Abstract
Purpose
Squamous cell carcinoma of the anal canal (SCCAC) is characterized by high locoregional failure (LRF) rates after sphincter-preserving definitive chemoradiation (CRT) and is typically associated with anogenital human papilloma virus infection. Because cetuximab enhances the effect of radiation therapy in human papilloma virus–associated oropharyngeal squamous cell carcinoma, we hypothesized that adding cetuximab to CRT would reduce LRF in SCCAC.
Methods
Sixty-one patients with stage I to III SCCAC received CRT including cisplatin, fluorouracil, and radiation therapy to the primary tumor and regional lymph nodes (45 to 54 Gy) plus eight once-weekly doses of concurrent cetuximab. The study was designed to detect at least a 50% reduction in 3-year LRF rate (one-sided α, 0.10; power 90%), assuming a 35% LRF rate from historical data.
Results
Poor risk features included stage III disease in 64% and male sex in 20%. The 3-year LRF rate was 23% (95% CI, 13% to 36%; one-sided P = .03) by binomial proportional estimate using the prespecified end point and 21% (95% CI, 7% to 26%) by Kaplan-Meier estimate in a post hoc analysis using methods consistent with historical data. Three-year rates were 68% (95% CI, 55% to 79%) for progression-free survival and 83% (95% CI, 71% to 91%) for overall survival. Grade 4 toxicity occurred in 32%, and 5% had treatment-associated deaths.
Conclusion
Although the addition of cetuximab to chemoradiation for SCCAC was associated with lower LRF rates than historical data with CRT alone, toxicity was substantial, and LRF still occurs in approximately 20%, indicating the continued need for more effective and less toxic therapies.
INTRODUCTION
Squamous cell carcinoma (SCC) of the anal canal (SCCAC) is a potentially curable disease with sphincter-sparing definitive chemoradiation (CRT) including concurrent radiation plus fluorouracil (FU) and mitomycin-C or cisplatin.1,2-6 Locoregional failure (LRF) occurs in up to approximately one third after CRT and is associated with significant morbidity, distant recurrence, and mortality.7 New approaches are needed to develop more effective therapies that result in improved local and systemic disease control.
SCCAC is a rare cancer, as defined by the US National Institutes of Health,8,9 with 1.8 cases annually per 100,000 in the United States, thereby creating additional challenges in designing and conducting clinical trials in this population. It accounts for only approximately 2% of all gastrointestinal cancers, with 8,080 new cases and 1,080 deaths expected in 2016 in the United States.10 On the other hand, SCCAC and other rare cancers collectively account for up to 25% of all cancers, which has prompted national and international efforts to coordinate clinical trials testing new approaches via the International Rare Cancer Initiative (ICRI).11-13 Approximately 1% of women and 28% of men with anal cancer also have HIV infection,14 indicating a need to also evaluate new treatment options for SCCAC in this context.
Concurrent with the development of the ICRI, the Eastern Cooperative Oncology Group (ECOG) and AIDS Malignancy Consortium (AMC), two of the ICRI’s participating organizations, began a collaboration in 2005 to coordinate the planning and conduct of prospective clinical trials designed to test new treatment approaches for SCCAC in patients with and without HIV infection. Our strategy focused on therapeutically exploiting the strong association between SCCAC and human papilloma virus (HPV) infection.15-17 The HPV-associated E5 protein amplifies the mitogenic signals mediated by the epidermal growth factor receptor (EGFR),18 which is broadly expressed in epithelial cancers, including SCC of the anogenital tract and oropharynx.19,20 Cetuximab is a chimeric IgG1 monoclonal antibody that binds EGFR with high specificity and with greater affinity than its ligands, thus blocking ligand-induced activation of EGFR.21 Cetuximab prolongs survival when used in combination with radiation therapy (RT) in patients with locally advanced SCC of the oropharynx,22,23 another cancer that is typically associated with HPV infection,24-26 but not other head and neck cancers not associated with HPV.27 Cetuximab also enhances the effectiveness of cisplatin in advanced head and neck carcinoma.28 We therefore hypothesized that the addition of cetuximab to CRT would improve locoregional control in patients with SCCAC and, as such, designed two trials that were concurrently conducted to determine the effectiveness of this combination in patients with HIV infection (AMC045) and without HIV infection (E3205). We herein report the results of E3205 and also report the results of AMC045 in a separate accompanying report.29 Both trials were single-arm phase II designs evaluating cetuximab plus the same CRT regimen of cisplatin, FU, and external beam RT. The treatment regimen in the two studies differed only in the use of two neoadjuvant cycles of cisplatin and FU in the E3205 trial on the basis of evidence at the time of a potential benefit for neoadjuvant chemotherapy.30 After accrual of the first 28 patients in the trial, the neoadjuvant chemotherapy was eliminated based on evolving data that it may not be beneficial.31
METHODS
Eligibility Criteria
Patients were required to have histologically confirmed anal canal or perianal (anal margin) squamous cell carcinoma (or tumors of nonkeratinizing histology, such as basaloid, transitional cell, or cloacogenic histology) and stage I (excluding well-differentiated stage I anal margin cancer), II (T2N0, T3N0), IIIA, or IIIB disease. After accrual of the first 28 patients, the eligibility criteria were modified to include only patients with high-risk stage II (T3N0), IIIA, or IIIB disease (thereby excluding patients with lower risk T1-2N0 disease who had previously been eligible). Other requirements included age 18 years or older; ECOG performance status 0 to 2; no prior potentially curative surgery, RT, or chemotherapy for this malignancy; no prior pelvic radiotherapy; no other concurrent malignancies (except for nonmelanomatous skin cancer); and adequate organ function (on the basis of laboratory values obtained within 2 weeks before registration). Exclusion criteria included prior history of rheumatic disorders, irritable bowel syndrome or inflammatory bowel disease, and other significant illnesses within the past 6 months, including active infection, uncontrolled diabetes or hypertension, or cerebrovascular or cardiovascular disease.
Study Objectives
The primary objective was LRF rate at 3 years. Secondary objectives included response rate and overall toxicity. Other clinical end points included progression-free survival (PFS), colostomy-free survival (CFS), and overall survival (OS).
Study End Point Definitions
LRF was defined as progression/relapse of disease in the anal canal and/or regional organs and/or regional lymph nodes. PFS was defined as time from registration to progression, relapse, or death from any cause. CFS was defined as date of registration until date that colostomy was required or death from any cause. OS was defined as time from registration until death from any cause. Response was classified according to Response Evaluation Criteria in Solid Tumors (RECIST, version 1.0) and required radiologic confirmation at least 4 weeks after initial objective response.32 Tumor assessments were made by physical examination and computerized tomography (CT of the abdomen and pelvis) at baseline, within 4 weeks of the completion of protocol treatment, every 6 months if patient was 1 to 4 years from registration, and annually thereafter. National Cancer Institute Common Adverse Events Criteria, version 3.0, was used to grade toxicity. Formalin-fixed paraffin-embedded tumor biopsy specimens were assessed for HPV DNA using previously described methods.33
Statistical Considerations
The study was designed to detect at least a 50% reduction in 3-year LRF rate (one-sided α, 0.10; power, 90%), the primary study end point, and assumed a 3-year LRF rate of 35% on the basis of historical data. For example, the 3-year LRF rate was 39% in the Anal Cancer Trial (ACT1)1 in which patients were treated with mitomycin-C/FU, and the 5-year LRF rate was 33% for the cisplatin/FU arm of the Radiation Therapy Oncology Group RTOG 9811 trial (on the basis of information available from a preliminary report of study results in 2006).34 In both reports, deaths from other causes were censored in estimating LRF rates by the Kaplan-Meier method. The study originally had an accrual goal of 45 eligible patients (50 total, assuming 10% ineligible), but was amended after accrual of the first 28 patients to eliminate the two cycles of neoadjuvant chemotherapy; accrual of 34 additional patients was planned (62 total), which retained the original 90% power to detect at least a 50% reduction in 3-year LRF rate.
For the primary study end point definition and analysis plan, patients were classified into two groups as a binary variable, including failure (defined as LRF, death due to disease or treatment within 3 years, or lost to follow-up without LRF within 3 years) or no failure, and evaluated by binomial proportion. The distributions of PFS, CFS, and OS were estimated using the Kaplan-Meier method, with 95% CIs calculated using Greenwood’s formula. Event rates at 3 years were evaluated, because prior studies indicated that most events related to SCCAC or its treatment occurred within 3 years.1,2-6 The cutoff date for the data analysis was November 4, 2015.
Informed Consent and Regulatory Approval
The study was developed and coordinated by the ECOG–American College of Radiology Imaging Network (ACRIN) Research Group, reviewed and approved by the Cancer Evaluation Therapy Program of the National Cancer Institute (E3205), and also reviewed and approved by the institutional review board at each participating institution (ClinicalTrials.gov identifier NCT00316888). All patients provided written informed consent.
Cetuximab, Chemotherapy, and Radiation Therapy
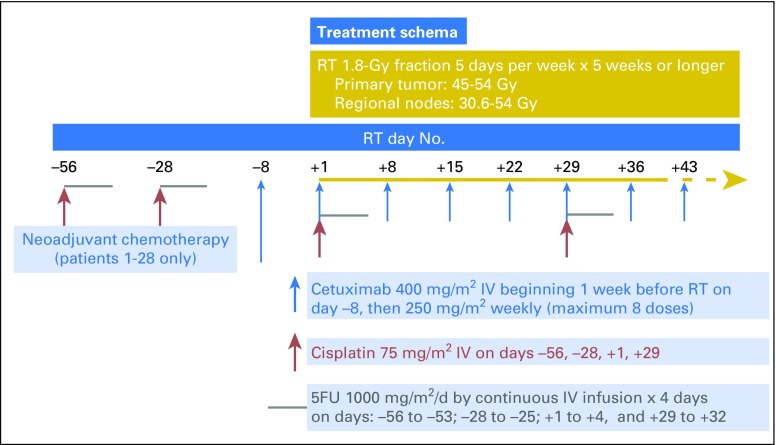
The treatment schema is shown in Figure 1. Criteria for treatment modifications are summarized in the next two paragraphs, with more detailed information provided in the Data Supplement. The protocol was suspended to accrual on November 3, 2008 after accrual of 28 patients, to eliminate the two cycles of neoadjuvant cisplatin and FU, and was reopened on August 18, 2009 with an additional modification to include only patients with more advanced stage (T3-4N0 or N1-3) disease.31
Fig 1.
Treatment schema. FU, fluorouracil; IV, intravenous; RT, radiation therapy.
Chemotherapy cycles were repeated every 4 weeks if the absolute neutrophil count was at least 1,200/μL and platelet count at least 100,000/μL, and doses of cisplatin and FU were reduced 25% or 50% depending on absolute neutrophil count or platelet nadir or for specific types of nonhematologic toxicity, including modifications of FU dosing (for diarrhea and/or stomatitis) or cisplatin dosing (for renal, neurologic, or ototoxicity). Cetuximab was discontinued if there was a grade 3 or 4 hypersensitivity reaction or grade 3 or higher cardiac toxicity and held if there was grade 3 skin rash or grade 2 or greater pulmonary toxicity.
Radiation therapy compliance was monitored by the Quality Assurance Review Center.35 Concurrent RT consisted of 1.8 Gy once per day 5 days per week for a minimum of 5 weeks and was based on prechemotherapy tumor volumes (minimum 45.0 Gy, maximum 54.0 Gy). Intensity modulated radiation therapy was used at the discretion of the treating physician according to the guidelines outlined in this protocol. The total dose of irradiation to the primary tumor was 45 Gy for T1 or T2 lesions, or between 50.4 Gy and 54.0 Gy for T3 or T4 lesions or T2 disease with clinical evidence of residual disease after 45 Gy. The total dose to the inguinal nodes was 30.6 Gy for N0 or N1 disease, or 50.4 to 54.0 Gy for N2 or N3 disease, for clinical evidence of residual disease after 45 Gy, or for any lymph node > 3 cm (see Data Supplement for additional specific details). RT (and cetuximab) were held for grade 3 or greater diarrhea (more than six stools per day above baseline); grade 4 vomiting (parenteral nutrition, intensive care, or hemodynamic collapse); or grade 4 skin ulceration or localized or generalized infection secondary to an area of confluent moist desquamation. RT was continued if there was moist desquamation in the absence of infection.
RESULTS
Patient Characteristics
A total of 63 patients were accrued between January 19, 2007 and May 29, 2012 at 22 sites and their affiliates, of whom one withdrew before beginning any therapy and another withdrew before beginning CRT (and after two cycles of neoadjuvant chemotherapy). The characteristics of the study population including 61 patients treated with CRT are outlined in Table 1. The median age was 56 years, and the distribution of stage I, II, and III disease was 5%, 31%, and 64%, respectively. Tumors were HPV positive in 25 (89%) of 28 specimens examined. Histology included SCC in 54 patients and other histologies in seven patients (five basaloid, one cloacogenic, one transitional cell). Poor risk features included male sex in 20%, T3 to 4 lesions in 54%, and positive regional nodes in 54%. Comparison of the characteristics between the first 28 patients treated with neoadjuvant chemotherapy (arm A) and the remaining 33 patients treated without neoadjuvant chemotherapy (arm B) revealed that patients in arm B were more likely to have advanced-stage disease (P = .006), as expected, because of the change in eligibility criteria when the trial was amended to eliminate neoadjuvant chemotherapy.
Table 1.
Patient Characteristics and Demographics

Treatment Administration
Information regarding chemotherapy dose intensity, radiation therapy administration, and dose modifications are summarized in Table 2. Treatment was completed as per protocol in 49 patients (79%), whereas 9 patients (15%) had adverse events preventing completion of therapy, and 4 patients (7%) withdrew before completion of therapy. All 28 patients in arm A assigned to neoadjuvant chemotherapy completed both cycles. Concurrent chemotherapy plus at least six cycles of cetuximab was completed in 24 (89%) of 27 patients in arm A and 26 (76%) of 34 patients treated in arm B. At least six doses of cetuximab were administered to 50 patients (82%), and 28 patients (46%) received eight doses.
Table 2.
Treatment Administered

Imaging and radiation treatment plans were acquired and reviewed at the Quality Assurance Review Center, with supervision by the radiation oncology study chair. In all cases there was no transection of gross tumor in either primary or involved lymph nodes. There were differences in contour of primary target volumes including the mesorectum and nodal drainage. RT was delivered per protocol in 69%, with minor deviations in 23% and major deviations in 8%. Although patients with major deviations all had appropriate primary tumor target coverage, each had difficulty meeting normal tissue constraints generally to the small bowel and femoral heads.
Adverse Events
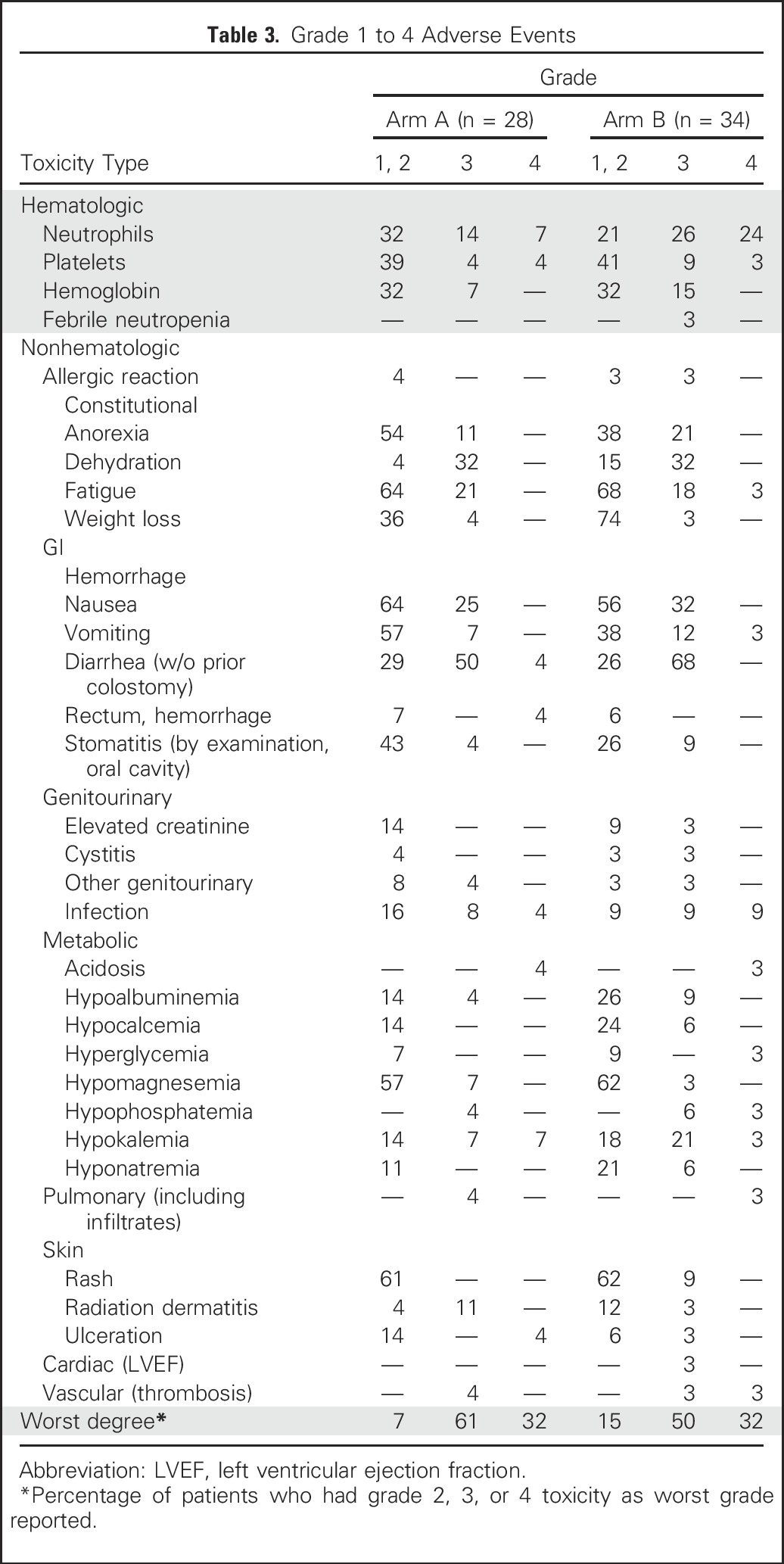
Adverse events are summarized in Table 3 for the 62 patients who received at least one dose of protocol chemotherapy and are shown separately for arms A and B. The most common grade 3 and 4 adverse events in arm B, occurring in 10% or more of patients, included diarrhea in 68% (68% grade 3, 0% grade 4), neutropenia in 50% (26% grade 3, 24% grade 4), nausea in 32% (32% grade 3, 0% grade 4), dehydration in 32% (32% grade 3, 0% grade 4), hypokalemia in 24% (21% grade 3, 3% grade 4), infection in 18% (9% grade 3, 9% grade 4), anemia in 15% (15% grade 3, 0% grade 4), thrombocytopenia in 12% (9% grade 3, 3% grade 4). The adverse event profile was generally similar in arm A compared with arm B, although there was more grade 3 and 4 neutropenia in arm B (50% v 21%). There were three deaths within 30 days of completing treatment, one of which was attributed by the treating physician to be possibly related to treatment (one patient in arm B with renal failure and pneumonitis), and two of which were not attributed to treatment (one patient in arm A [gastrointestinal bleeding] and one in in arm B [aspiration pneumonia]).
Table 3.
Grade 1 to 4 Adverse Events
Primary End Point: LRF at 3 Years
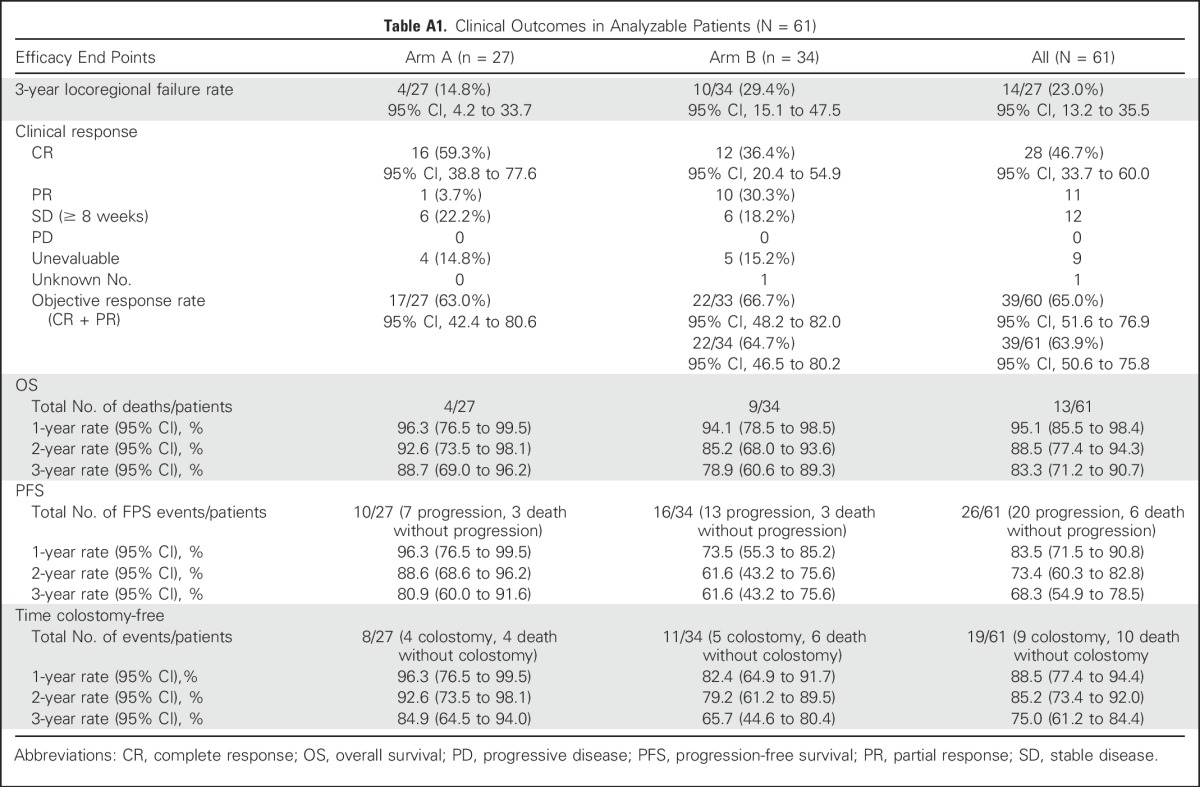
At 3 years, nine patients had a locoregional recurrence (15%), seven patients (11%) died without locoregional recurrence (two from disease or treatment within 3 years, five from other or unknown causes), and 45 surviving patients (74%) did not have an LRF event (of whom three were followed for < 3 years). The 3-year LRF rate was 23% (14 of 61 patients; 95% CI, 13% to 35%; one-sided P = .03) by binomial proportional estimate using the prespecified end point (LRF, death due to disease or treatment, or alive without LRF and followed < 3 years), and 21% (95% CI, 7% to 26%) by Kaplan-Meier estimate in a post hoc analysis using definitions and methods consistent with historical data,4
Secondary End Points: Objective Response and Other Clinical Outcomes
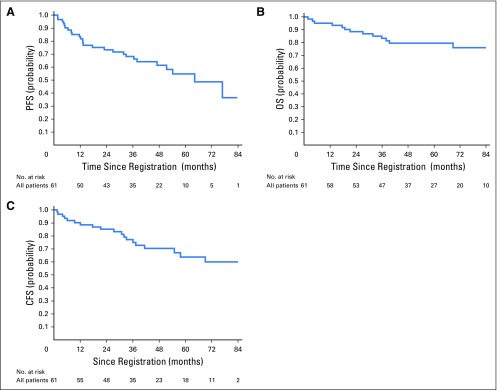
The objective response rate was 65% (95% CI, 52% to 77%) in both arms combined and was similar in arm A (63%; 95% CI, 42% to 81%) and arm B (67%; 95% CI, 20% to 55%). Complete response rates were higher in arm A (59%; 95% CI, 39% to 78%) than arm B (35%; 95% CI, 20% to 54%). At the time of the analysis, with a median follow-up time of 84 months (range, 25 to 97 months) for surviving patients, 13 patients died (including eight from anal cancer, five from other causes), 25 patients had a PFS event (including 20 with disease progression and five deaths from other causes), 14 patients had an LRF event (including nine locoregional recurrences and five deaths from other causes), and four patients had a colostomy. For the five patients who died of causes other than anal cancer, three deaths occurred during concurrent chemoradiotherapy as previously described, and two deaths occurred after completing therapy (acute myelogenous leukemia at 32 months and cardiac failure at 18 months). Kaplan-Meier estimates for PFS, OS, and CFS are shown in Figures 2A to 2C. Three-year event rates including both arms were 68% (95% CI, 55% to 79%) for PFS, 75% (95% CI, 61% to 84%) for CFS, and 83% (95% CI, 71% to 91%) for OS, and event rates at 1, 2, and 3 years and other outcomes by arm are shown in Appendix Table A1 (online only). Clinical outcomes were less favorable in arm B compared with arm A, likely due to change in the eligibility criterion to include higher-risk disease rather than omission of neoadjuvant chemotherapy.
Fig 2.
Kaplan-Meier estimates of (A) progression-free survival (PFS), (B) overall survival (OS), and (C) colostomy-free survival (CFS).
DISCUSSION
We performed a single-arm, nonrandomized phase II trial evaluating the anti-EGFR targeted agent cetuximab plus CRT including cisplatin, FU, and RT in immunocompetent patients with SCCAC (E3205). The trial was designed to determine whether adding cetuximab to CRT reduced LRF rates, which occurs in up to 35% of patients treated with CRT alone.3,4 We observed 3-year LRF rates of approximately 20% when similar definitions of LRF were used, suggesting cetuximab may have contributed to lower LRF rates observed in this trial and another trial evaluating the same regimen in patients with SCCAC associated with HIV infection (AMC045).29 Of important note, SCCAC has low rates of K-ras mutations,20,36, 37 which has been associated with resistance to cetuximab in colorectal cancer.38
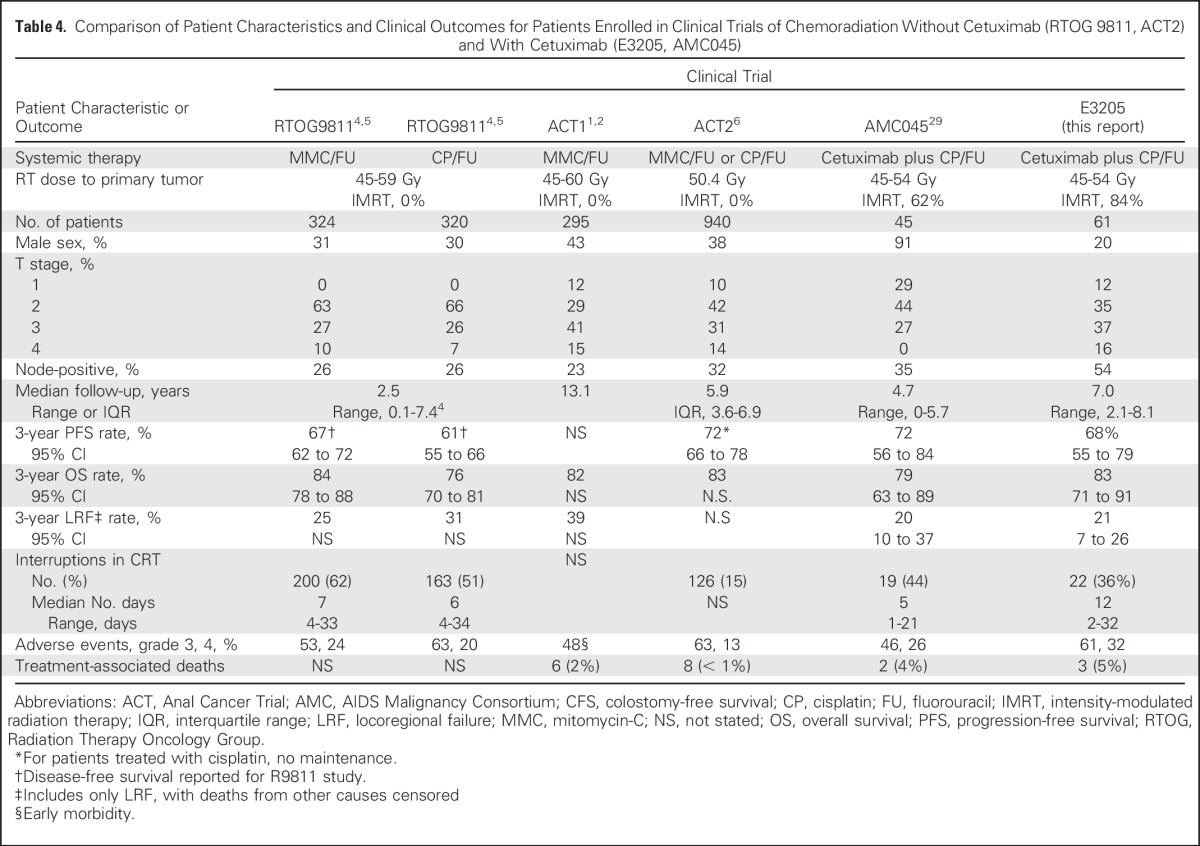
The patient characteristics and clinical outcomes from the phase II trials evaluating cetuximab-containing regimens (E3205, AMC045) and prior phase III trials evaluating CRT without cetuximab are shown in Table 4. Patients enrolled in the E3205 trial had higher rates of node-positive disease and larger (T3-4) tumors than prior phase III studies (RTOG 9811, ACT2), whereas patients enrolled in AMC45 had comparable rates of node-positive disease. Cross-trial comparison is limited by the lack of consistency regarding end point definitions and reporting of results and use of advanced imaging that may have resulted in upstaging in the more recent trials. Although some but not all phase I39-41 or II42-44 trials evaluating the combination of EGFR inhibitors (cetuximab or panitumumab) with CRT showed higher toxicity than expected with CRT alone, no other trials were specifically designed to evaluate whether the addition of EGFR inhibitors reduced LRF rates. Efficacy results from the UNICANCER trial with cetuximab were not encouraging,43 and the final results of the Vectibix for the Treatment of Anal Cancer (VITAL) trial with panitumumab are still awaited.44
Table 4.
Comparison of Patient Characteristics and Clinical Outcomes for Patients Enrolled in Clinical Trials of Chemoradiation Without Cetuximab (RTOG 9811, ACT2) and With Cetuximab (E3205, AMC045)
In conclusion, although these findings suggest that the addition of cetuximab to CRT for anal cancer may reduce LRF rates, toxicity was substantial, and LRF still occurs in approximately 20%, indicating the continued need for more effective and less toxic therapies. Although cetuximab improved clinical outcomes when added to RT in SCC of the oral cavity,22,23 similar benefit was not observed when cetuximab was added to cisplatin plus RT in this population,45 suggesting that the evaluation of cetuximab plus RT without chemotherapy may also be considered an option in future trials.
Appendix
Table A1.
Clinical Outcomes in Analyzable Patients (N = 61)
Footnotes
This study was coordinated by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants No. CA23318, CA66636, CA21115, CA180820, CA180794, CA14958, CA189859, CA15488, CA13650, CA17145, CA35267, CA189863, CA35431, CA189808, CA180864, CA35412, CA189956, and CA107868; the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services; Grant No. U10 CA029511 from the Quality Assurance Radiotherapy Committee; and Grant No. U24 CA180803 from the Imaging and Radiation Oncology Core.
The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Clinical trial information: NCT00316888.
See accompanying Editorial on page 699
AUTHOR CONTRIBUTIONS
Conception and design: Madhur K. Garg, Fengmin Zhao, Joseph A. Sparano, Joel Palefsky, Richard Whittington, Al B. Benson III
Administrative support: Thomas J. Fitzgerald
Provision of study materials or patients: Madhur K. Garg, Joseph A. Sparano, Edith P. Mitchell, Mary F. Mulcahy, Karin I. Armstrong, Nassim H. Nabbout, Shalom Kalnicki, Bassel F. El-Rayes, Adedayo A. Onitilo, Daniel J. Moriarty, Al B. Benson III
Collection and assembly of data: Madhur K. Garg, Fengmin Zhao, Joseph A. Sparano
Data analysis and interpretation: Madhur K. Garg, Fengmin Zhao, Joseph A. Sparano, Joel Palefsky, Edith P. Mitchell, Mary F. Mulcahy, Karin I. Armstrong, Nassim H. Nabbout, Shalom Kalnicki, Bassel F. El-Rayes, Adedayo A. Onitilo, Daniel J. Moriarty, Thomas J. Fitzgerald, Al B. Benson III
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cetuximab Plus Chemoradiotherapy in Immunocompetent Patients With Anal Carcinoma: A Phase II Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group Trial (E3205)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Madhur K. Garg
Speakers' Bureau: Varian Medical Systems, Covidien/Medtronic
Fengmin Zhao
No relationship to disclose
Joseph A. Sparano
Stock or Other Ownership: MetaStat
Consulting or Advisory Role: Genentech, Novartis, AstraZeneca, Celgene, Eli Lilly, Celldex Therapeutics, Pfizer, Prescient Therapeutics, Juno Therapeutics, Merrimack Pharmaceuticals
Research Funding: Prescient Therapeutics (Inst), Deciphera Pharmaceuticals (Inst), Genentech (Inst), Merck (Inst), Novartis (Inst), Merrimack Pharmaceuticals (Inst)
Joel Palefsky
Consulting or Advisory Role: Merck (Inst), Agenovir , Antiva Biosciences, Vax Genetics
Research Funding: Merck (Inst), Cel-Sci (Inst)
Travel, Accommodations, Expenses: Merck
Richard Whittington
Stock or Other Ownership: Mednax (I)
Edith P. Mitchell
Consulting or Advisory Role: Genentech, Novartis, Amgen
Research Funding: Genentech (Inst), Sanofi (Inst)
Mary F. Mulcahy
No relationship to disclose
Karin I. Armstrong
No relationship to disclose
Nassim H. Nabbout
No relationship to disclose
Shalom Kalnicki
Travel, Accommodations, Expenses: Varian Medical Systems
Bassel F. El-Rayes
Research Funding: Taiho Pharmaceutical (Inst), Bristol-Myers Squibb (Inst), Boston Biomedical (Inst), Cleave Biosciences (Inst), Genentech (Inst), AVEO Pharmaceuticals (Inst), Pfizer (Inst), Synta Pharmaceuticals (Inst)
Adedayo A. Onitilo
No relationship to disclose
Daniel J. Moriarty
Honoraria: Cardinal Health
Consulting or Advisory Role: Cardinal Health
Thomas J. Fitzgerald
No relationship to disclose
Al B. Benson III
Consulting or Advisory Role: Genentech, Sanofi, Bristol-Myers Squibb, Merck Serono, Merck/Schering-Plough, Spectrum Pharmaceuticals, Eli Lilly/ImClone Systems, Celgene, National Cancer Institute, Vicus Therapeutics, Pharmacyclics, Precision Therapeutics, Taiho Pharmaceutical, Bayer Healthcare Pharmaceuticals, Alchemia, Infinity Pharmaceuticals, Boehringer Ingelheim, Astellas Pharma, EMD Serono, Advanced Accelerator Applications, Guardant Health, Novartis, Opsona Therapeutics, Lexicon Pharmaceuticals, Guerbet, Helsinn Healthcare, Boston Biomedical
Research Funding: Genentech, Gilead Sciences, Amgen, Astellas Pharma, Bayer/Onyx Pharmaceuticals, Novartis, Alchemia, AVEO Pharmaceuticals, Infinity Pharmaceuticals, Merck Sharp & Dohme
REFERENCES
- 1.UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research Epidermoid anal cancer: Results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. Lancet. 1996;348:1049–1054. [PubMed] [Google Scholar]
- 2.Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I) Br J Cancer. 2010;102:1123–1128. doi: 10.1038/sj.bjc.6605605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: Results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–2539. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: A randomized controlled trial. JAMA. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 5.Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: Survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol. 2012;30:4344–4351. doi: 10.1200/JCO.2012.43.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): A randomised, phase 3, open-label, 2 × 2 factorial trial. Lancet Oncol. 2013;14:516–524. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 7.Shridhar R, Shibata D, Chan E, et al. Anal cancer: Current standards in care and recent changes in practice. CA Cancer J Clin. 2015;65:139–162. doi: 10.3322/caac.21259. [DOI] [PubMed] [Google Scholar]
- 8.Department of Health and Human Services (US), Office of Rare Diseases, National Institutes of Health: Annual report on the rare diseases research activities at the National Institutes of Health, FY 2005. http://rarediseases.info.nih.gov/asp/html/reports/fy2005/Annual_Report_FY_05_Final.pdf
- 9.National Cancer Institute Epidemiology and Genetics Research: Synergizing epidemiologic research on rare cancers, 2007. https://epi.grants.cancer.gov/events/rare-cancers/
- 10.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 11.Keat N, Law K, Seymour M, et al. International rare cancers initiative. Lancet Oncol. 2013;14:109–110. doi: 10.1016/S1470-2045(12)70570-3. [DOI] [PubMed] [Google Scholar]
- 12.Schott AF, Welch JJ, Verschraegen CF, et al. The National Clinical Trials Network: Conducting successful clinical trials of new therapies for rare cancers. Semin Oncol. 2015;42:731–739. doi: 10.1053/j.seminoncol.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogaerts J, Sydes MR, Keat N, et al. Clinical trial designs for rare diseases: Studies developed and discussed by the International Rare Cancers Initiative. Eur J Cancer. 2015;51:271–281. doi: 10.1016/j.ejca.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiels MS, Pfeiffer RM, Chaturvedi AK, et al. Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J Natl Cancer Inst. 2012;104:1591–1598. doi: 10.1093/jnci/djs371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams GR, Lu QL, Love SB, et al. Properties of HPV-positive and HPV-negative anal carcinomas. J Pathol. 1996;180:378–382. doi: 10.1002/(SICI)1096-9896(199612)180:4<378::AID-PATH697>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 16.Varnai AD, Bollmann M, Griefingholt H, et al. HPV in anal squamous cell carcinoma and anal intraepithelial neoplasia (AIN). Impact of HPV analysis of anal lesions on diagnosis and prognosis. Int J Colorectal Dis. 2006;21:135–142. doi: 10.1007/s00384-005-0777-7. [DOI] [PubMed] [Google Scholar]
- 17.Palefsky JM, Barrasso R. HPV infection and disease in men. Obstet Gynecol Clin North Am. 1996;23:895–916. doi: 10.1016/s0889-8545(05)70281-3. [DOI] [PubMed] [Google Scholar]
- 18.Tsai TC, Chen SL. The biochemical and biological functions of human papillomavirus type 16 E5 protein. Arch Virol. 2003;148:1445–1453. doi: 10.1007/s00705-003-0111-z. [DOI] [PubMed] [Google Scholar]
- 19.Lê LH, Chetty R, Moore MJ. Epidermal growth factor receptor expression in anal canal carcinoma. Am J Clin Pathol. 2005;124:20–23. doi: 10.1309/X4UADHVN317V2XMW. [DOI] [PubMed] [Google Scholar]
- 20.Paliga A, Onerheim R, Gologan A, et al. EGFR and K-ras gene mutation status in squamous cell anal carcinoma: A role for concurrent radiation and EGFR inhibitors? Br J Cancer. 2012;107:1864–1868. doi: 10.1038/bjc.2012.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blick SK, Scott LJ. Cetuximab: A review of its use in squamous cell carcinoma of the head and neck and metastatic colorectal cancer. Drugs. 2007;67:2585–2607. doi: 10.2165/00003495-200767170-00008. [DOI] [PubMed] [Google Scholar]
- 22.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 23.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–28. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 24.Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 25.Herrero R, Castellsagué X, Pawlita M, et al. Human papillomavirus and oral cancer: The International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 26.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 27.Termine N, Panzarella V, Falaschini S, et al. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: A meta-analysis (1988-2007) Ann Oncol. 2008;19:1681–1690. doi: 10.1093/annonc/mdn372. [DOI] [PubMed] [Google Scholar]
- 28.Burtness B, Goldwasser MA, Flood W, et al. :: Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: An Eastern Cooperative Oncology Group study J Clin Oncol 238646–8654.2005 [DOI] [PubMed] [Google Scholar]
- 29.2017;35:727–733. Sparano JA, Lee JY, Palefsky J, et al: Cetuximab plus chemoradiotherapy for HIV-associated anal carcinoma: A phase II AIDS malignancy consortium trial. J Clin Oncol. [Google Scholar]
- 30.Meropol NJ, Niedzwiecki D, Shank B, et al. Induction therapy for poor-prognosis anal canal carcinoma: A phase II study of the cancer and Leukemia Group B (CALGB 9281) J Clin Oncol. 2008;26:3229–3234. doi: 10.1200/JCO.2008.16.2339. [DOI] [PubMed] [Google Scholar]
- 31.Glynne-Jones R, Mawdsley S. Anal cancer: The end of the road for neoadjuvant chemoradiotherapy? J Clin Oncol. 2008;26:3669–3671. doi: 10.1200/JCO.2008.18.1651. [DOI] [PubMed] [Google Scholar]
- 32.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 33.Palefsky JM, Holly EA, Ralston ML, et al. Prevalence and risk factors for human papillomavirus infection of the anal canal in human immunodeficiency virus (HIV)-positive and HIV-negative homosexual men. J Infect Dis. 1998;177:361–367. doi: 10.1086/514194. [DOI] [PubMed] [Google Scholar]
- 34.Ajani JA, Winter KA, Gunderson LL, et al: Intergroup RTOG 98–11: A phase III randomized study of 5-fluorouracil (5-FU), mitomycin, and radiotherapy versus 5-fluorouracil, cisplatin and radiotherapy in carcinoma of the anal canal. J Clin Oncol 24, 2006 (suppl; abstr 4009)
- 35.Imaging and Radiation Oncology Core. http://www.qarc.org
- 36.Zampino MG, Magni E, Sonzogni A, et al. K-ras status in squamous cell anal carcinoma (SCC): It’s time for target-oriented treatment? Cancer Chemother Pharmacol. 2009;65:197–199. doi: 10.1007/s00280-009-1117-3. [DOI] [PubMed] [Google Scholar]
- 37.Martin V, Zanellato E, Franzetti-Pellanda A, et al. EGFR, KRAS, BRAF, and PIK3CA characterization in squamous cell anal cancer. Histol Histopathol. 2014;29:513–521. doi: 10.14670/HH-29.10.513. [DOI] [PubMed] [Google Scholar]
- 38.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 39.Olivatto LO, Vieira FM, Pereira BV, et al. Phase 1 study of cetuximab in combination with 5-fluorouracil, cisplatin, and radiotherapy in patients with locally advanced anal canal carcinoma. Cancer. 2013;119:2973–2980. doi: 10.1002/cncr.28045. [DOI] [PubMed] [Google Scholar]
- 40.Moreno V, García-Carbonero R, Maurel J, et al. Phase 1 study of cetuximab in combination with 5-fluorouracil, cisplatin, and radiotherapy in patients with locally advanced anal canal carcinoma. Cancer. 2014;120:454–456. doi: 10.1002/cncr.28449. [DOI] [PubMed] [Google Scholar]
- 41.Leon O, Guren MG, Radu C, et al. Phase I study of cetuximab in combination with 5-fluorouracil, mitomycin C and radiotherapy in patients with locally advanced anal cancer. Eur J Cancer. 2015;51:2740–2746. doi: 10.1016/j.ejca.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 42.Deutsch E, Lemanski C, Pignon JP, et al. Unexpected toxicity of cetuximab combined with conventional chemoradiotherapy in patients with locally advanced anal cancer: Results of the UNICANCER ACCORD 16 phase II trial. Ann Oncol. 2013;24:2834–2838. doi: 10.1093/annonc/mdt368. [DOI] [PubMed] [Google Scholar]
- 43.Levy A, Azria D, Pignon JP, et al. Low response rate after cetuximab combined with conventional chemoradiotherapy in patients with locally advanced anal cancer: Long-term results of the UNICANCER ACCORD 16 phase II trial. Radiother Oncol. 2015;114:415–416. doi: 10.1016/j.radonc.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Feliu J, Garcia-Carbonero R, Capdevilla J, et al: Phase II trial of panitumumab (P) plus mytomicin C (M), 5-fluorouracil (5-FU), and radiation (RT) in patients with squamous cell carcinoma of the anal canal (SCAC): Safety and efficacy profile—VITAL study, GEMCAD 09-02 clinical trial. J Clin Oncol 32, 2014 (suppl; abstr 4034)
- 45.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]