Abstract
The release of hemoglobin (Hb) with hemolysis causes vascular dysfunction. New evidence implicates Hb-induced NF-κB and hypoxia inducible factor (HIF) activation, which may be under the control of a Toll-like receptor (TLR)–signaling pathway. Nearly all TLR-signaling pathways activate the myeloid differentiation primary response gene–88 (MyD88) that regulates NF-κB. We hypothesized that the differing transition states of Hb influence endothelial cell permeability via NF-κB activation and HIF regulation through a MyD88-dependent pathway. In cultured human dermal microvascular endothelial cells (HMECs-1), we examined the effects of Hb in the ferrous (HbFe2+), ferric (HbFe3+), and ferryl (HbFe4+) transition states on NF-κB and HIF activity, HIF-1α and HIF-2α mRNA up-regulation, and monolayer permeability, in the presence or absence of TLR4, MyD88, NF-κB, or HIF inhibition, as well as superoxide dismutase (SOD) and catalase. Our data showed that cell-free Hb, in each transition state, induced NF-κB and HIF activity, up-regulated HIF-1α and HIF-2α mRNA, and increased HMEC-1 permeability. The blockade of either MyD88 or NF-κB, but not TLR4, attenuated Hb-induced HIF activity, the up-regulation HIF-1 and HIF-2α mRNA, and HMEC-1 permeability. The inhibition of HIF activity exerted less of an effect on Hb-induced monolayer permeability. Moreover, SOD and catalase attenuated NF-κB, HIF activity, and monolayer permeability. Our results demonstrate that Hb-induced NF-κB and HIF are regulated by two mechanisms, either MyD88 activation or Hb transition state–induced ROS formation, that influence HMEC-1 permeability.
Keywords: hemolytic disease, NF-κB, Toll-like receptors, MyD88, sickle-cell disease
Clinical Relevance
We have characterized a novel proinflammatory pathway by which free hemoglobin causes endothelial cell dysfunction and contributes to vascular diseases. This affects our understanding of the vascular toxicity associated with free hemoglobin, and provides new insights into therapies to improve the lifespan of patients with hemolytic disease.
Humans with chronic vascular inflammation and vasoconstriction develop progressive endothelial dysfunction and vascular diseases (e.g., pulmonary arterial hypertension, atherosclerosis, and sickle-cell disease) (1, 2). Vascular diseases develop in the face of chronic hemolysis, as occurs with hemoglobinopathies (including sickle-cell disease), or with the intermittent release of low to moderate levels of plasma-free hemoglobin (Hb), as may be seen in atherosclerosis with intraplaque hemorrhage. Low to moderate levels of plasma-free Hb may, in part, mediate these diseases (1–4). However, studies to date have not fully addressed the complete mechanisms responsible for Hb-induced vascular dysfunction.
The α2β2 tetramers of Hb transition safely and effectively between the relaxed (oxy) and tense (deoxy) conformational states, and the oxidation of Hb is controlled by red blood cell catalase, superoxide dismutase (SOD), and ferric Hb reductase. However, after their release from the red blood cell, Hb tetramers and dimers can be oxidized from ferrous (HbFe2+) to ferric (HbFe3+) (3–5). In theory, HbFe3+ in local tissue environments may react with hydrogen peroxide (H2O2) to transition between the ferryl (HbFe4+) and HbFe3+ states, leading to heme release, globin chain denaturation, and globin chain crosslinking (4, 5). Oxidized Hb species exhibit impaired plasma clearance because of their reduced affinity for haptoglobin protein, and are implicated as a primary cause of Hb-induced vascular toxicity (4, 5).
In vascular endothelial cells, NF-κB can control the regulation of hypoxia-inducible factor (HIF) (6, 7), and once activated, these two transcription factors together induce chronic inflammation, vasoconstriction, and endothelial permeability (7–9). To date, few studies have investigated whether Hb alters the endothelial activity of NF-κB (5, 10, 11) or HIF-1α (12–14). No studies have investigated Hb-induced NF-κB activation on HIF regulation and their combined effects on endothelial barrier function.
Interestingly, recent studies have suggested that free Hb or heme-induced NF-κB activation may be under the control of a Toll-like receptor (TLR)–signaling pathway (5, 15, 16). TLR receptors are expressed in many cell types, including the vascular endothelium, and play a key role in the innate immune response (17–20). Nearly all TLRs activate the myeloid differentiation primary response gene–88 (MyD88) pathway that regulates NF-κB (19). Thus, a mechanistic link between the free Hb induction of NF-κB, HIF regulation, and endothelial cell permeability may be associated with a TLR–MyD88–dependent signaling pathway.
We hypothesized that the differing transition states of Hb influence endothelial cell permeability via NF-κB activation, and subsequent HIF regulation, through a MyD88-dependent pathway, and that inhibition of the MyD88–NF-κB–HIF pathway would reduce endothelial leakage.
Our approach involved exposing human dermal microvascular endothelial cell (HMEC-1) monolayers to HbFe2+, HbFe3+, or HbFe4+ transition states lasting up to 24 hours, and to measure NF-κB and HIF activity, HIF-1α and HIF-2α mRNA synthesis, and monolayer permeability in comparison with untreated HMEC-1. We then treated HMEC-1 with either a TLR4 antagonist or short, interfering RNA (siRNA) against MyD88, NF-κB, or HIF, to delineate the role of the MyD88–NF-κB–HIF pathway in Hb-induced HMEC-1 permeability. Our results demonstrate that Hb-induced NF-κB regulates HIF-1α, HIF-2α, and HMEC-1 permeability, in part, via MyD88 activation, but not through TLR4.
Materials and Methods
Detailed methods are included in the online supplement.
Cell Line
HMECs-1 were obtained (catalogue number C-011-SC; Gibco, Grand Island, NY), cultured, and cultured in MCDB 131 (catalogue number 10372-019; Gibco), supplemented with 10% FBS, 0.1% gentamicin (catalogue number 15710-072; Invitrogen, Carlsbad, CA), 0.3% penicillin–streptomycin (catalogue number 15,070; Invitrogen), 0.1% antibiotic–antimycotic (catalogue number 15240-112; Invitrogen), and 0.1% polymyxin B sulfate (catalogue number 21850-029; Invitrogen). Cells were cultured under standard conditions (21% O2, 5% CO2, and balance N2), passaged 1:4, and used between Passages 8–20 for all experiments, unless otherwise stated. HMECs-1 were checked daily for changes in appearance of morphology, and before experiments, HMECs-1 were checked for dil-labeled low-density lipoprotein uptake by flow cytometry to verify an endothelial cell phenotype. Before HbA exposure to any experimental conditions, cells were washed with PBS, and fresh culture medium with 10% FBS was placed in each well.
Experimental Design and Cell Treatments
For all experiments, HMECs-1 were used from the same passage and grown during the same time frame in culture media containing 10% FBS. All experiments were completed in either a 24-well polystyrene plate (catalogue number 08-772-1; Fisher, Pittsburgh, PA) seeded at a concentration of 40,000 cells per well, or on 6.5-mm-diameter polyethylene terephthalate inserts (100,000 cells per insert) with a pore size of 0.4 μm (catalogue number 351,181; BD Falcon, San Jose, CA). Cells were untreated (i.e., control) or treated with HbFe2+, HbFe3+, or HbFe4+ (500 nM to 500 μM) between 4 and 24 hours (as noted in the figure legends) in the presence or absence of: (1) siRNA against MyD88, NF-κB (p65), or HIF-1α; (2) the TLR4 inhibitor, TAK-242 (catalogue number B1157; Biotek, Winooski, VT); (3) LPS, (4) CuZn SOD (150 units, catalogue number S9636; Sigma Chemical Co., St. Louis, MO), and catalase (150 units, catalogue number C3566; Sigma Chemical Co.); and (5) dimethyloxalyglycine (DMOG, 5–50 μg, catalogue number D3695; Sigma Chemical Co.). When appropriate, cells were also treated with mock Hb dialysis solution to ensure the obtained results were directly of Hb and oxidized Hb and not those of residual amounts of K3(Fe (2))6 or H2O2. With the exception of SOD and catalase, all inhibitors were added to the cell culture medium 24 hours before Hb treatment. SOD and catalase were added at the time of Hb treatments. After 24 hours of Hb exposure, NF-κB and HIF activity, HIF-1α and HIF-2α mRNA concentrations, and monolayer leakage were measured in triplicate. Unless noted, experiments were repeated at least three times on at least 2 separate days (n = 6).
Statistical Analysis
All experiments followed a randomized block design with the use of cells from at least three different cell preparations. Data are expressed as the means ± SEM of independent experiments. Significance between groups was determined by one-way ANOVA. Post hoc analyses were completed with Tukey-Kramer multiple-comparison tests. Statistical analysis was completed using the statistical software package JMP (version 5; SAS Institute, Cary, NC). Statistical significance was defined as P ≤ 0.05.
Results
Hb Oxidation
To test the stability of each iron oxidative transition state of Hb, we performed spectral analyses of HbFe2+, HbFe3+, or HbFe4+ (via sulf-met-Hb) to determine the specific states of Hb in the preparations immediately before and in culture media after 8, 18, and 24 hours of Hb incubation (Figures E1A–E1C in the online supplement). Our data showed that the two sources of H2O2 (namely, the addition of bolus H2O2 or glucose/glucose oxidase) induced Hb oxidation in all preparations. The preparation of HbFe4+ via the incubation of HbFe3+ with a 10-fold molar excess of H2O2 over heme demonstrated a 60:40 HbFe3+ to HbFe4+ ratio at time 0 hours, a 50:50 HbFe3+ to HbFe4+ ratio at 8 hours, and a 70:30 HbFe3+ to HbFe4+ ratio at 18 and 24 hours (Figure E1B). As a comparison, we also prepared HbFe4+ by incubating HbFe2+ with 10 units of glucose oxidase in glucose-rich media. Within 10 minutes of incubation with glucose oxidase, HbFe2+ was oxidized to a 50:50 HbFe3+ to HbFe4+ ratio. By 8 hours, the equilibrium of the 60:40 HbFe3+ to HbFe4+ ratio had been reached, and remained in place for 18 and 24 hours (Figure E1C). Unless otherwise stated, cell culture experiments used HbFe4+ prepared by incubating HbFe3+ with excess H2O2.
NF-κB and HIF Activation
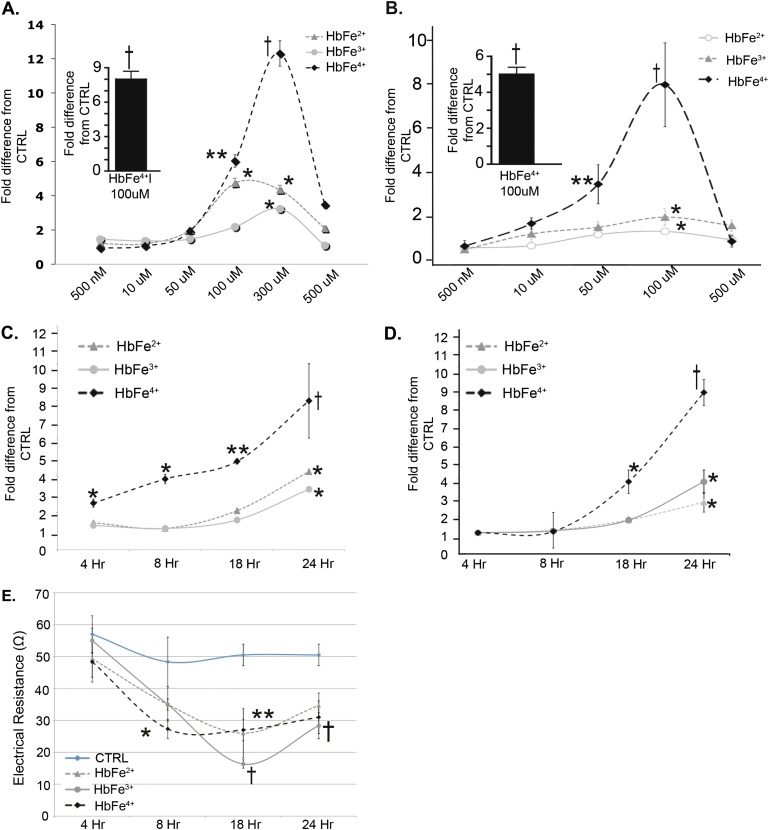
To determine whether free Hb induced NF-κB and HIF, HMECs-1 were exposed for 24 hours to HbFe2+, HbFe3+, or HbFe4+, and were evaluated for NF-κB and HIF activity. The data showed that all oxidative states of cell-free Hb activated NF-κB and HIF in a dose-dependent fashion (Figures 1A and 1B). HbFe4+ induced the greatest response, regardless of whether Hb was prepared with H2O2 or glucose oxidase (Figures 1A and 1B, insets). Equivalent volume solutions from mock preparations for HbFe3+ and HbFe4+ showed that NF-κB and HIF activity was derived from Hb rather than residual amounts of either K3Fe (2)6 and H2O2 in our Hb preparations (Figure E2). The NF-κB results were confirmed with ELISA (Figure E3). From these dose–response curves, we chose the most effective concentration (100 μM) for all subsequent cell culture experiments.
Figure 1.
Dose and time responses in human dermal microvascular endothelial cells (HMECs-1) stimulated with the ferrous (HbFe2+), ferric (HbFe3+), and ferryl (HbFe4+) states for (A) NF-κB activity and (B) Hypoxia-inducible factor (HIF) activity. Insets: Hb2+ incubated with glucose oxidase. (C) Time response for NF-κB activity in HMECs-1 stimulated with hemoglobin (Hb; 100 μM). (D) Time response for HIF activity of HMECs-1 stimulated with Hb (100 μM). (E) Time course for transendothelial electrical resistance (TER) in HMECs-1 (100 μM). *P < 0.05, versus control samples (CTRL; untreated cells). **P < 0.01, versus CTRL. †P < 0.001, versus CTRL.
Time Course of Hb-Induced NF-κB and HIF Activity
To determine whether Hb-induced NF-κB and HIF activity occurred in a time frame relevant to a mechanistic link between these transcription factors, we completed a time-course evaluation for their activity in HMEC-1 luciferase reporter cells and by Western blotting methods. NF-κB activity was increased in HMECs-1 as early as 4 hours, and continued to increase until 24 hours after incubation with Hb in the HbFe4+ state (Figure 1C). A trend toward increased HMEC-1 NF-κB activity (P = 0.1) was evident after 18 hours of incubation with HbFe3+, which was increased at 24 hours, whereas HbFe2+ increased NF-κB activity at 24 hours (Figure 1C). Western blot analysis confirmed that HbFe4+-treated HMECs-1 had increased nuclear p(65) at all time points, but not at the same magnitude at 18 or 24 hours (Figure E4). Interestingly, densitometry showed that HbFe3+ increased NF-κB activity at all time points, but peaked at 8 hours, with a nearly 6-fold increase. HbFe2+ induced an approximately twofold increase at 18 and 24 hours (Figure E4). Finally, HIF activity was increased at the 24-hour time point in HMECs-1 incubated with HbFe2+ and FeHb3+, and after 18 hours when incubated with HbFe4+ (Figure 1D).
Time Course of HMEC-1 Monolayer Permeability
To determine whether the oxidization states of Hb (100 μM) altered HMEC-1 permeability, transendothelial electrical resistance was evaluated between 4 and 24 hours of incubation with HbFe2+, HbFe3+, HbFe4+, or mock Hb preparations. Electrical resistance is inversely related to monolayer permeability. Changes in electrical resistance were noted starting at 8 hours and persisting for up to 24 hours (Figure 1E), and as expected, no differences were detected in cells treated with mock Hb solutions (Figure E5).
HIF-1α and HIF-2α mRNA
NF-κB is known to up-regulate HIF-1α and HIF-2α mRNA mechanistically (7, 8). An increased abundance of cytosolic HIF protein may also account for increased HIF activity. Thus, we measured HIF-1α and HIF-2α mRNA concentrations in HMEC-1 after 24 hours of Hb exposure. All three oxidative states of cell-free Hb increased the mRNA for HIF-1α and HIF-2α, with the greatest response induced by HbFe3+ and HbFe4+ (Figures 2A and 2B).
Figure 2.
Hb-induced effects on HIF-1α and HIF-2α mRNA. HMECs-1 were stimulated with 100 μM of HbFe2+, HbFe3+, or HbFe4+ states: (A) HIF-1α mRNA concentration and (B) HIF-2α mRNA concentration. #P < 0.02, versus CTRL (untreated cells). *P < 0.04, versus short, interfering RNA (siRNA)–transfected cells. Unless siRNA is specified in the treatment group, HMEC-1 incubation with HbFe2+, HbFe3+, or HbFe4+ oxidation states were completed in the absence of any targeted or scrambled siRNA. MyD88, myeloid differentiation primary response gene–88.
TLR4 Inhibition
TLRs are known to regulate NF-κB (21). TLR4 is the best-characterized of the TLR receptors, and was previously reported to be activated by heme in human embryonic kidney–293 or macrophage cell lines (18, 22). Thus, to determine whether NF-κB and HIF activation in our system was dependent on a TLR4-signaling pathway, HMECs-1 were treated with HbFe2+, HbFe3+, HbFe4+, or LPS (as a positive control) in the presence or absence of a TLR4 inhibitor, TAK-242. TLR4 inhibition exerted no significant effect on attenuating either Hb-induced NF-κB and HIF activity or HIF-1α and HIF-2α mRNA production (Figures E6A–E6D). As expected, TAK-242 decreased LPS-induced NF-κB activity (Figure E6A).
MyD88 Inhibition
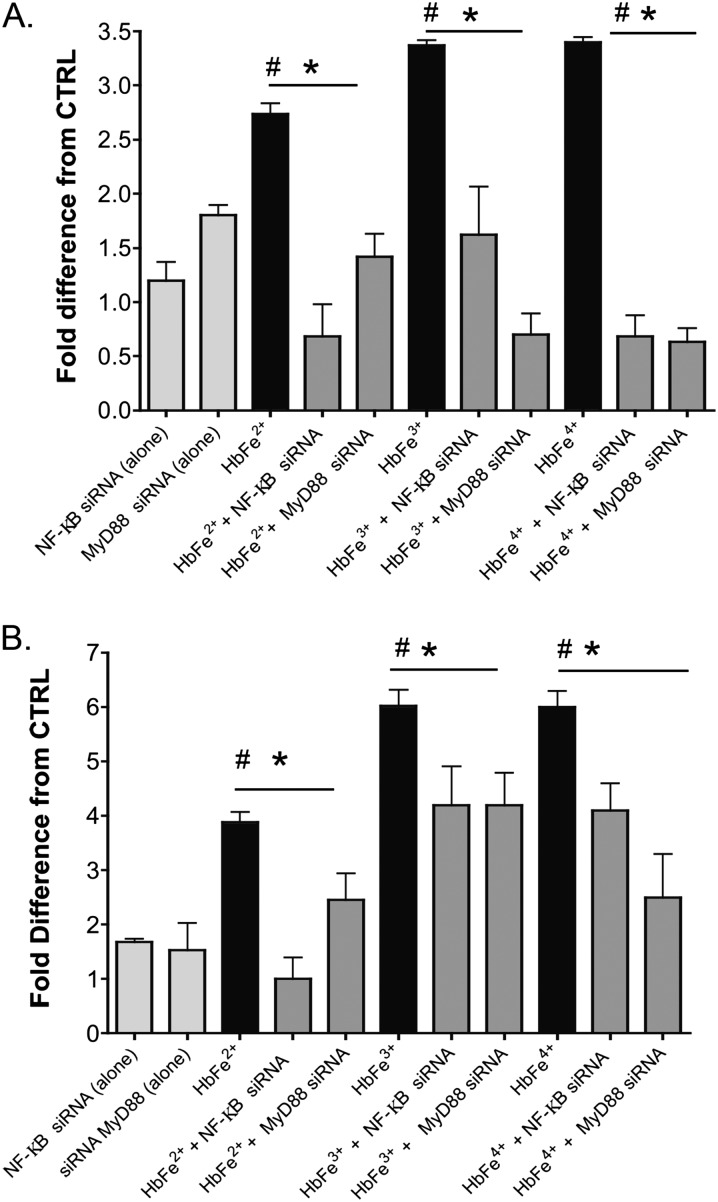
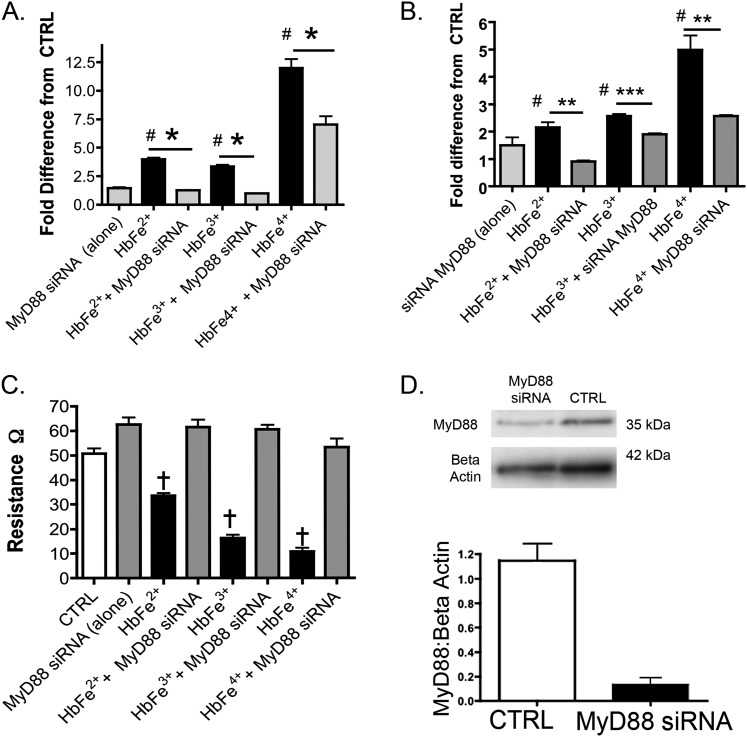
MyD88 is a universal adapter protein inherent to almost all TLR receptors, as well as to receptors for advanced glycation end products and interleukin-1β. Thus, we analyzed the impact of MyD88, using siRNA against MyD88, on Hb-induced NF-κB and HIF activity, HIF-1α and HIF-2α mRNA, and HMEC-1 electrical resistance. Our data showed that the knockdown of MyD88 attenuated the impact of all oxidative transition states of Hb on NF-κB and HIF activity (Figures 3A and 3B) and on HIF-1α and HIF-2α mRNA expression (Figure 2), and attenuated the decrease in HMEC-1 electrical resistance (Figure 3C).
Figure 3.
Effects of MyD88 inhibition on NF-κB, HIF activity, or transendothelial electrical resistance in HMECs-1 stimulated with 100 μM of HbFe2+, HbFe3+, or HbFe4+ states. To confirm siRNA knockdown, Western blots were completed on four to six independent cell treatments, and loading controls for β-actin were used from the same test blots. (A) NF-κB activity. (B) HIF activity. (C) TER. (D) Western blot of MyD88 in HMECs-1 treated with MyD88 siRNA. #P < 0.04, versus CTRL. *P < 0.01, versus Hb-induced activity in the absence MyD88 siRNA. **P < 0.02, versus Hb-induced activity in the absence MyD88 siRNA. ***P < 0.04, versus Hb-induced activity in the absence of MyD88 siRNA. †P < 0.001, versus CTRL or HMECs cotreated with Hb and MyD88 siRNA. CTRL refers to untreated cells.
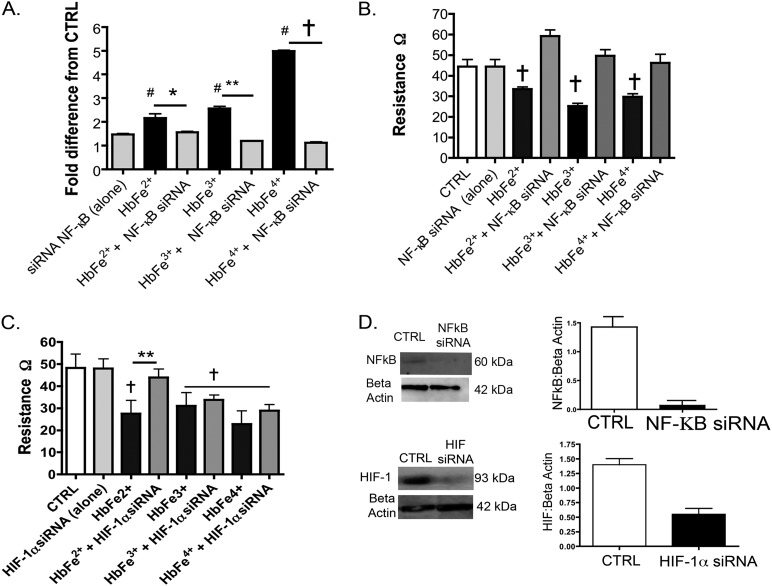
NF-κB and Prolyl Hydroxylase Inhibition
Next, we sought evidence to elucidate whether the influence of Hb on HIF activity was controlled by the NF-κB regulation of HIF-1α and HIF-2α mRNA synthesis, or by interference with prolyl hydroxylase (PH) activity. The PH class of enzymes tightly controls cytosolic HIF-1α and HIF-2α protein concentrations (10). Thus, we treated HMECs-1 with Hb species in the presence or absence of siRNA against NF-κB (p65) or a competitive inhibitor of the PH enzymes, DMOG. We measured HIF activity and HIF-1α and HIF-2α mRNA concentrations. Similar to MyD88 inhibition, the knockdown of p65 attenuated Hb-induced HIF activity (Figure 4A), HIF-1α and HIF-2α mRNA concentrations (Figure 2), and electrical resistance (Figure 4B). Interestingly, a unique synergistic HIF response existed between all oxidative states of Hb ([100 μM]) and DMOG (30 μg) (Figure E7).
Figure 4.
Effects of NF-κB (p65) and HIF inhibition on HMECs-1 stimulated with 100 μM of HbFe2+, HbFe3+, or HbFe4+ states. To confirm siRNA knockdown, Western blots were completed on three independent cell treatments, and loading controls for β-actin were used from the same test blots. (A) HIF activity. (B) TER with NF-κB siRNA. (C) TER with HIF siRNA. (D) Representative Western blot of siRNA against NF-κB (p65) or HIF. #P < 0.04, versus CTRL. *P < 0.04, versus Hb-induced activity versus with NF-κB or HIF siRNAs. **P < 0.02, versus Hb-induced activity with NF-κB or HIF siRNA. †P < 0.01, versus CTRL or HMECs cotreated with Hb and NF-κB siRNA. CTRL refers to untreated cells. Unless siRNA is specified in the treatment group, HMEC-1 incubation with HbFe2+, HbFe3+, or HbFe4+ oxidation states were completed in the absence of any targeted or scrambled siRNA.
HIF Inhibition
To elucidate the influence of NF-κB compared with HIF-regulated proteins on the HMEC-1 barrier function, we treated HMECs-1 with Hb species in the presence or absence of siRNA against HIF. Compared with NF-κB, the knockdown of HIF exerted less of an effect on electrical resistance in the HbFe3+ and HbFe4+ transition states (Figure 4C).
Antioxidant Treatments
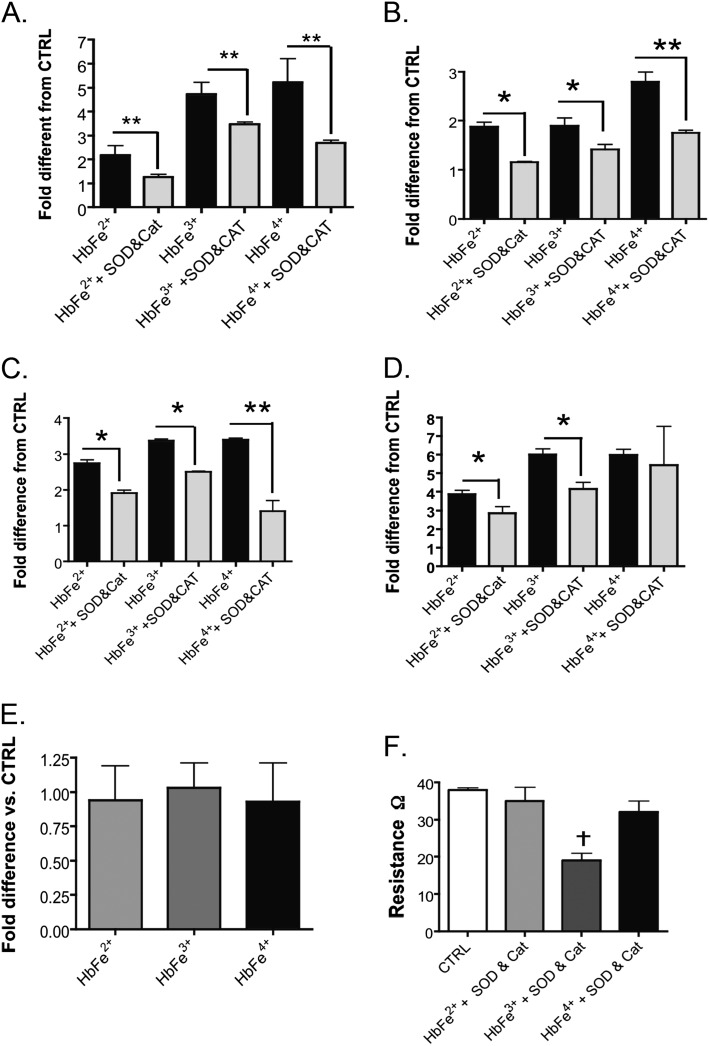
Cell-free Hb is well-established to promote the formation of reactive oxygen species (ROS) such as SOD and H2O2 in circulating cells, as well as in endothelial cells. Both SOD and H2O2 are known activators of HIF and NF-κB (7, 23). Thus, to determine the extent that Hb-induced ROS formation contributed to either HIF or NF-κB activation in our model system, HMECs-1 were cotreated with free Hb species and exogenous SOD and catalase. Treatment with SOD and catalase attenuated Hb-induced NF-κB and HIF activity in all three Hb transition states, as well as HIF-1α mRNA (Figures 5A–5D). Interestingly, less of an effect of SOD and catalase was evident in HIF-2α mRNA up-regulation. With the exception of HIF-2α mRNA, SOD and catalase exerted the greatest impact on the responses to Hb in the HbFe4+ state. To evaluate whether free Hb increased intracellular oxidative stress, we measured the glutathione (GSH) to GSH disulfide (GSSG) ratio in treated and untreated cells. Our results showed no significant changes in the GSH/GSSG ratio in HMECs-1 after 24 hours of exposure to any of the Hb species (Figure 5D). Interestingly, cotreatment with SOD and catalase inhibited Hb-induced permeability in the Fe2+ and Fe4+ states, but was not as effective in the Fe3+ state (Figure 5F).
Figure 5.
Effects of superoxide dismutase (SOD) and catalase (CAT) treatment on NF-κB and HIF activity, HIF-1α and HIF-2α mRNA, and electrical resistance in HMECs stimulated with 100 μM Hb, starting in either the HbFe2+, HbFe3+, or HbFe4+ states. (A) NF-κB activity. (B) HIF activity. (C) HIF-1α mRNA. (D) HIF-2α mRNA. (E) GSH/GSSG ratio as an indication of intracellular oxidative stress in HMECs. (F) TER. *P < 0.04, versus Hb-induced activity in the absence of SOD and CAT; **P < 0.02, versus Hb-induced activity in the absence of SOD and CAT. †P < 0.01, versus CTRL. CTRL refers to untreated cells.
Discussion
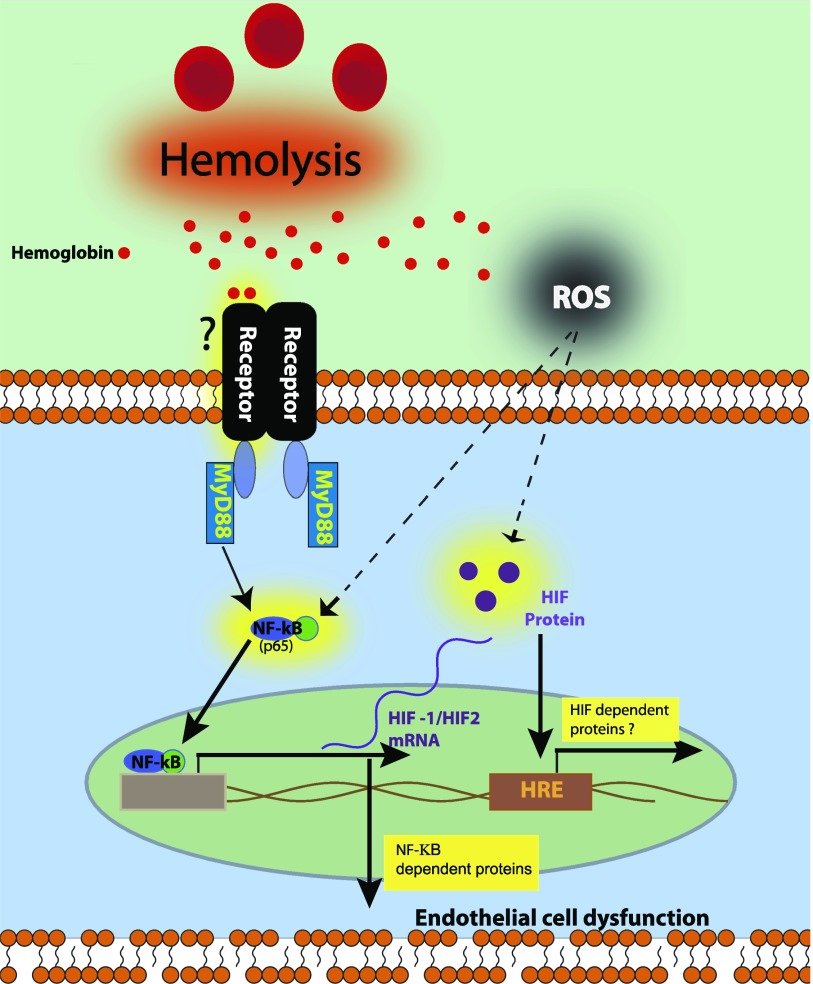
The principle observation of this study involves the novel finding that Hb induced an NF-κB–HIF signaling pathway in HMECs-1 via two main mechanisms, namely, either an MyD88-dependent pathway or a ROS-signaling event. Of interest, our results showed that NF-κB activity was a greater contributor to HMEC-1 monolayer permeability than was HIF activation. Further, our data showed that Hb-induced MyD88 signaling was independent of TLR4 (Figure 6). Thus, in addition to the pro-oxidant effects of Hb, our data support the concept that Hb can initiate an inflammatory response via a MyD88-dependent receptor such as TLR, pattern recognition receptors (PRRs), or the receptor for advanced glycation end products (4). These observations begin to define the complex interactions between signaling pathways and transcription factors that are initiated at the interface between free Hb and the endothelium that contribute to vascular diseases (Figure 6).
Figure 6.
Schematic depicts the mechanisms by which our data showed free Hb-induced NF-κB activity, HIF activity, and increased permeability in HMECs-1 through an MyD88-dependent mechanism or reactive oxygen species (ROS) produced from Hb transition states. Hemoglobin-induced MyD88 activation may occur through either a membrane-bound Toll-like receptor (TLR) or through a pattern recognition receptor (PRR) within the endothelial cell.
NF-κB is either a heterodimer or homodimer, and is formed by a family of five subunits, RelA (p65), RelB, cRel, p50, and p52 (7, 24). All subunits were previously shown to contribute to the regulation of HIF-1α and HIF-2α mRNA (6, 7), with the exception of the p52 regulation of HIF-1α mRNA (7). Our data showed that NF-κB preceded HIF activity, strengthening the conclusions of previous reports. Moreover, as expected, we did not achieve a complete blockade of Hb-induced HIF activity or HIF-1α and HIF-2α mRNA synthesis with p65 knockdown. Instead, p65 inhibition blocked Hb-induced permeability. Interestingly, changes in HMEC-1 electrical resistance began after 8 hours of incubation in HbFe2+, HbFe3+, and HbFe4+ transition states, but NF-κB activity was increased only in HbFe4+ cells. This suggests that in addition to NF-κB and HIF, free Hb may alter endothelial barrier function through other pathways, such as a p38 mitogen-activated protein kinase mechanism (22).
HIFs are heteroduplexes with α and β subunits, and coordinate the body’s response to hypoxia (9). As previously mentioned, HIF-1α and HIF-2α synthesis can be regulated through NF-κB, but their activity is controlled by PH enzymes that, under normal oxygen tensions, target HIF-α for degradation (7, 25). Our data showed a synergistic response in Hb-induced HIF activity when HMECs-1 were cotreated with DMOG, a competitive inhibitor of PH enzyme. This supports a notion that the principle mechanism by which Hb induces HIF is not through interference with PH enzymes, but through increased HIF cytosolic protein via NF-κB activation. However, because DMOG is a competitive inhibitor of PH enzymes, it does not entirely rule out the possibility that Hb alters PH activity.
Uncontrolled HIF activity can lead to endothelial cell dysfunction and pulmonary vascular leakage in vivo and in vitro (8, 26, 27). Our data showed that the knockdown of HIF-1α exerted a minimal effect on attenuating Hb-induced endothelial cell permeability. This provides evidence that Hb-induced endothelial permeability is regulated principally through NF-κB (p65) activation and controlled inflammatory proteins, such as TNF-α, as opposed to HIF-controlled proteins, such as vascular endothelial growth factor or endothelin-1. However, under hypoxic conditions, where HIF is not targeted for ubiquination, it may exert a greater effect on endothelial dysfunction. Thus, HIF activity may be relevant to causing endothelial cell dysfunction during vaso-occlusive episodes that cause tissue hypoxia in sickle-cell disease. We did not, however, specifically inhibit HIF-2α activity to determine its exact contribution to Hb-induced HMEC-1 permeability. Though it is unlikely, Hb-induced HMEC-1 permeability may have occurred through HIF-2α as opposed to p65.
Supporting our hypothesis, our data showed that MyD88 inhibition attenuated Hb-induced NF-κB and HIF activity, HIF-1α and HIF-2α mRNA synthesis, and HMEC-1 permeability. This describes a novel mechanistic signaling pathway between free Hb and the endothelium that disrupts endothelial barrier function properties and leads to increased endothelial cell permeability. TLR and PRR signaling cascades can be triggered by endogenous danger–associated molecular patterns (DAMPs) released by tissue damage, including ATP or hyaluronan, which can cause further endothelial cell dysfunction (19, 28). Thus our data, along with those of others, suggest that free Hb falls into the DAMP category as well.
TLR4 is activated by LPS, heat shock protein–60, and oxidized low-density lipoprotein (19, 28). In our cell-culture model, inhibition with the TLR4 receptor antagonist TAK-242 exerted no effect on Hb-induced NF-κB and HIF activation in any oxidative state. These data support those of Silva and colleagues (from 2009), which showed that HbFe4+ Hb activated a TLR receptor other then TLR4 (5). The exact TLR or PRR that Hb activates poses an important question to resolve, and we are actively seeking the answer.
As expected, in addition to MyD88 knockdown, SOD and catalase treatment attenuated Hb-induced NF-κB and HIF activity, HIF synthesis, and permeability. These data are congruent with the pro-oxidant characteristics of Hb, because extracellular free radical formations such as superoxide anion (O2·−) or H2O2 can contribute to Hb-induced NF-κB and HIF activity (12, 13, 16). It remains unclear why SOD and catalase did not exert as great an effect on attenuating permeability when the Hb began in the Fe3+ state. It is provocative to speculate that the HbFe3+ state is responsible for the MyD88 activation, and this poses an important question. Taking into account our data from all three Hb transitions states and considering the possibility they may occur simultaneously in the blood/interstitial milieu, future therapeutic strategies may involve targets to protect the endothelium against Hb toxicity with a combination of antioxidants and MyD88 inhibition.
In humans, circulating free Hb does not typically exceed 1–2 μM of heme, but under pathophysiological conditions, such as infections or in hemolytic anemia syndromes, it may persist at much higher concentrations (29). In our cell-culture system, Hb-induced NF-κB and HIF activation occurred between a low-level range of 10 and 300 μM (hyperhemolytic disease state concentrations). This suggests that HMECs-1 responded to Hb in a physiologically relevant range that could occur in moderate to high levels of red blood cell lyses, thus describing a potential role for Hb-induced endothelial permeability via an MyD88, NF-κB, and HIF signaling pathway. Furthermore, our data are consistent with other in vitro observations that Hb at the lower 10–100-μM concentrations exerts the greatest influence on NF-κB and HIF activity (5, 10, 14, 30). This may be particularly relevant in localized areas where erythrocytes are trapped, such as areas of vaso-occlusion during acute sickle-cell events, or in the unique setting of plaque rupture with hemorrhage in atherosclerotic vessels.
We recently reported that rats chronically infused with free Hb (12–35 mg/day) in the Fe2+ state, in which Hb infusions lasted up to 7 weeks, showed evidence for the progression of pulmonary vascular inflammation, intercellular adhesion molecule–1 (ICAM-1), and vascular disease (31). Both HMECs-1 and pulmonary vascular endothelial cells express TLR or IL-1Β receptors that can activate MyD88 (32). Because ICAM-1 is regulated by NF-κB activation, it is provocative to consider that increased lung ICAM-1 concentrations in our in vivo model may have occurred through an Hb-induced MyD88-dependent signaling pathway. However, endothelial cells from different organ beds and different locations within an organ behave differently (22), and therefore this will need to be verified in future studies.
In conclusion, the results of the present study showed that in HMECs-1 exposed for 24 hours to free Hb beginning in either the HbFe2+, HbFe3+, or HbFe4+ transition states, increased HMEC-1 monolayer permeability, HIF activity, and HIF protein synthesis via an NF-κB–dependent pathway controlled by two main mechanisms, either MyD88 activation or the Hb transition state, induced ROS formation. Our data support the concept that independent of the nitric oxide and pro-oxidant characteristics of Hb, Hb can act as a proinflammatory agent by activating an MyD88-dependent pathway.
Acknowledgments
Acknowledgments
The authors thank Dr. Paul Buehler for providing catalase-free Hb for these experiments; Thomas Weissmuller, M.D. (Resident, Brigham and Women’s Hospital, Boston, MA), for supplying the stably transfected HMEC-1 HIF reporter cells; and Joanne Maltzhan and Paul Eigenberger for proofreading and editing the manuscript.
Footnotes
This study was supported in part by a Giles F. Filley Award from the American Physiological Society and by National Institutes of Health grant 5P01HL014985-38.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0440OC on May 28, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. N Engl J Med. 2008;359:2254–2265. doi: 10.1056/NEJMra0804411. [DOI] [PubMed] [Google Scholar]
- 2.Kato GJ, Taylor JG., VI Pleiotropic effects of intravascular haemolysis on vascular homeostasis. Br J Haematol. 2010;148:690–701. doi: 10.1111/j.1365-2141.2009.08004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alayash AI. Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov. 2004;3:152–159. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 4.Buehler PW, D’Agnillo F. Toxicological consequences of extracellular hemoglobin: biochemical and physiological perspectives. Antioxid Redox Signal. 2010;12:275–291. doi: 10.1089/ars.2009.2799. [DOI] [PubMed] [Google Scholar]
- 5.Silva G, Jeney V, Chora A, Larsen R, Balla J, Soares MP. Oxidized hemoglobin is an endogenous proinflammatory agonist that targets vascular endothelial cells. J Biol Chem. 2009;284:29582–29595. doi: 10.1074/jbc.M109.045344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Uden P, Kenneth NS, Webster R, Müller HA, Mudie S, Rocha S. Evolutionary conserved regulation of HIF-1β by NF-κB. PLoS Genet. 2011;7:e1001285. doi: 10.1371/journal.pgen.1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor–1alpha by NF-kappaB. Biochem J. 2008;412:477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irwin DC, McCord JM, Nozik-Grayck E, Beckly G, Foreman B, Sullivan T, White M, Crossno J, Jr, Bailey D, Flores SC, et al. A potential role for reactive oxygen species and the HIF-1alpha–VEGF pathway in hypoxia-induced pulmonary vascular leak. Free Radic Biol Med. 2009;47:55–61. doi: 10.1016/j.freeradbiomed.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kupari M, Rapola J. Reversible pulmonary hypertension associated with vitamin C deficiency. Chest. 2012;142:225–227. doi: 10.1378/chest.11-1857. [DOI] [PubMed] [Google Scholar]
- 10.Ogasawara N, Oguro T, Sakabe T, Matsushima M, Takikawa O, Isobe K, Nagase F. Hemoglobin induces the expression of indoleamine 2,3-dioxygenase in dendritic cells through the activation of PI3K, PKC, and NF-kappaB and the generation of reactive oxygen species. J Cell Biochem. 2009;108:716–725. doi: 10.1002/jcb.22308. [DOI] [PubMed] [Google Scholar]
- 11.Simoni J, Simoni G, Lox CD, Prien SD, Shires GT. Modified hemoglobin solution, with desired pharmacological properties, does not activate nuclear transcription factor NF-kappa B in human vascular endothelial cells. Artif Cells Blood Substit Immobil Biotechnol. 1997;25:193–210. doi: 10.3109/10731199709118910. [DOI] [PubMed] [Google Scholar]
- 12.Buehler PW, D’Agnillo F, Hoffman V, Alayash AI. Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J Pharmacol Exp Ther. 2007;323:49–60. doi: 10.1124/jpet.107.126409. [DOI] [PubMed] [Google Scholar]
- 13.Manalo DJ, Buehler PW, Baek JH, Butt O, D’Agnillo F, Alayash AI. Acellular haemoglobin attenuates hypoxia-inducible factor–1alpha (HIF-1alpha) and its target genes in haemodiluted rats. Biochem J. 2008;414:461–469. doi: 10.1042/BJ20080313. [DOI] [PubMed] [Google Scholar]
- 14.Yeh LH, Alayash AI. Effects of cell-free hemoglobin on hypoxia-inducible factor (HIF-1alpha) and heme oxygenase (HO-1) expressions in endothelial cells subjected to hypoxia. Antioxid Redox Signal. 2004;6:944–953. doi: 10.1089/ars.2004.6.944. [DOI] [PubMed] [Google Scholar]
- 15.Lin S, Yin Q, Zhong Q, Lv FL, Zhou Y, Li JQ, Wang JZ, Su BY, Yang QW. Heme activates TLR4-mediated inflammatory injury via MyD88/TRIF signaling pathway in intracerebral hemorrhage. J Neuroinflammation. 2012;9:46. doi: 10.1186/1742-2094-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piazza M, Damore G, Costa B, Gioannini TL, Weiss JP, Peri F. Hemin and a metabolic derivative coprohemin modulate the TLR4 pathway differently through different molecular targets. Innate Immun. 2011;17:293–301. doi: 10.1177/1753425910369020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpenter S, O’Neill LA. Recent insights into the structure of Toll-like receptors and post-translational modifications of their associated signalling proteins. Biochem J. 2009;422:1–10. doi: 10.1042/BJ20090616. [DOI] [PubMed] [Google Scholar]
- 18.Nicolaou G, Erridge C. Toll-like receptor–dependent lipid body formation in macrophage foam cell formation. Curr Opin Lipidol. 2010;21:427–433. doi: 10.1097/MOL.0b013e32833cacd5. [DOI] [PubMed] [Google Scholar]
- 19.Opitz B, Eitel J, Meixenberger K, Suttorp N. Role of Toll-like receptors, NOD-like receptors and RIG-I–like receptors in endothelial cells and systemic infections. Thromb Haemost. 2009;102:1103–1109. doi: 10.1160/TH09-05-0323. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 22.Irwin DC, Tissot van Patot MC, Tucker A, Bowen R. Direct ANP inhibition of hypoxia-induced inflammatory pathways in pulmonary microvascular and macrovascular endothelial monolayers. Am J Physiol Lung Cell Mol Physiol. 2005;288:L849–L859. doi: 10.1152/ajplung.00294.2004. [DOI] [PubMed] [Google Scholar]
- 23.Van der Heiden K, Cuhlmann S, Luong A, Zakkar M, Evans PC. Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond) 2010;118:593–605. doi: 10.1042/CS20090557. [DOI] [PubMed] [Google Scholar]
- 24.Hayden MS, Ghosh SNF. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodríguez-Jiménez FJ, Moreno-Manzano V. Modulation of hypoxia-inducible factors (HIF) from an integrative pharmacological perspective. Cell Mol Life Sci. 2012;69:519–534. doi: 10.1007/s00018-011-0813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engebretsen BJ, Irwin D, Valdez ME, O’Donovan MK, Tucker A, van Patot MT. Acute hypobaric hypoxia (5486 M) induces greater pulmonary HIF-1 activation in Hilltop compared to Madison rats. High Alt Med Biol. 2007;8:312–321. doi: 10.1089/ham.2007.1031. [DOI] [PubMed] [Google Scholar]
- 27.Gao L, Chen Q, Zhou X, Fan L. The role of hypoxia-inducible factor 1 in atherosclerosis. J Clin Pathol. 2012;65:872–876. doi: 10.1136/jclinpath-2012-200828. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Mohan C. Toll-like receptor signaling pathways: therapeutic opportunities. Mediators Inflamm. 2010;2010:781235–781242. doi: 10.1155/2010/781235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Spolarics Z. Methemoglobin is a potent activator of endothelial cells by stimulating IL-6 and IL-8 production and E-selectin membrane expression. Am J Physiol Cell Physiol. 2003;285:C1036–C1046. doi: 10.1152/ajpcell.00164.2003. [DOI] [PubMed] [Google Scholar]
- 31.Buehler PW, Baek JH, Lisk C, Connor I, Sullivan T, Kominsky D, Majka S, Stenmark KR, Nozik-Grayck E, Bonaventura J, et al. Free hemoglobin induction of pulmonary vascular disease: evidence for an inflammatory mechanism. Am J Physiol Lung Cell Mol Physiol. 2012;303:L312–L326. doi: 10.1152/ajplung.00074.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK. Hypoxia inducible factor (HIF)–1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE. 2007;2:e1364. doi: 10.1371/journal.pone.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]