Abstract
A common pattern emerging from studies on the relationship between plant diversity and ecosystem functioning is that productivity increases with diversity. Most of these studies have been carried out in perennial grasslands, but many lasted only two growing seasons or reported data from a single year. Especially for perennial plant communities, however, the long-term effects of diversity are important. The question whether interactions between few species or among many species lead to increased productivity remained largely unanswered. So far, the main mechanism addressed is the increased input of nitrogen by nitrogen-fixing legumes. We report that other mechanisms can also generate strong increases of productivity with diversity. Results from 4 consecutive years of a plant diversity experiment without legumes show that a positive relationship between plant species richness and productivity emerged in the second year and strengthened with time. We show that increased nutrient use efficiency at high species richness is an important underlying mechanism. This mechanism had not been discussed in earlier studies. Furthermore, our results suggest that complementary nutrient uptake in space and time is important. Together, these mechanisms sustain consistently high productivity at high diversity.
Keywords: biodiversity, niche complementarity, nitrogen use efficiency, ecosystem functioning
The notion that the current loss of biodiversity may be detrimental to ecosystem functioning has led to major experiments in the last decade. Studies investigating the relationship between biodiversity and ecosystem functioning focused on the effects of losses of plant diversity on productivity (as a measure of ecosystem functioning) in grasslands. In these studies, productivity often declined with diversity loss (1), although several different patterns have been reported, including no response and idiosyncratic differences as plant diversity decreases (see ref. 2 for a review).
Both the patterns and the underlying mechanisms have been hotly debated (3–5, †, ‡). A positive relationship between diversity and productivity could arise through causal mechanisms such as facilitation or complementary resource use (6, 7). However, the same relationship between productivity and diversity could also be generated by chance, through a sampling or selection effect. More diverse plant communities have a higher chance of including a highly productive species that dominates the community (3, 4, 7). Complementarity and sampling effects may operate simultaneously, but can be separated by using the additive partitioning equation (8).
A positive effect of diversity on productivity was reported by several experiments, but most of these studies have been short term (<3 years) or reported results from a single growing season (9–18). In perennial grasslands, interactions between species occur over multiple years, but only three experiments reported results from a period >3 years. They showed that the positive effects of diversity increased several years after the start of the experiment. However, these experiments included legumes, which played an important role in overyielding (19–22). Apart from the effects of legumes, complementarity appeared to be important (20–22).
Resource partitioning may occur in time, space, and resource type (23–25). Facilitation may also be important: direct positive interactions have been demonstrated in many experiments (26). However, the main mechanism addressed in biodiversity–productivity experiments so far is nutrient enrichment by nitrogen fixers (27). Assessing the performance of individual species is crucial for understanding which mechanisms are responsible for the positive effect of diversity on productivity. Interspecific interactions like niche differentiation, facilitation, and frequency-dependent growth may promote high diversity by increasing the performance of rare species (28, 29). Under these mechanisms, a range of species (including rare ones) may show increased performance with increasing diversity, thus increasing total productivity (30, 31). However, few studies have determined the performance of individual species within long-term diversity–ecosystem functioning experiments (22, 32). We investigated overyielding among eight plant species. These species include two functional types: grasses and dicots. Legumes were not included. If a single resource, like nitrogen, is limiting productivity and species are complementary in nutrient use because differences in phenology, rooting depth, and other functional characteristics, then total plant nitrogen should be greater in mixtures (9). In the search for underlying mechanisms, we focused on differences between species in nutrient uptake and nutrient use at different levels of species richness and their contribution to the relationship between productivity and species richness over time.
Methods
Plots were established on an arable field in Wageningen, The Netherlands, in early spring 2000. In each plot, the topsoil was removed to a depth of 45 cm. At this depth, the mineral sand layer below the arable soil was reached. Wooden frames measuring 1 × 1 × 0.5 m (length × width × height) were placed in each hole and filled with a mixture of pure sand and soil from an old field (3:1). Seeds were laid to germinate in the greenhouse, and seedlings were planted after 3 weeks. In total, 144 seedlings were planted per plot in a substitutive design (i.e., each plot had the same total seedling density). During the first 3 months, plots were watered regularly to avoid desiccation of the seedlings. The experiment constituted 102 plots of 1 m2 distributed over six replicated blocks. Distance between plots was 1 m, and blocks were 2 m apart. Each block contained monocultures of all species, four mixtures of two and four species, and an eight-species mixture. The mixtures of two and four species were assembled by constrained random selection from the species pool. Selecting a certain composition twice was not allowed in this procedure (16). Composition was maintained throughout the experiment by removing seedlings of all other species at monthly intervals during each growing season. See ref. 33 for further details about weeding.
Species were selected from a pool of four grass species (Agrostis capillaris L., Anthoxanthum odoratum L., Festuca rubra L., and Holcus lanatus L.) and four dicot species (Centaurea jacea L., Leucanthemum vulgare Lamk., Plantago lanceolata L., and Rumex acetosa L.). Nomenclature follows van der Meijden (34). Species will further be referred to by their genus names. All species are C3 perennials and commonly coexist in European hay meadows.
The analysis is based on data of aboveground biomass. Total aboveground net primary productivity was measured by harvesting all plant material after the vegetation had reached peak-standing biomass. Because all aboveground tissue is new each year and every species is present throughout the growing season, aboveground biomass at the end of the growing season gives a reasonable estimate of total plot production. In August 2000, 2001, 2002, and 2003, plants were clipped to 2.5 cm above the soil surface, sorted to species, and dried for at least 48 h at 70°C before weighing. To avoid confounding edge effects, plots were divided into a center of 60 × 60 cm and a surrounding edge. Only data from the centers were used for the analysis.
Most measures of complementarity actually measure overyielding. It is defined as the production of mixtures exceeding expectations based on monoculture performance. We used several indices that address overyielding. In each of them, we used monoculture biomass within the same block instead of using the mean monoculture biomass to generate expected values (9, 21).
The relative yield total (RYT) measures overyielding by summing the relative yields of all species in a mixture. The relative yield of a species is calculated by dividing its biomass in a mixture by its monoculture biomass. RYT > 1 indicates complementarity. RYT was originally proposed as the most appropriate measure of niche complementarity (35) and is one of the most common metrics for assessing overyielding (27). It is a robust measure when planting densities give constant final yield, sufficient time has been allowed for interactions to develop, and indices are calculated on a yield per area basis rather than as yield per individual (21, 36, 37). These criteria were met for all data throughout our experiment. However, RYT does not identify a relationship between biomass in monoculture and mixture performance (the selection effect). For these purposes, the additive partitioning method can be used (8). In this method, the net effect is measured as the observed biomass in a mixture minus the expected biomass, which is based on monoculture biomass. This net effect can be partitioned into a complementarity effect and a selection effect. A positive complementarity effect occurs if species yields in a mixture are on average higher than expected on the basis of the weighted average monoculture yield of the component species. Note that the term complementarity effect actually refers to an effect caused by differentiation in resource use and/or facilitative interactions. Distinguishing between complementary resource use and facilitation is difficult (8). The selection effect is the standard statistical covariance effect, in this case between biomass in monoculture and change in relative yield in mixtures.
To determine the contribution of individual species, we also determined a net effect per species. Its calculation is similar to the net effect in the additive partitioning method (8).
In 2002, all aboveground plant samples were ground and digested with sulfuric acid, salicylic acid, hydrogen peroxide, and selenium (38). Nitrogen and phosphorus concentrations were measured colorimetrically by using a segmented flow analyzer (SKALAR SAN Plus System, Breda, The Netherlands). Potassium was measured by flame atomic emission spectroscopy (Varion SpectrAA-600, Bergen op Zoom, The Netherlands). Aboveground nutrient pools were determined per species by multiplying nutrient concentration by aboveground biomass. The amount of aboveground nitrogen of each species was used to calculate RYT values for aboveground nitrogen instead of biomass. RYT-N indicates complementarity in nitrogen uptake (9, 23). We determined the amount of aboveground biomass per unit of aboveground nitrogen as a measure of nutrient use efficiency.
All relationships with species richness were determined for each year by using a univariate general linear model (GLM) with block as random factor and log2 of species richness as a covariate. The effect of time was determined at each species richness level by using GLM repeated measures with block as factor. In this procedure, we used the Huynh–Feldt degrees of freedom adjustment when the sphericity assumption was violated (spss 10.0, SPSS, Chicago). Differences between years were determined by using pair-wise comparisons or linear contrasts for time within this procedure. Biomass and RYT data were ln-transformed when necessary. Values from the additive partitioning method were square root transformed when necessary but preserved their original positive and negative signs (8). Differences from expected values (one for RYT, zero for the other measures) were determined by using t tests at each species richness level for each year.
Results
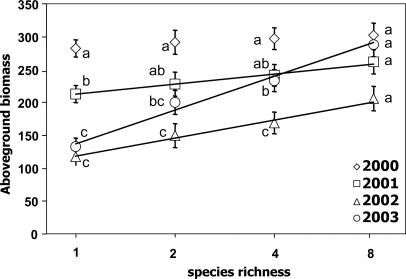
There was no relationship between species richness and aboveground biomass in the first year, but from the second year (2001) onwards, species richness enhanced productivity. Overall, aboveground productivity decreased with time from 2000 until 2002, although this decrease was not significant at the highest level of species richness. In 2003, especially four and eight species mixtures showed an increase of productivity compared to 2002 (Fig. 1). This resulted in a stronger increase of productivity with species richness, as indicated by significant interaction between year and species richness (repeated measures 2001–2003, time × log2 richness, F2,93 = 11.59; P = 3.2E-05).
Fig. 1.
Annual above-ground biomass (g/m2) as a function of plant species richness from 2000 to 2003. The log linear relationship with species richness was significant for 2001–2003 (2001, F1,95 = 9.54, P = 0.003; 2002, F1,95 = 21.72, P = 1.03E-05; 2003, F1,95 = 28.60, P = 6.12E-07). Different letters denote significant (P < 0.05) differences between years at that species-richness level (Sidak-corrected multiple pair-wise comparisons within general linear model repeated measures). Data show means ± SE.
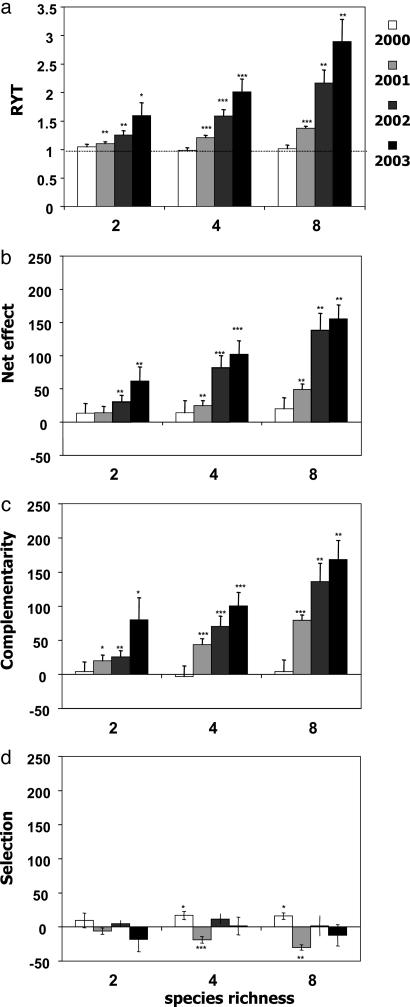
RYT was not different from one in 2000, but was significantly higher than one at each species richness level in the following years. In each of these years, RYT increased log-linearly with species richness and increased linearly with time at each species richness level (Fig. 2a). The net effect and the complementarity effect, calculated by using the additive partition method, showed similar patterns as RYT. The net effect increased with species richness in 2001, 2002, and 2003, and the complementarity effect increased with species richness in 2001 and 2002. Both effects increased with time at each species richness level (Fig. 2 b and c). This observation already indicates that the complementarity effect prevailed. Selection effects were generally small. A positive selection effect occurred in four and eight species mixtures in 2000, whereas the same mixtures showed a negative selection effect in 2001. The selection effect decreased with species richness in 2001. No relationship with time could be detected (Fig. 2d).
Fig. 2.
Results of different measures of complementarity from 2000 to 2003. (a) RYT increased with species richness in 2001, 2002, and 2003 and increased with time at each level of species richness (P < 0.05). (b) The net effect increased with species richness in 2002 and 2003, and increased with time at each level of species richness (P < 0.05). (c) The complementarity effect increased with species richness in 2001 and 2002, and increased with time at each level of species richness (P < 0.05). (d) The selection effect decreased with species richness in 2001 (P < 0.05). Asterisks indicate significant difference from one (RYT) or zero. ***, P < 0.001; **, P < 0.01; *, P < 0.05. Data show means ± SE.
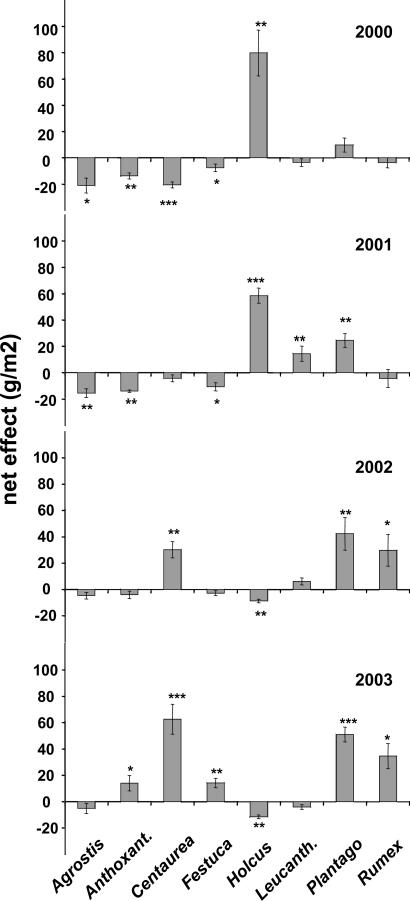
The net effects per species strongly shifted with time. In 2000, only one species (Holcus) performed better than expected at the highest level of species richness, whereas four species (Agrostis, Anthoxanthum, Centaurea, and Festuca) performed worse. However, the contribution of Holcus declined with time and turned negative in the last 2 years. Simultaneously, all species, except Leucanthemum, increased their contribution. In 2003, five species contributed significantly more to mixture biomass than expected based on their monoculture performance (Fig. 3).
Fig. 3.
Net effect of each species, shown for the eight species mixtures from 2000 to 2003. Asterisks indicate significant differences from zero. ***, P < 0.001; **, P < 0.01; *, P < 0.05. Data show means ± SE.
The ratio of nitrogen (N) to phosphorus (P) in aboveground biomass is considered a useful predictor of N or P limitation. Values of N/P < 14 are generally considered to indicate N limitation (39, 40). In 2002, N/P ratios ranged from 3.54 ± 0.09 (Leucanthemum) to 6.05 ± 0.15 for Rumex in our experiment. Considering these low values, plants are assumed to be N limited. Therefore, we focus on nitrogen in our analysis. Total aboveground nitrogen showed patterns similar to plant biomass in 2002: Centaurea and Plantago showed a log-linear increase of total aboveground N with species richness (P < 0.05 and P < 0.001, respectively), whereas Holcus showed a log-linear decrease (P < 0.01). The other species showed no relationship between aboveground N and species richness. As a result, the total amount of aboveground nitrogen per plot increased with species richness (P < 0.01). This was confirmed by applying the RYT approach to aboveground amounts of N. At each level of species richness, RYT-N values were significantly (P < 0.05) higher than one.
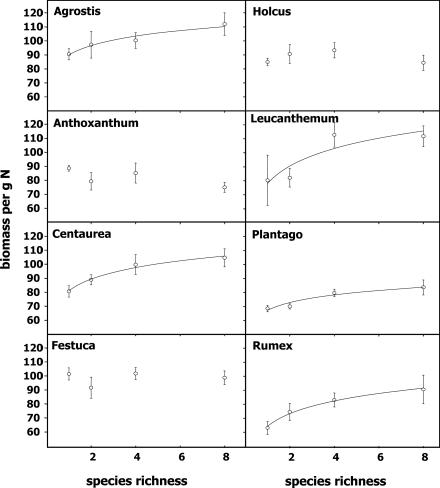
We used the amount of aboveground biomass produced per unit aboveground nitrogen as a measure of nitrogen use efficiency (NUE). Interestingly, this measure also increased with species richness for several species. All dicot species and one grass species (Agrostis) showed this pattern. The other three grass species showed a neutral relationship between the amount of biomass per unit nitrogen and species richness (Fig. 4). Phosphorus and potassium budgets showed similar patterns, although less pronounced.
Fig. 4.
Amount of aboveground biomass (gram) per gram of nitrogen, shown for each species in 2002. Agrostis, F = 4.92, P < 0.05; Centaurea, F = 8.00, P < 0.01; Leucanthemum, F = 6.17, P < 0.05; Plantago, F = 12.31, P < 0.01; Rumex, F = 10.30, P < 0.01; Anthoxanthum, Festuca, and Holcus were nonsignificant. Data show means ± SE.
We used the expected values for aboveground biomass, total nitrogen, and NUE based on the monocultures to partition the net effect of each species in the eight-species mixtures (see Fig. 3) into the effects of increased NUE and those of complementarity (i.e., increased amounts of aboveground nitrogen). The contribution of increased NUE was calculated as YOi - (NOi × NUEMi), where YOi and NOi are the biomass and the total amount of nitrogen of species i in the mixture, respectively, and NUEMi is the NUE of the species in monoculture. Similarly, the contribution of complementarity is calculated as (NOi - NMi/n) × NUEMi, where NMi is the amount of nitrogen of species i in monoculture, and n is the number of species in the mixture. Summed over all species, complementarity accounted for 58% of the increase in productivity in eight species mixtures, and the increased NUE accounted for 42%.
Discussion
In our experiment, a positive relationship between species richness and plant productivity appeared in the second year and became increasingly positive as the experiment continued. This pattern has also been shown in the first biodiversity field experiment in Cedar Creek, which included legumes (19). Our results confirm that similar patterns can arise in the absence of legumes.
Different indices suggested that complementarity in resource use and/or facilitative interactions were the main driver(s) of increased productivity at higher levels of species richness. RYT values increased with species richness and with time. In 2003, two-species mixtures had an average value of 1.60. An extensive review found that >95% of two and three species mixtures without legumes had RYT <1.3 (23, 43). RYT of eight-species mixtures approached unusual values of 3.0 in our experiment. Complementarity effects also increased both with species richness and time and were significantly greater than zero since 2001. Selection effects were small and often nonsignificant. In 2003, Centaurea dominated mixtures, but because of its high monoculture biomass, the contribution of Centaurea was mainly attributed to the selection effect. Still, its increase may be caused by increased complementarity in resource use. The selection effect is not independent of complementarity: if resource partitioning also facilitates more productive species, some complementarity may be attributed to the selection effect. As such, the complementarity effect we report may be conservative as a measure of positive interactions (21, 42).
Similar to an earlier experiment (43), the importance of the individual species changed during the development of the experimental plots. In the first year, competitive exclusion dominated the interactions in the experimental plant communities. Holcus appeared to out-compete the other species because its high contribution to mixture biomass coincided with negative net effects of several other species. Probably, its higher growth rate (44) allowed Holcus to effectively forage for nutrients in large parts of the initially unoccupied soil volume, at least in the upper layer. The depletion of nutrients in the upper layer probably forced especially the dicot species to increase their rooting depth (45–47). From 2001, the biomass of Holcus strongly decreased, and other species became dominant. We propose that Holcus is gradually out-competed by species that invested in roots adapted to low nutrient availability (i.e., long-lived roots, ref. 50), because nutrient availability probably declined after plant roots had occupied and depleted most of the soil and nutrients allocated aboveground were continuously removed from the plots by harvesting aboveground biomass. It can only survive in monoculture because all other species are continuously removed from those plots.
With the decline of Holcus, interspecific interactions that promote diversity became more important. This is illustrated by the percentage of species contributing to increased productivity at high species richness increasing from 12.5% in 2000 to >60% in 2003. Especially, dicot species started to show positive net effects, which may be the result of complementarity in rooting depth between the dominant grass species and these dicots (23, 24, 49). This is confirmed by the increase of aboveground N with species richness shown by two of these species (Centaurea and Plantago) and the positive RYT-N values in 2002. These positive RYT-N values are in contrast to an earlier study, which also calculated complementarity based on total nitrogen, and found no signs of increased nitrogen amounts despite significant over-yielding in terms of biomass (9). This finding indicates that, in our study, plant growth is mainly nitrogen limited, whereas competition for other resources probably plays an important role in the other study system (9).
Complementarity in rooting depth is likely to be important, but temporal differences in nutrient uptake may also have significant effects (24, 50). For two species present in our experiment (Anthoxanthum and Plantago), it has been shown that they differ in rooting depth, and also in their main periods of nitrogen uptake (51). Plants may also facilitate each other (26), but we found no evidence for facilitative interactions.
Importantly, several species used nutrients more efficiently at high species richness, as shown by the increase of aboveground biomass per unit nitrogen with species richness. This is a mechanism that, to our knowledge, has not been presented in other studies. Its contribution to increased productivity at high diversity is substantial, making up >40% of the total net effect on productivity. However, this shift in NUE is difficult to explain. An earlier study on aboveground resource use in experimental grasslands showed that grass species responded to increased light competition at high diversity by investing more biomass in stems to increase in height (11). Because stems generally have a higher C/N ratio than leaves, this response may lead to an increase of the amount of aboveground biomass per unit nitrogen. This increase might explain the response shown by the grass species Agrostis (see Fig. 4). In contrast to the grasses, the dicot species did not show an increase in height in that experiment (11). Moreover, most dicot species that showed an increase of NUE with diversity in our experiment (Fig. 4) also showed increased biomass and increased nutrient yields at high diversity. For these species, the availability of nutrients appears to have increased because of complementary interactions. As a response to that increase, they may have invested more biomass into stems (52). Similar to the grasses increasing in height, their aboveground C/N ratio increased as these dicots invested relatively more biomass into flowering stems and less into leaves.
Conclusions
In the year of establishment, interspecific interactions were dominated by competitive exclusion, as shown by the strong expansion of one fast-growing species and the decline of many other species. Already in the second year, however, productivity increased with species richness. Our results show that this positive relationship between species richness and productivity strengthens with time in perennial communities. The patterns observed were caused by increased niche differentiation and/or facilitation, as shown by increasing values of RYT and the complementarity effect, and the number of plant species that contributed to increased productivity at higher levels of species richness. In our experiment, these patterns cannot be attributed to increased nitrogen input because of the presence of legumes. Detailed nutrient analysis revealed two alternative underlying mechanisms. First, complementarity in nutrient uptake probably enabled the diverse communities to acquire greater amounts of nutrients. In addition, increased nutrient-use efficiency of several species at higher levels of species richness was very important. The increase of nutrient-use efficiency probably is the result of changes in the biomass allocation patterns of species. This mechanism provides a contribution to our understanding of the mechanisms of diversity effects on ecosystem functioning.
Acknowledgments
We thank Gerlinde De Deyn, Maurits Gleichman, Eelke Jongejans, Frans Möller, Louis de Nijs, Ciska Raaijmakers, Henk van Roekel, and Jan van Walsem for practical assistance. Two anonymous reviewers gave very helpful suggestions on an earlier version of the manuscript. This work was supported by Stimulation Program Biodiversity of the Netherlands Organization for Scientific Research.
Author contributions: J.v.R. and F.B. designed research; J.v.R. performed research; J.v.R. and F.B. analyzed data; and J.v.R. and F.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: RYT, relative yield total; NUE, nitrogen use efficiency.
Footnotes
Huston, M. A., Aarssen, L. W., Austin, M. P., Cade, B. S., Fridley, J. D., Garnier, E., Grime, J. P., Lauenroth, W. K., Thompson, K. & Wardle, D. A. (2000) Science 289, 1255a (abstr.).
Hector, A., Schmid, B., Beierkuhnlein, C., Caldeira, M. C., Diemer, B., Dimitrakopoulos, P. G., Finn, J. A., Freitas, H., Giller, P. S., Good, J., et al. (2000) Science 289, 1255a (abstr.).
References
- 1.Loreau, M., Naeem, S. & Inchausti, P. (2002) Biodiversity and Ecosystem Functioning: Synthesis and Perspectives (Oxford Univ. Press, Oxford).
- 2.Schmid, B., Joshi, J. & Schläpfer, F. (2002) in Functional Consequences of Biodiversity: Experimental Progress and Theoretical Extensions, eds. Kinzig, A., Tilman, D. & Pacala, S. W. (Princeton Univ. Press, Princeton, NJ), pp. 120-150.
- 3.Aarssen, L. W. (1997) Oikos 80, 183-184. [Google Scholar]
- 4.Huston, M. A. (1997) Oecologia 110, 449-460. [DOI] [PubMed] [Google Scholar]
- 5.Loreau, M., Naeem, S., Inchausti, P., Bengtsson, J., Grime, J. P., Hector, A., Hooper, D. U., Huston, M. A., Raffaelli, D. G., Schmid, B., et al. (2001) Science 294, 804-808. [DOI] [PubMed] [Google Scholar]
- 6.Loreau, M. (2000) Oikos 91, 3-17. [Google Scholar]
- 7.Tilman, D., Lehman, C. L. & Thomson, K. T. (1997) Proc. Natl. Acad. Sci. USA 94, 1857-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loreau, M. & Hector, A. (2001) Nature 412, 72-76. [DOI] [PubMed] [Google Scholar]
- 9.Hooper, D. U. (1998) Ecology 79, 704-719. [Google Scholar]
- 10.Hector, A., Schmid, B., Beierkuhnlein, C., Caldeira, M. C., Diemer, M., Dimitrakopoulos, P. G., Finn, J. A., Freitas, H., Giller, P. S., Good, J., et al. (1999) Science 286, 1123-1127. [DOI] [PubMed] [Google Scholar]
- 11.Spehn, E. M., Joshi, J., Schmid, B., Diemer, B. & Körner, C. (2000) Func. Ecol. 14, 326-337. [Google Scholar]
- 12.Leps, J., Brown, V. K., Diaz Len, T. A., Gormsen, D., Hedlund, K., Kailova, J., Korthals, G. W., Mortimer, S. R., Rodriquez-Barrueco, C., Roy, J., et al. (2001) Oikos 92, 123-134. [Google Scholar]
- 13.Dukes, J. S. (2001) Oikos 94, 468-480. [Google Scholar]
- 14.Hector, A., Bazeley-White, E., Loreau, M., Otway, S. J. & Schmid, B. (2002) Ecol. Lett. 5, 502-511. [Google Scholar]
- 15.Špaèková, I. & Lepš, J. (2001) Ecol. Lett. 4, 585-594. [Google Scholar]
- 16.van Ruijven, J. & Berendse, F. (2003) Ecol. Lett. 6, 170-175. [Google Scholar]
- 17.Fridley, J. D. (2002) Oecologia 132, 271-277. [DOI] [PubMed] [Google Scholar]
- 18.Fridley, J. D. (2003) J. Ecol. 91, 396-406. [Google Scholar]
- 19.Tilman, D., Reich, P. B., Knops, J., Wedin, D., Mielke, T. & Lehman, C. L. (2001) Science 294, 843-846. [DOI] [PubMed] [Google Scholar]
- 20.Mulder, C. P. H., Jumpponen, A., Högberg, P. & Huss-Danell, K. (2002) Oecologia 133, 412-421. [DOI] [PubMed] [Google Scholar]
- 21.Hooper, D. U. & Dukes, J. S. (2004) Ecol. Lett. 7, 95-105. [Google Scholar]
- 22.Hille Ris Lambers, J., Harpole, W. S., Tilman, D., Knops, J. & Reich, P. B. (2004) Ecol. Lett. 7, 661-668. [Google Scholar]
- 23.Berendse, F. (1981) Oecologia 48, 334-341. [DOI] [PubMed] [Google Scholar]
- 24.McKane, R. B., Grigal, D. F. & Russelle, M. P. (1990) Ecology 71, 1126-1132. [Google Scholar]
- 25.McKane, R. B., Johnson, L. C., Shaver, G. R., Nadelhoffer, K. J., Rastetter, E. B., Fry, B., Giblin, A. E., Kielland, K., Kwiatkowski, B. L., Laundre, J. A., et al. (2002) Nature 415, 68-71. [DOI] [PubMed] [Google Scholar]
- 26.Callaway, R. M. (1995) Bot. Rev. 61, 306-349. [Google Scholar]
- 27.Fridley, J. D. (2001) Oikos 93, 514-526. [Google Scholar]
- 28.Chesson, P. (2000) Annu. Rev. Ecol. Syst. 31, 343-367. [Google Scholar]
- 29.Bruno, J. F., Stachowicz, J. J. & Bertness, M. D. (2003) Trends Ecol. Evol. 18, 119-125. [Google Scholar]
- 30.Petchey, O. L. (2000) Am. Nat. 155, 696-702. [DOI] [PubMed] [Google Scholar]
- 31.Mouquet, N., Moore, J. L. & Loreau, M. (2002) Ecol. Lett. 5, 56-65. [Google Scholar]
- 32.Craine, J. M., Reich, P. B., Tilman, D., Ellsworth, D., Fargione, J., Knops, J. & Naeem, S. (2003) Ecol. Lett. 6, 623-630. [Google Scholar]
- 33.van Ruijven, J., De Deyn, G. B. & Berendse, F. (2003) Ecol. Lett. 6, 910-918. [Google Scholar]
- 34.van der Meijden, R. (1990) Heukels' Flora van Nederland (Wolters–Noordhoff, Groningen, The Netherlands).
- 35.de Wit, C. T. & van den Bergh, J. P. (1965) Neth. J. Agr. Sci 13, 212-221. [Google Scholar]
- 36.de Wit, C. T. (1960) Verslagen Landbouwkundig Onderzoek 66, 1-82. [Google Scholar]
- 37.Jolliffe, P. A. (2000) J. Ecol. 88, 371-385. [Google Scholar]
- 38.Novozamsky, I., Houba, V. J. G., van Eck, R. & van Vark, W. (1983) Comm. Soil Sci. Plant Anal. 14, 239-249. [Google Scholar]
- 39.Koerselman, W. & Meuleman, A. F. M. (1996) J. Appl. Ecol. 33, 1441-1450. [Google Scholar]
- 40.Olde Venterink, H., Wassen, M. J., Verkroost, A. W. M. & de Ruiter, P. C. (2003) Ecology 84, 2191-2199. [Google Scholar]
- 41.Trenbath, B. R. (1974) Adv. Agron. 26, 177-210. [Google Scholar]
- 42.Petchey, O. L. (2003) Oikos 101, 323-330. [Google Scholar]
- 43.Niklaus, P. A., Leadley, P. W., Schmid, B. & Körner, C. (2001) Ecol. Monogr. 71, 341-356. [Google Scholar]
- 44.Grime, J. P. & Hunt, R. (1975) J. Ecol. 63, 393-422. [Google Scholar]
- 45.Berendse, F. (1982) Oecologia 53, 50-55. [DOI] [PubMed] [Google Scholar]
- 46.Wardle, D. A. & Peltzer, D. A. (2003) Oikos 100, 497-506. [Google Scholar]
- 47.Jumpponen, A., Högberg, P., Huss-Danell, K. & Mulder, C. P. H. (2002) Funct. Ecol. 16, 454-461. [DOI] [PubMed] [Google Scholar]
- 48.van der Krift, A. J. & Berendse, F. (2002) Funct. Ecol. 16, 198-203. [Google Scholar]
- 49.Fitter, A. H. (1986) Oecologia 69, 594-599. [DOI] [PubMed] [Google Scholar]
- 50.Hooper, D. U. & Vitousek, P. M. (1998) Ecol. Monogr. 68, 121-149. [Google Scholar]
- 51.Berendse, F. (1981) Ph.D. dissertation (Utrecht University, Utrecht, The Netherlands).
- 52.Müller, I., Schmid, B. & Weiner, J. (2000) Perspect. Plant Ecol. Evol. Sys. 3, 115-127. [Google Scholar]