Abstract
The heart consumes more energy per gram than any other organ, and the creatine kinase (CK) reaction serves as its prime energy reserve. Because chemical energy is required to fuel systolic and diastolic function, the question of whether the failing heart is “energy starved” has been debated for decades. Despite the central role of the CK reaction in cardiac energy metabolism, direct measures of CK flux in the beating human heart were not previously possible. Using an image-guided molecular assessment of endogenous ATP turnover, we directly measured ATP flux through CK in normal, stressed, and failing human hearts. We show that cardiac CK flux in healthy humans is faster than that estimated through oxidative phosphorylation and that CK flux does not increase during a doubling of the heart rate-blood pressure product by dobutamine. Furthermore, cardiac ATP flux through CK is reduced by 50% in mild-to-moderate human heart failure (1.6 ± 0.6 vs. 3.2 ± 0.9 μmol/g of wet weight per sec, P < 0.0005). We conclude that magnetic resonance strategies can now directly assess human myocardial CK energy flux. The deficit in ATP supplied by CK in the failing heart is cardiac-specific and potentially of sufficient magnitude, even in the absence of a significant reduction in ATP stores, to contribute to the pathophysiology of human heart failure. These findings support the pursuit of new therapies that reduce energy demand and/or augment energy transfer in heart failure and indicate that cardiac magnetic resonance can be used to assess their effectiveness.
Keywords: heart failure, magnetic resonance spectroscopy, metabolism
ATP provides the chemical energy that fuels myocardial contractile function. Relatively large rates of ATP synthesis are required to sustain normal systolic and diastolic function. The “energy starvation” hypothesis of heart failure suggests that inadequate ATP supply underlies the contractile dysfunction present in heart failure (1, 2). Large-scale clinical trials demonstrating that pharmacologic agents such as beta-blockers and angiotensin-converting enzyme inhibitors that reduce metabolic demand improve outcomes in heart failure, whereas those such as positive inotropic agents that increase energetic demand worsen outcomes (3) are consistent with the energy-starvation hypothesis. However, the ability to test the energy-starvation hypothesis has been limited, in part, by an inability to directly measure ATP synthesis in the human heart.
Creatine kinase (CK) is central to mammalian energy metabolism and serves as the prime energy reserve of the heart. CK reversibly converts ADP and creatine phosphate (PCr) to ATP and creatine (Cr). This reaction allows tight control of ADP and ATP concentrations in cardiac and skeletal muscle as well as in brain, providing a rapid source of ATP during ischemia and burst activity in skeletal muscle (4-6). It is also hypothesized that the CK reaction serves as an intracellular spatial energy shuttle, facilitating the transfer of high-energy phosphates from the mitochondria (where ATP is produced) to the cytosol (where ATP is used) and facilitating the return of products to the mitochondria for rephosphorylation (5, 7).
Endogenous human cardiac CK metabolite levels have been measured in biopsies (4, 8) and by in vivo magnetic resonance spectroscopy (MRS) (9-13). Modest reductions in myocardial CK metabolite levels have been reported in humans at rest with moderate-to-severe heart failure (4, 8, 14-17). However, metabolite pool sizes do not directly index flux or energy turnover. Dynamic measures of energy metabolite turnover have been possible with saturation transfer MRS in animals (18, 19), but flux measurements in the human heart have been unobtainable because of the complexity, sensitivity, and inefficiency of standard methods. We recently developed and validated a four-angle saturation transfer (FAST) method for rapidly measuring CK flux rate by using phosphorus (31P) MRS (20) and, separately, noninvasive methods for quantifying myocardial metabolite concentrations (11). Here we combine these techniques to obtain direct measurements of energy turnover in the human heart. The data provide insight into the long-standing but important question of whether the failing human heart is energy-deficient.
Materials and Methods
Human Subjects. All studies were approved by The Johns Hopkins Institutional Review Board on human investigation. Informed consent was obtained after explanation of the study and protocol. Normal subjects were <50 years old with no history of hypertension, diabetes, or heart disease. Heart failure subjects included 4 women and 13 men with a mean age of 46 ± 10 (mean ± SD) years, identified by clinical history. Left ventricular ejection fraction was <40%, and significant coronary disease was excluded by x-ray contrast angiography in all patients. One patient was in New York Heart Association (NYHA) heart failure class I, seven in class II, seven in class III, one in class III-IV, and one in class IV.
Stress Studies. To determine whether increased energy demand during stress increases the rate of ATP synthesis through CK over a physiologic range of stress, cardiac CK flux was measured in six normal subjects at rest and during i.v. dobutamine infusion that doubled the rate-pressure product. After baseline metabolic measurements were taken, dobutamine was administered by i.v. continuous infusion with increments in dose every 3-5 min until the (heart-rate) × (systolic blood pressure) product increased to twice that at baseline. CK flux measurements were repeated at the doubled rate-pressure product.
Image-Guided, Cardiac 31P MRS Quantification of CK Flux. Imageguided 31P MRS was performed on a clinical 1.5-T General Electric Signa MRI scanner with subjects positioned prone on a 6-cm surface receive coil. A one-dimensional chemical-shift imaging sequence was applied (1-cm resolution) with B1-field independent rotation phase-cycled (BIRP) adiabatic pulses (21) to provide exact flip angles of 15° or 60°. Square pulses at 2% of the BIRP power level were applied to selectively saturate the γ-phosphate of ATP (γ-ATP) at -2.7 ppm and to provide a control irradiation at +2.7 ppm relative to the PCr resonance. FAST measurements consisted of two pairs of measurements of the PCr signal with flip angles of 15° and 60°: one pair with γ-ATP saturated and the other pair with control irradiation (total scan time ≈38 min; repetition time (TR) = 1 sec) (20). The forward CK rate constant kfor was derived from the classic relationship kfor T′1 = 1 - M′o/Mo corrected for spillover irradiation, where T′1 is the spin-lattice relaxation time in the presence of saturating irradiation and M′o/Mo is the fully relaxed fractional reduction in PCr. Specifically, we used equations 5, 6, and 9 of ref. 20. The CK forward flux rate was calculated from the classic equation CK flux = kfor·[PCr]. In all studies at rest, a fifth 31P MRS data set was acquired with a 60° pulse and without selective saturation to measure both the spillover of the saturation and the ATP and PCr concentrations (scan time ≈6 min, TR = 1 sec). In all but the dobutamine studies, this procedure was followed by a 1H MRS acquisition with the same detector coil (scan time ≈4 min, TR = 2 sec) to provide a water signal as an internal concentration reference (11).
In the dobutamine studies, the FASTest protocol (20) was applied after doubling of the rate-pressure product was achieved. This protocol involved two 60° acquisitions with γ-ATP and control irradiation (scan time ≈13 min) during stress. At least one additional 15° acquisition with selective saturation was made to confirm that the longitudinal relaxation times did not change with stress (20).
After the examination, the patient was replaced by a large, phosphate-containing phantom, and fully relaxed 31P and 1H MR spectra were acquired to calibrate concentration measurements (11). PCr and ATP concentrations were determined by both the water- and phosphate-reference methods (10, 11, 22), except for the normal dobutamine studies, when only the phosphate-reference method was used (10, 22). Comparison of measurements using both methods showed no significant difference. Metabolite measurements were saturation-corrected and ATP concentration was corrected for blood contamination (10, 11, 14, 22).
Results
Representative image-guided, spatially localized, cardiac 31P FAST spectra from a normal subject at rest are shown in Fig. 1b. The reduction in PCr signal with ATP saturated is directly proportional to the forward flux rate through the CK reaction in the human heart (20). The metabolite signals recorded in the absence of any saturating irradiation are proportional to the metabolite concentrations (11). The metabolite concentrations and the unidirectional forward flux rate, kfor, of ATP synthesis through CK were determined for cardiac and chest skeletal muscle in 16 normal subjects at rest. The results are summarized in Table 1. CK flux in chest muscle is similar to that previously reported from leg muscle (20). CK flux in the beating heart is ≈50% that of resting skeletal muscle.
Fig. 1.
MRI/MRS detection of CK energy flux in the human heart. Shown are cardiac MRI and 31P spectra from FAST studies of a normal subject acquired at rest (a and b) and during dobutamine stress (c), and of a 37-year-old patient with NYHA class III heart failure at rest (d and e). Horizontal white lines in images denote the source of the spectra in the anterior myocardium. The white box shows the detector coil location. Arrows on the spectra identify the frequency of the saturating irradiation tuned to the γ-ATP resonance (spectra on right) and in a symmetric control location, relative to PCr (spectra on left). With γ-ATP saturated, the PCr resonance decreases (oblique lines) in direct proportion to the forward CK flux because the saturated γ-ATP signal is unable to contribute to the PCr signal by the reverse reaction: the greater the flux the greater the decrease. Dobutamine stress had a dramatic 2-fold effect on cardiac workload (rate-pressure product), but did not dramatically alter CK flux in the heart (b and c). At rest, the flux in the patient is lower (e). In each study, four such 31P data sets are acquired with saturation and two different flip angles (20); then a 31P and a 1H data set are acquired without saturation to measure concentrations (10, 11, 22). The spectral scale is chemical shift in ppm.
Table 1. Metabolite levels and ATP turnover in normal subjects and patients with heart failure.
| Tissue | PCr, μmol/g wet wt | ATP, μmol/g wet wt | kfor, sec−1 | CK flux, μmol/g wet wt per sec | n |
|---|---|---|---|---|---|
| Chest wall | |||||
| Normal | 27 ± 8* | 8.2 ± 2.4 | 0.22 ± 0.07† | 5.7 ± 2.2† | 14‡ |
| Heart failure | 26 ± 8*§ | 8.4 ± 3.4§ | 0.21 ± 0.08§ | 5.1 ± 2.2§¶ | 13∥ |
| Cardiac muscle Normal | |||||
| Rest | 10.1 ± 1.3 | 5.7 ± 1.3 | 0.32 ± 0.07 | 3.2 ± 0.9 | 16 |
| Dobutamine | 9.9 ± 1.2 | 5.6 ± 1.4 | 0.33 ± 0.09 | 3.3 ± 1.2 | 6 |
| Heart failure | 8.3 ± 2.6** | 5.2 ± 1.3 | 0.21 ± 0.07†† | 1.6 ± 0.6†† | 17 |
Data are mean ± SD. *, P < 0.0001 vs. heart in same subjects; †, P < 0.003 vs. heart in same subject; ‡, chest muscle was not unambiguously identified in two normal subjects; §, P = not significant vs. chest in normal subjects; ¶, P < 0.002 vs. heart in same subjects; ∥, chest muscle was not unambiguously identified in four heart failure patients; **, P = 0.03 vs. normal; ††, P < 0.0005 vs. normal.
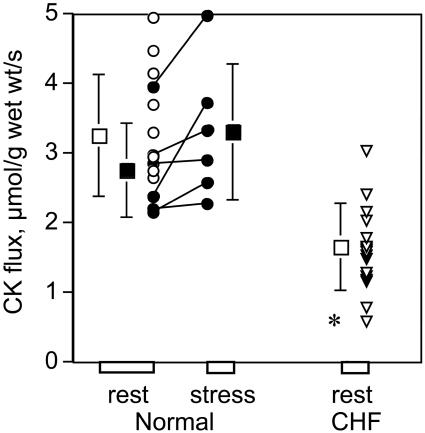
Dobutamine stress doubled the rate-pressure product in normal subjects, from 7,820 ± 521 (±SD) to 16,300 ± 1,260 mmHg·beats/min. This should increase myocardial oxygen consumption and ATP synthesis through oxidative phosphorylation (Ox-Phos) by the same proportion (23, 24). Although dobutamine caused a modest increase in kfor of borderline statistical significance in the same six subjects (P = 0.06), CK flux was not significantly changed during dobutamine infusion in normal subjects (Table 1, Fig. 2). Whereas mean myocardial CK flux is slightly higher at 3.3 ± 1.2 vs. 2.8 ± 0.7 μmol/g per sec (P = 0.22), this increase is not statistically significant and is not proportionate to the doubled rate-pressure product. The finding that CK flux does not significantly increase with cardiac work-load over a physiologic range in humans is different from that seen in isolated rat hearts (25) but similar to prior studies performed in vivo in sheep, pigs, and dogs (18, 19, 26, 27).
Fig. 2.
The forward cardiac CK flux is reduced in chronic heart failure (CHF). Forward myocardial CK flux is measured in normal subjects at rest (at left), during dobutamine stress (in the center, filled symbols), and in patients with NYHA class I-IV CHF (at right). Square symbols with vertical error bars denote means ± SD. *, P < 0.0005 vs. normal subjects at rest.
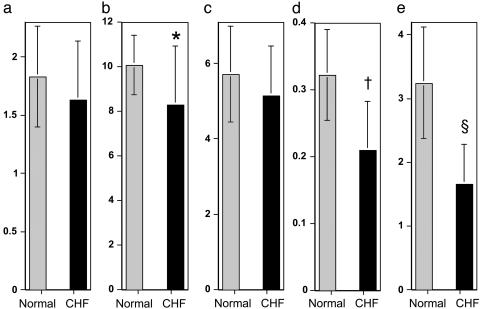
A significant (18%) decline in cardiac PCr concentration was observed in CHF patients (P < 0.03) whereas ATP concentration was unchanged (Table 1). More importantly, the cardiac kfor was reduced by 37%, and ATP flux through CK was reduced by 50% from that in normal subjects (Table 1, Fig. 2). Thus, dramatic reductions in ATP flux through CK are observed in human heart failure even in the absence of a detectable decline in ATP concentration (Fig. 3). Reduced CK flux in CHF is apparently cardiac-specific because CK flux in chest muscle was comparable to that of normal subjects (5.1 ± 2.2 vs. 5.7 ± 2.2 μmol/g per sec, P = 0.56). If cardiac PCr content is reduced in proportion to the severity of heart failure (16), and because flux is the product of PCr concentration and kfor, even greater reductions in cardiac CK flux are anticipated in more severe CHF than that studied here.
Fig. 3.
Cardiac CK flux is significantly decreased in human CHF. (a) Myocardial PCr/ATP. (b) PCr concentration ([PCr]) (μmol/g of wet weight). (c) ATP concentration ([ATP]) (μmol/g of wet weight). (d) CK forward rate constant, kfor (sec-1). (e) ATP synthesis through CK (μmol/g of wet weight per sec), for normal subjects (gray bars) and patients with heart failure (black bars). Note that the reduction in CK flux with heart failure is disproportionate to the reduction in metabolite levels. *, P = 0.03; †, P < 0.0005; §, P < 0.0005.
Discussion
These direct measures of ATP synthesis through CK in the human heart demonstrate a deficit in energy supply in clinical heart failure. This reduction in ATP synthesis through CK is cardiac-specific and occurs in mild-to-moderate heart failure before a significant reduction in ATP can be detected.
Most myocardial ATP production occurs in the mitochondria through Ox-Phos, and most ATP utilization occurs at the myofibrils. The CK reaction spans these sites and contributes to rapidly buffering ATP and to maintaining high ADP concentration at the former location and high ATP concentration at the latter (5, 7, 28, 29). The CK enzyme isoforms are compartmentalized and include a mitochondrial form that is increasingly expressed after birth and several constitutive cytoplasmic forms typically associated with the myofibrils (30). Our 31P MRS approach cannot resolve CK turnover in the different subcellular compartments, but it does provide a measure of the average net forward CK flux involving all of the detectable PCr (20, 31, 32). Because the diffusion of PCr and Cr is more rapid than that of ATP and ADP, it is hypothesized that the CK system may also provide a shuttle mechanism for transporting high-energy phosphates to the myofibrils and ADP to the mitochondria for rephosphorylation (5, 7, 30). Our finding that CK turnover in human heart is lower than in resting skeletal muscle is consistent with the latter's higher PCr content and in vitro enzyme activity (33), reflecting, perhaps, the wider range of energy utilization between rest and exercise and the greater distance between mitochondria and myofibrils in skeletal muscle.
Although myocardial CK flux in the normal heart is slower than that in resting skeletal muscle, it is still faster than that through Ox-Phos. With prior indirect estimates of ATP production through Ox-Phos (see Appendix), the rate of myocardial ATP production through CK at rest (≈3.2 μmol/g of wet weight per sec, from Table 1) is still 7-10 times that of ATP production through Ox-Phos (0.3-0.4 μmol/g of wet weight per sec). Although this factor is lower than predicted from in vitro studies (25, 30, 34), it is comparable to that measured in larger intact animals (18, 35). This factor is consistent with the CK reaction being at or near equilibrium in the normal human heart.
The finding that net CK flux is not significantly changed by stress indicates that sufficient CK activity exists at baseline to meet the additional demands of moderate physiologic stress in the normal heart. It also means that the ATP flux supplied by the CK reaction is not unlimited. The ratio of cardiac CK flux to Ox-Phos, an index of CK reserve, falls from 7- to 10-fold at rest and to 3- to 5-fold in normal humans during an adrenergic stress associated with hemodynamic effects similar to those of nonexhaustive exercise. Thus, although prior in vitro studies suggested a 20-fold reserve of CK (30) (measured as the ratio of ATP flux through CK to that of Ox-Phos), our direct measures in the human heart demonstrate that the ratio is much lower and only 3- to 5-fold during a stress that is comparable to nonexhaustive aerobic exercise.
Our direct in vivo measures of CK flux in the human heart provide important information that cannot be obtained from in vitro studies. These data demonstrate that ATP regeneration through the CK reaction is greatly reduced in heart failure and that this reduction is cardiac-specific (Table 1) and occurs before a significant reduction in ATP concentration. Prior in vitro human data acquired at the time of transplantation in patients with end-stage heart failure (36) demonstrated reductions in in vitro CK enzyme activity and in PCr and Cr content. However, those findings may not reflect the changes in in vivo CK flux or other metabolic parameters present in healthier patients with milder or earlier stages of heart failure, such as those studied here.
Although ATP content may be reduced in human CHF (8, 36), the reduction is, at most, modest (<25% in prior reports and Table 1), and ATP concentration is still much greater than the levels that would limit most energy-requiring reactions (Km) (1, 2). Because the release of ADP is a rate-limiting step for the actomyosin-ATPase reaction, an increase in concentration of free ADP could reduce the rate of cross-bridge cycling, impair diastolic function, and lower the free energy of ATP hydrolysis (ΔG∼ATP) (2). Prior studies of heart failure in animal models have not clearly answered this question, with reports showing that ADP concentration is increased (35), unchanged (37-40), or decreased (41-43). Our data shed light on this controversy and, moreover, do not support a higher ADP concentration in human CHF. Intracellular free ADP concentration is typically calculated from the CK equilibrium reaction [ADP] = ([ATP][free Cr])/([PCr][H+]Keq) where Keq is 1.66 × 109 liters/mol for a Mg2+ concentration of 1.0 mmol/liter, the cytosolic volume taken as 0.725 ml/g of wet weight (44), and intracellular pH as 7.05. When our 31P MRS data (Table 1) are combined with recent 1H MRS measures showing reduced total Cr in comparable patients with heart failure (16.1 μmol/g of wet weight in CHF vs. 27.6 μmol/g of wet weight in healthy subjects) (13), myocardial free-ADP concentration calculated from the CK equilibrium reaction is 50% lower in failing hearts (≈45 μmol/liter) than in normal hearts (≈92 μmol/liter, see Appendix). These in vivo estimates of ADP concentration argue against an increased ADP concentration in human heart failure and are consistent with the reduced ADP concentration observed in several animal studies noted above (41-43) as well as with in vitro data from end-stage human heart failure (36).
Our data can provide a basis for calculating the free energy available from ATP hydrolysis (ΔG∼ATP) in human heart failure, if inorganic phosphate (Pi) concentration is known. We as well as others (10, 14, 16, 17, 45) were unable to reliably quantify myocardial Pi concentration because the Pi resonance is small and not resolved from that of blood 2,3-diphosphoglycerate in most heart failure subjects (Fig. 1). If myocardial Pi concentration is similar in normal and failing human hearts (≈1 μmol/g of wet weight), then our data (Table 1 and ADP concentration) suggest that ΔG∼ATP is not lower in failing (61.4 kJ/mol) than in normal (59.7 kJ/mol) hearts and, therefore, cannot account for the mechanical dysfunction. If, however, Pi concentration is severalfold higher in heart failure (e.g., ≈3 μmol/g of wet weight) than in normal hearts (≈1 μmol/g of wet weight), then ΔG∼ATP would trend lower (59 kJ/mol) in failing human hearts. The recent availability of higher-field MRS systems offering increased spectral resolution and sensitivity may permit quantification in vivo of cardiac Pi concentration in the near future, which would enable, with the present data, a calculation of ΔG∼ATP to resolve this question.
Although ATP depletion, increased ADP concentration, and lower ΔG∼ATP are metabolic factors that, theoretically, could impair ventricular function, we do not find evidence for these factors in our human heart-failure studies. Rather, reduced CK flux is the most prominent metabolic abnormality we observe in human heart failure. The 50% reduction in CK flux reported here with mild-to-moderate human heart failure, coupled with a possible 40% increase in ATP synthesis through Ox-Phos observed recently in an animal model of heart failure (41) and the available human data (46, 47) (see Appendix), suggest that the ratio of CK flux to Ox-Phos is only 2-3 in CHF patients at rest. It is, therefore, likely that this ratio approaches unity during exercise or adrenergic stress in patients with mild-to-moderate CHF and at rest in patients with very severe CHF. As it happens, these situations typically coincide with the onset of symptoms in heart failure patients. Thus, although it was long thought that the human heart had considerable CK excess and that only dramatic (>90%) reductions could impact contractile function, the combination of lower than anticipated CK flux in the normal heart, the lack of increase during stress, and the 50% reduction at rest in mild-to-moderate heart failure are evidence that CK capacity is severely compromised in human heart failure.
What are the consequences of lower CK flux in human heart failure? As a spatial ATP buffer, CK is important for maintaining high cytosolic ATP concentration at the site of utilization and low ADP concentration at the site of de novo ATP synthesis. CK also buffers ATP over time (6, 48), and this buffering effect is likely important when energy demands vary widely during the cardiac cycle. In normal skeletal muscle, PCr acts as a rapid, transient source to replenish ATP by the CK reaction after each contraction, with changes in PCr observed on a millisecond time scale (6). Cyclic changes in PCr during the cardiac-contraction cycle have also been described in some (49-52), but not all (53-55) reports. To the extent that cardiac muscle shares some properties with skeletal muscle (6), rapid provision of ATP by CK with contraction would represent a potentially important mechanism by which a significant reduction in CK flux in heart failure could limit temporal buffering and, thereby, limit contractile ATP supply and function. In this light it is important to reconsider the current evidence on CK reserve as indexed by the time-averaged ratio of net CK flux to de novo ATP synthesis which our data predict would approach unity in some human heart failure settings. Because peak energy demand with contraction must exceed the time-averaged value of energy use or production over the entire cardiac cycle, a time-averaged ratio of CK flux to de novo synthesis, approaching unity in heart failure is likely inadequate to meet the peak ATP needs associated with contraction.
Why is CK flux decreased in human heart failure? Because the myocardial PCr and ATP concentrations are 3- to 10-fold higher than their respective Km for the CK reaction, the modest 18% reduction in PCr concentration and insignificant decline in ATP concentration cannot account for reduced CK flux in human heart failure. However, because ADP concentration is close to its Km, the calculated reduction in ADP concentration in failure could lower CK flux. To estimate the potential contribution of altered metabolite pools to CK flux, CK flux was predicted from the formal CK rate equation, as previously described (56). In the absence of changes in Vmax or any other metabolite levels, a 50% reduction in ADP concentration would result in a 39% reduction in predicted CK flux (56). Thus, lower ADP concentration is sufficient to account for most of the 50% reduction in CK flux in human heart failure. Others have noted a loss of CK activity in failing animal hearts (39) as well as in patients with end-stage heart failure (36). Because a combination of reduced CK Vmax (36), and altered ADP concentration and other metabolite pool sizes observed here would result in a 64% reduction in predicted CK flux, it is likely that lower Vmax also contributes to lower CK flux in patients with mild-to-moderate heart failure. A lower ADP concentration, in turn, is attributable to a greater reduction in total and free Cr than in PCr (Table 1 and refs. 13 and 36). Because Cr is not synthesized in myocytes, the lower Cr concentration is likely because of a reduction in Cr-transport protein (57). Although a decline in Cr content in heart failure prevents an increase in ADP concentration (41), a dramatic decline (13, 36) could reduce ADP concentration below normal values to the level that reduces CK flux seen here.
In conclusion, although many factors may contribute to the onset and progression of human heart failure, reduced ATP production through CK would, regardless of its underlying cause, certainly have a direct, adverse affect on myocardial function if it reduced energy supply to a level insufficient to meet demand. We have now shown that it is possible to move beyond metabolite levels to in vivo molecular-imaging studies of human myocardial energy turnover under physiologic conditions, including evaluations of the energetic response to intervention. Our studies reveal that the failing human heart has a deficit in energy supply. The reduction in ATP turnover through CK is cardiac-specific and out of proportion to the reduction in metabolite ratios and pool sizes. We find that reduced ATP supplied by the CK reaction, rather than an increase in ADP concentration, may contribute to dysfunction. The results support the use of heartfailure treatment strategies that reduce metabolic demand as opposed to those that stimulate contractility and deplete energy reserves. The results also provide the foundation for new strategies that augment energy delivery and/or improve metabolic/mechanical coupling.
Acknowledgments
We thank Drs. Steven P. Schulman, Edward K. Kasper, and Kenneth L. Baughman for referring heart failure patients for study; Drs. Ronald Ouwerkerk and Fabao Gao for providing technical assistance; Michele Waldron for nursing assistance; and Dr. Robert Shulman for critical suggestions and thoughtful discussions. This work was supported by National Institutes of Health Grants HL61912 and HL56882 and by funds from the Donald W. Reynolds Cardiovascular Clinical Research Center at The Johns Hopkins Hospital.
Appendix
ATP synthesis through Ox-Phos has previously been estimated from measures of myocardial oxygen consumption, which in turn has been consistently measured by several noninvasive approaches at ≈0.085 ml/g of wet weight per min in normal subjects at rest (24, 58). Similar values have been obtained in patients with dilated cardiomyopathy and heart failure by using invasive (0.075 ml/g of wet weight per min) (46) and noninvasive (0.079 ml/g of wet weight per min) (47) techniques, respectively. Assuming a ratio of 3 moles of ATP produced for each mole of oxygen consumed, this method yields a mean ATP rate through Ox-Phos of 0.33 μmol/g per sec. A comparable mean ATP rate of ≈0.43 μmol/g per sec has been derived from invasive measures (59).
CK flux was calculated from the classic relationship CK flux = kfor·[PCr], where kfor is the pseudo-first-order rate constant measured by saturation transfer techniques. In addition, CK flux = k2·[PCr]·[ADP], where [PCr] and [ADP] are the respective concentrations of the metabolites, and k2 is the second-order CK rate constant (25, 26, 60). Intracellular free ADP concentration was calculated from the CK equilibrium reaction: [ADP] = ([ATP][free Cr])/([PCr][H+]Keq), where Keq is 1.66 × 109 liters/mol for a Mg2+ concentration of 1.0 mmol/liter, the cytosolic volume is taken as 0.725 ml/g of wet weight (44), and intracellular pH is taken as 7.05. The free-energy change of ATP hydrolysis [-ΔG∼ATP (kJ/mol)] is determined from the formula ΔG∼ATP = ΔG° + RT log([ADP][Pi]/[ATP]); where ΔG° is the standard free-energy change, R is the universal gas constant, and T is the absolute temperature (61).
Author contributions: R.G.W., G.G., and P.A.B. designed research; R.G.W., G.G., and P.A.B. performed research; R.G.W. and P.A.B. analyzed data; and R.G.W., G.G., and P.A.B. wrote the paper.
Abbreviations: CHF, chronic heart failure; CK, creatine kinase; Cr, creatine; MRS, magnetic resonance spectroscopy; FAST, four-angle saturation transfer; NYHA, New York Heart Association; Ox-Phos, oxidative phosphorylation; PCr, creatine phosphate.
References
- 1.Ingwall, J. S. (1993) Circulation 87, VII58-VII62. [Google Scholar]
- 2.Katz, A. M. (1998) Cardiol. Clin. 16, 633-644. [DOI] [PubMed] [Google Scholar]
- 3.Poole-Wilson, P. A. (2002) J. Am. Med. Assoc. 287, 1578-1580. [DOI] [PubMed] [Google Scholar]
- 4.Ingwall, J. S., Kramer, M. F., Fifer, M. A., Lorell, B. H., Shemin, R., Grossman, W. & Allen, P. D. (1985) N. Engl. J. Med. 313, 1050-1054. [DOI] [PubMed] [Google Scholar]
- 5.Wallimann, T. (1994) Curr. Biol. 4, 42-46. [DOI] [PubMed] [Google Scholar]
- 6.Chung, Y., Sharman, R., Carlsen, R., Unger, S. W., Larson, D. & Jue, T. (1998) Am. J. Physiol. 274, C846-C852. [DOI] [PubMed] [Google Scholar]
- 7.Bessman, S. P. & Carpenter, C. L. (1985) Annu. Rev. Biochem. 54, 831-862. [DOI] [PubMed] [Google Scholar]
- 8.Starling, R. C., Hammer, D. F. & Altschuld, R. A. (1998) Mol. Cell. Biochem. 180, 171-177. [PubMed] [Google Scholar]
- 9.Weiss, R. G., Bottomley, P. A., Hardy, C. J. & Gerstenblith, G. (1990) N. Engl. J. Med. 323, 1593-1600. [DOI] [PubMed] [Google Scholar]
- 10.Yabe, T., Mitsunami, K., Inubushi, T. & Kinoshita, M. (1995) Circulation 92, 15-23. [DOI] [PubMed] [Google Scholar]
- 11.Bottomley, P. A., Atalar, E. & Weiss, R. G. (1996) Magn. Reson. Med. 35, 664-670. [DOI] [PubMed] [Google Scholar]
- 12.Bottomley, P. A. & Weiss, R. G. (1998) Lancet 351, 714-718. [DOI] [PubMed] [Google Scholar]
- 13.Nakae, I., Mitsunami, K., Omura, T., Yabe, T., Tsutamoto, T., Matsuo, S., Takahashi, M., Morikawa, S., Inubushi, T., Nakamura, Y., et al. (2003) J. Am. Coll. Cardiol. 42, 1587-1593. [DOI] [PubMed] [Google Scholar]
- 14.Hardy, C. J., Weiss, R. G., Bottomley, P. A. & Gerstenblith, G. (1991) Am. Heart J. 122, 795-801. [DOI] [PubMed] [Google Scholar]
- 15.Conway, M. A., Allis, J., Ouwerkerk, R., Niioka, T., Rajagopalan, B. & Radda, G. K. (1991) Lancet 338, 973-976. [DOI] [PubMed] [Google Scholar]
- 16.Neubauer, S., Krahe, T., Schindler, R., Horn, M., Hillenbrand, H., Entzeroth, C., Mader, H., Kromer, E. P., Riegger, G. A. J., Lackner, K., et al. (1992) Circulation 86, 1810-1818. [DOI] [PubMed] [Google Scholar]
- 17.Beer, M., Seyfarth, T., Sandstede, J., Landschutz, W., Lipke, C., Kostler, H., von Kienlin, M., Harre, K., Hahn, D. & Neubauer, S. (2002) J. Am. Coll. Cardiol. 40, 1267-1274. [DOI] [PubMed] [Google Scholar]
- 18.Katz, L. A., Swain, J. A., Portman, M. A. & Balaban, R. S. (1989) Am. J. Physiol. 256, H265-H274. [DOI] [PubMed] [Google Scholar]
- 19.Ye, Y., Wang, C., Zhang, J., Cho, Y. K., Gong, G., Murakami, Y. & Bache, R. J. (2001) Am. J. Physiol. 281, H376-H386. [DOI] [PubMed] [Google Scholar]
- 20.Bottomley, P. A., Ouwerkerk, R., Lee, R. F. & Weiss, R. G. (2002) Magn. Reson. Med. 47, 850-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottomley, P. A. & Ouwerkerk, R. (1993) J. Magn. Reson. 103A, 242-244. [Google Scholar]
- 22.Bottomley, P. A., Hardy, C. J. & Roemer, P. B. (1990) Magn. Reson. Med. 14, 425-434. [DOI] [PubMed] [Google Scholar]
- 23.Tamaki, N., Magata, Y., Takahashi, N., Kawamoto, M., Torizuka, T., Yonekura, Y., Tadamura, E., Okuda, K., Ono, S., Nohara, R., et al. (1993) Eur. J. Nucl. Med. 20, 231-237. [DOI] [PubMed] [Google Scholar]
- 24.Sun, K. T., Yeatman, L. A., Buxton, D. B., Chen, K., Johnson, J. A., Huang, S.-C., Kofoed, K. F., Weismueller, S., Czernin, J., Phelps, M. E., et al. (1998) J. Nucl. Med. 39, 272-280. [PubMed] [Google Scholar]
- 25.Bittl, J. A. & Ingwall, J. S. (1985) J. Biol. Chem. 260, 3512-3517. [PubMed] [Google Scholar]
- 26.Martin, J. F., Guth, B. D., Griffey, R. F. & Hoekenga, D. E. (1989) Magn. Reson. Med. 11, 64-72. [DOI] [PubMed] [Google Scholar]
- 27.Portman, M. A. & Ning, X.-H. (1992) Am. J. Physiol. 263, C453-C460. [DOI] [PubMed] [Google Scholar]
- 28.Dzeja, P. P. & Terzic, A. (2003) J. Exp. Biol. 206, 2039-2047. [DOI] [PubMed] [Google Scholar]
- 29.Saks, V. A., Ventura-Clapier, R., Leverve, X., Rossi, A. & Rigoulet, M. (1998) Mol. Cell. Biochem. 184, 3-9. [PubMed] [Google Scholar]
- 30.Saks, V. A. (1980) in Heart Creatine Kinase, eds. Jacobus, W. E. & Ingwall, J. S. (Williams & Wilkins, Baltimore), pp. 109-124.
- 31.Ouwerkerk, R. & Bottomley, P. A. (2001) J. Magn. Reson. 148, 425-435. [DOI] [PubMed] [Google Scholar]
- 32.Ouwerkerk, R. & Bottomley, P. A. (2001) J. Magn. Reson. 149, 282-286. [DOI] [PubMed] [Google Scholar]
- 33.Wallimann, T., Wyss, M., Brdiczka, D. & Nicolay, K. (1992) Biochem. J. 281, 21-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neubauer, S., Hamman, B. L., Perry, S. B., Bittl, J. A. & Ingwall, J. S. (1988) Circ. Res. 63, 1-15. [DOI] [PubMed] [Google Scholar]
- 35.Murakami, Y., Zhang, J., Eijgelshoven, J. H. J., Chen, W., Carlyle, W. C., Zhang, Y., Gong, G. & Bache, R. J. (1999) Am. J. Physiol. 276, H892-H900. [DOI] [PubMed] [Google Scholar]
- 36.Nascimben, L., Ingwall, J. S., Pauletto, P., Friedrich, J., Gwathmey, J. K., Saks, V. A., Pessina, A. C. & Allen, P. D. (1996) Circulation 94, 1894-1901. [DOI] [PubMed] [Google Scholar]
- 37.Liao, R., Nascimben, L., Friedrich, J., Gwathmey, J. K. & Ingwall, J. S. (1996) Circ. Res. 78, 893-902. [DOI] [PubMed] [Google Scholar]
- 38.Spindler, M., Saupe, K. W., Tian, R., Ahmed, S., Matlib, M. A. & Ingwall, J. S. (1999) J. Mol. Cell. Cardiol. 31, 2175-2189. [DOI] [PubMed] [Google Scholar]
- 39.Ye, Y., Gong, G., Ochiai, K., Liu, J. & Zhang, J. (2001) Circulation 103, 1570-1576. [DOI] [PubMed] [Google Scholar]
- 40.Gong, G., Liu, J., Liang, P., Guo, T., Hu, Q., Ochiai, K., Hou, M., Ye, Y., Wu, X., Mansoor, A., et al. (2003) Am. J. Physiol. 285, H541-H548. [DOI] [PubMed] [Google Scholar]
- 41.Shen, W., Asai, K., Uechi, M., Mathier, M. A., Shannon, R. P., Vatner, S. F. & Ingwall, J. S. (1999) Circulation 100, 2113-2118. [DOI] [PubMed] [Google Scholar]
- 42.Neubauer, S., Horn, M., Naumann, A., Tian, R., Hu, K., Laser, M., Friedrich, J., Gaudron, P., Schnackerz, K., Ingwall, J. S., et al. (1995) J. Clin. Invest. 95, 1092-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nascimben, L., Friedrich, J., Liao, R., Pauletto, P., Pessina, A. C. & Ingwall, J. S. (1995) Circulation 91, 1824-1833. [DOI] [PubMed] [Google Scholar]
- 44.Vinnakota, K. C. & Bassingthwaighte, J. B. (2004) Am. J. Physiol. 286, H1742-H1749. [DOI] [PubMed] [Google Scholar]
- 45.Neubauer, S., Horn, M., Cramer, M., Harre, K., Newell, J. B., Peters, W., Pabst, T., Ertl, G., Hahn, D., Ingwall, J. S., et al. (1997) Circulation 96, 2190-2196. [DOI] [PubMed] [Google Scholar]
- 46.Sundram, P., Reddy, H. K., McElroy, P. A., Janicki, J. S. & Weber, K. T. (1990) Am. Heart J. 119, 891-898. [DOI] [PubMed] [Google Scholar]
- 47.Beanlands, R. S. B., Bach, D. S., Raylman, R., Armstrong, W. F., Wilson, V., Montieth, M., Moore, C. K., Bates, E. & Schwaiger, M. (1993) J. Am. Coll. Cardiol. 22, 1389-1398. [DOI] [PubMed] [Google Scholar]
- 48.Heineman, F. W. & Balaban, R. S. (1990) J. Clin. Invest. 85, 843-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wikman-Coffelt, J., Sievers, R. E., Coffelt, R. J. & Parmley, W. W. (1983) Am. J. Physiol. 245, H354-H362. [DOI] [PubMed] [Google Scholar]
- 50.Fossel, E. T., Morgan, H. E. & Ingwall, J. S. (1980) Proc. Natl. Acad. Sci. USA 77, 3654-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ingwall, J. S. (1982) Am. J. Physiol. 242, H729-H744. [DOI] [PubMed] [Google Scholar]
- 52.Kusuoka, H., Inoue, M., Tsuneoka, Y., Watari, H., Hori, M. & Abe, H. (1985) Jpn. Circ. J. 49, 1099-1107. [DOI] [PubMed] [Google Scholar]
- 53.Kantor, H. L., Briggs, R. W., Metz, K. R. & Balaban, R. S. (1986) Am. J. Physiol. 251, H171-H175. [DOI] [PubMed] [Google Scholar]
- 54.Koretsky, A. P., Wang, S., Murphy-Boesch, J., Klein, M. P., James, T. L. & Weiner, M. W. (1983) Proc. Natl. Acad. Sci. USA 80, 7491-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robitaille, P. M., Merkle, H., Lew, B., Path, G., Hendrich, K., Lindstrom, P., From, A. H. L., Garwood, M., Bache, R. J. & Ugurbil, K. (1990) Magn. Reson. Med. 16, 91-116. [DOI] [PubMed] [Google Scholar]
- 56.Bittl, J. A., DeLayre, J. & Ingwall, J. S. (1987) Biochemistry 26, 6083-6090. [DOI] [PubMed] [Google Scholar]
- 57.Neubauer, S., Remkes, H., Spindler, M., Horn, M., Wiesmann, F., Prestle, J., Walzel, B., Ertl, G., Hasenfuss, G. & Wallimann, T. (1999) Circulation 100, 1847-1850. [DOI] [PubMed] [Google Scholar]
- 58.Gerber, B. L., Wijns, W., Vanoverschelde, J. J., Heyndrickx, G. R., DeBruyne, B., Bartunek, J. & Melin, J. A. (1999) J. Am. Coll. Cardiol. 34, 1939-1946. [DOI] [PubMed] [Google Scholar]
- 59.Ganz, W., Tamura, K., Marcus, H. S., Ronoso, R., Yoshida, S. & Swan, H. J. C. (1971) Circulation 44, 181-195. [DOI] [PubMed] [Google Scholar]
- 60.Cave, A. C., Ingwall, J. S., Friedrich, J., Liao, R., Saupe, K. W., Apstein, C. S. & Eberli, F. R. (2000) Circulation 101, 2090-2096. [DOI] [PubMed] [Google Scholar]
- 61.Gibbs, C. (1985) J. Mol. Cell. Cardiol. 17, 727-731. [DOI] [PubMed] [Google Scholar]