.1998.275.1.L110. [DOI] [PubMed] [Google Scholar]
- 33.Clement A, Edeas M, Chadelat K, Brody JS. Inhibition of lung epithelial cell proliferation by hyperoxia: posttranscriptional regulation of proliferation-related genes. J Clin Invest. 1992;90:1812–1818. doi: 10.1172/JCI116056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grant MM, Koo HC, Rosenfeld W. Oxygen affects human endothelial cell proliferation by inactivation of fibroblast growth factors. Am J Physiol. 1992;263:L370–L375. doi: 10.1152/ajplung.1992.263.3.L370. [DOI] [PubMed] [Google Scholar]
- 35.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1073–L1084. doi: 10.1152/ajplung.00347.2006. [DOI] [PubMed] [Google Scholar]
- 36.Irwin D, Helm K, Campbell N, Imamura M, Fagan K, Harral J, Carr M, Young KA, Klemm D, Gebb S, et al. Neonatal lung side population cells demonstrate endothelial potential and are altered in response to hyperoxia-induced lung simplification. Am J Physiol Lung Cell Mol Physiol. 2007;293:L941–L951. doi: 10.1152/ajplung.00054.2007. [DOI] [PubMed] [Google Scholar]
- 37.Belik J, Jankov RP, Pan J, Tanswell AK. Chronic O2 exposure enhances vascular and airway smooth muscle contraction in the newborn but not adult rat. J Appl Physiol. 2003;94:2303–2312. doi: 10.1152/japplphysiol.00820.2002. [DOI] [PubMed] [Google Scholar]
- 38.Baker CD, Ryan SL, Ingram DA, Seedorf GJ, Abman SH, Balasubramaniam V. Endothelial colony-forming cells from preterm infants are increased and more susceptible to hyperoxia. Am J Respir Crit Care Med. 2009;180:454–461. doi: 10.1164/rccm.200901-0115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yee M, White RJ, Awad HA, Bates WA, McGrath-Morrow SA, O’Reilly MA. Neonatal hyperoxia causes pulmonary vascular disease and shortens life span in aging mice. Am J Pathol. 2011;178:2601–2610. doi: 10.1016/j.ajpath.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filippone M, Bonetto G, Corradi M, Frigo AC, Baraldi E. Evidence of unexpected oxidative stress in airways of adolescents born very pre-term. Eur Respir J. 2012;40:1253–1259. doi: 10.1183/09031936.00185511. [DOI] [PubMed] [Google Scholar]
- 41.Buczynski BW, Yee M, Paige Lawrence B, O’Reilly MA. Lung development and the host response to influenza A virus are altered by different doses of neonatal oxygen in mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1078–L1087. doi: 10.1152/ajplung.00026.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Londhe VA, Sundar IK, Lopez B, Maisonet TM, Yu Y, Aghai ZH, Rahman I. Hyperoxia impairs alveolar formation and induces senescence through decreased histone deacetylase activity and up-regulation of p21 in neonatal mouse lung. Pediatr Res. 2011;69:371–377. doi: 10.1203/PDR.0b013e318211c917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nanduri J, Makarenko V, Reddy VD, Yuan G, Pawar A, Wang N, Khan SA, Zhang X, Kinsman B, Peng YJ, et al. Epigenetic regulation of hypoxic sensing disrupts cardiorespiratory homeostasis. Proc Natl Acad Sci USA. 2012;109:2515–2520. doi: 10.1073/pnas.1120600109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boros SJ, Matalon SV, Ewald R, Leonard AS, Hunt CE. The effect of independent variations in inspiratory-expiratory ratio and end expiratory pressure during mechanical ventilation in hyaline membrane disease: the significance of mean airway pressure. J Pediatr. 1977;91:794–798. doi: 10.1016/s0022-3476(77)81044-5. [DOI] [PubMed] [Google Scholar]
- 45.Normand IC, Reynolds EO, Strang LB, Wigglesworth JS. Flow and protein concentration of lymph from lungs of lambs developing hyaline membrane disease. Arch Dis Child. 1968;43:334–339. doi: 10.1136/adc.43.229.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escobedo MB, Hilliard JL, Smith F, Meredith K, Walsh W, Johnson D, Coalson JJ, Kuehl TJ, Null DM, Jr, Robotham JL. A baboon model of bronchopulmonary dysplasia: I. Clinical features. Exp Mol Pathol. 1982;37:323–334. doi: 10.1016/0014-4800(82)90045-4. [DOI] [PubMed] [Google Scholar]
- 47.Coalson JJ, Kuehl TJ, Escobedo MB, Hilliard JL, Smith F, Meredith K, Null DM, Jr, Walsh W, Johnson D, Robotham JL. A baboon model of bronchopulmonary dysplasia: II. Pathologic features. Exp Mol Pathol. 1982;37:335–350. doi: 10.1016/0014-4800(82)90046-6. [DOI] [PubMed] [Google Scholar]
- 48.Delemos RA, Coalson JJ, Gerstmann DR, Kuehl TJ, Null DM., Jr Oxygen toxicity in the premature baboon with hyaline membrane disease. Am Rev Respir Dis. 1987;136:677–682. doi: 10.1164/ajrccm/136.3.677. [DOI] [PubMed] [Google Scholar]
- 49.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med. 1999;160:1333–1346. doi: 10.1164/ajrccm.160.4.9810071. [DOI] [PubMed] [Google Scholar]
- 50.Awasthi S, Coalson JJ, Yoder BA, Crouch E, King RJ. Deficiencies in lung surfactant proteins A and D are associated with lung infection in very premature neonatal baboons. Am J Respir Crit Care Med. 2001;163:389–397. doi: 10.1164/ajrccm.163.2.2004168. [DOI] [PubMed] [Google Scholar]
- 51.Yoder BA, Siler-Khodr T, Winter VT, Coalson JJ. High-frequency oscillatory ventilation: effects on lung function, mechanics, and airway cytokines in the immature baboon model for neonatal chronic lung disease. Am J Respir Crit Care Med. 2000;162:1867–1876. doi: 10.1164/ajrccm.162.5.9912145. [DOI] [PubMed] [Google Scholar]
- 52.Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol. 2002;282:L811–L823. doi: 10.1152/ajplung.00325.2001. [DOI] [PubMed] [Google Scholar]
- 53.Afshar S, Gibson LL, Yuhanna IS, Sherman TS, Kerecman JD, Grubb PH, Yoder BA, McCurnin DC, Shaul PW. Pulmonary no synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2003;284:L749–L758. doi: 10.1152/ajplung.00334.2002. [DOI] [PubMed] [Google Scholar]
- 54.Yoder BA, Coalson JJ, Winter VT, Siler-Khodr T, Duffy LB, Cassell GH. Effects of antenatal colonization with ureaplasma urealyticum on pulmonary disease in the immature baboon. Pediatr Res. 2003;54:797–807. doi: 10.1203/01.PDR.0000091284.84322.16. [DOI] [PubMed] [Google Scholar]
- 55.McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, Kerecman JD, Albertine KH, Winter VT, Coalson JJ, et al. Inhaled no improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2005;288:L450–L459. doi: 10.1152/ajplung.00347.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomson MA, Yoder BA, Winter VT, Martin H, Catland D, Siler-Khodr TM, Coalson JJ. Treatment of immature baboons for 28 days with early nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2004;169:1054–1062. doi: 10.1164/rccm.200309-1276OC. [DOI] [PubMed] [Google Scholar]
- 57.Pierce RA, Albertine KH, Starcher BC, Bohnsack JF, Carlton DP, Bland RD. Chronic lung injury in preterm lambs: disordered pulmonary elastin deposition. Am J Physiol. 1997;272:L452–L460. doi: 10.1152/ajplung.1997.272.3.L452. [DOI] [PubMed] [Google Scholar]
- 58.Albertine KH, Jones GP, Starcher BC, Bohnsack JF, Davis PL, Cho SC, Carlton DP, Bland RD. Chronic lung injury in preterm lambs: disordered respiratory tract development. Am J Respir Crit Care Med. 1999;159:945–958. doi: 10.1164/ajrccm.159.3.9804027. [DOI] [PubMed] [Google Scholar]
- 59.Bland RD, Albertine KH, Carlton DP, Kullama L, Davis P, Cho SC, Kim BI, Dahl M, Tabatabaei N. Chronic lung injury in preterm lambs: abnormalities of the pulmonary circulation and lung fluid balance. Pediatr Res. 2000;48:64–74. doi: 10.1203/00006450-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 60.Bland RD, Ling CY, Albertine KH, Carlton DP, MacRitchie AJ, Day RW, Dahl MJ. Pulmonary vascular dysfunction in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2003;285:L76–L85. doi: 10.1152/ajplung.00395.2002. [DOI] [PubMed] [Google Scholar]
- 61.Shimada S, Raju TN, Vidyasagar D, Maeta H, Bhat R. Chest radiographic course after exogenous surfactant therapy in baboons with respiratory distress syndrome. Crit Care Med. 1990;18:969–973. doi: 10.1097/00003246-199009000-00013. [DOI] [PubMed] [Google Scholar]
- 62.Kinsella JP, Gerstmann DR, Clark RH, Null DM, Jr, Morrow WR, Taylor AF, deLemos RA. High-frequency oscillatory ventilation versus intermittent mandatory ventilation: early hemodynamic effects in the premature baboon with hyaline membrane disease. Pediatr Res. 1991;29:160–166. doi: 10.1203/00006450-199102000-00012. [DOI] [PubMed] [Google Scholar]
- 63.Nilsson R. The artificially ventilated preterm rabbit neonate as experimental model of hyaline membrane disease. Acta Anaesthesiol Scand. 1982;26:89–103. doi: 10.1111/j.1399-6576.1982.tb01733.x. [DOI] [PubMed] [Google Scholar]
- 64.Kroon AA, Wang J, Kavanagh BP, Huang Z, Kuliszewski M, van Goudoever JB, Post M. Prolonged mechanical ventilation induces cell cycle arrest in newborn rat lung. PLoS ONE. 2011;6:e16910. doi: 10.1371/journal.pone.0016910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mokres LM, Parai K, Hilgendorff A, Ertsey R, Alvira CM, Rabinovitch M, Bland RD. Prolonged mechanical ventilation with air induces apoptosis and causes failure of alveolar septation and angiogenesis in lungs of newborn mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L23–L35. doi: 10.1152/ajplung.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bland RD, Ertsey R, Mokres LM, Xu L, Jacobson BE, Jiang S, Alvira CM, Rabinovitch M, Shinwell ES, Dixit A. Mechanical ventilation uncouples synthesis and assembly of elastin and increases apoptosis in lungs of newborn mice: prelude to defective alveolar septation during lung development? Am J Physiol Lung Cell Mol Physiol. 2008;294:L3–L14. doi: 10.1152/ajplung.00362.2007. [DOI] [PubMed] [Google Scholar]
- 67.Bland RD, Mokres LM, Ertsey R, Jacobson BE, Jiang S, Rabinovitch M, Xu L, Shinwell ES, Zhang F, Beasley MA. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2007;293(5):L1099–L1110. doi: 10.1152/ajplung.00217.2007. [DOI] [PubMed] [Google Scholar]
- 68.Hilgendorff A, Parai K, Ertsey R, Juliana Rey-Parra G, Thébaud B, Tamosiuniene R, Jain N, Navarro EF, Starcher BC, Nicolls MR, et al. Neonatal mice genetically modified to express the elastase inhibitor elafin are protected against the adverse effects of mechanical ventilation on lung growth. Am J Physiol Lung Cell Mol Physiol. 2012;303:L215–L227. doi: 10.1152/ajplung.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bose CL, Dammann CE, Laughon MM. Bronchopulmonary dysplasia and inflammatory biomarkers in the premature neonate. Arch Dis Child Fetal Neonatal Ed. 2008;93:F455–F461. doi: 10.1136/adc.2007.121327. [DOI] [PubMed] [Google Scholar]
- 70.Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med. 2006;11:354–362. doi: 10.1016/j.siny.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Ogden BE, Murphy SA, Saunders GC, Pathak D, Johnson JD. Neonatal lung neutrophils and elastase/proteinase inhibitor imbalance. Am Rev Respir Dis. 1984;130:817–821. doi: 10.1164/arrd.1984.130.5.817. [DOI] [PubMed] [Google Scholar]
- 72.Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC, Yull FE, Prince LS. NF-κB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol. 2011;187:2740–2747. doi: 10.4049/jimmunol.1101495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bhattacharya S, Go D, Krenitsky DL, Huyck HL, Solleti SK, Lunger VA, Metlay L, Srisuma S, Wert SE, Mariani TJ, et al. Genome-wide transcriptional profiling reveals connective tissue mast cell accumulation in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2012;186:349–358. doi: 10.1164/rccm.201203-0406OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carlton DP, Albertine KH, Cho SC, Lont M, Bland RD. Role of neutrophils in lung vascular injury and edema after premature birth in lambs. J Appl Physiol. 1997;83:1307–1317. doi: 10.1152/jappl.1997.83.4.1307. [DOI] [PubMed] [Google Scholar]
- 75.Nupponen I, Pesonen E, Andersson S, Mäkelä A, Turunen R, Kautiainen H, Repo H. Neutrophil activation in preterm infants who have respiratory distress syndrome. Pediatrics. 2002;110:36–41. doi: 10.1542/peds.110.1.36. [DOI] [PubMed] [Google Scholar]
- 76.Rosen D, Lee JH, Cuttitta F, Rafiqi F, Degan S, Sunday ME. Accelerated thymic maturation and autoreactive t cells in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2006;174:75–83. doi: 10.1164/rccm.200511-1784OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ueda K, Cho K, Matsuda T, Okajima S, Uchida M, Kobayashi Y, Minakami H, Kobayashi K. A rat model for arrest of alveolarization induced by antenatal endotoxin administration. Pediatr Res. 2006;59:396–400. doi: 10.1203/01.pdr.0000200796.86858.ca. [DOI] [PubMed] [Google Scholar]
- 78.Kramer BW, Moss TJ, Willet KE, Newnham JP, Sly PD, Kallapur SG, Ikegami M, Jobe AH. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med. 2001;164:982–988. doi: 10.1164/ajrccm.164.6.2103061. [DOI] [PubMed] [Google Scholar]
- 79.Varughese R, Nayak JL, LoMonaco M, O’Reilly MA, Ryan RM, D’Angio CT. Effects of hyperoxia on tumor necrosis factor alpha and Grobeta expression in newborn rabbit lungs. Lung. 2003;181:335–346. doi: 10.1007/s00408-003-1036-8. [DOI] [PubMed] [Google Scholar]
- 80.Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, Lukashev D, Bittmann I, Sitkovsky MV. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;3:e174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson MR, Choudhury S, Goddard ME, O'Dea KP, Nicholson AG, Takata M. High tidal volume upregulates intrapulmonary cytokines in an in vivo mouse model of ventilator-induced lung injury. J Appl Physiol. 2003;95:1385–1393. doi: 10.1152/japplphysiol.00213.2003. [DOI] [PubMed] [Google Scholar]
- 82.Wilson MR, Choudhury S, Takata M. Pulmonary inflammation induced by high-stretch ventilation is mediated by tumor necrosis factor signaling in mice. Am J Physiol Lung Cell Mol Physiol. 2005;288:L599–L607. doi: 10.1152/ajplung.00304.2004. [DOI] [PubMed] [Google Scholar]
- 83.Yi M, Jankov RP, Belcastro R, Humes D, Copland I, Shek S, Sweezey NB, Post M, Albertine KH, Auten RL, et al. Opposing effects of 60% oxygen and neutrophil influx on alveologenesis in the neonatal rat. Am J Respir Crit Care Med. 2004;170:1188–1196. doi: 10.1164/rccm.200402-215OC. [DOI] [PubMed] [Google Scholar]
- 84.Hillman NH, Moss TJ, Kallapur SG, Bachurski C, Pillow JJ, Polglase GR, Nitsos I, Kramer BW, Jobe AH. Brief, large tidal volume ventilation initiates lung injury and a systemic response in fetal sheep. Am J Respir Crit Care Med. 2007;176:575–581. doi: 10.1164/rccm.200701-051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brew N, Hooper SB, Allison BJ, Wallace MJ, Harding R. Injury and repair in the very immature lung following brief mechanical ventilation. Am J Physiol Lung Cell Mol Physiol. 2011;301:L917–L926. doi: 10.1152/ajplung.00207.2011. [DOI] [PubMed] [Google Scholar]
- 86.Velten M, Heyob KM, Rogers LK, Welty SE. Deficits in lung alveolarization and function after systemic maternal inflammation and neonatal hyperoxia exposure. J Appl Physiol. 2010;108:1347–1356. doi: 10.1152/japplphysiol.01392.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ikegami M, Jobe AH. Postnatal lung inflammation increased by ventilation of preterm lambs exposed antenatally to Escherichia coli endotoxin. Pediatr Res. 2002;52:356–362. doi: 10.1203/00006450-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 88.Bry K, Whitsett JA, Lappalainen U. Il-1beta disrupts postnatal lung morphogenesis in the mouse. Am J Respir Cell Mol Biol. 2007;36:32–42. doi: 10.1165/rcmb.2006-0116OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32:311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 90.Lukkarinen H, Hogmalm A, Lappalainen U, Bry K. Matrix metalloproteinase-9 deficiency worsens lung injury in a model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol. 2009;41:59–68. doi: 10.1165/rcmb.2008-0179OC. [DOI] [PubMed] [Google Scholar]
- 91.Harju K, Ojaniemi M, Rounioja S, Glumoff V, Paananen R, Vuolteenaho R, Hallman M. Expression of toll-like receptor 4 and endotoxin responsiveness in mice during perinatal period. Pediatr Res. 2005;57:644–648. doi: 10.1203/01.PDR.0000156212.03459.A9. [DOI] [PubMed] [Google Scholar]
- 92.Kallapur SG, Jobe AH, Ikegami M, Bachurski CJ. Increased IP-10 and MIG expression after intra-amniotic endotoxin in preterm lamb lung. Am J Respir Crit Care Med. 2003;167:779–786. doi: 10.1164/rccm.2203030. [DOI] [PubMed] [Google Scholar]
- 93.Miller JD, Benjamin JT, Kelly DR, Frank DB, Prince LS. Chorioamnionitis stimulates angiogenesis in saccular stage fetal lungs via CC chemokines. Am J Physiol Lung Cell Mol Physiol. 2010;298:L637–L645. doi: 10.1152/ajplung.00414.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med. 2002;8:702–710. doi: 10.1038/nm721. [DOI] [PubMed] [Google Scholar]
- 95.Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–244. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 96.Bourbon J, Boucherat O, Chailley-Heu B, Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr Res. 2005;57:38R–46R. doi: 10.1203/01.PDR.0000159630.35883.BE. [DOI] [PubMed] [Google Scholar]
- 97.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- 98.Tambunting F, Beharry KD, Waltzman J, Modanlou HD. Impaired lung vascular endothelial growth factor in extremely premature baboons developing bronchopulmonary dysplasia/chronic lung disease. J Investig Med. 2005;53:253–262. doi: 10.2310/6650.2005.53508. [DOI] [PubMed] [Google Scholar]
- 99.Coalson JJ, Winter V, deLemos RA. Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am J Respir Crit Care Med. 1995;152:640–646. doi: 10.1164/ajrccm.152.2.7633720. [DOI] [PubMed] [Google Scholar]
- 100.De Paepe ME, Mao Q, Powell J, Rubin SE, DeKoninck P, Appel N, Dixon M, Gundogan F. Growth of pulmonary microvasculature in ventilated preterm infants. Am J Respir Crit Care Med. 2006;173:204–211. doi: 10.1164/rccm.200506-927OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.DeLisser HM, Helmke BP, Cao G, Egan PM, Taichman D, Fehrenbach M, Zaman A, Cui Z, Mohan GS, Baldwin HS, et al. Loss of PECAM-1 function impairs alveolarization. J Biol Chem. 2006;281:8724–8731. doi: 10.1074/jbc.M511798200. [DOI] [PubMed] [Google Scholar]
- 102.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1alpha-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol. 1999;209:254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- 103.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- 104.Grover TR, Asikainen TM, Kinsella JP, Abman SH, White CW. Hypoxia-inducible factors hif-1alpha and hif-2alpha are decreased in an experimental model of severe respiratory distress syndrome in preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1345–L1351. doi: 10.1152/ajplung.00372.2006. [DOI] [PubMed] [Google Scholar]
- 105.Hosford GE, Olson DM. Effects of hyperoxia on VEGF, its receptors, and HIF-2alpha in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L161–L168. doi: 10.1152/ajplung.00285.2002. [DOI] [PubMed] [Google Scholar]
- 106.Huang Y, Kempen MB, Munck AB, Swagemakers S, Driegen S, Mahavadi P, Meijer D, van Ijcken W, van der Spek P, Grosveld F, et al. Hypoxia-inducible factor 2α plays a critical role in the formation of alveoli and surfactant. Am J Respir Cell Mol Biol. 2012;46:224–232. doi: 10.1165/rcmb.2011-0024OC. [DOI] [PubMed] [Google Scholar]
- 107.Asikainen TM, Schneider BK, Waleh NS, Clyman RI, Ho WB, Flippin LA, Gunzler V, White CW. Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung. Proc Natl Acad Sci USA. 2005;102:10212–10217. doi: 10.1073/pnas.0504520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Asikainen TM, Chang LY, Coalson JJ, Schneider BK, Waleh NS, Ikegami M, Shannon JM, Winter VT, Grubb P, Clyman RI, et al. Improved lung growth and function through hypoxia-inducible factor in primate chronic lung disease of prematurity. Faseb J. 2006;20:1698–1700. doi: 10.1096/fj.06-5887fje. [DOI] [PubMed] [Google Scholar]
- 109.Healy AM, Morgenthau L, Zhu X, Farber HW, Cardoso WV. VEGF is deposited in the subepithelial matrix at the leading edge of branching airways and stimulates neovascularization in the murine embryonic lung. Dev Dyn. 2000;219:341–352. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1061>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 110.Zeng X, Wert SE, Federici R, Peters KG, Whitsett JA. VEGF enhances pulmonary vasculogenesis and disrupts lung morphogenesis in vivo. Dev Dyn. 1998;211:215–227. doi: 10.1002/(SICI)1097-0177(199803)211:3<215::AID-AJA3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 111.McGrath-Morrow SA, Cho C, Cho C, Zhen L, Hicklin DJ, Tuder RM. Vascular endothelial growth factor receptor 2 blockade disrupts postnatal lung development. Am J Respir Cell Mol Biol. 2005;32:420–427. doi: 10.1165/rcmb.2004-0287OC. [DOI] [PubMed] [Google Scholar]
- 112.Iosef C, Alastalo TP, Hou Y, Chen C, Adams ES, Lyu SC, Cornfield DN, Alvira CM. Inhibiting NF-κB in the developing lung disrupts angiogenesis and alveolarization. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1023–L1036. doi: 10.1152/ajplung.00230.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bland RD, Mokres LM, Ertsey R, Jacobson BE, Jiang S, Rabinovitch M, Xu L, Shinwell ES, Zhang F, Beasley MA. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1099–L1110. doi: 10.1152/ajplung.00217.2007. [DOI] [PubMed] [Google Scholar]
- 115.Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 116.McGowan SE. Extracellular matrix and the regulation of lung development and repair. FASEB J. 1992;6:2895–2904. [PubMed] [Google Scholar]
- 117.McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol. 1997;59:43–62. doi: 10.1146/annurev.physiol.59.1.43. [DOI] [PubMed] [Google Scholar]
- 118.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 119.Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol. 2000;23:320–326. doi: 10.1165/ajrcmb.23.3.3906. [DOI] [PubMed] [Google Scholar]
- 120.Nakamura T, Liu M, Mourgeon E, Slutsky A, Post M. Mechanical strain and dexamethasone selectively increase surfactant protein C and tropoelastin gene expression. Am J Physiol Lung Cell Mol Physiol. 2000;278:L974–L980. doi: 10.1152/ajplung.2000.278.5.L974. [DOI] [PubMed] [Google Scholar]
- 121.Guarino N, Teramoto H, Shima H, Oue T, Puri P. Effect of mechanical ventilation on the pulmonary expression and production of elastin in nitrofen-induced diaphragmatic hernia in rats. J Pediatr Surg. 2002;37:1253–1257. doi: 10.1053/jpsu.2002.34974. [DOI] [PubMed] [Google Scholar]
- 122.Jalali S, del Pozo MA, Chen K, Miao H, Li Y, Schwartz MA, Shyy JY, Chien S. Integrin-mediated mechanotransduction requires its dynamic interaction with specific extracellular matrix (ECM) ligands. Proc Natl Acad Sci USA. 2001;98:1042–1046. doi: 10.1073/pnas.031562998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Joyce BJ, Wallace MJ, Pierce RA, Harding R, Hooper SB. Sustained changes in lung expansion alter tropoelastin mRNA levels and elastin content in fetal sheep lungs. Am J Physiol Lung Cell Mol Physiol. 2003;284:L643–L649. doi: 10.1152/ajplung.00090.2002. [DOI] [PubMed] [Google Scholar]
- 124.Hussain N, Wu F, Christian C, Kresch MJ. Hyperoxia inhibits fetal rat lung fibroblast proliferation and expression of procollagens. Am J Physiol. 1997;273:L726–L732. doi: 10.1152/ajplung.1997.273.4.L726. [DOI] [PubMed] [Google Scholar]
- 125.Chen CM, Wang LF, Chou HC, Lang YD, Lai YP. Up-regulation of connective tissue growth factor in hyperoxia-induced lung fibrosis. Pediatr Res. 2007;62:128–133. doi: 10.1203/PDR.0b013e3180987202. [DOI] [PubMed] [Google Scholar]
- 126.Chetty A, Cao GJ, Severgnini M, Simon A, Warburton R, Nielsen HC. Role of matrix metalloprotease-9 in hyperoxic injury in developing lung. Am J Physiol Lung Cell Mol Physiol. 2008;295:L584–L592. doi: 10.1152/ajplung.00441.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bruce MC, Schuyler M, Martin RJ, Starcher BC, Tomashefski JF, Jr, Wedig KE. Risk factors for the degradation of lung elastic fibers in the ventilated neonate: implications for impaired lung development in bronchopulmonary dysplasia. Am Rev Respir Dis. 1992;146:204–212. doi: 10.1164/ajrccm/146.1.204. [DOI] [PubMed] [Google Scholar]
- 128.Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, Edwards DK, III, Gluck L. Elastase and alpha 1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome: role of inflammation in the pathogenesis of bronchopulmonary dysplasia. J Clin Invest. 1983;72:656–666. doi: 10.1172/JCI111015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kumarasamy A, Schmitt I, Nave AH, Reiss I, van der Horst I, Dony E, Roberts JD, Jr, de Krijger RR, Tibboel D, Seeger W, et al. Lysyl oxidase activity is dysregulated during impaired alveolarization of mouse and human lungs. Am J Respir Crit Care Med. 2009;180:1239–1252. doi: 10.1164/rccm.200902-0215OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bruce MC, Wedig KE, Jentoft N, Martin RJ, Cheng PW, Boat TF, Fanaroff AA. Altered urinary excretion of elastin cross-links in premature infants who develop bronchopulmonary dysplasia. Am Rev Respir Dis. 1985;131:568–572. doi: 10.1164/arrd.1985.131.4.568. [DOI] [PubMed] [Google Scholar]
- 131.Masumoto K, de Rooij JD, Suita S, Rottier R, Tibboel D, de Krijger RR. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases during normal human pulmonary development. Histopathology. 2005;47:410–419. doi: 10.1111/j.1365-2559.2005.02228.x. [DOI] [PubMed] [Google Scholar]
- 132.Schulz CG, Sawicki G, Lemke RP, Roeten BM, Schulz R, Cheung PY. Mmp-2 and mmp-9 and their tissue inhibitors in the plasma of preterm and term neonates. Pediatr Res. 2004;55:794–801. doi: 10.1203/01.PDR.0000120683.68630.FB. [DOI] [PubMed] [Google Scholar]
- 133.Tambunting F, Beharry KD, Hartleroad J, Waltzman J, Stavitsky Y, Modanlou HD. Increased lung matrix metalloproteinase-9 levels in extremely premature baboons with bronchopulmonary dysplasia. Pediatr Pulmonol. 2005;39:5–14. doi: 10.1002/ppul.20135. [DOI] [PubMed] [Google Scholar]
- 134.Altiok O, Yasumatsu R, Bingol-Karakoc G, Riese RJ, Stahlman MT, Dwyer W, Pierce RA, Bromme D, Weber E, Cataltepe S. Imbalance between cysteine proteases and inhibitors in a baboon model of bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2006;173:318–326. doi: 10.1164/rccm.200503-425OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yasumatsu R, Altiok O, Benarafa C, Yasumatsu C, Bingol-Karakoc G, Remold-O'Donnell E, Cataltepe S. Serpinb1 upregulation is associated with in vivo complex formation with neutrophil elastase and cathepsin g in a baboon model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L619–L627. doi: 10.1152/ajplung.00507.2005. [DOI] [PubMed] [Google Scholar]
- 136.Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, Karoubi G, Courtman DW, Zucco L, Granton J, Stewart DJ. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res. 2006;98:209–217. doi: 10.1161/01.RES.0000200180.01710.e6. [DOI] [PubMed] [Google Scholar]
- 137.Southwood M, Jeffery TK, Yang X, Upton PD, Hall SM, Atkinson C, Haworth SG, Stewart S, Reynolds PN, Long L, et al. Regulation of bone morphogenetic protein signalling in human pulmonary vascular development. J Pathol. 2008;214:85–95. doi: 10.1002/path.2261. [DOI] [PubMed] [Google Scholar]
- 138.Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, et al. Hyperoxia modulates TGF-beta/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2007;292:L537–L549. doi: 10.1152/ajplung.00050.2006. [DOI] [PubMed] [Google Scholar]
- 139.Suzuki Y, Montagne K, Nishihara A, Watabe T, Miyazono K. BMPs promote proliferation and migration of endothelial cells via stimulation of VEGF-A/VEGFR2 and angiopoietin-1/Tie2 signalling. J Biochem. 2008;143:199–206. doi: 10.1093/jb/mvm215. [DOI] [PubMed] [Google Scholar]
- 140.de Jesus Perez VA, Alastalo TP, Wu JC, Axelrod JD, Cooke JP, Amieva M, Rabinovitch M. Bone morphogenetic protein 2 induces pulmonary angiogenesis via Wnt-beta-catenin and Wnt-RhoA-Rac1 pathways. J Cell Biol. 2009;184:83–99. doi: 10.1083/jcb.200806049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Colarossi C, Chen Y, Obata H, Jurukovski V, Fontana L, Dabovic B, Rifkin DB. Lung alveolar septation defects in Ltbp-3-null mice. Am J Pathol. 2005;167:419–428. doi: 10.1016/S0002-9440(10)62986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sun J, Chen H, Chen C, Whitsett JA, Mishina Y, Bringas P, Jr, Ma JC, Warburton D, Shi W. Prenatal lung epithelial cell-specific abrogation of Alk3-bone morphogenetic protein signaling causes neonatal respiratory distress by disrupting distal airway formation. Am J Pathol. 2008;172:571–582. doi: 10.2353/ajpath.2008.070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen H, Zhuang F, Liu YH, Xu B, Del Moral P, Deng W, Chai Y, Kolb M, Gauldie J, Warburton D, et al. TGF-beta receptor II in epithelia versus mesenchyme plays distinct roles in the developing lung. Eur Respir J. 2008;32:285–295. doi: 10.1183/09031936.00165407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gauldie J, Galt T, Bonniaud P, Robbins C, Kelly M, Warburton D. Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J Pathol. 2003;163:2575–2584. doi: 10.1016/s0002-9440(10)63612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhao J, Sime PJ, Bringas P, Jr, Gauldie J, Warburton D. Adenovirus-mediated decorin gene transfer prevents TGF-beta-induced inhibition of lung morphogenesis. Am J Physiol. 1999;277:L412–L422. doi: 10.1152/ajplung.1999.277.2.L412. [DOI] [PubMed] [Google Scholar]
- 146.Zhao J, Sime PJ, Bringas P, Jr, Tefft JD, Buckley S, Bu D, Gauldie J, Warburton D. Spatial-specific TGF-beta1 adenoviral expression determines morphogenetic phenotypes in embryonic mouse lung. Eur J Cell Biol. 1999;78:715–725. doi: 10.1016/s0171-9335(99)80040-5. [DOI] [PubMed] [Google Scholar]
- 147.Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- 148.Gauldie J, Kolb M, Ask K, Martin G, Bonniaud P, Warburton D. Smad3 signaling involved in pulmonary fibrosis and emphysema. Proc Am Thorac Soc. 2006;3:696–702. doi: 10.1513/pats.200605-125SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bland RD, Xu L, Ertsey R, Rabinovitch M, Albertine KH, Wynn KA, Kumar VH, Ryan RM, Swartz DD, Csiszar K, et al. Dysregulation of pulmonary elastin synthesis and assembly in preterm lambs with chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1370–L1384. doi: 10.1152/ajplung.00367.2006. [DOI] [PubMed] [Google Scholar]
- 150.Kuang PP, Zhang XH, Rich CB, Foster JA, Subramanian M, Goldstein RH. Activation of elastin transcription by transforming growth factor-beta in human lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 2007;292:L944–L952. doi: 10.1152/ajplung.00184.2006. [DOI] [PubMed] [Google Scholar]
- 151.Zhao Y, Gilmore BJ, Young SL. Expression of transforming growth factor-beta receptors during hyperoxia-induced lung injury and repair. Am J Physiol. 1997;273:L355–L362. doi: 10.1152/ajplung.1997.273.2.L355. [DOI] [PubMed] [Google Scholar]
- 152.Jobe AH, Kramer BW, Moss TJ, Newnham JP, Ikegami M. Decreased indicators of lung injury with continuous positive expiratory pressure in preterm lambs. Pediatr Res. 2002;52:387–392. doi: 10.1203/00006450-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 153.Enhörning G, Robertson B. Lung expansion in the premature rabbit fetus after tracheal deposition of surfactant. Pediatrics. 1972;50:58–66. [PubMed] [Google Scholar]
- 154.Cutz E, Enhörning G, Robertson B, Sherwood WG, Hill DE. Hyaline membrane disease: effect of surfactant prophylaxis on lung morphology in premature primates. Am J Pathol. 1978;92:581–594. [PMC free article] [PubMed] [Google Scholar]
- 155.Jobe A, Ikegami M, Glatz T, Yoshida Y, Diakomanolis E, Padbury J. Duration and characteristics of treatment of premature lambs with natural surfactant. J Clin Invest. 1981;67:370–375. doi: 10.1172/JCI110044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980;1:55–59. doi: 10.1016/s0140-6736(80)90489-4. [DOI] [PubMed] [Google Scholar]
- 157.Gitlin JD, Soll RF, Parad RB, Horbar JD, Feldman HA, Lucey JF, Taeusch HW. Randomized controlled trial of exogenous surfactant for the treatment of hyaline membrane disease. Pediatrics. 1987;79:31–37. [PubMed] [Google Scholar]
- 158.Liechty EA, Donovan E, Purohit D, Gilhooly J, Feldman B, Noguchi A, Denson SE, Sehgal SS, Gross I, Stevens D, et al. Reduction of neonatal mortality after multiple doses of bovine surfactant in low birth weight neonates with respiratory distress syndrome. Pediatrics. 1991;88:19–28. [PubMed] [Google Scholar]
- 159.Friedman CA, Menchaca RC, Baker MC, Rivas CK, Laberge RN, Rios EH, Haider SH, Romero EJ, Eason EB, Fraley JK, et al. Bubble nasal continuous positive airway pressure, early surfactant treatment, and rapid extubation are associated with decreased incidence of bronchopulmonary dysplasia in very low birth weight newborns: efficacy and safety considerations. Respir Care. 2012 doi: 10.4187/respcare.01998. [DOI] [PubMed] [Google Scholar]
- 160.Greenough A, Ahmed N.Perinatal prevention of bronchopulmonary dysplasia J Perinat MedIn press [DOI] [PubMed] [Google Scholar]
- 161.Jaarsma AS, Braaksma MA, Geven WB, Van Oeveren W, Oetomo SB. Early activation of inflammation and clotting in the preterm lamb with neonatal RDS: comparison of conventional ventilation and high frequency oscillatory ventilation. Pediatr Res. 2001;50:650–657. doi: 10.1203/00006450-200111000-00019. [DOI] [PubMed] [Google Scholar]
- 162.Gilbert WM, Eby-Wilkens E, Plopper C, Whitsett JA, Tarantal AF. Fetal monkey surfactants after intra-amniotic or maternal administration of betamethasone and thyroid hormone. Obstet Gynecol. 2001;98:466–470. doi: 10.1016/s0029-7844(01)01417-x. [DOI] [PubMed] [Google Scholar]
- 163.Moss TJ, Mulrooney NP, Nitsos I, Ikegami M, Jobe AH, Newnham JP. Intra-amniotic corticosteroids for preterm lung maturation in sheep. Am J Obstet Gynecol. 2003;189:1389–1395. doi: 10.1067/s0002-9378(03)00622-7. [DOI] [PubMed] [Google Scholar]
- 164.Antonow-Schlorke I, Helgert A, Gey C, Coksaygan T, Schubert H, Nathanielsz PW, Witte OW, Schwab M. Adverse effects of antenatal glucocorticoids on cerebral myelination in sheep. Obstet Gynecol. 2009;113:142–151. doi: 10.1097/AOG.0b013e3181924d3b. [DOI] [PubMed] [Google Scholar]
- 165.Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J Physiol. 2003;547:117–123. doi: 10.1113/jphysiol.2002.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bal MP, de Vries WB, van der Leij FR, van Oosterhout MF, Berger RM, Baan J, van der Wall EE, van Bel F, Steendijk P. Neonatal glucocorticosteroid treatment causes systolic dysfunction and compensatory dilation in early life: studies in 4-week-old prepubertal rats. Pediatr Res. 2005;58:46–52. doi: 10.1203/01.PDR.0000163617.01673.9A. [DOI] [PubMed] [Google Scholar]
- 167.de Vries WB, van der Leij FR, Bakker JM, Kamphuis PJ, van Oosterhout MF, Schipper ME, Smid GB, Bartelds B, van Bel F. Alterations in adult rat heart after neonatal dexamethasone therapy. Pediatr Res. 2002;52:900–906. doi: 10.1203/00006450-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 168.Kamphuis PJ, de Vries WB, Bakker JM, Kavelaars A, van Dijk JE, Schipper ME, van Oosterhout MF, Croiset G, Heijnen CJ, van Bel F, et al. Reduced life expectancy in rats after neonatal dexamethasone treatment. Pediatr Res. 2007;61:72–76. doi: 10.1203/01.pdr.0000249980.95264.dd. [DOI] [PubMed] [Google Scholar]
- 169.Halliday HL. Postnatal steroids and chronic lung disease in the newborn. Paediatr Respir Rev. 2004;5:S245–S248. doi: 10.1016/s1526-0542(04)90046-2. [DOI] [PubMed] [Google Scholar]
- 170.Halliday HL. Use of steroids in the perinatal period. Paediatr Respir Rev. 2004;5(Suppl A):S321–S327. doi: 10.1016/s1526-0542(04)90057-7. [DOI] [PubMed] [Google Scholar]
- 171.Chytil F. Retinoids in lung development. FASEB J. 1996;10:986–992. doi: 10.1096/fasebj.10.9.8801181. [DOI] [PubMed] [Google Scholar]
- 172.Fraslon C, Bourbon JR. Retinoids control surfactant phospholipid biosynthesis in fetal rat lung. Am J Physiol. 1994;266:L705–L712. doi: 10.1152/ajplung.1994.266.6.L705. [DOI] [PubMed] [Google Scholar]
- 173.Grummer MA, Thet LA, Zachman RD. Expression of retinoic acid receptor genes in fetal and newborn rat lung. Pediatr Pulmonol. 1994;17:234–238. doi: 10.1002/ppul.1950170406. [DOI] [PubMed] [Google Scholar]
- 174.Massaro GD, Massaro D. Retinoic acid treatment partially rescues failed septation in rats and in mice. Am J Physiol Lung Cell Mol Physiol. 2000;278:L955–L960. doi: 10.1152/ajplung.2000.278.5.L955. [DOI] [PubMed] [Google Scholar]
- 175.Massaro GD, Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med. 1997;3:675–677. doi: 10.1038/nm0697-675. [DOI] [PubMed] [Google Scholar]
- 176.Massaro GD, Massaro D, Chan WY, Clerch LB, Ghyselinck N, Chambon P, Chandraratna RA. Retinoic acid receptor-beta: an endogenous inhibitor of the perinatal formation of pulmonary alveoli. Physiol Genomics. 2000;4:51–57. doi: 10.1152/physiolgenomics.2000.4.1.51. [DOI] [PubMed] [Google Scholar]
- 177.McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol. 2000;23:162–167. doi: 10.1165/ajrcmb.23.2.3904. [DOI] [PubMed] [Google Scholar]
- 178.Veness-Meehan KA, Pierce RA, Moats-Staats BM, Stiles AD. Retinoic acid attenuates O2-induced inhibition of lung septation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L971–L980. doi: 10.1152/ajplung.00266.2001. [DOI] [PubMed] [Google Scholar]
- 179.Shenai JP, Kennedy KA, Chytil F, Stahlman MT. Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. J Pediatr. 1987;111:269–277. doi: 10.1016/s0022-3476(87)80086-0. [DOI] [PubMed] [Google Scholar]
- 180.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, Bauer CR, et al. National Institute of Child Health and Human Development Neonatal Research Network. Vitamin a supplementation for extremely-low-birth-weight infants. N Engl J Med. 1999;340:1962–1968. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 181.MacRitchie AN, Albertine KH, Sun J, Lei PS, Jensen SC, Freestone AA, Clair PM, Dahl MJ, Godfrey EA, Carlton DP, et al. Reduced endothelial nitric oxide synthase in lungs of chronically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1011–L1020. doi: 10.1152/ajplung.2001.281.4.L1011. [DOI] [PubMed] [Google Scholar]
- 182.Tzao C, Nickerson PA, Steinhorn RH, Noble BK, Swartz DD, Russell JA. Type I nitric oxide synthase is decreased in the fetal pulmonary circulation of hypertensive lambs. Pediatr Pulmonol. 2002;33:437–442. doi: 10.1002/ppul.10105. [DOI] [PubMed] [Google Scholar]
- 183.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase-deficient mouse. Am J Physiol Lung Cell Mol Physiol. 2003;284:L964–L971. doi: 10.1152/ajplung.00421.2002. [DOI] [PubMed] [Google Scholar]
- 184.Bland RD, Albertine KH, Carlton DP, MacRitchie AJ. Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. Am J Respir Crit Care Med. 2005;172:899–906. doi: 10.1164/rccm.200503-384OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tang JR, Seedorf G, Balasubramaniam V, Maxey A, Markham N, Abman SH. Early inhaled nitric oxide treatment decreases apoptosis of endothelial cells in neonatal rat lungs after vascular endothelial growth factor inhibition. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1271–L1280. doi: 10.1152/ajplung.00224.2007. [DOI] [PubMed] [Google Scholar]
- 186.Stuart RB, Ovadia B, Suzara VV, Ross PA, Thelitz S, Fineman JR, Gutierrez JA. Inhaled nitric oxide increases surfactant protein gene expression in the intact lamb. Am J Physiol Lung Cell Mol Physiol. 2003;285:L628–L633. doi: 10.1152/ajplung.00264.2002. [DOI] [PubMed] [Google Scholar]
- 187.Mercier JC, Hummler H, Durrmeyer X, Sanchez-Luna M, Carnielli V, Field D, Greenough A, Van Overmeire B, Jonsson B, Hallman M, et al. EUNO Study Group. Inhaled nitric oxide for prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. Lancet. 2010;376:346–354. doi: 10.1016/S0140-6736(10)60664-2. [DOI] [PubMed] [Google Scholar]
- 188.Cole FS, Alleyne C, Barks JD, Boyle RJ, Carroll JL, Dokken D, Edwards WH, Georgieff M, Gregory K, Johnston MV, et al. NIH Consensus Development Conference statement: inhaled nitric-oxide therapy for premature infants. Pediatrics. 2011;127:363–369. doi: 10.1542/peds.2010-3507. [DOI] [PubMed] [Google Scholar]
- 189.O’Reilly M, Thébaud B. Cell-based strategies to reconstitute lung function in infants with severe bronchopulmonary dysplasia. Clin Perinatol. 2012;39:703–725. doi: 10.1016/j.clp.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Aslam M, Baveja R, Liang OD, Fernandez-Gonzalez A, Lee C, Mitsialis SA, Kourembanas S. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.van Haaften T, Byrne R, Bonnet S, Rochefort GY, Akabutu J, Bouchentouf M, Rey-Parra GJ, Galipeau J, Haromy A, Eaton F, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Reiss I, Landmann E, Heckmann M, Misselwitz B, Gortner L. Increased risk of bronchopulmonary dysplasia and increased mortality in very preterm infants being small for gestational age. Arch Gynecol Obstet. 2003;269:40–44. doi: 10.1007/s00404-003-0486-9. [DOI] [PubMed] [Google Scholar]
- 193.Walsh MC, Yao Q, Horbar JD, Carpenter JH, Lee SK, Ohlsson A. Changes in the use of postnatal steroids for bronchopulmonary dysplasia in 3 large neonatal networks. Pediatrics. 2006;118:e1328–e1335. doi: 10.1542/peds.2006-0359. [DOI] [PubMed] [Google Scholar]
- 194.Bose C, Van Marter LJ, Laughon M, O’Shea TM, Allred EN, Karna P, Ehrenkranz RA, Boggess K, Leviton A Extremely Low Gestational Age Newborn Study Investigators. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics. 2009;124:e450–e458. doi: 10.1542/peds.2008-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Binet ME, Bujold E, Lefebvre F, Tremblay Y, Piedboeuf B. Role of gender in morbidity and mortality of extremely premature neonates. Am J Perinatol. 2012;29:159–166. doi: 10.1055/s-0031-1284225. [DOI] [PubMed] [Google Scholar]
- 196.Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, Ment LR, Gruen JR Neonatal Genetics Study Group. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117:1901–1906. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 197.Hallman M, Haataja R. Genetic influences and neonatal lung disease. Semin Neonatol. 2003;8:19–27. doi: 10.1016/s1084-2756(02)00196-3. [DOI] [PubMed] [Google Scholar]
- 198.Hilgendorff A, Heidinger K, Pfeiffer A, Bohnert A, Konig IR, Ziegler A, Merz C, Frey G, Chakraborty T, Gortner L, et al. Association of polymorphisms in the mannose-binding lectin gene and pulmonary morbidity in preterm infants. Genes Immun. 2007:8671–677. doi: 10.1038/sj.gene.6364432. [DOI] [PubMed] [Google Scholar]