Abstract
Bronchopulmonary dysplasia (BPD) is a major cause of morbidity in premature infants receiving oxygen therapy. Currently, sildenafil is being examined clinically to improve pulmonary function in patients with BPD. Based on the pharmacological action of sildenafil, the elevation of cyclic guanosine 3′,5′-monophosphate (cGMP) in lung tissue is considered to underlie its beneficial effects, but this mechanism is not understood at the molecular level. Here, we examined the possibility that sildenafil helps the pulmonary system adapt to hyperoxic stress. To induce BPD, fetal rats were exposed to LPS before delivery, and neonates were exposed to hyperoxia, followed by intraperitoneal injections of sildenafil. Alveolarization was impaired in rats exposed to hyperoxia, and alveolarization significantly recovered with sildenafil. An immunohistochemical examination revealed that sildenafil effectively increased vascular distribution in lung tissue. Furthermore, the oxygen sensor hypoxia-inducible factor (HIF)–1/2α and the angiogenic factor vascular endothelial growth factor (VEGF) were highly expressed in the lungs of sildenafil-treated rats. In human small-airway epithelial cells, HIF-1/2α and its downstream genes, including VEGF, were confirmed to be induced by sildenafil at both the protein and mRNA levels. Mechanistically, cGMP in airway cells accumulated after sildenafil treatment because of interfering phosphodiesterase Type 5, and subsequently cGMP activated HIF-mediated hypoxic signaling by stimulating the phosphoinositide 3-kinase (PI3K)–v-akt murine thymoma viral oncogene homolog 1 (AKT)–mammalian target of rapamycin (mTOR) pathway. This study provides a better understanding about the mode of action for sildenafil, and suggests that HIF can be a potential target for treating patients with BPD.
Keywords: BPD, sildenafil, AKT/mTOR pathway, HIF-1α, VEGF
Clinical Relevance
Sildenafil has therapeutic potential in bronchopulmonary dysplasia (BPD), and this effect is likely attributable to the activation of the hypoxia-inducible factor (HIF) signaling pathway. We also suggest that the activation of HIF offers a possible strategy for the treatment or prevention of BPD.
Despite advancements in perinatal care, bronchopulmonary dysplasia (BPD) remains the major cause of morbidity in premature babies (1). BPD lungs exhibit large alveoli with reduced septation and poor vascularity, sometimes in association with pulmonary hypertension (2, 3). Hyperoxic therapy and mechanical ventilation are required to supply oxygen to premature babies with respiratory failure. However, these therapeutic modalities paradoxically impair postnatal lung development, and aggravate BPD symptoms (4, 5). Lung inflammation is also considered a causative factor in BPD because prenatal chorioamnionitis and postnatal respiratory tract infections are associated with disease progress (6–8). However, the mechanism underlying the impaired alveolarization and vascularization in BPD remains to be clarified.
Vascular endothelial growth factor (VEGF) is highly expressed in alveolar septa, where it mediates airway–vascular coupling reactions (9). VEGF not only mediates neovascularization, but also promotes alveolar cell proliferation (10). VEGF thereby plays critical roles in lung morphogenesis toward a normal ventilation–perfusion match (9, 11), and its deregulation leads to impaired lung development. VEGF has been reported to be down-regulated in the lungs of patients with BPD (12), and intratracheal VEGF gene therapy was found to improve alveolarization in rat lungs with BPD (13–15).
Nitric oxide (NO) is also required for lung development (16). NO per se mediates neovascularization and also enforces the angiogenic action of VEGF in lung tissue (17). Moreover, the inhalation of NO gas reduces the risk of BPD in premature infants with respiratory insufficiency, and NO-producing medications have been developed for BPD therapy (17, 18). In particular, sildenafil was developed to mimic the pharmacological action of NO by increasing intracellular cyclic guanosine 3′,5′-monophosphate (cGMP) concentrations. Ladha and colleagues (19) demonstrated that sildenafil improves alveolar growth and ameliorates BPD-associated pulmonary hypertension in rats with BPD, and sildenafil was also reported to ameliorate BPD symptoms by augmenting the neovascularization mediated by VEGF and angiopoietin-1 (20). Currently, sildenafil is undergoing clinical trials as a BPD therapeutic regimen (21, 22). However, the mechanism underlying the effects of sildenafil remains to be investigated.
The production of VEGF and NO is regulated by hypoxia-inducible factor (HIF), and recently HIF was suggested to participate in normal lung development. It is down-regulated in the lungs of primates with BPD (23–25). HIF is a transcription factor that orchestrates the expressions of several hundred genes essential for cellular adaptation to hypoxia (26). It helps cells survive under hypoxic conditions, and facilitates the supply of more blood to hypoxic areas by expressing VEGF and NOS (27). HIF has three isoforms, namely, HIF-1α, HIF-2α, and HIF-3α. HIF-1α and HIF-2α act as transcription factors, whereas HIF-3α does not. HIFs are composed of oxygen-dependent α-subunits and a common β-subunit, which is termed the aryl-hydrocarbon receptor nuclear translocator (ARNT). HIF-1 and HIF-2 possess different α-subunits (HIF-1α and HIF-2α, respectively). The α-subunits are hydroxylated at their proline resides by prolyl-hydroxylase (PHD), which also recruits von Hippel-Lindau protein, and leads to the ubiquitination and proteasomal degradation of α-subunits (28–30). Under hypoxic conditions, oxygen-dependent hydroxylation is blocked, and HIF-αs become stable and active.
In the present study, we addressed two questions: (1) Does sildenafil improve lung development in a BPD animal model, and (2) Does HIF mediate the effect of sildenafil? This study provides a better understanding about the mode of action for sildenafil, and suggests that HIF may be a potential target for treating patients with BPD.
Materials and Methods
Cell Culture
Primary human small airway epithelial cells (HSAEp-C) and cancer cells originating from human alveolar epithelium (A549) were purchased from Promo Cell (Heidelberg, Germany) and the American Type Culture Collection (Manassas, VA), respectively. HSAEp-C and A549 cells were cultured in an epithelial medium provided by the supplier and in RPMI-1640, respectively, with 10% heat-inactivated FBS. Cells were kept in a 5% CO2 humidified atmosphere at 37°C. Agents were administered to the medium 1 hour before normoxic (20% oxygen) or hyperoxic (85% oxygen) incubation.
RT-PCR
Total RNAs were isolated from cells using Trizol (Invitrogen, Carlsbad, CA). Semiquantitative and quantitative RT-PCR was preformed to analyze mRNA concentrations, as previously described (31). Detailed methods are described in the online supplement, and primer sequences are summarized in Tables E1 and E2 in the online supplement.
Western Blotting
Cells were lysed in an SDS sample buffer, and the lysates were denatured at 95°C for 10 minutes. Proteins were separated on SDS/polyacrylamide gels and transferred to Immunobilon-P membranes (Millipore, Bedford, MA). The membranes were sequentially incubated with primary and secondary antibodies, and the immune complexes were visualized by chemiluminescence (please refer to the online supplement).
Immunohistochemistry
Paraffin-embedded lung sections (4 μm) were deparaffinized and sequentially incubated with a primary antibody against HIF-1/2α or CD31 and a biotin-conjugated secondary antibody. Immune complexes were visualized using streptavidin–Alexa Fluor 488 fluorescence probes for HIF-1/2α, or using diaminobenzidine for CD31. Nuclei were stained with 4′,6-diamidino-2-phenylindole (please refer to the online supplement for further details).
cGMP Assay
Concentrations of cGMP were measured using an immunoassay kit provided by Amersham Pharmacia Biotech (Piscataway, NJ). Concentrations of cGMP were determined using standard solutions (please refer to the online supplement for details).
Rat BPD Model
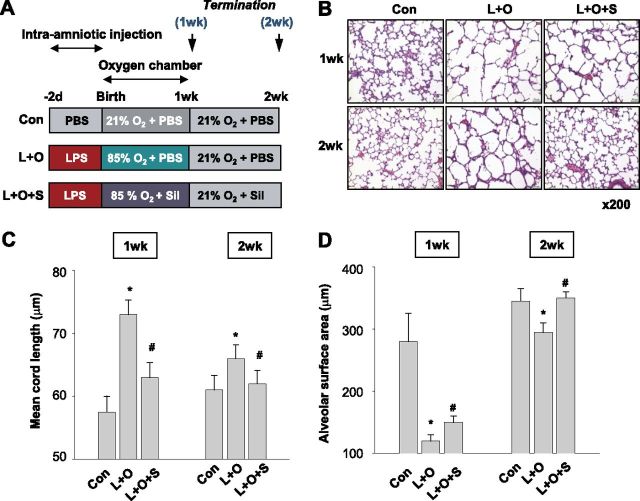
Rat pups were spontaneously delivered 2 to 2.5 days after an intra-amniotic injection of LPS (0.5 μg) or PBS. Pups were randomly divided into three groups: the control group, receiving a saline injection and room air exposure; the L + O group, receiving an LPS injection and 85% oxygen exposure; and the L + O + S group, receiving an LPS injection, 85% oxygen exposure, and sildenafil treatment (100 μg/g daily, intraperitoneal). After exposure to hyperoxia for 1 week, pups were killed immediately (1 wk) or 1 week later (2 wk). Control pups were kept continuously in room air (please refer to the online supplement). The experimental design is summarized in Figure 1A. The experimental protocol was approved by the Animal Care and Use Committee of Seoul National University Bundang Hospital.
Figure 1.
Sildenafil promotes the alveolarization of lungs with bronchopulmonary dysplasia (BPD) induced by LPS pretreatment and hyperoxic respiration. (A) Experimental design for inducing bronchopulmonary dysplasia in neonatal rats. Rats were subjected to the indicated conditions, and killed 2 or 3 weeks after birth. Five rats were allocated for each experiment. (B) Microscopic examination of neonatal rat lung tissues stained with hematoxylin and eosin. Neonatal rats were subjected to normal (Con), LPS-pretreated hyperoxic (L + O), or LPS-pretreated, sildenafil-treated hyperoxic (L + O + S) conditions for 1 week, or were allowed to recover in room air for 1 week. (C and D) Mean cord lengths and alveolar surface areas were analyzed in neonatal rat lungs subjected to the indicated conditions. Both parameters were analyzed in photographs of hematoxylin and eosin–stained tissues by means of morphometric analysis. The values of mean cord length and alveolar surface area are presented as the means ± SDs of five experiments. The Mann-Whitney U-test was used for the analysis, and all comparisons were two-sided. Sil, sildenafil. *P < 0.05, versus the control group. #P < 0.05, versus the L + O group.
Lung Morphometry
Morphometric examinations were performed in six fields of two distal lung sections for each pup. Photographs were analyzed as previously described (32). All measurements were performed by a single observer. Tissue volume densities (VDT) were determined, using a 10 × 10 grid (29-μm side length). Mean cord length (Lm) provided an estimate of the distance from one airspace wall to an adjacent airspace wall, and was determined by counting airspace walls using an 84-line array (24-μm side length). Alveolar surface area (SA) was calculated according to the formula SA = 4 × VDT × lung volume/Lm (please refer to the online supplement).
Statistical Analysis
Measured values are expressed as means ± SDs, and one-way ANOVA was used for comparisons. Post hoc analysis was performed according to least significant differences, and P < 0.05 was considered statistically significant.
Results
Sildenafil Enhances Alveolarization of Neonatal Lungs in BPD Rats
To determine the pharmacological effects of sildenafil in rats with BPD, sildenafil was cotreated to neonatal rats with lung injury induced by LPS/O2 treatment, as shown in Figure 1A. Consequently, LPS/O2 treatment disturbed alveolarization in the lungs, and sildenafil recovered this impaired alveolarization (Figure 1B). Alveolarization was morphometrically evaluated by measuring alveolar cord lengths and surface areas. Hematoxylin and eosin–stained sections were randomly photographed, and transparent gridded sheets were overlaid on pictures. Mean cord length increased by 28% or 8% in neonatal rats treated with LPS/O2 for 1 or 2 weeks, respectively, versus control rats (Figure 1C). Likewise, alveolar surface area decreased by 61% or 14% in neonatal rats treated with LPS/O2 for 1 or 2 weeks, respectively (Figure 1D). Interestingly, sildenafil normalized both parameters for alveolarization.
Sildenafil Promotes Vascular Formation and HIF-1/2α Expression in BPD Rats
Given that airway development is coupled with vessel formation, we checked lung vascularity by immunostaining vascular endothelia with anti-CD31. Many vessels were present in control lungs at 1 week after birth, and these structurally matured 1 week later (Figure 2A). In contrast, vessels in the L + O group not only decreased in number, but appeared structurally immature. In sildenafil-treated rats, the vascular numbers and structure in lungs recovered. The CD31 concentrations in lung tissues were rechecked by Western blotting, and the results further supported our immunohistochemical results (Figure 2B). Because VEGF determines vessel formation during lung development, we also analyzed VEGF concentrations in neonatal lungs. VEGF was found to be down-regulated in the L + O group, and up-regulated by sildenafil (Figure 2B), suggesting that VEGF-mediated angiogenesis may underlie sildenafil-induced alveolarization. Based on its molecular weight (∼ 25 kD) and the information from previous literature (33), the detected protein was assumed to be VEGF-A188. Because HIF-1α and HIF-2α both function to stimulate angiogenesis by inducing VEGF, we analyzed the expression of HIF-1α and HIF-2α in rat lungs via immunofluorescence. Interestingly, both HIF-αs were expressed in the nuclei of alveolar cells in neonatal lung tissues, but not in adult lung tissues (Figure E1). In neonatal lungs, the numbers of HIF-1/2α-positive cells were substantially reduced by LPS/O2 treatment, and these were recovered by sildenafil (Figure 2C). To confirm HIF-1/2α expression, we analyzed concentrations according to Western blotting, and found HIF-1/2α to be induced by sildenafil (Figure 2B). All these effects of sildenafil were more noticeable in the 2-week group. These results encouraged us to hypothesize that sildenafil ameliorates BPD via the sildenafil–HIF–VEGF axis.
Figure 2.
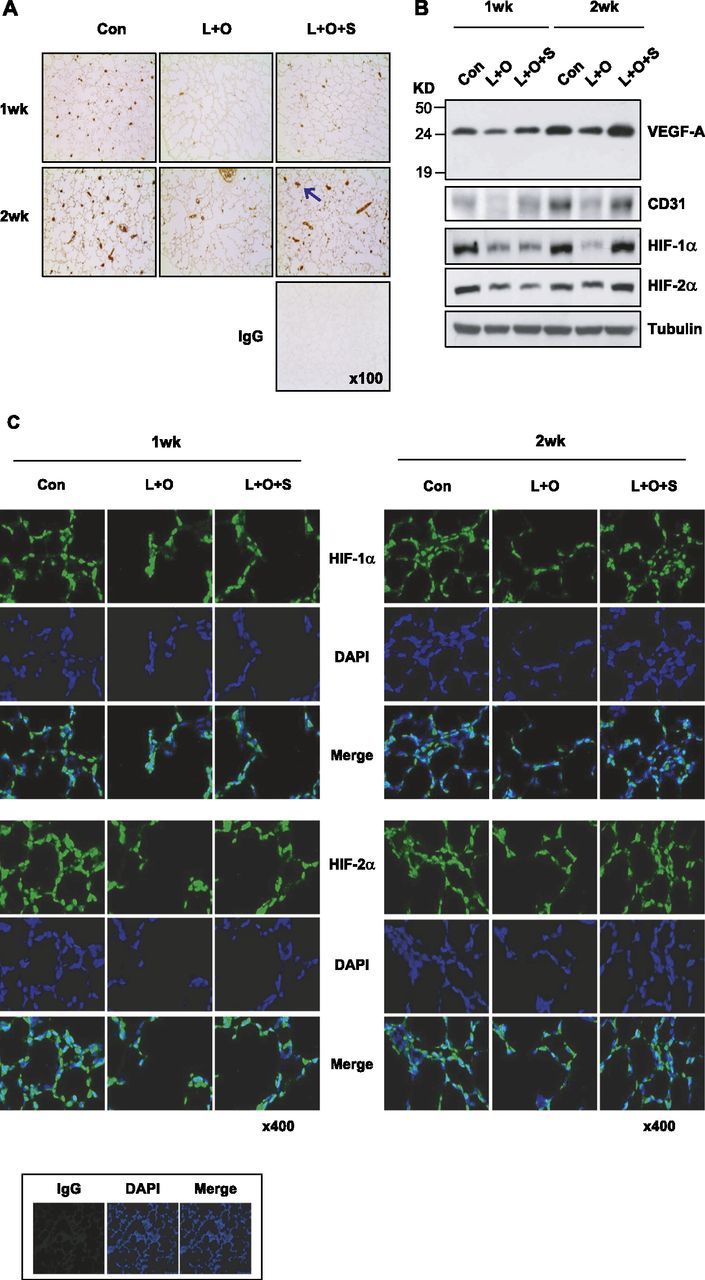
Sildenafil recovers angiogenesis and hypoxia-inducible factor (HIF)–1/2α expression in the lungs of LPS/hyperoxia-treated neonatal rats. (A) Vessels in lung tissue were immunostained with anti-CD31 antibody. An arrow indicates one of the CD31-positive vessels. Nonimmunized rabbit serum was used as a nonspecific IgG control. (B) Vascular endothelial growth factor (VEGF), CD31, and HIF-1/2α expression in neonatal rat lungs. Lung-tissue homogenates (20 μg protein per lane) were subjected to Western blotting with specific antibodies, and β-tubulin was used as a loading control. (C) Immunofluorescent staining of HIF-1/2α in lung tissues. Tissue sections were incubated with anti–HIF-1α or anti–HIF-2α antibody, and immune complexes were visualized using Alexa Fluor 488 green. 4′,6-Diamidino-2-phenylindole was used to stain nuclei, and nonimmunized rabbit serum was used as a nonspecific IgG control.
Sildenafil Up-Regulates HIF Target Genes in Lung Epithelial Cells
To understand how sildenafil recovers HIF-1/2α expression in neonatal lungs, we examined whether sildenafil stimulates the expression of HIF-1/2α in pulmonary cells. When human small airway epithelial (HSAEp) cells were treated with sildenafil, HIF-1/2α proteins were expressed in a dose-dependent manner, even under normoxia (Figure 3A). We next examined time courses, and found that HIF-1/2α proteins began to be induced 8–12 hours after sildenafil treatment in both HSAEp and A549 (a cancer cell line originating from alveolar epithelium) cells (Figure 3B). However, sildenafil did not affect the expression of ARNT, which dimerizes with HIF-1/2α. To evaluate whether the sildenafil-induced HIF-1/2αs are transcriptionally active, concentrations of the mRNAs of VEGF and other HIF target genes (pyruvate dehydrogenase kinase, isozyme 1 and carbonic anhydrase 9) were assayed by semiquantitative RT-PCR. All mRNAs were found to be induced time-dependently by sildenafil (Figure 3C). However, the kinetic change of HIF-1α mRNA was obscure because of PCR overcycling. We rechecked mRNA concentrations using quantitative RT-PCR, and confirmed that sildenafil enhances the transcriptions of HIF target genes. In addition, the concentration of HIF-1α mRNA was found to be increased by sildenafil (Figure 3D). To know which of the two HIF-α isoforms is responsible for sildenafil-induced VEGF expression, we knocked down each isoform in HSAEp cells. As a result, the induction of VEGF was attenuated by silencing either HIF-1α or HIF-2α (Figure 3E), which indicates that HIF-αs are likely to contribute to the induction of VEGF by sildenafil. Based on these results, sildenafil is suggested to activate the HIF signaling pathway by up-regulating HIF-1/2αs at the posttranscriptional level.
Figure 3.
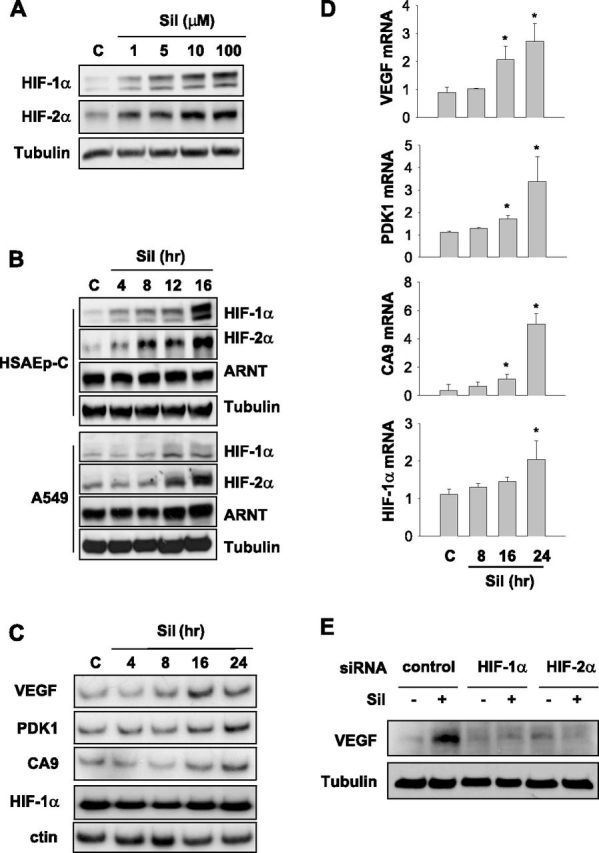
Sildenafil increases HIF-1/2 signaling pathways in human small airway epithelial cells. (A) Sildenafil up-regulated HIF-1α and HIF-2α in a dose-dependent manner. Human small airway epithelial (HSAEp-C) cells were incubated with the indicated concentrations of sildenafil for 16 hours, and the expression of HIF-1/2α was evaluated by Western blotting. (B) Sildenafil up-regulated HIF-1/2α proteins in a time-dependent manner. HSAEp-C cells or cancer cells originating from human alveolar epithelium (A549) were incubated with 100 μM sildenafil for the indicated times. The expressions of HIF-1/2α or aryl hydrocarbon receptor nuclear translocator (ARNT) were analyzed by Western blotting. (C) Semiquantitative RT-PCR of HIF-target gene transcripts in HSAEp-C cells. Cells were incubated with 100 μM sildenafil for the indicated times, and total RNAs were extracted. The mRNA concentrations of VEGF, pyruvate dehydrogenase kinase (PDK), carbonic anhydrase 9 (CA9), and HIF-1α were analyzed by RT-PCR with deoxycytidine 5′-[α-32P] triphosphate and autoradiographed. The result is representative of three experiments. (D) Quantitative RT-PCR of HIF-target gene transcripts in HSAEp-C cells. The mRNAs of VEGF, PDK, CA9, and HIF-1α were reverse-transcribed, and the complementary DNAs were applied to the real-time PCR system. An 18S RNA signal was used to normalize the signal of each HIF-target transcript. *P < 0.05 versus control samples (n = 4). (E) HSAEp-C cells were transfected with the small interfering (si)RNA targeting HIF-1α or HIF-2α, and then incubated with 100 μM sildenafil for 24 hours. Total cell lysates were subjected to Western blotting to analyze the indicated protein concentrations.
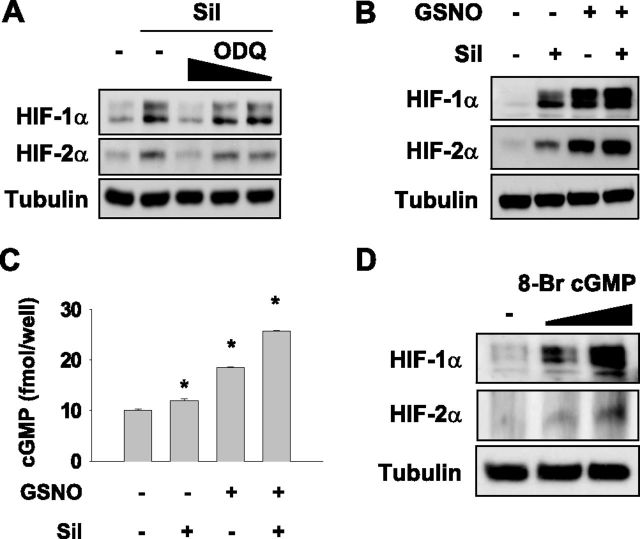
Sildenafil Induces HIF-1/2α Expression by Increasing cGMP
Given that sildenafil increases intracellular cGMP by inhibiting phosphodiesterase-5, we examined whether cGMP mediates the sildenafil induction of HIF-1/2α. When cGMP production was blocked by 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ) (an inhibitor of soluble guanylyl cyclase generating cGMP), the sildenafil-induced HIF-1/2α expression was abolished in HSAEp cells (Figure 4A). When we increased cGMP using S-nitrosoglutathione (GSNO, a NO-releasing substance), GSNO alone obviously induced HIF-1/2αs, and in combination with sildenafil further increased their concentrations (Figure 4B). Concomitantly, intracellular cGMP concentrations were elevated by sildenafil or GSNO, and more so by their combination (Figure 4C). To confirm that cGMP regulates HIF-1/2α expression, we administered 8-bromo-cGMP (a membrane-permeable cGMP analogue) to HSAEp cells, and found that 8-bromo-cGMP was sufficient to induce HIF-1/2α (Figure 4D). Taken together, these results suggest that sildenafil stimulates the expression of HIF-1/2α via the NO/cGMP signaling pathway.
Figure 4.
The cyclic guanosine 3′,5′-monophosphate (cGMP) pathway is involved in the sildenafil-induced expression of HIF-1/2α. (A) HSAEp-C cells were cotreated with 100 μM sildenafil and 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ) (10, 20, or 50 μM) for 16 hours, and HIF-1/2α expression was then analyzed by Western blotting. (B) HSAEp-C cells were treated with 100 μM sildenafil and/or 100 nM S-nitrosoglutathione (GSNO) for 8 hours, and HIF-1/2α expression was analyzed by Western blotting. (C) HSAEp-C cells were seeded in 12-well plates at 106 cells/ml, and treated with 100 μM sildenafil and/or 200 μM GSNO for 16 hours. Cells were lysed to assay intracellular cGMP concentrations. (D) HSAEp-C cells were treated with 0.5 or 1 μM 8-Br-cGMP for 16 hours, and HIF-1/2α expression was analyzed by Western blotting.
Sildenafil Stimulates the Synthesis of HIF-1/2α Proteins
To understand how sildenafil regulates HIF-1/2α expression, we first checked whether the degradation of HIF-1/2α was affected by sildenafil. For this purpose, we stabilized HIF-1/2α proteins under hypoxia, and then initiated their degradation by reoxygenation with 20% oxygen. Both HIF-1/2α proteins disappeared within 15 minutes after oxygenation, regardless of sildenafil treatment (Figure 5A). The half-lives of HIF-1/2α proteins were estimated using protein band intensities, but no differences were observed between the control and sildenafil-treated groups (Figure 5B). Next, we checked de novo syntheses of HIF-1/2α proteins. After removing remnants of HIF-1/2α using cycloheximide, we initiated the de novo synthesis of HIF-1/2α proteins by sequentially washing out cycloheximide and adding N-(benzyloxycarbonyl)leucinylleucinylleucinal (MG132) (an HIF-α stabilizer). HIF-1/2α proteins were found to be expressed earlier and at higher concentrations in sildenafil-treated cells than in control cells (Figure 5C). Protein concentrations were densitometrically analyzed (Figure 5D). These results indicate that sildenafil induces HIF-1/2α proteins by facilitating their synthesis. To examine whether sildenafil induces the accumulation of HIF-1/2α in nuclei, HSAEp cells were subjected to the indicated conditions and prepared for the extraction of nuclear proteins. Nuclear HIF-1/2α proteins were accumulated by MG132, and their concentrations were higher in the presence of sildenafil. However, LPS exerted no direct effect on protein concentrations. To mimic the conditions of in vivo hyperoxia, we incubated cells in a hyperoxic chamber. The sildenafil-induced expression of nuclear HIF-1/2α proteins was not significantly affected by LPS or ambient oxygen tension (Figure 5E). Moreover, the expressions of HIF-1α and its target mRNAs were not augmented by LPS, and sildenafil was able to induce them even under hyperoxic conditions (Figure E2).
Figure 5.
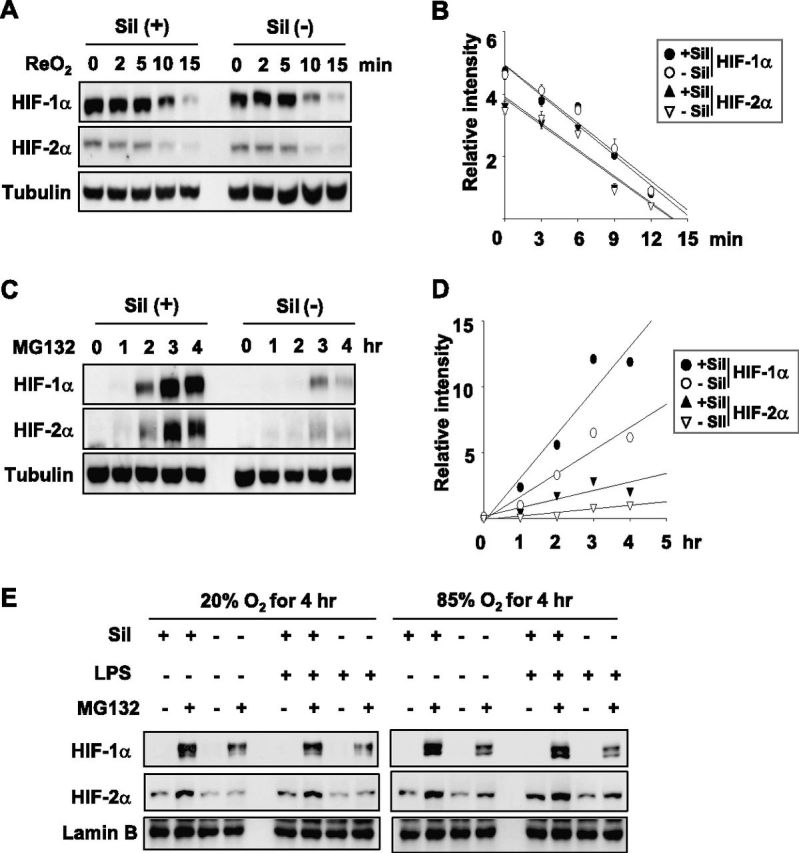
Sildenafil induces HIF-1/2α at the translational level. (A) Sildenafil did not affect the stability of HIF-1/2α. HSAEp-C cells were incubated during a hypoxic condition (1% oxygen) for 8 hours with or without 100 μM of sildenafil, and then incubated under a normoxic condition (20% oxygen) for the indicated times. HIF-1/2α protein concentrations were analyzed by Western blotting, and tubulin was used as a loading control. (B) Band intensities of HIF-1/2α in A were quantified using the ImageJ program and plotted. Half-lives of HIF-1/2α were calculated from first-order decay curves. Points represent the means ± SDs of three experiments. (C) Sildenafil increased the synthesis of HIF-1/2α at the translational level. HSAEp-C cells were treated with 100 μM cycloheximide for 1 hour. After washing, cells were further treated with N-(benzyloxycarbonyl)leucinylleucinylleucinal (MG132) (10 μM) only or MG132 (10 μM) + sildenafil (100 μM) for the indicated times. Protein concentrations of HIF-1/2α or tubulin were analyzed by immunoblotting. (D) Band intensities of HIF-1/2α in C were quantified using the ImageJ program (http://rsbweb.nih.gov). Points represent the means ± SDs of three experiments. (E) HSAEp-C cells were treated with sildenafil (100 μM), LPS (100 ng/ml), and/or MG132 (10 μM) for 4 hours at 20% or 85% oxygen. Nuclear proteins were loaded on SDS/PAGE gels and blotted with antibodies against the indicated proteins. Lamin B was used as a loading control for nuclear proteins.
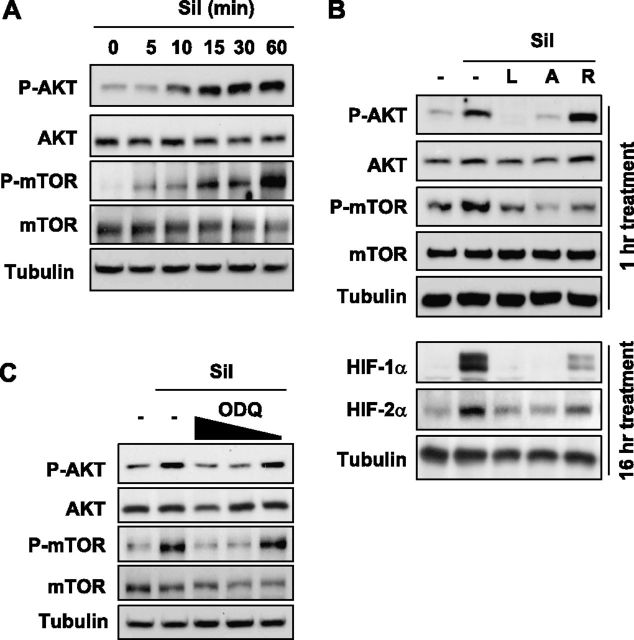
Sildenafil Activated the PI3K/AKT/mTOR Signaling Pathway
Basically, protein synthesis is determined by two distinct factors: the amount of mRNA, and the translational rate. Because the HIF-1α mRNA concentration was already found to be increased by sildenafil, we checked whether sildenafil stimulates the translational process for HIF-α proteins. PI3K was shown to phosphorylate AKT, phospho-AKT phosphorylates mTOR, and finally phospho-mTOR initiates the translation of HIF-1α protein (34, 35). Therefore, we tested the possible involvement of the PI3K/AKT/mTOR signaling pathway in the sildenafil-induced synthesis of HIF-1/2α proteins. To determine the time required for sildenafil to activate AKT, we treated HSAEp cells with sildenafil for different times. Cellular concentrations of phospho-AKT and phospho-mTOR were found to increase time-dependently in the presence of sildenafil (Figure 6A). Based on the results, we decided to fix the incubation time at 1 hour in the subsequent experiment. Furthermore, the sildenafil-induced AKT phosphorylation was blocked by LY294002 (a PI3K inhibitor) and AktIV (an AKT inhibitor), and the mTOR phosphorylation was blocked by these inhibitors and by rapamycin (an mTOR inhibitor). This result verifies that sildenafil activates the PI3K/AKT/mTOR signaling pathway. When each component of the PI3K/AKT/mTOR pathway was inhibited, the expression of HIF-1/2α was also attenuated (Figure 6B), which indicates that sildenafil facilitates the synthesis of HIF-1/2α proteins through the PI3K/AKT/mTOR pathway. Moreover, activation of the PI3K/AKT/mTOR pathway by sildenafil was blocked by ODQ (Figure 6C). Therefore, the activation by sildenafil of the PI3K/AKT/mTOR pathway is likely to be mediated by increased cGMP.
Figure 6.
Sildenafil-dependent induction of HIF-1/2α was Akt/mTOR signaling–dependent. (A) Sildenafil activated the AKT/mTOR signaling pathway. HSAEp-C cells were incubated with 100 μM sildenafil for the indicated times, and then harvested to determine protein concentrations by Western blotting. (B) HSAEp-C cells were incubated with 100 μM sildenafil in the absence or presence of various inhibitors for the indicated times C, control; L, 50 μM LY 294002; A, 10 μM AKTIV; R, 1 μM rapamycin; P, 50 μM PD98059. Protein concentrations of phospho-AKT (P-AKT), AKT, phospho-mTOR (P-mTOR), and mTOR (above) or HIF-1/2α proteins (below) were evaluated by Western blotting. (C) HSAEp-C cells were incubated with sildenafil or sildenafil + ODQ. Cells were preincubated with ODQ (50, 20, and 10 μM) for 2 hours, and then further incubated with 100 μM sildenafil for 1 hour. Protein concentrations were evaluated by Western blotting.
Discussion
This study shows that sildenafil promotes the postnatal development of the alveolar and vascular systems in rats with BPD, and that these effects are associated with the inductions of HIF-1/2α and VEGF. In alveolar epithelial cells, sildenafil activates the NO/cGMP signaling pathway, which activates the PI3K/AKT/mTOR pathway, which facilitates the de novo synthesis of HIF-1/2α proteins, which induces VEGF, which is responsible for angiogenesis and alveolarization. Portions of this sildenafil action and HIF-1/2α regulation have been investigated elsewhere. However, to the best of our knowledge, few studies have been performed to demonstrate the mode of action for sildenafil in terms of BPD therapy. We believe that this study provides a basis for the development of new therapeutic modalities in the treatment of BPD.
Preterm infants delivered before 30 weeks of gestation run a high risk of BPD, which involves poor alveolarization and aberrant lung microvasculature (36, 37). Intrauterine infection and amniotic inflammation are also considered high risk factors of BPD. When infants with BPD suffer from respiratory distress, an oxygen supply via mechanical ventilation is required to relieve hypoxic symptoms. Paradoxically, oxygen therapy during the perinatal period increases the risk of BPD and aggravates its symptoms (38). In a previous study, we developed a BPD rat model based on an intra-amniotic LPS injection and whole-body exposure to hyperoxia, and in the present study we used this model to examine the effects of sildenafil on BPD.
Lung small airways and alveoli are codeveloped with vessels, and are coupled structurally and functionally to match the ventilation/perfusion ratio. Thus, the retardation of vascular growth during the perinatal period leads to impaired alveolarization. In fact, angiogenic molecules, such as VEGF/VEGFR2 (Flk-1), angiopoietin (Ang)-1, Ang-2, and tyrosine kinase with immunoglobulin-like and EGF-like domain 2, are up-regulated during alveolarization in mice, and remain continuously at high concentrations, even after the lungs mature (39, 40). Of these, VEGF has been most intensively investigated in relation to lung development. VEGF is secreted by small airway epithelial cells, and then targets VEGF receptors (flk-1/kinase insert domain receptor and flt-1) in endothelial cells and alveolar epithelial cells (9). Here we found that alveolarization, vascularity, and VEGF production decline in rats with BPD, and effectively recover with the use of sildenafil. Our results also support the idea of airway–vessel coupling in lung development.
A recent study demonstrated that HIF-1/2αs are deregulated in the lungs of BPD baboons by mechanical ventilation and in preterm infants with respiratory distress (41). In addition, the HIF inducers FG-4095 and dimethyloxalylgycine, both of which stabilize HIF-1/2α by inhibiting PHDs, have been reported to improve pulmonary compliance limited by BPD, and to stimulate airway branching and vessel formation (24, 42). Likewise, we found that sildenafil acts to induce HIF-1/2α in alveolar epithelial cells, and that by doing so it promotes alveolarization and vessel formation. Given our results and previous reports, HIF constitutes a compelling pharmaceutical target to improve lung function in infants with BPD.
Because HIF-1/2α proteins are rapidly degraded under aerobic conditions, their concentrations in the lung are expected to decline along with respiration. Indeed, Grover and colleagues (41) and Asikainen and colleagues (25) demonstrated that HIF-1α and VEGF concentrations in baboon and lamb lungs significantly decreased after term delivery. In contrast, HIF-2α was found to be continuously expressed in the lungs after birth. Given their differential expressions after birth, HIF-1/2α may play different roles in postnatal lung development, which remain to be understood. In the present study, we did not measure HIF-1/2α concentrations in lungs during the prenatal stage, but during the postnatal stage. Despite postnatal breathing, both HIF-αs were detected in the surface of air sacs where oxygen tension is highest in the body. However, both HIF-1/2αs disappeared in adult rat lungs. HIF-1/2αs may be regulated via a unique mechanism in neonatal lungs.
In the present study, sildenafil increased HIF-1/2α synthesis, which elevated their normoxic expression up to immunologically detectable concentrations. Furthermore, we found that the effect of sildenafil involves the PI3K/AKT/mTOR pathway. A similar scenario regarding the normoxic expression of HIF-1α was suggested by Zhong and colleagues (43), who questioned why HIF-1α protein is well expressed in prostate cancer cells during normoxia. These researchers identified a novel pathway responsible for the normoxic expression of HIF-1α, that is, the PI3K/AKT/mTOR pathway, which can enhance HIF-1α translation to a rate exceeding its rate of degradation (44, 45). Many lines of evidence suggest that this mode of HIF-1α expression is closely linked to tissue remodeling and tumorigenesis, but the results of the present study suggest for the first time, to the best of our knowledge, that the PI3K-signaling HIF axis participates in lung development. On the other hand, we note that HIF-1/2α concentrations are also determined by PHD-mediated protein degradation. Because PHDs are oxygen-dependent enzymes, their activities could increase during hyperoxia, probably nullifying the effects of sildenafil on HIF-1/2α expression. However, considering that hyperoxia induces oxidative stress, PHDs may become less active during hyperoxia because they can be inactivated by reactive oxygen species (46). Since PHD activity is differentially regulated by oxygen and reactive oxygen species, we cannot predict whether PHD activity increases or decreases during hyperoxia. At least in our experimental settings, oxygen and ROS may offset each other in PHD regulation because HIF-1/2α degradation rates were not altered by hyperoxia.
Sildenafil was originally developed to treat male erectile dysfunction (47). Sildenafil binds and inactivates PDE5, leading to an elevation of cGMP in penile smooth muscle cells. Pulmonary vascular smooth muscle cells also express PDE5, and like penile smooth muscle cells, are also relaxed by sildenafil, which is why sildenafil is currently used to relieve pulmonary arterial hypertension (48, 49). Moreover, PDE5 is also expressed in the alveolar walls of neonatal rat lungs. This report provides a biochemical rationale for the improved alveolarization in neonatal lungs by sildenafil. However, PDE5 concentrations in lungs decrease with age, and are fourfold lower in adult lungs than in neonatal lungs (50). Similarly, NO synthases (Types I and III) are also regulated age-dependently in rat lungs. NO synthase concentrations in rat lungs increase with fetal development and peak during the neonatal period, but then abruptly collapse after lung maturation (51). The age-dependent expressions of NO synthases and PDE5 provided us with a clue regarding the answer to a question posed in this study, “Why are HIF-1/2αs expressed in neonatal lungs but not in adult lungs?” Given that cGMP-generating and cGMP-removing systems are both maximally expressed in neonatal lungs, the dynamic regulation of NO/cGMP signaling would appear to be required for lung development during the perinatal period. Our findings suggest that HIF-1/2αs are up-regulated by NO/cGMP signaling, which encourages us to speculate that dynamic NO/cGMP signaling up-regulates HIF-1/2α in neonatal lungs. However, further experiments are required to clarify the mechanism involved.
We focused on the effects of sildenafil on HIF-1/2α expression, but did not examine the mechanism underlying HIF-1/2α suppression in the lungs of rats with BPD. Of the multiple steps determining HIF-1/2α expression, the step that is deregulated in BPD lungs remains unknown. Nonetheless, we can speculate that the HIF-deregulating process continues during hyperoxia. Although sildenafil was daily injected into rats during hyperoxia, HIF-1/2α and VEGF were not effectively induced at the 1-week time point. However, sildenafil strongly induced HIF-1/2α and VEGF while rats recovered in room air. Therefore, a BPD-driving process during hyperoxia may overwhelm the effect of sildenafil on HIF-1/2α expression.
In conclusion, our findings suggest that sildenafil has therapeutic potential in BPD, and that this effect is probably attributable to the activation of the HIF signaling pathway. Mechanistically, sildenafil appears to facilitate the de novo synthesis of HIF-1/2αs by sequentially activating NO/cGMP signaling and the PI3K/AKT/mTOR pathway. Furthermore, HIF activation appears to offer a possible strategy for the treatment or prevention of BPD.
Footnotes
This work was supported by grant A100236 from the Korean Ministry of Health and Welfare Research Fund 2010.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0043OC on October 11, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A, Kleinman R, Klijanowicz A, Martinez F, Ozdemir A. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med 2003;168:356–396. [DOI] [PubMed] [Google Scholar]
- 2.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med 2009;14:2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alagappan A, Malloy M. Systemic hypertension in very low-birth weight infants with bronchopulmonary dysplasia: incidence and risk factors. Am J Perinatol 1998;15:3–8. [DOI] [PubMed] [Google Scholar]
- 4.Northway WH, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline membrane disease: bronchopulmonary dysplasia. N Engl J Med 1967;276:357–368. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH. Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Physiol 2007;292:L1073–L1084. [DOI] [PubMed] [Google Scholar]
- 6.Speer CP. Inflammation and bronchopulmonary dysplasia: a continuing story. Semin Fetal Neonatal Med 2006;11:354–362. [DOI] [PubMed] [Google Scholar]
- 7.Speer CP. Role of inflammation in the evolution of bronchopulmonary dysplasia. Drug Discov Today Dis Mech 2006;3:409–414. [Google Scholar]
- 8.Speer CP. Role of inflammation in the pathogenesis of acute and chronic neonatal lung disease. In: Bancalari E, editor. The newborn lung: questions and controversies in neonatology series. Pulmonary volume, 1st edition. Philadelphia: Saunders; 2008. pp 166–186.
- 9.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol 2006;290:L209–L221. [DOI] [PubMed] [Google Scholar]
- 10.Oladipupo S, Hu S, Kovalski J, Yao J, Santeford A, Sohn RE, Shohet R, Maslov K, Wang LV, Arbeit JM. VEGF is essential for hypoxia-inducible factor–mediated neovascularization but dispensable for endothelial sprouting. Proc Natl Acad Sci USA 2011;108:13264–13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown KR, England KM, Goss KL, Snyder JM, Acarregui MJ. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am J Physiol Lung Cell Mol Physiol 2001;281:L1001–L1010. [DOI] [PubMed] [Google Scholar]
- 12.Patrik L, Ari R, Olavi Y, Lasse V, Sture A. Vascular endothelial growth factor in human preterm lung. Am J Respir Crit Care Med 1999;159:1429–1433. [DOI] [PubMed] [Google Scholar]
- 13.Wilson WL, Mullen M, Olley PM, Rabinovitch M. Hyperoxia-induced pulmonary vascular and lung abnormalities in young rats and potential for recovery. Pediatr Res 1985;19:1059–1067. [DOI] [PubMed] [Google Scholar]
- 14.Shaffer SG, O’Neill D, Bradt SK, Thibeault DW. Chronic vascular pulmonary dysplasia associated with neonatal hyperoxia exposure in the rat. Pediatr Res 1987;21:14–20. [DOI] [PubMed] [Google Scholar]
- 15.Randell SH, Mercer RR, Young SL. Neonatal hyperoxia alters the pulmonary alveolar and capillary structure of 40-day-old rats. Am J Pathol 1990;136:1259–1266. [PMC free article] [PubMed] [Google Scholar]
- 16.Thébaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation 2005;112:2477–2486. [DOI] [PubMed] [Google Scholar]
- 17.Bhandari V, Choo-Wing R, Lee CG, Yusuf K, Nedrelow JH, Ambalavanan N, Malkus H, Homer RJ, Elias JA. Developmental regulation of NO-mediated VEGF-induced effects in the lung. Am J Respir Cell Mol Biol 2008;39:420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinhorn R, Porta N. Use of inhaled nitric oxide in the preterm infant. Curr Opin Pediatr 2007;19:137–144. [DOI] [PubMed] [Google Scholar]
- 19.Ladha F, Bonnet S, Eaton F. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med 2005;172:750–756. [DOI] [PubMed] [Google Scholar]
- 20.Koneru S, Varma Penumathsa S, Thirunavukkarasu M, Vidavalur R, Zhan L, Singal PK, Engelman RM, Das DK, Maulik N. Sildenafil-mediated neovascularization and protection against myocardial ischaemia reperfusion injury in rats: role of VEGF/angiopoietin-1. J Cell Mol Med 2008;12:2651–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinhorn R, Kinsella J, Pierce C, Butrous G, Dilleen M, Oakes M. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension of newborn. J Pediatr 2009;155:841–847. [DOI] [PubMed] [Google Scholar]
- 22.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics 2006;117:1077–1083. [DOI] [PubMed] [Google Scholar]
- 23.Jung F, Palmer LA, Zhou N, Johns RA. Hypoxic regulation of inducible nitric oxide synthase via hypoxia inducible factor–1 in cardiac myocytes. Circ Res 2000;86:319–325. [DOI] [PubMed] [Google Scholar]
- 24.van Tuyl M, Liu J, Wang J, Kuliszewski M, Tibboel D, Post M. Role of oxygen and vascular development in epithelial branching morphogenesis of the developing mouse lung. Am J Physiol Lung Cell Mol Physiol 2005;288:167–178. [DOI] [PubMed] [Google Scholar]
- 25.Asikainen TM, Ahmad A, Schneider BK, White CW. Effect of preterm birth on hypoxia-inducible factors and vascular endothelial growth factor in primate lungs. Pediatr Pulmonol 2005;40:538–546. [DOI] [PubMed] [Google Scholar]
- 26.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE 2005;306:re12. [DOI] [PubMed] [Google Scholar]
- 27.Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L. A hypoxia responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med 1995;182:1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivan M. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 2001;292:464–468. [DOI] [PubMed] [Google Scholar]
- 29.Jaakkola P. Targeting of HIF-alpha to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001;292:468–472. [DOI] [PubMed] [Google Scholar]
- 30.Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci USA 2001;98:9630–9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeo EJ, Chun YS, Cho YS, Kim jh, Lee JC, Kim MS, Park JW. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst 2003;95:516–525. [DOI] [PubMed] [Google Scholar]
- 32.Lorenz R, Weibel ER. Morphometry of the human lung. Biom Z 1966;8:143–144. [Google Scholar]
- 33.Bacic M, Edwards NA, Merrill MJ. Differential expression of vascular endothelial growth factor (vascular permeability factor) forms in rat tissues. Growth Factors 1995;12:11–15. [DOI] [PubMed] [Google Scholar]
- 34.Kim WY, Oh SH, Woo JK, Hong WK, Lee HY. Targeting heat shock protein 90 overrides the resistance of lung cancer cells by blocking radiation-induced stabilization of hypoxia-inducible factor–1alpha. Cancer Res 2009;69:1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pez F, Dayan F, Durivault J, Kaniewski B, Aimond G, Le Provost GS, Deux B, Clézardin P, Sommer P, Pouysségur J, et al. The HIF-1–inducible lysyl oxidase activates HIF-1 via the AKT pathway in a positive regulation loop and synergizes with HIF-1 in promoting tumor cell growth. Cancer Res 2011;71:1647–1657. [DOI] [PubMed] [Google Scholar]
- 36.Jobe AH, Ikegami M. Antenatal infection/inflammation and postnatal lung maturation and injury. Respir Res 2001;2:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi CW, Kim BI, Hong JS, Kim EK, Kim HS, Choi JH. Bronchopulmonary dysplasia in a rat model induced by intra-amniotic inflammation and postnatal hyperoxia: morphometric aspects. Pediatr Res 2009;65:323–337. [DOI] [PubMed] [Google Scholar]
- 38.Mourani PM, Ivy DD, Gao D, Abman SH. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med 2004;170:1006–1013. [DOI] [PubMed] [Google Scholar]
- 39.Karmpaliotis D, Kosmidou I, Ingenito EP, Hong K, Malhotra A, Sunday ME, Haley KJ. Angiogenic growth factors in the pathophysiology of a murine model of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2002;283:L585–L595. [DOI] [PubMed] [Google Scholar]
- 40.Parera MC, van Dooren M, van Kempen M, de Krijger R, Grosveld F, Tibboel D, Rottier R. Distal angiogenesis: a new concept for lung vascular morphogenesis. Am J Physiol Lung Cell Mol Physiol 2005;288:L141–L149. [DOI] [PubMed] [Google Scholar]
- 41.Grover TR, Asikainen TM, Kinsella JP, Abman SH, White CW. Hypoxia-inducible factors HIF-1alpha and HIF-2alpha are decreased in an experimental model of severe respiratory distress syndrome in preterm lambs. Am J Physiol Lung Cell Mol Physiol 2007;292:L1345–L1351. [DOI] [PubMed] [Google Scholar]
- 42.Asikainen TM, Schneider BK, Waleh NS, Clyman RI, Ho WB, Flippin LA, Günzler V, White CW. Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung. Proc Natl Acad Sci USA 2005;102:10212–10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong H, Agani F, Baccala AA, Laughner E, Rioseco-Camacho N, Isaacs WB, Simons JW, Semenza GL. Increased expression of hypoxia inducible factor–1alpha in rat and human prostate cancer. Cancer Res 1998;58:5280–5284. [PubMed] [Google Scholar]
- 44.Fukuda R, Hirota K, Fan F, Jung Y, Ellis L, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1–mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem 2002;277:38205–38211. [DOI] [PubMed] [Google Scholar]
- 45.Saji M, Ringel MD. The PI3K–AKT–mTOR pathway in initiation and progression of thyroid tumors. Mol Cell Endocrinol 2010;321:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cash TP, Pan Y, Simon MC. Reactive oxygen species and cellular oxygen sensing. Free Radic Biol Med 2007;43:1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsertsvadze A, Yazdi F, Fink HA, MacDonald R, Wilt TJ, Bella AJ, Ansari MT, Garritty C, Soares-Weiser K, Daniel R, et al. Oral sildenafil citrate (Viagra) for erectile dysfunction: a systematic review and meta-analysis of harms. Urology 2009;74:831–836. [DOI] [PubMed] [Google Scholar]
- 48.Turko IV, Ballard SA, Francis SH, Corbin JD. Inhibition of cyclic GMP-binding cyclic GMP-specific phosphodiesterase (Type 5) by sildenafil and related compounds. Mol Pharmacol 1999;56:124–130. [DOI] [PubMed] [Google Scholar]
- 49.Kuschner WG. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2006;354:1091–1093. [PubMed] [Google Scholar]
- 50.Sanchez LS, de la Monte SM, Filippov G, Jones RC, Zapol WM, Bloch KD. Cyclic-GMP–binding, cyclic-GMP–specific phosphodiesterase (PDE5) gene expression is regulated during rat pulmonary development. Pediatr Res 1998;43:163–168. [DOI] [PubMed] [Google Scholar]
- 51.North AJ, Star RA, Brannon TS, Ujiie K, Wells LB, Lowenstein CJ, Snyder SH, Shaul PW. Nitric oxide synthase Type I and Type III gene expression are developmentally regulated in rat lung. Am J Physiol 1994;266:L635–L641. [DOI] [PubMed] [Google Scholar]