Abstract
Purpose
Radiation therapy is a critical component in the care of patients with non–small-cell lung cancer (NSCLC), yet cardiac injury after treatment is a significant concern. Therefore, we wished to elucidate the incidence of cardiac events and their relationship to radiation dose to the heart.
Patients and Materials
Study eligibility criteria included patients with stage II to III NSCLC treated on one of four prospective radiation therapy trials at two centers from 2004 to 2013. All cardiac events were reviewed and graded per Common Terminology Criteria for Adverse Events (v4.03). The primary end point was the development of a grade ≥ 3 cardiac event.
Results
In all, 125 patients met eligibility criteria; median follow-up was 51 months for surviving patients. Median prescription dose was 70 Gy, 84% received concurrent chemotherapy, and 27% had pre-existing cardiac disease. Nineteen patients had a grade ≥ 3 cardiac event at a median of 11 months (interquartile range, 6 to 24 months), and 24-month cumulative incidence was 11% (95% CI, 5% to 16%). On multivariable analysis (MVA), pre-existing cardiac disease (hazard ratio [HR], 2.96; 95% CI, 1.07 to 8.21; P = .04) and mean heart dose (HR, 1.07/Gy; 95% CI, 1.02 to 1.13/Gy; P = .01) were significantly associated with grade ≥ 3 cardiac events. Analyzed as time-dependent variables on MVA analysis, both disease progression (HR, 2.15; 95% CI, 1.54 to 3.00) and grade ≥ 3 cardiac events (HR, 1.76; 95% CI, 1.04 to 2.99) were associated with decreased overall survival. However, disease progression (n = 71) was more common than grade ≥ 3 cardiac events (n = 19).
Conclusion
The 24-month cumulative incidence of grade ≥ 3 cardiac events exceeded 10% among patients with locally advanced NSCLC treated with definitive radiation. Pre-existing cardiac disease and higher mean heart dose were significantly associated with higher cardiac event rates. Caution should be used with cardiac dose to minimize risk of radiation-associated injury. However, cardiac risks should be balanced against tumor control, given the unfavorable prognosis associated with disease progression.
INTRODUCTION
Non–small-cell lung cancer (NSCLC) frequently presents with locally advanced, unresectable disease, and standard fractionation radiation has been the backbone of definitive treatment for more than 30 years.1 Despite improved outcomes with the addition of concurrent chemotherapy,2 locoregional relapse remains common and is associated with inferior overall survival (OS).3 Radiation dose escalation has been associated with increased tumor control,4 leading to a number of promising phase II trials.5-7 Ultimately, the Radiation Therapy Oncology Group (RTOG) 0617 (High-Dose or Standard-Dose Radiation Therapy and Chemotherapy With or Without Cetuximab in Treating Patients With Newly Diagnosed Stage III Non-Small Cell Lung Cancer That Cannot Be Removed by Surgery) phase III dose-escalation trial compared 74 Gy with the standard 60 Gy, both delivered with concurrent chemotherapy.8 In an unexpected result, the high-dose arm had worse OS. On multivariable analysis, increasing radiation dose to the heart was independently associated with worse survival, and some hypothesize that under-reported cardiac events may have contributed to the inferior results in the experimental arm.9
The association of increasing exposure to ionizing radiation with long-term cardiac toxicity is well established. With decades of follow-up, increases in rates of cardiac disease with ionizing radiation exposure to the heart have been noted in long-term survivors of breast cancer10,11 and lymphoma.12,13 However, the subacute effects of radiation on the heart, such as those most likely to impact outcomes in patients with locally advanced NSCLC, have not been well elucidated. This is a research area of critical unmet need, especially given the uncertainty following RTOG 0617.
In this article, we analyzed the combined long-term results of four consecutive, prospective radiation therapy trials for locally advanced NSCLC. We hypothesized that the cardiac event rate in this population is higher than previously reported. We provide a comprehensive patient-level assessment of pretreatment cardiac status along with a detailed evaluation of cardiac events. We aimed to assess the patient- and treatment-related factors associated with clinically significant cardiac events and to determine the impact of these events on OS.
PATIENTS AND MATERIALS
Patients
Between 2004 and 2013, 125 patients with medically inoperable or unresectable stage II to III NSCLC were treated with informed consent on one of four consecutive trials approved by institutional review boards at two centers (University of Michigan and Ann Arbor Veterans Affairs Hospital). All patients had biopsy-confirmed NSCLC. Computed tomography (CT) of the chest and upper abdomen, positron emission tomography (PET) –CT scans and brain imaging (CT or magnetic resonance imaging) were required for staging. Eligible patients had Karnofsky performance score (KPS) ≥ 60. Patients with any component of small-cell carcinoma, those with stage I disease, or those treated with stereotactic body radiation therapy were ineligible for this study.
Treatment
Details of the specific prospective trials are summarized in Appendix Table A1 (online only). All trials included patients treated with hypofractionated radiation (> 2 Gy/fraction). Radiation was delivered over 6 weeks with concurrent chemotherapy as tolerated. Patients treated on the adaptive dose-escalation protocol underwent treatment on the basis of midtreatment PET-CT response similar to that in RTOG 1106 (Study of Positron Emission Tomography and Computed Tomography in Guiding Radiation Therapy in Patients With Stage III Non-Small Cell Lung Cancer).14 Heart constraints included volume receiving 40 Gy < 100% (V40Gy < 100%) and V65Gy < 33%. All normal tissue constraints were evaluated in biologically equivalent 2-Gy fractions. Tumor planning target volume (PTV) coverage took priority over cardiac constraints. History, physical examination, and chest imaging (CT) were completed every 3 to 6 months after treatment. Response was determined by Response Evaluation Criteria in Solid Tumors (RECIST) criteria.15
End Points
The primary end point of this study was the development of a first grade ≥ 3 cardiac event after treatment. Cardiac events were graded prospectively on each prospective trial on the basis of Common Terminology Criteria for Adverse Events (CTCAE, version 3). In this study, all previous CTCAE cardiac events were reviewed, confirmed, and updated to the most recent version of the CTCAE (v4.03; Appendix Table A2, online only). In addition, cardiac events not previously attributed to radiation were documented, graded, and included in the analysis. All events were confirmed by two independent physicians without knowledge of the treatment plan or cardiac radiation dose. Secondary outcomes included grade ≥ 2 cardiac events, progression-free survival (PFS) and OS.
Dosimetric Analysis
All previous plans underwent uniform dose calculation using modern photon dose calculations (Analytical Anisotropic Algorithm, Varian Medical Systems). To account for variable fractionation schemes, biologic effective corrections to equivalent dose in 2 Gy fractions (EQD2Gy) were performed by using the linear quadratic model (assuming α/β ratio = 2.5 for heart consistent with previous studies [2.0],16 experimental data on myocardium [2.4 to 2.9], capillary vasculature [1.8 to 2.8],17,18 and clinical estimates of pericardium [2.5]19). Heart contours were reviewed against a validated cardiac atlas and edited as necessary.20
Covariables
Sex, diabetes, and current smoking status were analyzed as binary variables. KPS, age, and systolic blood pressure were analyzed as continuous variables. Pre-existing cardiac disease was defined as the presence or absence of either pre-existing ischemic heart disease (acute myocardial infarction, coronary artery bypass grafting procedure, angioplasty or stent placement, or a diagnosis of coronary artery disease [CAD]) or clinical diagnosis of congestive heart failure (CHF). For those without pre-existing cardiac disease, Framingham risk scores (hard coronary heart disease 10-year risk) were calculated and analyzed as a continuous variable.21 Cardiac radiation dose was analyzed as a continuous variable with a focus on parameters identified as clinically significant in RTOG 0617 (percent volume receiving 5 Gy [V5Gy], 30 Gy [V30Gy], and 50 Gy [V50Gy], and mean heart dose).8
Statistical Analysis
Descriptive statistics were used to characterize baseline patient and treatment characteristics. Cumulative incidence curves of the development of a first grade ≥ 3 cardiac event were generated accounting for noncardiac death as a competing risk. Univariable and multivariable Cox proportional hazard models were used to analyze the difference in the hazard of cardiac events by baseline patient- and treatment-related characteristics. Concordance index (C-index) values were calculated to quantify the discriminatory ability of cardiac dose terms with respect to cardiac events. Fine and Gray competing risk regression was used to generate models to predict cumulative incidence of grade ≥ 3 cardiac events. The Kaplan-Meier method was used to estimate OS and PFS. For all analyses, two-sided P values of < .05 were considered statistically significant and values < .1 were considered a marginal association. Analyses were performed using R (version 3.3.1).
RESULTS
Baseline Patient and Treatment Characteristics
The median age was 66 years (range, 40 to 92 years), 98% had a KPS ≥ 70, and the vast majority were current (52%) or former (42%) smokers (Table 1). Twenty seven percent of patients (n = 34) had pre-existing cardiac disease before treatment started; all 34 had CAD and five had concurrent CHF. For those without cardiac disease (n = 71), the median Framingham risk score was 13% (range, 0.2% to 37%), and 15% (n = 19) had a Framingham risk score ≥ 20%.
Table 1.
Patient, Tumor, and Treatment Characteristics
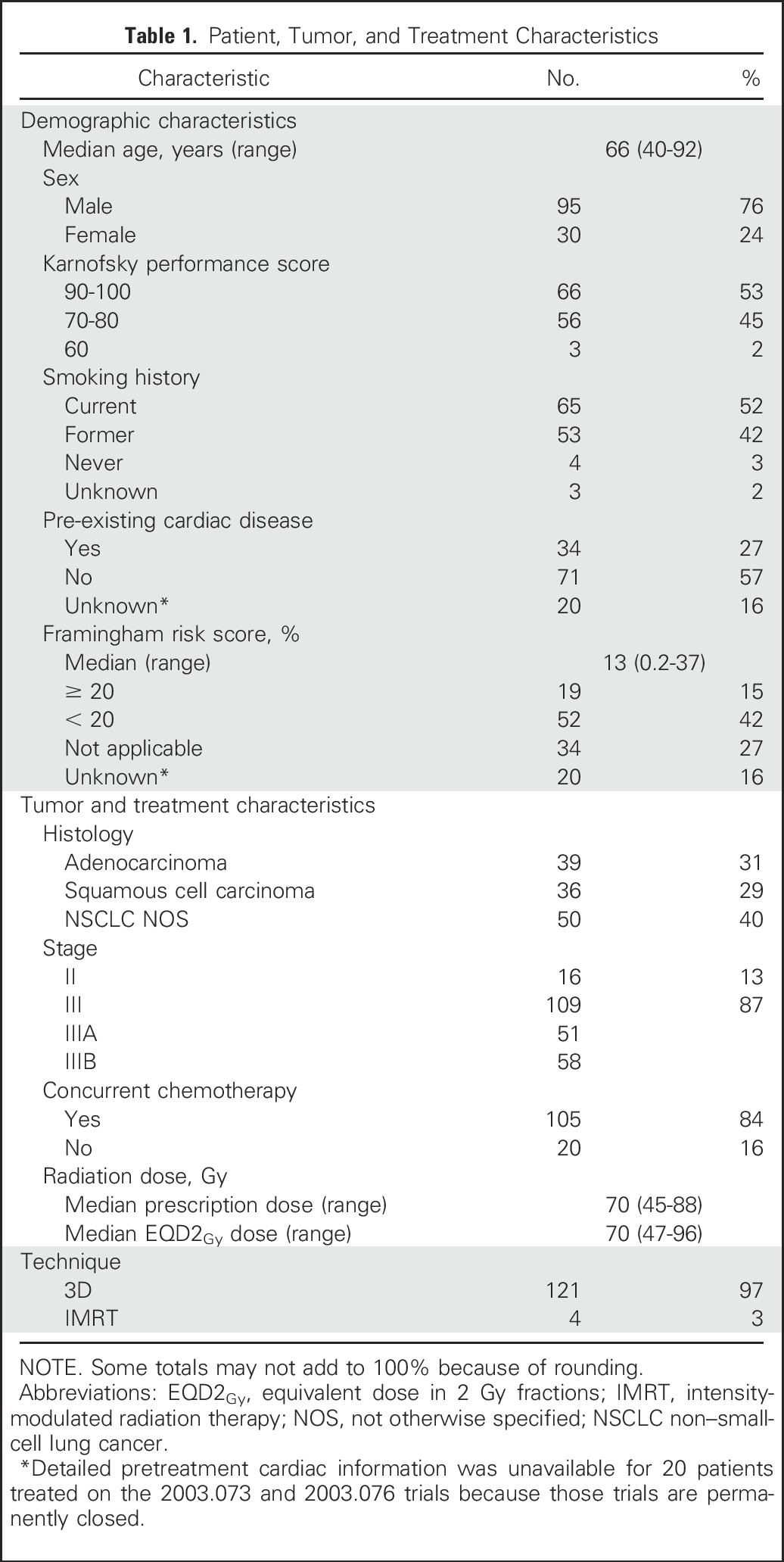
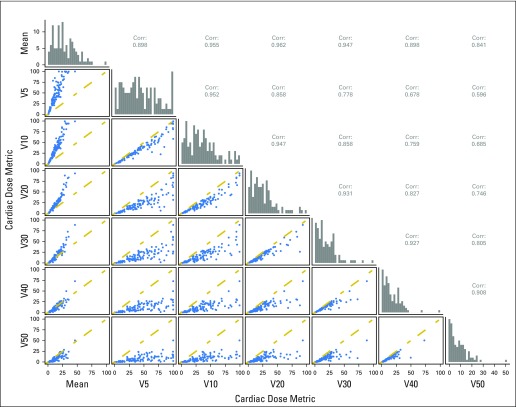
Eighty-four percent of the study population received concurrent chemotherapy and, assuming an α/β ratio of 10 for tumor, the median EQD2Gy prescription dose was 70 Gy (range, 47 to 96 Gy). Ninety-seven percent of patients were treated with 3D-conformal radiation and 3% with intensity-modulated radiation therapy (IMRT). The median mean cardiac dose was 11 Gy (range, 0.3 to 46 Gy; interquartile range [IQR], 7 to 19 Gy). Cardiac volumetric dose parameters (V5Gy to V50Gy) were highly correlated (Appendix Fig A1, online only).
Cardiac Events
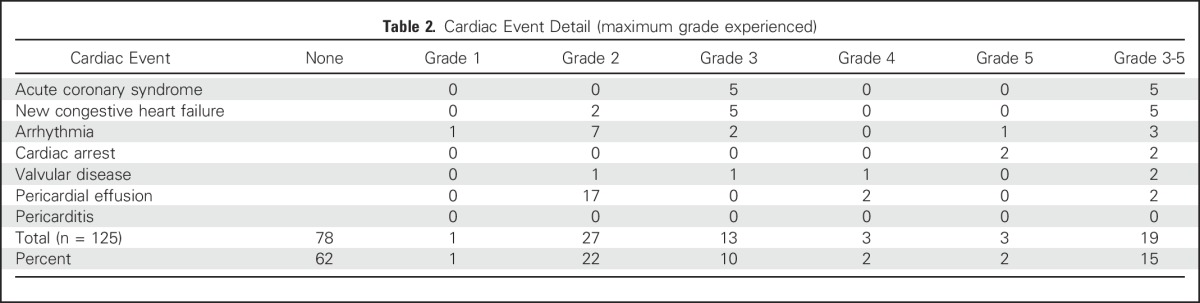
The median follow-up was 23 months for the entire cohort and 51 months for patients alive at last contact. Nineteen patients experienced a grade ≥ 3 cardiac event after treatment at a median of 11 months (range, 0.4 to 63 months; IQR, 6 to 24 months) after treatment. Thirteen patients had a grade 3 cardiac event primarily consisting of acute coronary syndrome events (n = 5) or new diagnoses of CHF (n = 5). There were three grade 4 events: two patients underwent emergent surgical intervention for severe and symptomatic pericardial effusions 13 and 23 months after treatment, and one patient underwent emergent balloon valvuloplasty for severe aortic stenosis 19 months after treatment. There were three grade 5 events: two patients had fatal cardiac arrest 14 and 29 months after treatment, both in the setting of pretreatment multivessel CAD, and one patient had a fatal ventricular arrhythmia 1 month after treatment in the setting of pretreatment multivessel CAD and CHF. No patient with a grade ≥ 3 cardiac event had additional grade 4 or 5 esophageal or pulmonary events. Twenty-eight patients had grade 1 to 2 cardiac events, primarily asymptomatic pericardial effusions (Table 2).
Table 2.
Cardiac Event Detail (maximum grade experienced)
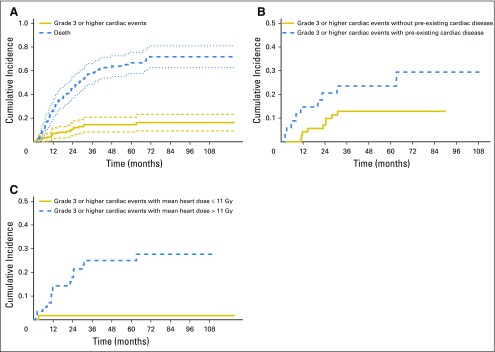
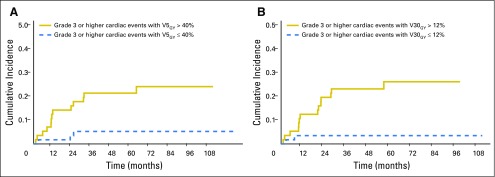
Accounting for noncardiac death as a competing risk, the 12- and 24-month cumulative incidence of grade ≥ 3 cardiac events was 9% (95% CI, 3% to 12%) and 11% (95% CI, 5% to 16%), respectively (Fig 1A). Among those with pre-existing cardiac disease, the 12- and 24-month cumulative incidence was 15% (95% CI, 3% to 27%) and 21% (95% CI, 7% to 35%) compared with 4% (95% CI, 0% to 9%) and 7% (95% CI, 1% to 13%) in those without this history (P = .08; Fig 1B). The median mean heart dose was 11 Gy. The 12- and 24-month cumulative incidence of grade ≥ 3 cardiac events was 14% (95% CI, 5% to 24%) and 18% (95% CI, 8% to 28%) in those with mean heart dose above 11 Gy and 2% (95% CI, 0% to 5%) and 2% (95% CI, 0% to 5%) in those with mean heart dose below 11 Gy (P < .01; Fig 1C). Similar findings were noted for patients above and below median V5Gy (40%) and V30Gy (12%; Appendix Fig A2, online only).
Fig 1.
Cumulative incidence of grade ≥ 3 cardiac events. (A) Actual cumulative incidence of grade ≥ 3 cardiac events (with noncardiac death as competing risk) for the total cohort, (B) for those with and without pre-existing cardiac disease, and (C) for those with greater than or less than the median mean heart dose of 11 Gy.
Predictors of Cardiac Events
On univariable analysis, male sex, diabetes, pre-existing cardiac disease, and Framingham risk score were at least marginally associated with increased risk of grade ≥ 3 cardiac events (P < .1). Moreover, mean heart dose, V5Gy and V30Gy were each significantly associated with an increased hazard of cardiac events (P < .01) with similar C-indices (approximately 0.7; Table 3).
Table 3.
Univariable and Multivariable Analysis for Time to Earliest Grade ≥ 3 Cardiac Event
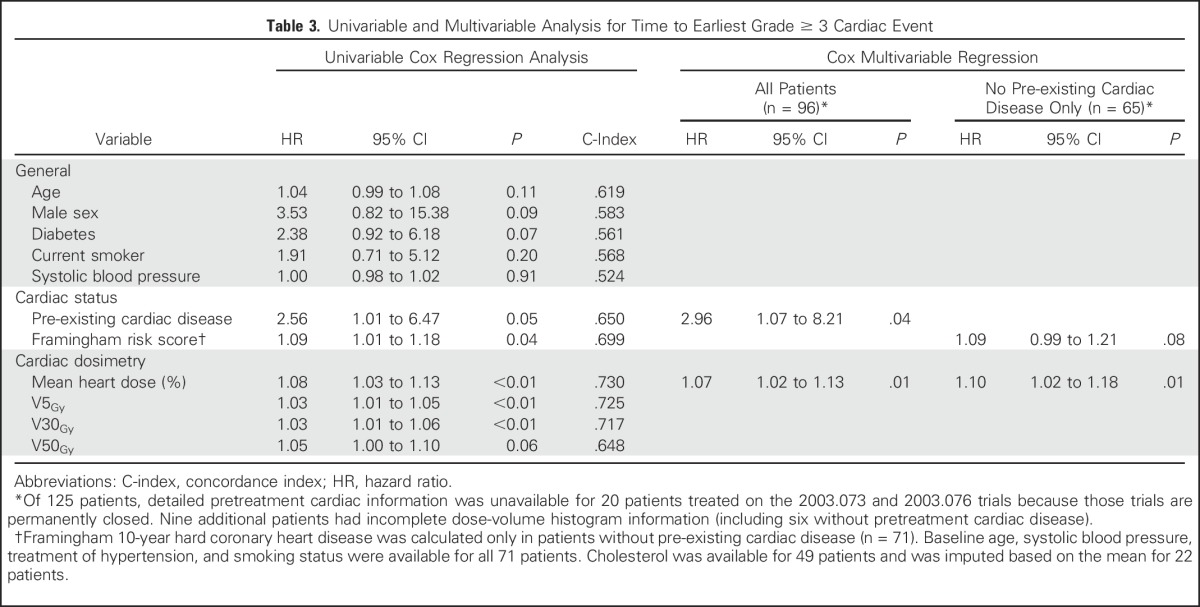
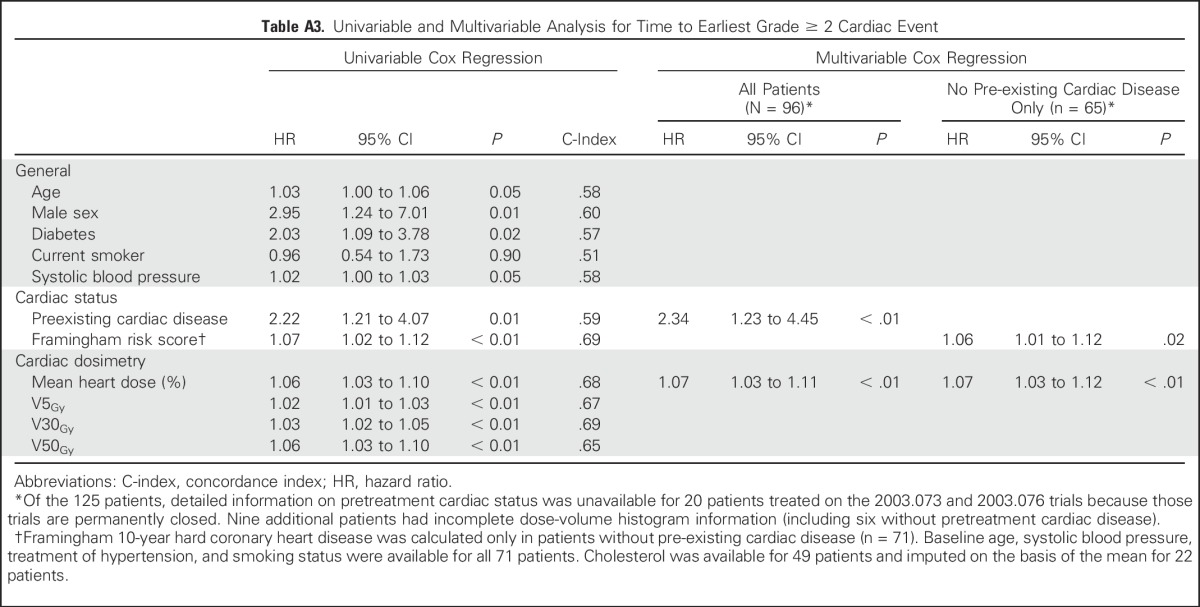
Cox stepwise multivariable regression analysis (MVA) identified pre-existing cardiac disease (hazard ratio [HR], 2.96; 95% CI, 1.07 to 8.21; P = .04) and higher mean heart dose (HR, 1.07/Gy; 95% CI, 1.02 to 1.13/Gy; P = .01) as significant predictors of grade ≥ 3 cardiac events. No evidence of effect modification with cardiac dose and pre-existing cardiac disease was demonstrated (P = .80 for interaction). In a subpopulation MVA including only those without pre-existing cardiac disease (n = 65), increased mean heart dose remained significantly associated with grade ≥ 3 cardiac events (HR, 1.10/Gy; 95% CI, 1.02 to 1.18/Gy; P = .01) and Framingham risk score was marginally associated with an increased hazard (HR, 1.09 per 1% increase in Framingham risk score; 95% CI, 0.99 to 1.21 per 1% increase in Framingham risk score; P = .08). Similar to grade ≥ 3 cardiac events, grade ≥ 2 events were associated with pre-existing cardiac disease and radiation dose to the heart on MVA (Appendix Table A3, online only).
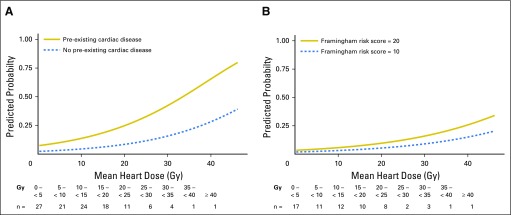
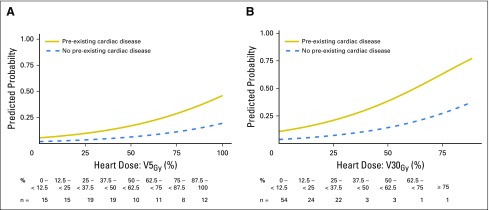
Among patients with pre-existing cardiac disease, mean heart doses of 5 and 12 Gy associated with a Fine and Gray competing risk regression model predicted 24-month grade ≥ 3 cardiac event rates of 10% and 15%, respectively (Fig 2A). Among those without pre-existing cardiac disease, the model-predicted mean heart dose thresholds increased to 23 Gy and 29 Gy, respectively, for equivalent 24-month 10% and 15% cardiac event rates. Analysis of V5Gy and V30Gy provided similar results (Appendix Fig A3, online only). Among patients without pre-existing cardiac disease, model-predicted cardiac event rates were lower than those for patients with overt cardiac disease, even with a Framingham risk score of 20% (Fig 2B).
Fig 2.
Model-predicted cumulative incidence of a grade ≥ 3 cardiac event within 24 months of treatment by increasing mean heart dose (A) for those with and without pre-existing cardiac disease and (B) for the subpopulation of only those without baseline cardiac disease. Representative patient Framingham risk scores of 10 and 20 are shown.
Cardiac Events and OS
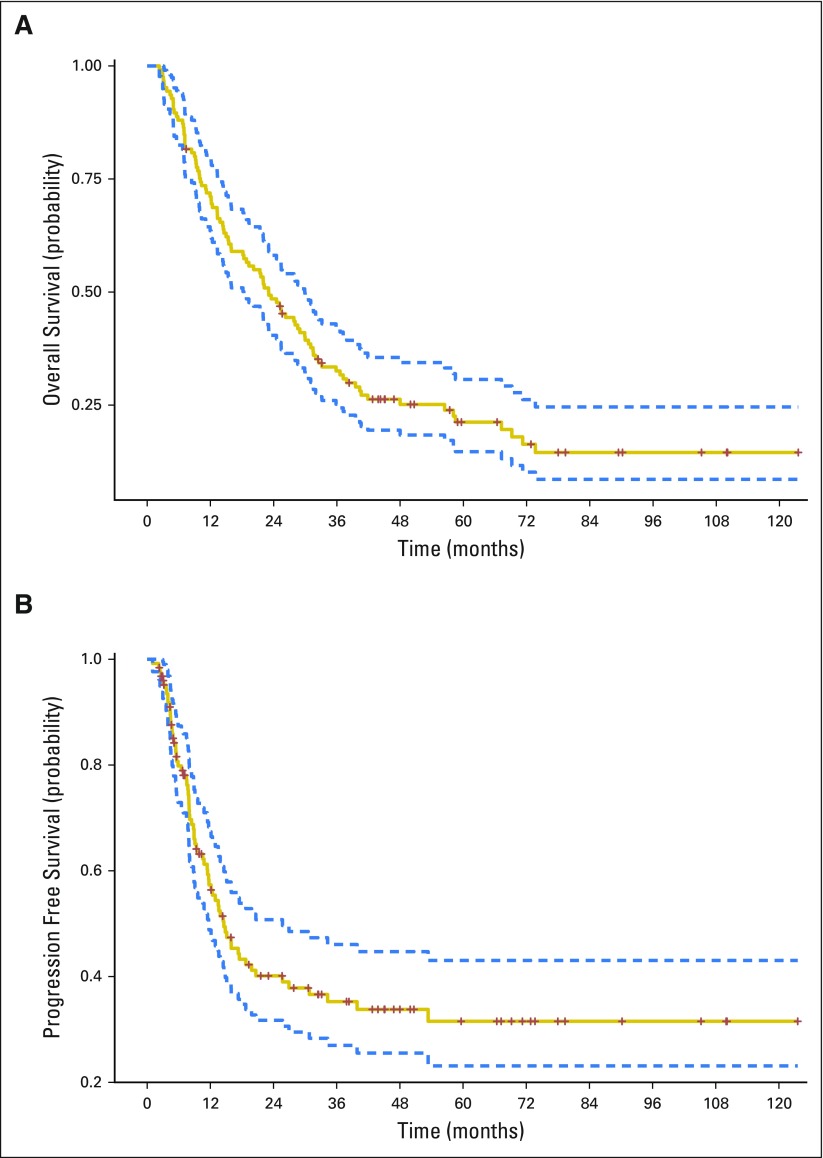
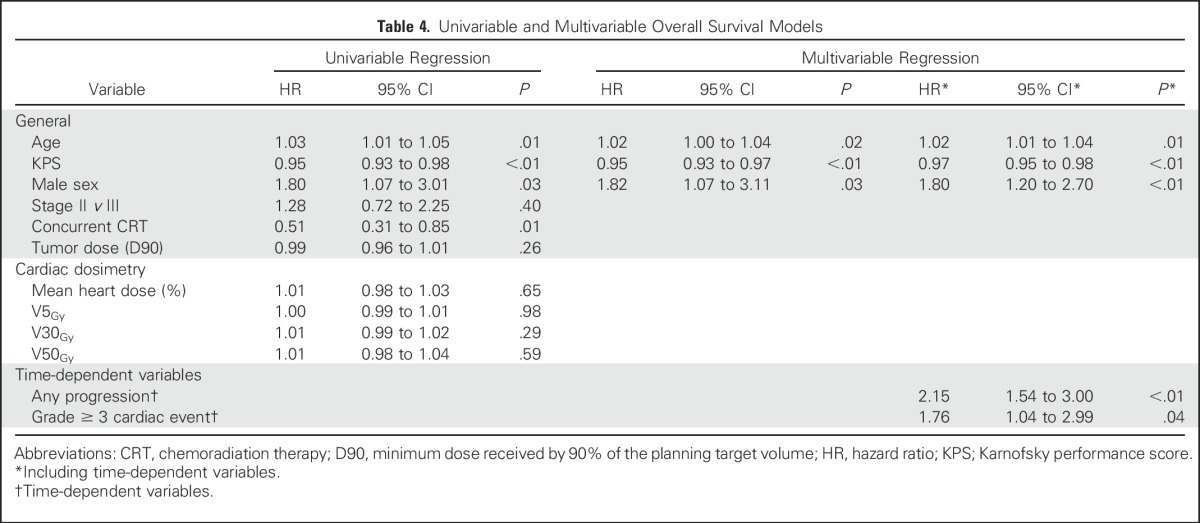
The median OS and PFS were 23.1 months and 14.6 months, respectively (Appendix Fig A4, online only). Younger age, higher pretreatment KPS, and female sex were associated with increased OS on MVA (Table 4). Cardiac dose was not significantly associated with OS, either on univariable analysis or MVA with or without the inclusion of tumor dose. When analyzed as time-dependent variables in MVA analysis, both disease progression (HR, 2.15; 95% CI, 1.54 to 3.00; P < .01) and grade ≥ 3 cardiac events (HR, 1.76; 95% CI, 1.04 to 2.99; P = .04) were associated with increased hazard of death. Disease progression (n = 71), however, was far more common in the cohort than grade ≥ 3 cardiac events (n = 19). In contrast to grade ≥ 3 events, time-dependent grade ≥ 2 events were not significantly associated with OS on MVA (HR, 1.29; 95% CI, 0.90 to 1.84; P = .17).
Table 4.
Univariable and Multivariable Overall Survival Models
DISCUSSION
In a detailed review of patients with locally advanced NSCLC treated on four prospective radiation therapy trials, clinically significant cardiac events were common and often within the window of median expected survival for this patient population. The 24-month cumulative incidence of grade ≥ 3 cardiac events was 11% (95% CI, 5% to 16%), with events occurring at a median of 11 months after treatment (IQR, 6 to 24 months). Our event rate exceeds the baseline coronary risk of approximately 2% to 3% per year in a population with a similar Framingham risk score distribution and prevalence of pre-existing CAD. To the best of our knowledge, this represents one of the largest detailed studies performed to date of cardiac morbidity and mortality in patients with locally advanced NSCLC treated on prospective protocols with dose-escalated radiation therapy. Our data provide useful insight regarding patient- and treatment-related factors associated with increased risk of developing clinically significant cardiac events.
On MVA, pre-existing cardiac disease was significantly associated with a near three-fold increase in the likelihood of developing a grade ≥ 3 cardiac event. Pre-existing cardiac disease was common in our cohort. More than one in four patients (27%) had overt CAD, a finding consistent with other studies. Janssen-Heijnen et al22 reviewed nearly 4,000 patients in the Netherlands with lung cancer and noted that a diagnosis of concomitant cardiovascular disease was 23%, nearly twice that of the general population. In our patients with pre-existing cardiac disease, the 24-month cumulative incidence of grade ≥ 3 cardiac events after treatment was 21% (95% CI, 7% to 35%). Such a rate far exceeds thresholds for aggressive preventative treatment in the general population, which range from 7.5% to 20% risk of composite cardiac or cardiovascular events over 10 years.21,23 Thus, patients with pre-existing cardiac disease may warrant establishment of care with a cardiologist with frequent, close monitoring after treatment. Further investigation into preventive management strategies should be explored in this high-risk population. Moreover, individualized, more stringent cardiac dosimetric constraints may be needed in those with pre-existing cardiac disease, especially given our finding of the cardiac dose-effect on grade ≥ 3 cardiac events.
Independent of pretreatment cardiac status, cardiac radiation dose was significantly associated with increased risk of grade ≥ 3 cardiac events on MVA with a hazard of 1.07/Gy increase in mean heart dose. A recent retrospective series from Washington University in St. Louis24 and preliminary data from University of North Carolina prospective trials have suggested a similar cardiac dose-response relationship with cardiac events.25 In retrospect, our previous heart dose constraints were liberal; V40Gy < 100% exceeds our model-predicted thresholds for a 15% incidence of grade ≥ 3 cardiac events at 24 months. By comparison, cardiac constraints in ongoing trials such as RTOG 1308 (Comparing Photon Therapy To Proton Therapy To Treat Patients With Lung Cancer) are more stringent (eg, V30Gy < 50%), and achieving these lower targets may lead to lower cardiac event rates.
Our patients were treated almost exclusively with 3D-conformal radiation therapy. In a recent secondary analysis, the RTOG 0617 group reported that patients treated with IMRT had significantly lower cardiac doses compared with those treated with 3D-conformal treatment (eg, V40Gy, 6.8% v 11.4%; P < .01) and that V40Gy was inversely associated with OS on adjusted analysis.26 It is therefore possible that IMRT may be an attractive treatment option for reducing risk of cardiac events. Proton therapy may also be an attractive treatment option because cardiac dosimetry benefits have previously been demonstrated.27 It is important to note that in addition to mean heart dose, V5Gy and V30Gy were essentially equally valid parameters on the basis of the C-indices, and clinicians should focus on these metrics as well.
It is increasingly evident that radiation-induced cardiac mortality has an earlier time course than previously appreciated.28,29 We now understand that dose-escalated radiation therapy can have a detrimental effect on survival in locally advanced NSCLC,8 especially in the context of concurrent chemotherapy.30 In our cohort, time-dependent MVA suggests that grade ≥ 3 cardiac events negatively impact survival (HR, 1.76) after accounting for age, KPS, sex, and disease progression. We provide several plausible explanations by which an increased yet under-reported cardiac event rate on the 74 Gy arm of RTOG 0617 may have led to a decreased OS. A lack of stratification by pre-existing cardiac disease may have led to treatment arm imbalances, thus negatively impacting survival. Moreover, higher heart dose on the dose-escalated arm may have directly increased the cardiac event rate and contributed to the inferior results. Of note, similar to RTOG 0617, certain cardiac dose metrics increased hazard of death in our series, but the association was not statistically significant (eg, V30Gy; HR,1.01; P = .29) likely a result of the limited power of our study.
Our study has several other limitations. It is possible that not all cardiac events were completely captured. However, all patients were enrolled on prospective clinical trials with regularly scheduled follow-up. In addition, 75 of 125 patients were treated in the Veterans Affairs Health System which offers comprehensive medical care, thus increasing the likelihood of reliably capturing cardiac events. We acknowledge that the incidence of cardiac events will likely continue to rise with further follow-up. We also recognize that others have estimated a slightly higher α/β ratio for heart than 2.5.31 Using an α/β of 3.5 does not change our associations, but does increase our reported EQD2Gy heart doses by approximately 3%. Finally, larger confirmatory studies with uniform fractionation and chemotherapy are required to confirm and broadly interpret our findings.
In conclusion, we have demonstrated that the cumulative incidence of grade ≥ 3 cardiac events exceeds 10% within 24 months among patients with locally advanced NSCLC treated with definitive radiation therapy. Pre-existing cardiac disease markedly increased the risk of post-treatment cardiac events. Moreover, higher mean heart doses, V5Gy and V30Gy, were each independently associated with an increased cardiac event rate. In light of these findings, future studies of thoracic radiation therapy should stratify on the basis of pre-existing cardiac status to enable a more meaningful analysis of cardiac events. Our results also suggest that stricter cardiac dose constraints may be necessary, especially among patients with pre-existing cardiac disease.
At our own institution, we are evaluating the use of cardiac magnetic resonance imaging to noninvasively identify early pathologic changes in cardiac function and to investigate the utility of novel imaging biomarkers. We are also evaluating potential blood biomarkers of cardiac damage or stress such as troponin and N-terminal prohormone brain natriuretic peptide.32 Finally, our ongoing adaptive definitive lung protocol incorporates more stringent cardiac dose constraints on the basis of pretreatment cardiac status to evaluate the feasibility and outcomes of personalized dose escalation. Although this study focused on grade ≥ 3 cardiac events, progression of disease was far more common in our study population. By minimizing cardiac and other toxicities, the full benefit of intensive chemoradiation can be realized.
Appendix
Fig A1.
The cardiac dose correlation (Corr.) matrix demonstrates the correlation between mean heart dose and volume receiving 5 Gy (V5Gy) to V50Gy (%). The distribution of each variable is shown on the diagonal.
Fig A2.
Cumulative incidence of grade ≥ 3 cardiac events (volume receiving 5 Gy [V5Gy] and V30Gy). Actual cumulative incidence of grade ≥ 3 cardiac events (with noncardiac death as competing risk) (A) for those greater than or less than the median V5Gy of 40% (P < .01) and (B) for those greater than or less than the median V30Gy of 12% (P < .01).
Fig A3.
Predicted probability of grade ≥ 3 cardiac events within 24 months. Model-predicted cumulative incidence of a grade ≥ 3 cardiac event within 24 months of treatment for those with and without pre-existing cardiac disease with increasing (A) V5Gy and (B) V30Gy.
Fig A4.
K-M estimated for (A) overall survival and (B) progression-free survival.
Table A1.
Detail of Prospective Trials
Table A2.
Detail of Common Terminology Criteria for Adverse Events (version 4.03) Cardiac Event Grading
Table A3.
Univariable and Multivariable Analysis for Time to Earliest Grade ≥ 2 Cardiac Event
Footnotes
Supported in part by Grants No. P01 CA059827 and R01 CA142840 from the National Institutes of Health.
Clinical trial information: NCT00603057, NCT01190527.
AUTHOR CONTRIBUTIONS
Conception and design: Robert T. Dess, Grace Sun, Matthew J. Schipper, Shruti Jolly
Financial support: Feng-Ming Kong, Randall K. Ten Haken, Theodore S. Lawrence
Provision of study materials or patients: Gregory P. Kalemkerian
Collection and assembly of data: Robert T. Dess, Yilun Sun, Martha M. Matuszak, Grace Sun, Latifa Bazzi, Shruti Jolly
Data analysis and interpretation: Robert T. Dess, Yilun Sun, Martha M. Matuszak, Payal D. Soni, Venkatesh L. Murthy, Jason W.D. Hearn, Feng-Ming Kong, Gregory P. Kalemkerian, James A. Hayman, Randall K. Ten Haken, Theodore S. Lawrence, Matthew J. Schipper, Shruti Jolly
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Robert T. Dess
No relationship to disclose
Yilun Sun
No relationship to disclose
Martha M. Matuszak
Research Funding: Varian Medical Systems
Grace Sun
No relationship to disclose
Payal D. Soni
No relationship to disclose
Latifa Bazzi
No relationship to disclose
Venkatesh L. Murthy
Stock or Other Ownership: AbbVie, Abbott Laboratories, Baxter, Becton Dickinson, Biogen Idec, Bristol-Myers Squibb, Cardinal Health, DaVita, Depomed, Express Scripts, Johnson & Johnson, McKesson, Medtronic, Merck, Pfizer, Roche, Zimmer-Biomet
Honoraria: Bracco Diagnostic, Ionetix
Consulting or Advisory Role: Bracco Diagnostic, Ionetix
Speakers’ Bureau: Bracco Diagnostic
Research Funding: INVIA Medical Imaging Solutions
Travel, Accommodations, Expenses: Ionetix
Jason W. D. Hearn
No relationship to disclose
Feng-Ming Kong
Honoraria: Varian Medical Systems
Speakers’ Bureau: Varian Medical Systems
Research Funding: Varian Medical Systems
Travel, Accommodations, Expenses: Varian Medical Systems
Gregory P. Kalemkerian
Research Funding: Merck, OncoMed Pharmaceuticals, Pfizer, Astex Pharmaceuticals, GlaxoSmithKline, Millennium Pharmaceuticals
James A. Hayman
No relationship to disclose
Randall K. Ten Haken
Travel, Accommodations, Expenses: Varian Medical Systems
Theodore S. Lawrence
No relationship to disclose
Matthew J. Schipper
Consulting or Advisory Role: Armune BioScience, Hygieia Sciences
Shruti Jolly
No relationship to disclose
REFERENCES
- 1.Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung: Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980;45:2744–2753. doi: 10.1002/1097-0142(19800601)45:11<2744::aid-cncr2820451108>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Aupérin A, Le Péchoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 3.Machtay M, Bae K, Movsas B, et al: Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: An analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 82:425-434, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martel MK, Ten Haken RK, Hazuka MB, et al. Estimation of tumor control probability model parameters from 3-D dose distributions of non-small cell lung cancer patients. Lung Cancer. 1999;24:31–37. doi: 10.1016/s0169-5002(99)00019-7. [DOI] [PubMed] [Google Scholar]
- 5.Bradley JD, Moughan J, Graham MV, et al: A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: Phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys 77:367-372, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stinchcombe TE, Lee CB, Moore DT, et al. Long-term follow-up of a phase I/II trial of dose escalating three-dimensional conformal thoracic radiation therapy with induction and concurrent carboplatin and paclitaxel in unresectable stage IIIA/B non-small cell lung cancer. J Thorac Oncol. 2008;3:1279–1285. doi: 10.1097/JTO.0b013e31818b1971. [DOI] [PubMed] [Google Scholar]
- 7.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 8.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faivre-Finn C. Dose escalation in lung cancer: Have we gone full circle? Lancet Oncol. 2015;16:125–127. doi: 10.1016/S1470-2045(15)70001-X. [DOI] [PubMed] [Google Scholar]
- 10.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 11.Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 12.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 13.Hancock SL, Donaldson SS, Hoppe RT. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol. 1993;11:1208–1215. doi: 10.1200/JCO.1993.11.7.1208. [DOI] [PubMed] [Google Scholar]
- 14.RTOG Foundation, Clinical Trials, Protocol Table, Study Details: RTOG 1106/ACRIN 6697, Randomized phase II trial of individualized adaptive radiotherapy using during treatment FDG-PET/CT and modern technology in locally advanced non-small lung cancer (NSCLC). 2016 [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Taylor CW, Nisbet A, McGale P, et al: Cardiac exposures in breast cancer radiotherapy: 1950s-1990s. Int J Radiat Oncol Biol Phys 69:1484-1495, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Gillette EL, McChesney SL, Hoopes PJ: Isoeffect curves for radiation-induced cardiomyopathy in the dog. Int J Radiat Oncol Biol Phys 11:2091-2097, 1985 [DOI] [PubMed] [Google Scholar]
- 18.Stewart JR, Fajardo LF, Gillette SM, et al: Radiation injury to the heart. Int J Radiat Oncol Biol Phys 31:1205-1211, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Cosset JM, Henry-Amar M, Girinski T, et al. Late toxicity of radiotherapy in Hodgkin’s disease: The role of fraction size. Acta Oncol. 1988;27:123–129. doi: 10.3109/02841868809090332. [DOI] [PubMed] [Google Scholar]
- 20.Feng M, Moran JM, Koelling T, et al: Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 79:10-18, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 285:2486-2497, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Janssen-Heijnen ML, Schipper RM, Razenberg PP, et al. Prevalence of co-morbidity in lung cancer patients and its relationship with treatment: A population-based study. Lung Cancer. 1998;21:105–113. doi: 10.1016/s0169-5002(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 23.Stone NJ, Robinson JG, Lichtenstein AH, et al: 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 63:2889-2934, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Speirs CK, DeWees TA, Rehman S, et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol. 2017;12:293–301. doi: 10.1016/j.jtho.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 25.Wang K, Eblan MJ, Zagar TM, et al: Cardiac toxicity after radiation for stage III non-small cell lung cancer: Pooled analysis of several prospective dose-escalation trials delivering 70 to 90 Gy. Int J Radiat Oncol Biol Phys 96:S130, 2016 [Google Scholar]
- 26.Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG Oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56–62. doi: 10.1200/JCO.2016.69.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang JY, Zhang X, Wang X, et al: Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 65:1087-1096, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Marks LB, Zagar TM, Kaidar-Person O: Reassessing the time course for radiation-induced cardiac mortality in patients with breast cancer. Int J Radiat Oncol Biol Phys 97:303-305, 2017 [DOI] [PubMed] [Google Scholar]
- 29.Marks LB, Yu X, Prosnitz RG, et al: The incidence and functional consequences of RT-associated cardiac perfusion defects. Int J Radiat Oncol Biol Phys 63:214-223, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Ramroth J, Cutter DJ, Darby SC, et al: Dose and fractionation in radiation therapy of curative intent for non-small cell lung cancer: Meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys 96:736-747, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schultz-Hector S, Sund M, Thames HD. Fractionation response and repair kinetics of radiation-induced heart failure in the rat. Radiother Oncol. 1992;23:33–40. doi: 10.1016/0167-8140(92)90303-c. [DOI] [PubMed] [Google Scholar]
- 32.Gomez DR, Yusuf SW, Munsell MF, et al. Prospective exploratory analysis of cardiac biomarkers and electrocardiogram abnormalities in patients receiving thoracic radiation therapy with high-dose heart exposure. J Thorac Oncol. 2014;9:1554–1560. doi: 10.1097/JTO.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]