Abstract
Purpose
The significance of radiotherapy (RT) –associated cardiac injury for stage III non–small-cell lung cancer (NSCLC) is unclear, but higher heart doses were associated with worse overall survival in the Radiation Therapy Oncology Group (RTOG) 0617 study. We assessed the impact of heart dose in patients treated at our institution on several prospective dose-escalation trials.
Patients and Methods
From 1996 to 2009, 127 patients with stage III NSCLC (Eastern Cooperative Oncology Group performance status, 0 to 1) received dose-escalated RT to 70 to 90 Gy (median, 74 Gy) in six trials. RT plans and cardiac doses were reviewed. Records were reviewed for the primary end point: symptomatic cardiac events (symptomatic pericardial effusion, acute coronary syndrome, pericarditis, significant arrhythmia, and heart failure). Cardiac risk was assessed by noting baseline coronary artery disease and calculating the WHO/International Society of Hypertension score. Competing risks analysis was used.
Results
In all, 112 patients were analyzed. Median follow-up for surviving patients was 8.8 years. Twenty-six patients (23%) had one or more events at a median of 26 months to first event (effusion [n = 7], myocardial infarction [n = 5], unstable angina [n = 3], pericarditis [n = 2], arrhythmia [n = 12], and heart failure [n = 1]). Heart doses (eg, heart mean dose; hazard ratio, 1.03/Gy; P = .002,), coronary artery disease (P < .001), and WHO/International Society of Hypertension score (P = .04) were associated with events on univariable analysis. Heart doses remained significant on multivariable analysis that accounted for baseline risk. Two-year competing risk–adjusted event rates for patients with heart mean dose < 10 Gy, 10 to 20 Gy, or ≥ 20 Gy were 4%, 7%, and 21%, respectively. Heart doses were not associated with overall survival.
Conclusion
Cardiac events were relatively common after high-dose thoracic RT and were independently associated with both heart dose and baseline cardiac risk. RT-associated cardiac toxicity after treatment of stage III NSCLC may occur earlier than historically understood, and heart doses should be minimized.
INTRODUCTION
Radiotherapy (RT) –associated heart toxicity has long been recognized in patients with breast cancer or Hodgkin lymphoma, with increases in cardiovascular events and deaths typically noted 10 or more years after treatment.1-6 However, the clinical relevance of RT-associated heart disease for patients with stage III non–small-cell lung cancer (NSCLC) is unclear. Conventional wisdom holds that there are few long-term survivors to experience toxicity, given the typically long latency of RT-associated heart injury and poor prognosis. On the other hand, though most RT-associated cardiac events in patients with breast cancer or lymphoma occur many years after RT, there may also be an increased rate at earlier intervals.6-9 In addition, patients with lung cancer are more likely to have comorbid risk factors such as smoking and pre-existing cardiac disease that lower their reserve and predispose them to earlier events.
Several studies report increased cardiac deaths with postoperative RT after surgery for stage I to III NSCLC.10-12 Evidence for cardiac injury after definitive RT is more limited.13,14 Recently, the Radiation Therapy Oncology Group (RTOG) 0617 (A Randomized Phase III Comparison of Standard-Dose [60 Gy] Versus High-Dose [74 Gy] Conformal Radiotherapy With Concurrent and Consolidation Carboplatin Plus Paclitaxel ± Cetuximab {IND #103444} in Patients With Stage IIIA and IIIB Non–Small-Cell Lung Cancer) trial reported inferior overall survival (OS) with dose-escalated chemoradiation to 74 Gy compared with standard 60 Gy for stage III NSCLC. Heart radiation dose was associated with worse OS with a median follow-up of 2 years, suggesting a contribution of radiation-induced cardiac morbidity relatively soon after treatment.15
From 1996 to 2009, the University of North Carolina (UNC) participated in six single or multi-institution prospective phase I and II trials that investigated various chemotherapeutic regimens with dose-escalated RT in the definitive treatment of stage III NSCLC.16-22 Here we review these studies to assess the impact of cardiac doses on subsequent cardiac events.
PATIENTS AND METHODS
Study Design
This was a post hoc analysis pooling several prospective trials. OS and progression-free survival (PFS) were prospectively assessed. Records were retrospectively reviewed to assess toxicity. The primary end point was symptomatic cardiac events (see Evaluation of Cardiac Toxicity), including symptomatic pericardial effusion, myocardial infarction, unstable angina, pericarditis, significant arrhythmia, and heart failure. Secondary end points were OS and new pericardial effusion.
Patient Population and Treatment
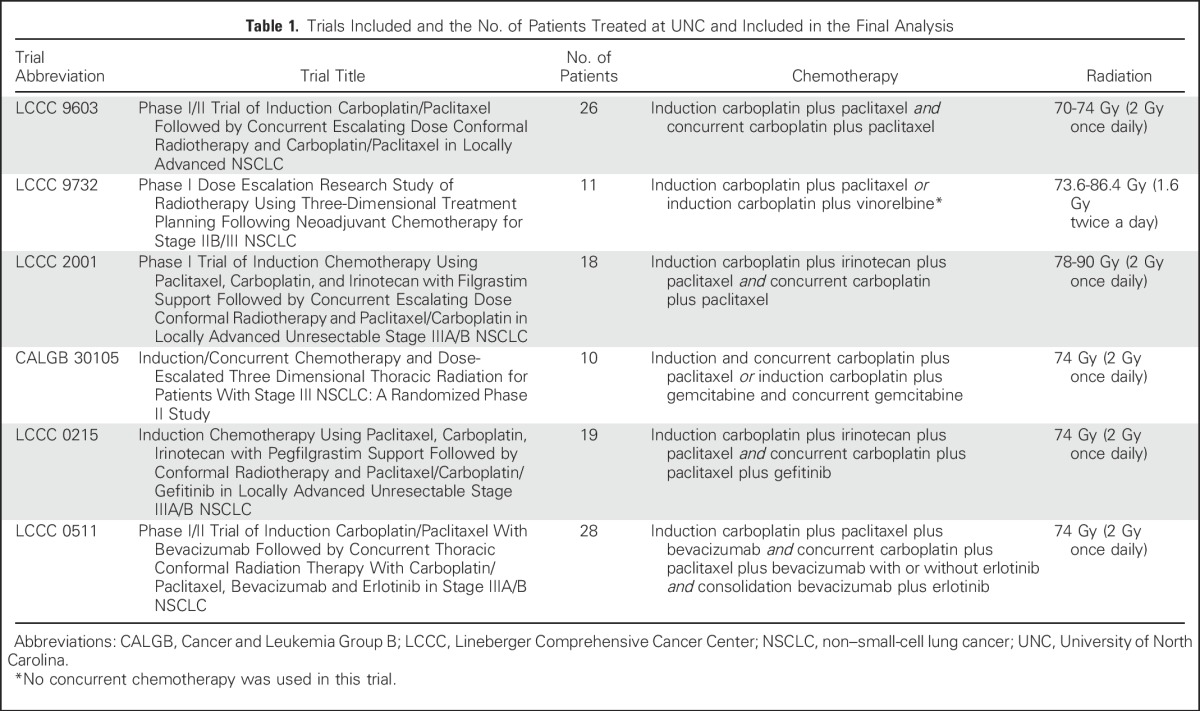
In all, 127 patients were treated at UNC with dose-escalated RT to 70 to 90 Gy in six institutional review board–approved prospective phase I or II trials from 1996 to 2009 (Table 1). Patients had stage III NSCLC, Eastern Cooperative Oncology Group performance status of 0 to 1, and received three-dimensional (3D) conformal RT, typically using a four-field technique with some elective nodal coverage (eg, large opposed anterior/posterior fields and smaller off-spinal cord oblique fields). Intensity-modulated radiation therapy (IMRT) was not used. Three of six trials used a dose-escalation design; two of them did not observe any dose-limiting toxicities and one observed dose-limiting toxicities at the final planned dose level. The other three trials prescribed 74 Gy. Four of six trials did not mandate cardiac dose limits, one trial limited heart volume receiving 40 Gy (V40Gy) to < 100%, and one trial limited the left ventricle (LV) V40Gy to < 100%. All patients received induction, and most patients received concurrent chemotherapy. Further details are available in prior publications.16-22 Patients were excluded if they did not complete RT to ≥ 70 Gy (n = 9) or had inaccessible RT plans (n = 6), leaving 112 patients for this analysis.
Table 1.
Trials Included and the No. of Patients Treated at UNC and Included in the Final Analysis
Dosimetric Assessment
The delivered 3D RT dose distributions for each patient (created using the departmental treatment planning software Plan-UNC)23 were reviewed. Lungs were delineated using automatic thresholding, excluding gross tumor. The esophagus was delineated from the cricoid to the gastroesophageal junction. The heart was delineated by one investigator (K.W.) per previously published methods24 and independently reviewed for accuracy and consistency by a second investigator (M.J.E.). Dose-volume histograms were generated for organs at risk. Parameters for analysis were prespecified largely on the basis of RTOG 0617 and included heart mean dose, heart volume receiving ≥ 30 Gy (V30Gy), heart volume receiving ≥ 5 Gy (V5Gy), LV mean dose, LV V30Gy, LV V5Gy, lung mean dose, lung V5Gy, lung V20Gy, esophagus mean dose, esophagus maximum dose, and esophagus V60Gy.15
Evaluation of Cardiac Toxicity
All available patient records were reviewed to assess the primary end point: symptomatic cardiac events. Radiographic studies and echocardiograms were reviewed for the secondary end point of pericardial effusion (either symptomatic or asymptomatic). Malignant pericardial effusions were not counted as events. Six symptomatic cardiac events were defined by an attending cardiologist (B.C.J.):
Symptomatic pericardial effusion: effusions presenting with shortness of breath, confirmed on echocardiogram as hemodynamically significant and/or requiring procedural intervention;
Myocardial infarction: chest pain with increased cardiac biomarkers or as otherwise noted in the medical record;
Unstable angina: chest pain without biomarker increase but with ischemia on stress test or significant stenosis on cardiac catheterization;
Pericarditis: radiographic-, echocardiographic-, or electrocardiogram-confirmed pericardial inflammation along with a presentation with shortness of breath or chest pain;
Significant arrhythmia: new onset arrhythmia requiring either medical or procedural intervention;
Heart failure: shortness of breath with new diagnosis of echocardiogram-confirmed heart failure or hospitalization for existing heart failure unassociated with other defined events.
To assess baseline cardiac risk, the WHO/International Society of Hypertension (WHO/ISH) risk score was calculated. This score uses age, sex, smoking status, diabetes, and systolic blood pressure to estimate 10-year risk of a cardiovascular event in five strata (0 to < 10%, 10% to < 20%, 20% to < 30%, 30% to < 40%, and ≥ 40%).25 Baseline risk was also assessed by noting whether patients had previously diagnosed coronary artery disease.
Statistical Analysis
The Kaplan-Meier method was used to estimate OS, PFS, and cumulative incidence of cardiac events (treating deaths as censored). A competing risks regression analysis (Fine and Gray method)26 was then used to model the cumulative incidence function of cardiac events while accounting for the significant competing risk of death. Univariable analysis was used to test the association of covariates with cardiac events, with subdistribution hazard ratios (HRs)27 reported. In addition to analyzing heart dose as a continuous variable, patients were also divided into three heart dose strata, and univariable analyses were performed for each two-strata pair (treating the lower stratum as the referent). Multivariable analysis was used to test potentially significant covariates (limited to two covariates per analysis, given the relatively few events). A two-tailed P of < .05 was considered significant. Analysis was performed using SAS (SAS Institute, Cary, NC).
RESULTS
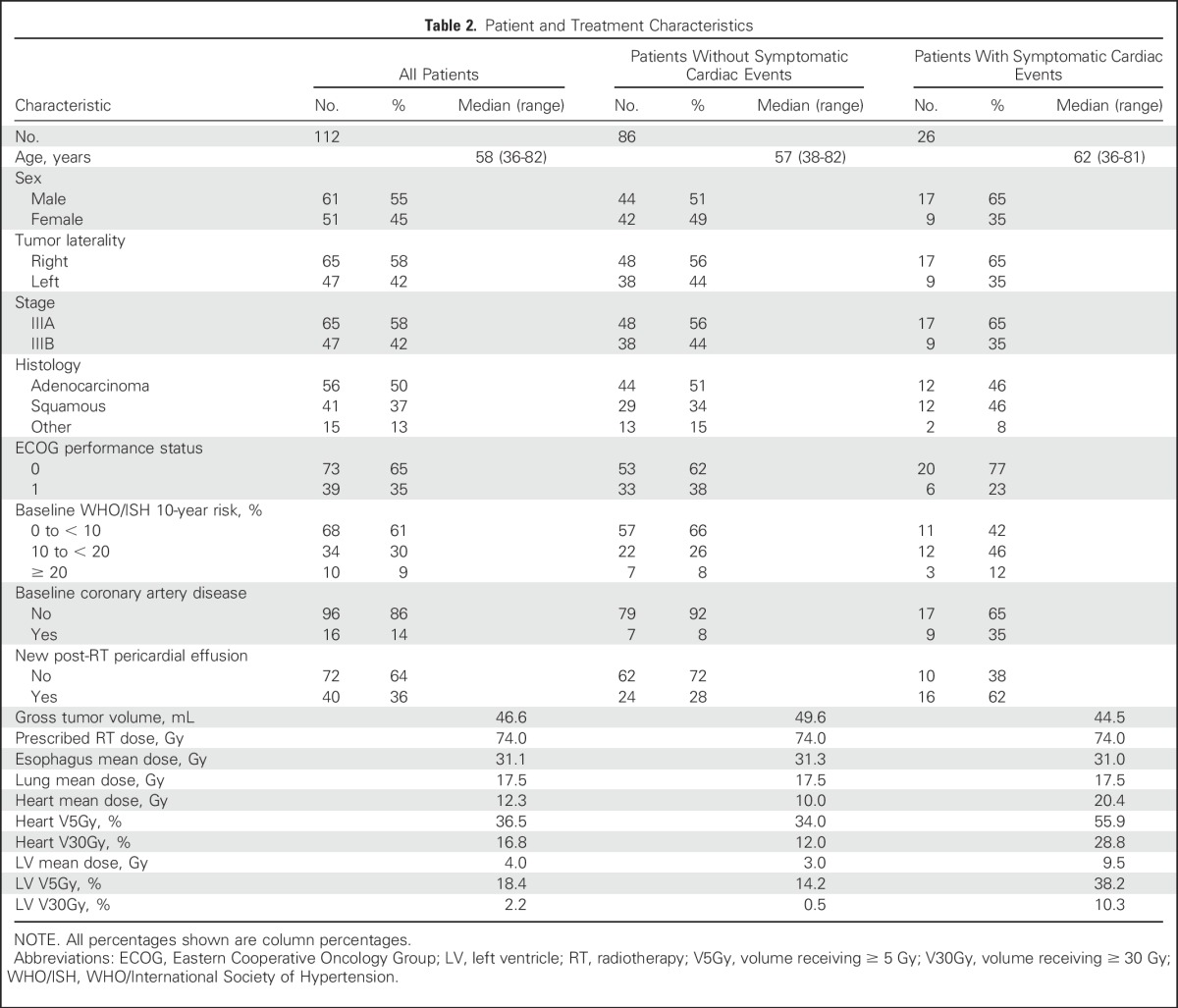
Patient characteristics are listed in Table 2. Median follow-up for the 11 surviving patients was 8.8 years (range, 2.3 to 17.3 years). Median follow-up for the 39 patients without disease progression was 6.3 years (range, 0.3 to 17.3 years). Most patients (72%) received 74 Gy. All patients received induction chemotherapy, 90% received concurrent chemotherapy, and 25% received consolidation chemotherapy.
Table 2.
Patient and Treatment Characteristics
Symptomatic Cardiac Events
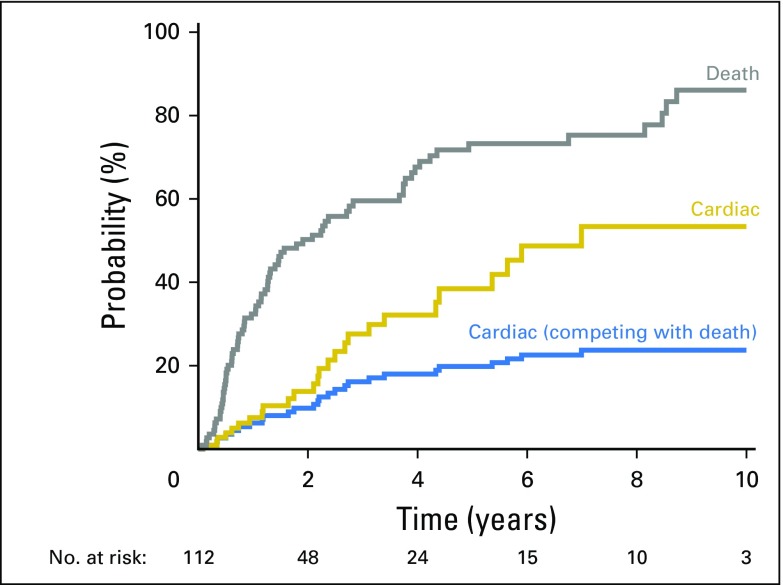
Twenty-six patients (23%) had one or more symptomatic cardiac events at a median of 26 months to first event (range, 1 to 84 months); these included symptomatic pericardial effusion (n = 7), myocardial infarction (n = 5), unstable angina (n = 3), pericarditis (n = 2), significant arrhythmia (n = 12), and heart failure (n = 1). Details are provided in Table 3. Figure 1 shows the overall cumulative incidence of death and symptomatic cardiac events. The 2- and 4-year rates of symptomatic cardiac events were 14% and 32%. After adjustment for the competing risk of death, 2- and 4-year rates of symptomatic cardiac events were 10% and 18%.
Table 3.
Patients With Symptomatic Cardiac Events
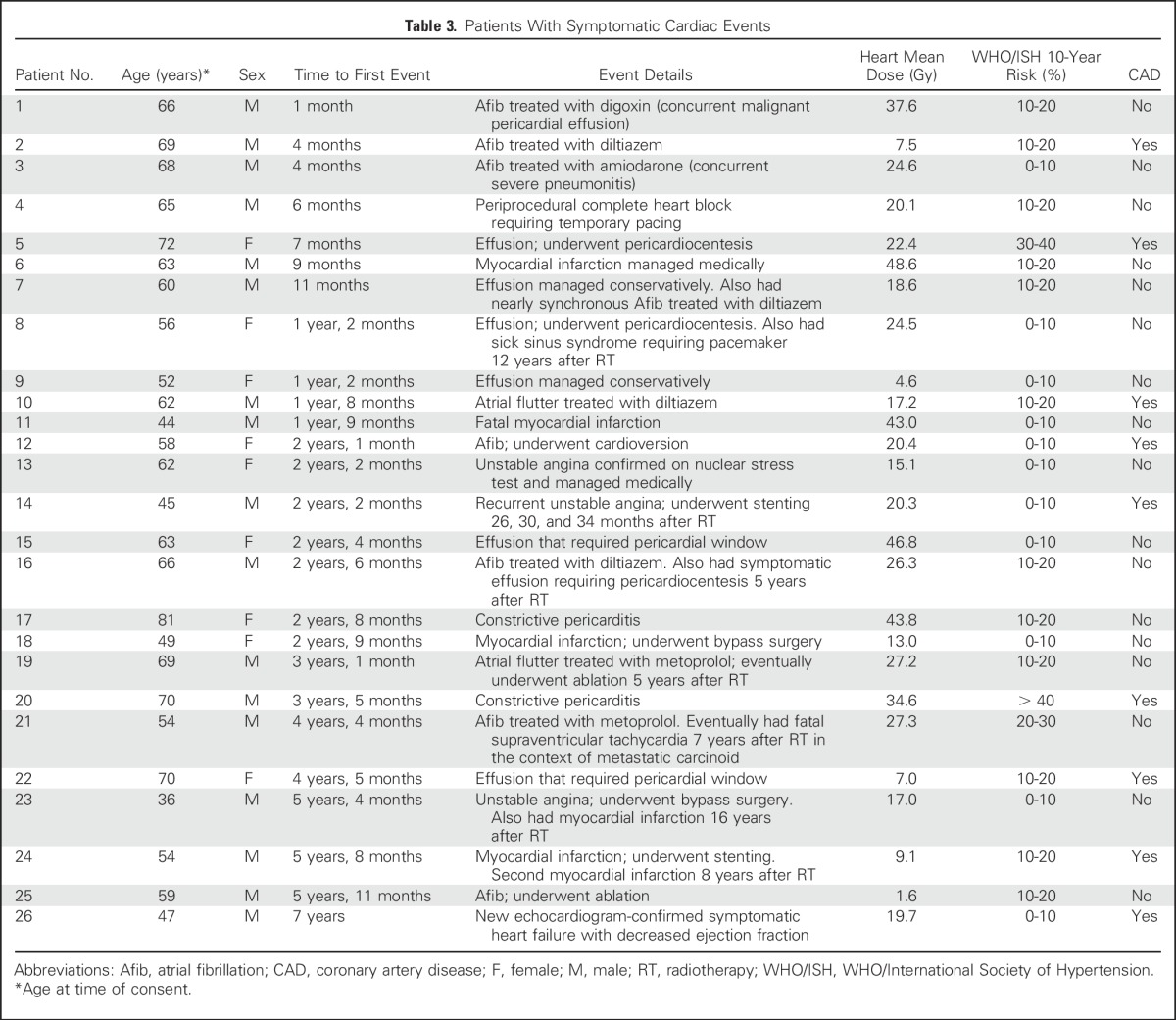
Fig 1.
Cumulative incidence plot of death (gray), symptomatic cardiac events (gold), and symptomatic cardiac events adjusted for the competing risk of death (blue).
Characteristics of patients with and without symptomatic cardiac events are listed in Table 2. Patients with events seemed to have higher heart doses than patients without events (heart mean dose, 20 Gy v 10 Gy; V5Gy, 56% v 34%; V30Gy, 29% v 12%). Patients with events also seemed to have higher rates of baseline coronary artery disease (CAD; 35% v 8%) and higher WHO/ISH risk scores. Univariable and multivariable analyses accounting for time to event and the competing risk of death for these characteristics are listed in Table 4. On univariable analysis, events were significantly associated with all heart-related dosimetric variables, including heart mean dose (P = .002), heart V5Gy (P < .001), heart V30Gy (P = .001), LV mean dose (P = .03), LV V5Gy (P = .001), and LV V30Gy (P = .03), as well as both CAD (P < .001) and WHO/ISH risk (P = .04). On multivariable analysis pairing heart doses with either CAD or WHO/ISH score, heart mean dose, heart V5Gy, heart V30Gy, and LV V5Gy remained significantly associated with events. LV mean dose and LV V30Gy remained significant when paired with WHO/ISH score but were only borderline significant when paired with CAD.
Table 4.
Competing Risk-Adjusted Analyses for Symptomatic Cardiac Events
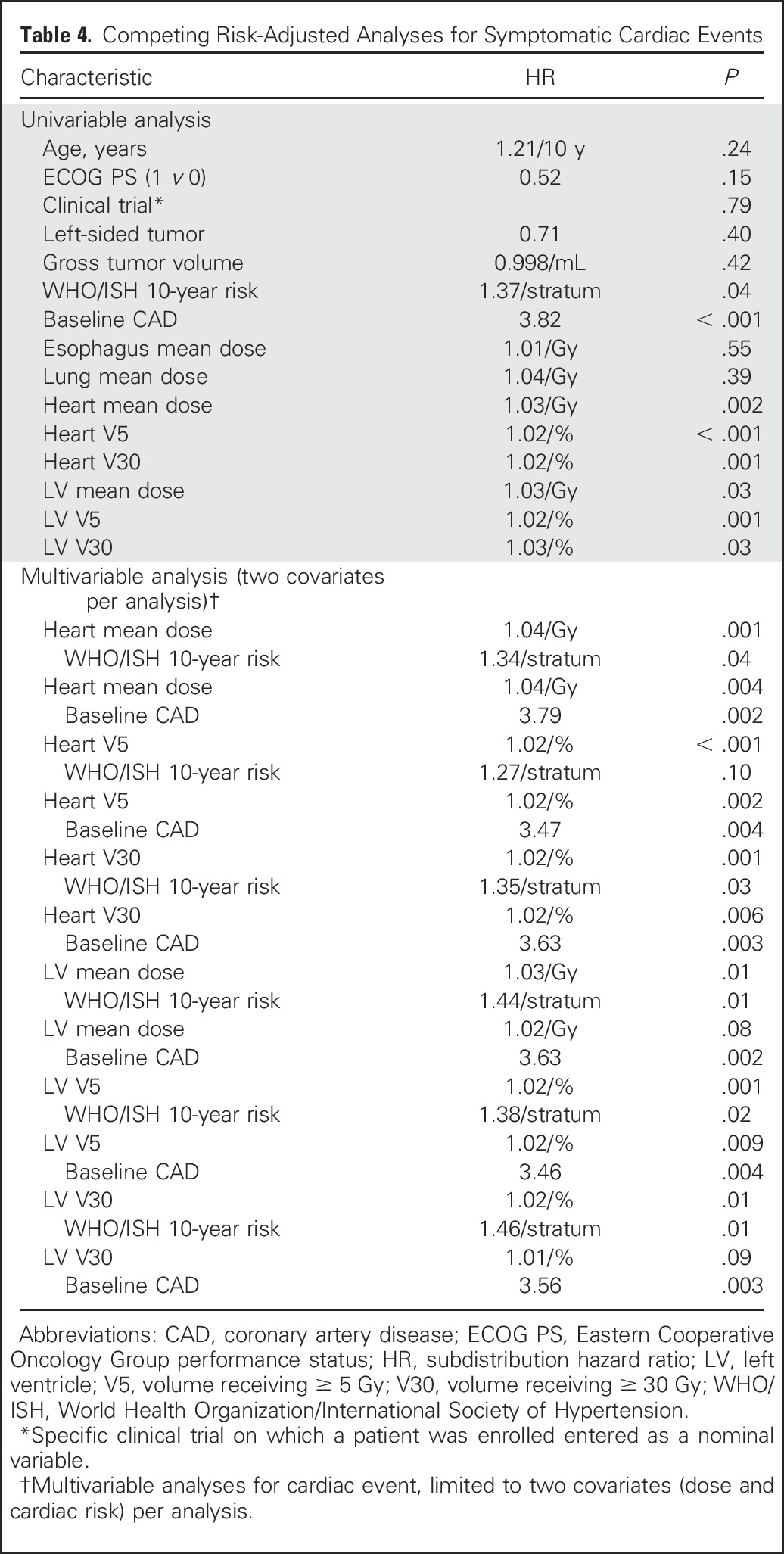
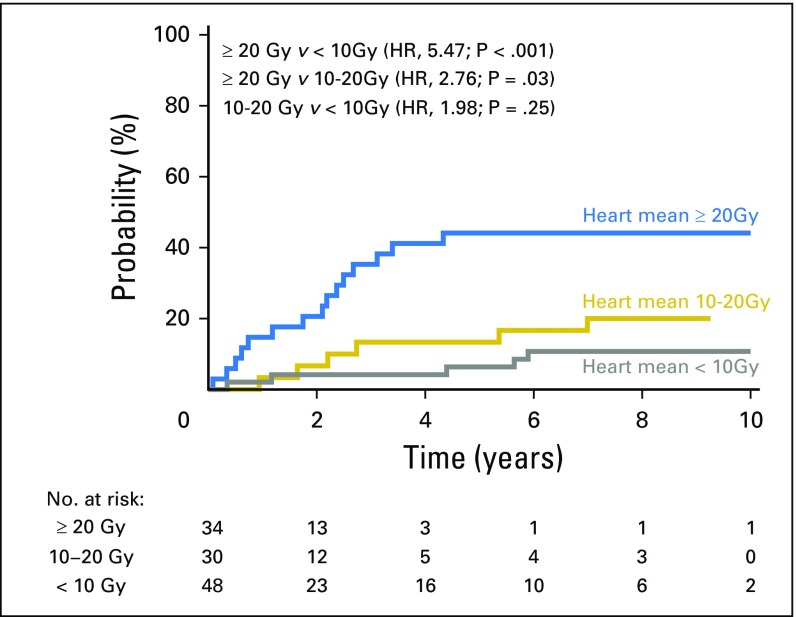
Figure 2 shows the cumulative incidence of symptomatic cardiac events adjusted for the competing risk of death for three heart mean dose strata: < 10 Gy, 10 to 20 Gy, and ≥ 20 Gy (created by choosing cut points around the median heart dose). Competing risk-adjusted event rates for patients with heart mean dose < 10 Gy, 10 to 20 Gy, and ≥ 20 Gy were 4%, 7%, and 21%, respectively at 2 years and 4%, 13%, and 41%, respectively, at 4 years. Patients with heart mean dose ≥ 20 Gy had a significantly higher rate of cardiac events than patients with heart mean dose < 10 Gy (HR, 5.47; P < .001) or 10 to 20 Gy (HR, 2.76; P = .03). Event rate did not differ significantly between patients with heart mean dose 10 to 20 Gy v < 10 Gy (HR, 1.98; P = .25). Crude event rates were 10% (five of 48 patients), 20% (six of 30 patients), and 44% (15 of 34 patients), for heart mean dose < 10 Gy, 10 to 20 Gy, and ≥ 20 Gy, respectively.
Fig 2.
Cumulative incidence of competing risk–adjusted symptomatic cardiac events in patients with heart mean dose ≥ 20 Gy (blue), 10 to 20 Gy (gold), and < 10 Gy (gray).
Pericardial Effusion
After RT, 40 patients (36%) had new pericardial effusions including symptomatic effusions. Most were discovered incidentally on surveillance computed tomography scans at a median 11 months (range, 1 to 206 months) after RT. There was a borderline significant association between pericardial effusion and V5Gy (P = .09) but no statistically significant association with other parameters.
Survival
Median OS was 18.5 months and median PFS was 14.7 months. There was no statistically significant association between OS and heart doses. Two-year OS for patients with heart mean dose < 10 Gy, 10 to 20 Gy, and ≥ 20 Gy was 50%, 40%, and 44%, respectively (log rank P = .73).
DISCUSSION
In 112 patients with stage III NSCLC treated on six prospective dose-escalation trials with long follow-up, there was an association between clinically significant cardiac events and both radiation doses to the heart and baseline cardiac risk. Although cardiac events were likely multifactorial, the association with heart dose was strong, and it persisted when controlling for baseline cardiac risk. These data may inform researchers and clinicians regarding the potential significance of cardiac toxicity in this setting and also have implications for RT technique and estimates of risk based on heart dosimetry.
The major question we addressed is whether cardiac toxicity is important in the definitive treatment of stage III NSCLC. A common perception is that significant RT-associated heart toxicity occurs 10 or more years after treatment on the basis of studies in breast cancer and Hodgkin lymphoma.1-6 Those patients are comparably younger with more favorable prognosis, and thus RT practices have evolved toward cardiac avoidance for these diagnoses. This is not the case for patients with stage III NSCLC in whom median survival is generally less than 2 years and for whom pneumonitis and esophagitis are considered the major toxicities. This is reflected by the lack of cardiac constraints for most of the trials included in our study, as well as by the RTOG 0617 protocol itself, which allowed up to 100% of the heart to receive 40 Gy (V40Gy < 100%) and which states “Normal tissue constraints shall be prioritized in the following order for treatment planning: 1 = spinal cord, 2 = lungs, 3 = esophagus, 4 = brachial plexus, and 5 = heart.”15
Like RTOG 0617, our findings challenge the perception that minimizing heart dose is not important in the treatment of patients with stage III NSCLC. Although prognosis is poor in these patients, they generally receive higher heart doses and may also have more comorbidities and smoking history, thus increasing risk and perhaps shortening the latency between RT and resultant heart disease. In this study, 23% of patients had symptomatic cardiac events, most within 5 years of treatment. Mean heart dose was 20 Gy and V30Gy was 29% in patients with events—well within the RTOG 0617 limit (V40Gy < 100%), and those of other modern protocols including V40Gy < 100% in RTOG 1306 (A Randomized Phase II Study of Individualized Combined Modality Therapy for Stage III Non–Small-Cell Lung Cancer [NSCLC]), and V30Gy < 50% in RTOG 1308 (Phase III Randomized Trial Comparing Overall Survival After Photon Versus Proton Chemoradiotherapy for Inoperable Stage II to IIIB NSCLC).15,28,29 Even with adjustment for the competing risk of death, patients with mean heart doses ≥ 20 Gy had a 21% 2-year event rate. We did not find an association between heart dose and survival, perhaps because of the small sample size and the overpowering competing risk of death from lung cancer. Nevertheless, our data support minimization of heart radiation exposure whenever possible to doses lower than commonly recommended9,15,28,29 in patients with stage III NSCLC to reduce risks of toxicity.
Several studies report increased cardiac toxicity with postoperative RT for lung cancer.10-12 However, these studies did not show a dose-toxicity relationship and might not apply to patients managed nonoperatively with chemoradiation; evidence is limited in this setting. Allan et al30 recently presented data indicating a possible association between cardiac events and heart dose in patients treated to approximately 63 Gy. Hardy et al14 also showed an association between RT and cardiac toxicity, but patients with stage IV disease were included and nearly half had surgery. In contrast, Schytte et al13 found no association between heart doses and cardiac events or survival in patients with stage I to III disease treated with RT with or without chemotherapy. Similarly, Tucker et al31 also reported no association between heart doses and survival in patients with stage III disease undergoing chemoradiation but did not report toxicity. Most of the patients in those studies received conventional doses of 60 to 66 Gy and were not enrolled in prospective trials. Patients included in this analysis all received ≥ 70 Gy and had protocol-specified follow-up. Thus, our findings and those from RTOG 0617 support the notion that RT-associated heart injury may be relevant, particularly in the setting of dose escalation.
It is unclear how radiation may increase the risk of early cardiac toxicity, but given the diversity of event types, the mechanism is likely multifactorial. As described by Darby et al,7 RT may cause both accelerated late atherosclerosis of major vessels and early microvascular changes. Evidence suggesting early microvascular changes comes from animal models32,33 and from clinical studies. Marks et al34 reported a 27% rate of new perfusion defects detected with nuclear imaging only 6 months after RT. Similar findings have been reported by others.35-37 Hatakenaka et al38 also reported early LV dysfunction on cardiac magnetic resonance imaging scanning after chemoradiation for esophageal cancer. Although early microvascular changes may increase susceptibility to late coronary artery disease, they could also contribute to early cardiac toxicity. Another possible mechanism is the development of pericardial effusions that occurred in 36% of patients at a median 11 months after treatment, consistent with prior reports.39 Effusions that are initially insignificant may eventually become symptomatic or contribute to the later development of other cardiac toxicities. Finally, there was a high rate of significant arrhythmia. Although these events are perhaps more likely to be precipitated by concurrent medical illnesses, the effect of RT on nervous system structures is well documented in other settings40; injury to cardiac nerve pathways could conceivably lead to aberrant conduction and increase the risk of arrhythmia. This is supported by a recent article on almost 2,000 long-term survivors of Hodgkin lymphoma in whom ischemia and arrhythmia were the two most common initial cardiac events.41
Our study suggests a dose dependence for RT-associated cardiotoxicity. The growing dose, volume, and outcome data from our study and similar studies provide additional information to guide RT treatment planning. Ideally doses are delivered only to the target, but the physical nature of radiation beams makes this impossible. Incidental irradiation of surrounding normal tissues is unavoidable. Thus, the planning process essentially balances where this incidental dose is delivered. Risk of cardiac toxicity likely increases continuously with heart dose, but the degree to which heart dose can be minimized is limited by competing priorities of tumor control and doses to other organs, especially the lung. Further studies are needed to determine the ideal balance between these priorities. In our opinion, tumor coverage should rarely be compromised to meet a heart dose constraint. However, it would be reasonable to try to limit heart mean dose to < 20 Gy (lower if possible) on the basis of the high event rate we observed in patients exceeding this dose (21% at 2 years and 41% at 4 years). Sophisticated radiation treatment planning techniques (eg, IMRT) and charged particle therapy with protons or carbon ions may provide increased flexibility to generate more conformal treatment plans and reduce heart dose, which could potentially improve the clinical outcomes in patients with stage III NSCLC.42-44
Our study has several limitations. First, toxicity end points were retrospectively assessed. However, patients were followed prospectively on protocols, which may increase the completeness of post-treatment evaluations.45 Nevertheless, there may have been undocumented cardiac events, and our results may therefore actually underestimate their frequency. Second, the number of events was low, which limits the examination of multiple covariates, but this is still one of the largest studies of its type to address this important clinical question. Third, assessing baseline cardiac risk via the WHO/ISH risk score and baseline CAD is imperfect, but it is still a reasonable and practical approach similar to those used by others.3,8 Fourth, patients were treated over many years on multiple trials, which introduces treatment heterogeneity. Different chemotherapy regimens may contribute differentially to cardiac toxicity, although we did not find associations between specific trials (and therefore chemotherapy regimens) and events. Furthermore, agents used in these trials are not considered particularly cardiotoxic,46 although they could potentially enhance radiation-induced cardiotoxicity. Fifth, patients were treated using high-dose RT (≥ 70 Gy), often without cardiac constraints, and thus our findings might not be applicable for patients treated to conventional doses or with IMRT in the modern era. Nevertheless, our findings support the presence of a relationship between dose-volume and toxicity, and our data can assist in redefining reasonable cardiac constraints (which for lung cancer have not changed appreciably for many years). Sixth, multiple types of events were included in the primary end point, and some (such as arrhythmia) may be more subject to confounding by concurrent illnesses. However, when excluding arrhythmia from the primary end point, the results were essentially unchanged. Finally, the high competing risk of death as a result of cancer is a significant confounder when analyzing a comparably rare toxicity, but this issue plagues essentially all similar studies in patients with such serious illnesses.
In conclusion, clinically significant cardiac events after high-dose thoracic RT for stage III NSCLC were associated with heart dose and cardiac risk and occurred fairly early after treatment. Consistent with the current emphasis on reducing radiation heart exposure for other malignancies, heart doses should be similarly minimized in patients with stage III NSCLC.
Footnotes
Supported in part by Grant No. CA69579 from the National Institutes of Health.
See accompanying Editorial on page 1381
AUTHOR CONTRIBUTIONS
Conception and design: Kyle Wang, Michael J. Eblan, Mark A. Socinski, Thomas E. Stinchcombe, Lawrence B. Marks
Financial support: Mark A. Socinski, Thomas E. Stinchcombe
Provision of study materials or patients: Mark A. Socinski, Thomas E. Stinchcombe
Collection and assembly of data: Kyle Wang, Michael J. Eblan, Matthew Lipner, Panayiotis Mavroidis, Carrie B. Lee, Mark A. Socinski, Thomas E. Stinchcombe, Lawrence B. Marks
Data analysis and interpretation: Kyle Wang, Michael J. Eblan, Allison M. Deal, Matthew Lipner, Timothy M. Zagar, Yue Wang, Panayiotis Mavroidis, Brian C. Jensen, Julian G. Rosenman, Mark A. Socinski, Thomas E. Stinchcombe, Lawrence B. Marks
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cardiac Toxicity After Radiotherapy for Stage III Non–Small-Cell Lung Cancer: Pooled Analysis of Dose-Escalation Trials Delivering 70 to 90 Gy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Kyle Wang
No relationship to disclose
Michael J. Eblan
Research Funding: La Jolla Pharmaceutical (I)
Patents, Royalties, Other Intellectual Property: Patent application with University of North Carolina titled “Circulating Tumors Cells as Predictive Biomarker for Response to Radiation With or Without Chemotherapy” (US Provisional Application No. 62/161,595)
Allison M. Deal
No relationship to disclose
Matthew Lipner
No relationship to disclose
Timothy M. Zagar
Honoraria: Vision RT
Consulting or Advisory Role: Vision RT, Medtronic
Research Funding: Vision RT (Inst)
Travel, Accommodations, Expenses: Vision RT
Yue Wang
No relationship to disclose
Panayiotis Mavroidis
No relationship to disclose
Carrie B. Lee
Travel, Accommodations, Expenses: Novartis
Brian C. Jensen
Patents, Royalties, Other Intellectual Property: University of North Carolina/University of California at San Francisco, patent pending for an alpha receptor agonist in the treatment of cardiomyopathy
Julian G. Rosenman
No relationship to disclose
Mark A. Socinski
No relationship to disclose
Thomas E. Stinchcombe
No relationship to disclose
Lawrence B. Marks
Research Funding: Elekta, Morphomics, Accuray (Inst)
Travel, Accommodations, Expenses: Varian Medical Systems and Elekta
REFERENCES
- 1.Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447–453. doi: 10.1200/JCO.1994.12.3.447. [DOI] [PubMed] [Google Scholar]
- 2.Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 3.Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst. 2007;99:365–375. doi: 10.1093/jnci/djk064. [DOI] [PubMed] [Google Scholar]
- 4.van Nimwegen FA, Schaapveld M, Janus CP, et al. Cardiovascular disease after Hodgkin lymphoma treatment: 40-year disease risk. JAMA Intern Med. 2015;175:1007–1017. doi: 10.1001/jamainternmed.2015.1180. [DOI] [PubMed] [Google Scholar]
- 5.van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016;34:235–243. doi: 10.1200/JCO.2015.63.4444. [DOI] [PubMed] [Google Scholar]
- 6.Hancock SL, Tucker MA, Hoppe RT. Factors affecting late mortality from heart disease after treatment of Hodgkin’s disease. JAMA. 1993;270:1949–1955. [PubMed] [Google Scholar]
- 7.Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: Current knowledge and future prospects. Int J Radiat Oncol Biol Phys. 2010;76:656–665. doi: 10.1016/j.ijrobp.2009.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 9.Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys. 2010;76:S77–S85. doi: 10.1016/j.ijrobp.2009.04.093. [DOI] [PubMed] [Google Scholar]
- 10.Dautzenberg B, Arriagada R, Chammard AB, et al. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma: Groupe d’Etude et de Traitement des Cancers Bronchiques. Cancer. 1999;86:265–273. doi: 10.1002/(sici)1097-0142(19990715)86:2<265::aid-cncr10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Lally BE, Detterbeck FC, Geiger AM, et al. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2007;110:911–917. doi: 10.1002/cncr.22845. [DOI] [PubMed] [Google Scholar]
- 12.Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: The adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72:695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 13.Schytte T, Hansen O, Stolberg-Rohr T, et al. Cardiac toxicity and radiation dose to the heart in definitive treated non-small cell lung cancer. Acta Oncol. 2010;49:1058–1060. doi: 10.3109/0284186X.2010.504736. [DOI] [PubMed] [Google Scholar]
- 14.Hardy D, Liu CC, Cormier JN, et al. Cardiac toxicity in association with chemotherapy and radiation therapy in a large cohort of older patients with non-small-cell lung cancer. Ann Oncol. 2010;21:1825–1833. doi: 10.1093/annonc/mdq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Socinski MA, Rosenman JG, Halle J, et al. Dose-escalating conformal thoracic radiation therapy with induction and concurrent carboplatin/paclitaxel in unresectable stage IIIA/B nonsmall cell lung carcinoma: A modified phase I/II trial. Cancer. 2001;92:1213–1223. doi: 10.1002/1097-0142(20010901)92:5<1213::aid-cncr1440>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Marks LB, Garst J, Socinski MA, et al. Carboplatin/paclitaxel or carboplatin/vinorelbine followed by accelerated hyperfractionated conformal radiation therapy: Report of a prospective phase I dose escalation trial from the Carolina Conformal Therapy Consortium. J Clin Oncol. 2004;22:4329–4340. doi: 10.1200/JCO.2004.02.165. [DOI] [PubMed] [Google Scholar]
- 18.Socinski MA, Morris DE, Halle JS, et al. Induction and concurrent chemotherapy with high-dose thoracic conformal radiation therapy in unresectable stage IIIA and IIIB non-small-cell lung cancer: A dose-escalation phase I trial. J Clin Oncol. 2004;22:4341–4350. doi: 10.1200/JCO.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 19.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 20.Stinchcombe TE, Morris DE, Lee CB, et al. Induction chemotherapy with carboplatin, irinotecan, and paclitaxel followed by high dose three-dimension conformal thoracic radiotherapy (74 Gy) with concurrent carboplatin, paclitaxel, and gefitinib in unresectable stage IIIA and stage IIIB non-small cell lung cancer. J Thorac Oncol. 2008;3:250–257. doi: 10.1097/JTO.0b013e3181653cf4. [DOI] [PubMed] [Google Scholar]
- 21.Lee CB, Stinchcombe TE, Moore DT, et al. Late complications of high-dose (>/=66 Gy) thoracic conformal radiation therapy in combined modality trials in unresectable stage III non-small cell lung cancer. J Thorac Oncol. 2009;4:74–79. doi: 10.1097/JTO.0b013e3181915028. [DOI] [PubMed] [Google Scholar]
- 22.Socinski MA, Stinchcombe TE, Moore DT, et al. Incorporating bevacizumab and erlotinib in the combined-modality treatment of stage III non-small-cell lung cancer: Results of a phase I/II trial. J Clin Oncol. 2012;30:3953–3959. doi: 10.1200/JCO.2012.41.9820. [DOI] [PubMed] [Google Scholar]
- 23.Tewell MA, Adams R. The PLUNC 3D treatment planning system: A dynamic alternative to commercially available systems. Med Dosim. 2004;29:134–138. doi: 10.1016/j.meddos.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Feng M, Moran JM, Koelling T, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendis S, Lindholm LH, Mancia G, et al. World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: Assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J Hypertens. 2007;25:1578–1582. doi: 10.1097/HJH.0b013e3282861fd3. [DOI] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 27.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: Competing risks and multi-state models. Stat Med. 2007;26:2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 28.Radiation Therapy Oncology Group (RTOG) Foundation: RTOG 1306 Protocol Information: A Randomized Phase II Study of Individualized Combined Modality Therapy for Stage III Non-Small Cell Lung Cancer (NSCLC). Version date May 16, 2016. https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1306
- 29.Radiation Therapy Oncology Group (RTOG) Foundation: RTOG 1308 Protocol Information: Phase III Randomized Trial Comparing Overall Survival After Photon Versus Proton Chemoradiotherapy for Inoperable Stage II-IIIB NSCLC. Version date October 5, 2015. https://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=1308
- 30.Allan E, Williams TM, Grecula JC, et al. Mortality, cardiac toxicity, and radiation dose to the heart in patients treated with curative intent fractionated radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 93:3, 2015 (suppl; abstr 1012) [Google Scholar]
- 31.Tucker SL, Liu A, Gomez D, et al. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother Oncol. 2016;119:495–500. doi: 10.1016/j.radonc.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Lauk S, Kiszel Z, Buschmann J, et al. Radiation-induced heart disease in rats. Int J Radiat Oncol Biol Phys. 1985;11:801–808. doi: 10.1016/0360-3016(85)90314-1. [DOI] [PubMed] [Google Scholar]
- 33.Schultz-Hector S, Sund M, Thames HD. Fractionation response and repair kinetics of radiation-induced heart failure in the rat. Radiother Oncol. 1992;23:33–40. doi: 10.1016/0167-8140(92)90303-c. [DOI] [PubMed] [Google Scholar]
- 34.Marks LB, Yu X, Prosnitz RG, et al. The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys. 2005;63:214–223. doi: 10.1016/j.ijrobp.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 35.Gyenes G, Fornander T, Carlens P, et al. Morbidity of ischemic heart disease in early breast cancer 15-20 years after adjuvant radiotherapy. Int J Radiat Oncol Biol Phys. 1994;28:1235–1241. doi: 10.1016/0360-3016(94)90500-2. [DOI] [PubMed] [Google Scholar]
- 36.Gyenes G, Fornander T, Carlens P, et al. Myocardial damage in breast cancer patients treated with adjuvant radiotherapy: A prospective study. Int J Radiat Oncol Biol Phys. 1996;36:899–905. doi: 10.1016/s0360-3016(96)00125-3. [DOI] [PubMed] [Google Scholar]
- 37.Seddon B, Cook A, Gothard L, et al. Detection of defects in myocardial perfusion imaging in patients with early breast cancer treated with radiotherapy. Radiother Oncol. 2002;64:53–63. doi: 10.1016/s0167-8140(02)00133-0. [DOI] [PubMed] [Google Scholar]
- 38.Hatakenaka M, Yonezawa M, Nonoshita T, et al. Acute cardiac impairment associated with concurrent chemoradiotherapy for esophageal cancer: Magnetic resonance evaluation. Int J Radiat Oncol Biol Phys. 2012;83:e67–e73. doi: 10.1016/j.ijrobp.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 39.Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2008;70:707–714. doi: 10.1016/j.ijrobp.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 40.Dropcho EJ. Neurotoxicity of radiation therapy. Neurol Clin. 2010;28:217–234. doi: 10.1016/j.ncl.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Maraldo MV, Giusti F, Vogelius IR, et al. Cardiovascular disease after treatment for Hodgkin’s lymphoma: An analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol. 2015;2:e492–e502. doi: 10.1016/S2352-3026(15)00153-2. [DOI] [PubMed] [Google Scholar]
- 42.Chun SG, Hu C, Choy H, et al. Comparison of 3-D conformal and intensity modulated radiation therapy outcomes for locally advanced non-small cell lung cancer in NRG Oncology/RTOG 0617. Int J Radiat Oncol Biol Phys 93:3s, 2015 (suppl; abstr 2) [Google Scholar]
- 43.Movsas B, Hu C, Sloan J, et al. Quality of life analysis of a radiation dose-escalation study of patients with non-small-cell lung cancer: A secondary analysis of the Radiation Therapy Oncology Group 0617 Randomized Clinical Trial. JAMA Oncol. 2016;2:359–367. doi: 10.1001/jamaoncol.2015.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giaddui T, Chen W, Yu J, et al. Establishing the feasibility of the dosimetric compliance criteria of RTOG 1308: Phase III randomized trial comparing overall survival after photon versus proton radiochemotherapy for inoperable stage II-IIIB NSCLC. Radiat Oncol. 2016;11:66. doi: 10.1186/s13014-016-0640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J Clin Epidemiol. 2001;54:217–224. doi: 10.1016/s0895-4356(00)00305-x. [DOI] [PubMed] [Google Scholar]
- 46.Nolan MT, Lowenthal RM, Venn A, et al. Chemotherapy-related cardiomyopathy: A neglected aspect of cancer survivorship. Intern Med J. 2014;44:939–950. doi: 10.1111/imj.12532. [DOI] [PubMed] [Google Scholar]