Abstract
Proteins and peptides in Drosophila melanogaster seminal fluid induce mated females to increase their rates of egg deposition. One seminal-fluid protein, ovulin (Acp26Aa), stimulates an early step in the egg-laying process, the release of oocytes by the ovary. Ovulin, upon transfer to females, is cleaved sequentially within the mated female's reproductive tract. Here, we show that systemic ectopic expression of ovulin is sufficient to stimulate ovulation in unmated females. By using this assay to assess the functionality of ovulin's cleavage products, we find that two of the four cleavage products of ovulin can stimulate ovulation independently. Thus, ovulin's cleavage in mated females is not destructive and instead may liberate additional functional products with potential to modulate ovulation independently.
Keywords: mating, processing, prohormone, seminal proteins
Even after mating, a male can continue to affect the reproductive success of his mate. One way this effect is accomplished results from the male's donation of seminal proteins. For example, in Drosophila melanogaster, ≈83 accessory gland proteins (Acps) are produced in the male's accessory glands (refs. 1 and 2; reviewed in ref. 3). In the mated female, Acps increase egg production, ovulation, and egg deposition; decrease receptivity to remating; promote the storage of sperm; and decrease the lifespan of the mated female (reviewed in refs. 3-8). Individual Acps have been shown to cause specific changes in mated females: Acp26Aa (ovulin) and Acp70A (sex peptide) affect the egg-laying process (9-15), Acp70A affects receptivity (12-15), Acp36DE is needed for sperm storage (16, 17), and Acp62F has been suggested to contribute to the decrease in the mated female's life span (18).
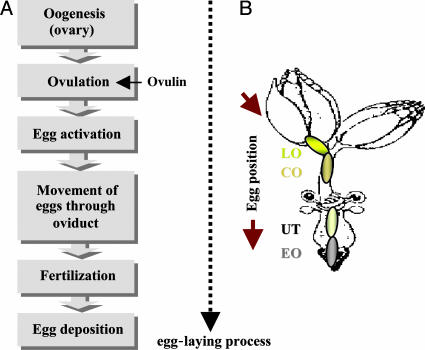
Here, we focus on ovulin, an Acp that stimulates ovulation. Egg-laying in Drosophila is a multistep process (ref. 10; reviewed in ref. 19). First, eggs produced in the ovaries must pass checkpoints during oogenesis (refs. 20-22; reviewed in ref. 23). Mature oocytes are then released from ovaries (ovulation), a process that also triggers oocyte activation (24). Oocytes move through the oviducts to the uterus, where they can be fertilized by sperm released from storage, and, finally, they are released from the uterus and deposited onto the substratum (Fig. 1A). Mates of males who do not provide ovulin show decreased ovulation and egg deposition (9-11). Within the mated female, some ovulin from the male enters the circulatory system (hemolymph) and some localizes to the base of the ovary (10, 25). Thus, ovulin could conceivably induce ovulation in the female by direct interaction with neuromuscular targets along the upper region (lateral oviducts) of the genital tract or indirectly by affecting the activity of the neuroendocrine system (26).
Fig. 1.
The ovulation and egg-laying process in mated females. (A) Genetic studies of a knockout mutant showed that ovulin was necessary only at the step corresponding to the initial release of oocytes during ovulation (10). (B) Positions of eggs in the female reproductive tract at the time of dissection. The stages are determined based on the position of the posterior end of the egg within the oviducts. Eggs at the lateral oviduct (LO) position are partly in the ovary and partly in the lateral oviducts. Eggs at the common oviduct (CO) position are completely out of the ovary and within the common oviduct. Eggs at the uterus (UT) position are in the uterus, which is also the fertilization site. Eggs at the external opening (EO) position (the external opening of the reproductive tract) are in the process of being deposited onto the food surface (10). The drawing in B is adapted from ref. 55 with modifications.
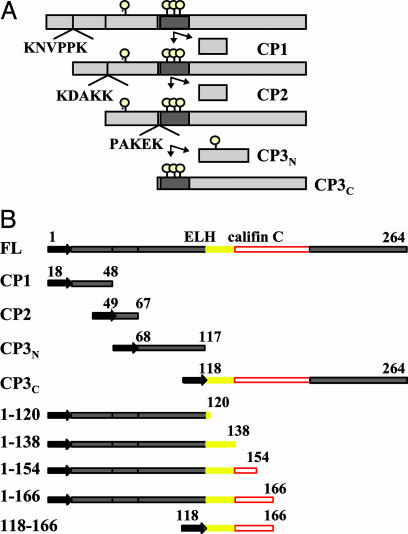
The sequence of ovulin suggests that it is a prohormone. The primary translation product is 264 aa long, including a predicted signal sequence of 18 aa that is expected to be cleaved to allow secretion of ovulin (27). Ovulin contains amino acid sequences that resemble prohormone cleavage sites in other organisms [e.g., egg laying hormone ELH, Aplysia (28-32); oxytocin, cattle (33); and tachykinin-related peptide, Drosophila (34)]. Shortly after it enters the female's reproductive tract, ovulin is proteolytically processed (25, 27, 35). A map of cleavage products (CPs) of ovulin suggests that three sequential cleavages occur at ovulin residues that match prohormone cleavage consensus. The first cleavage is after Lys-48, the second is after Lys-68, and the third is after either Lys-115 or Lys-117 (35) (see Fig. 2). The C-terminal CP of ovulin contains two small regions (residues 120-137 and 154-188) of similarity (46% amino acid identity) to Aplysia californica egg-laying hormones that regulate egg deposition. These mollusk hormones, ELH and califin C, are also processed from prohormone precursors. The cleavage products of ELH and califin C are released into the Aplysia reproductive tract, where they stimulate the release of oocytes (29, 36, 37).
Fig. 2.
Ovulin's cleavage pattern and fragments tested for ovulation activity. (A) Schematic diagrams of ovulin processing and its potential CPs in the female reproductive tract. The potential cleavage sites are represented by bars. The circles above each diagram represent potential O-linked glycosylation sites (27). Gray bars represent the region of similarity to ELH/califin C (10). The putative cleavage site sequences are also shown (adapted from ref. 35 with minor modifications). (B) Nine constructs were created to ectopically express subregions of ovulin. Constructs CP1-CP3 are designed to generate naturally occurring CPs of ovulin (after cleavage of the attached signal sequence). Constructs 1-120, 1-138, 1-154, 1-166, and 118-166 are truncations as described in the text. These nine constructs were used to identify the active region(s) of ovulin. Yellow indicates the region of similarity to ELH; red indicates the region of similarity to califin C. FL, Full-length ovulin.
One possible mechanism for regulating the activity of seminal peptides within the female reproductive tract is to process them from an inactive to an active form (as occurs for the Aplysia hormones ELH and califin C). Alternatively, processing could be inactivational, particularly given that some Acps are harmful to the female (38). Here we test whether full-length ovulin or its predicted cleavage products and its ELH- and califin-similar regions are sufficient to induce ovulation. We found that ectopic expression of full-length ovulin and at least two of its cleavage products stimulate ovulation. Thus, the cleavage products of ovulin can act independently, and the cleavage seen in mated females is not degradative.
Materials and Methods
Fly Stocks. Flies were kept in a 12-h light/dark cycle at 23°C ± 2°C. Transgenic stocks for upstream activation sequence (UAS)-ovulinfull-length were described in Park and Wolfner (35); transgenic stocks for UAS-ovulin subregions are described below. Expression was induced by a heat-inducible GAL4 driver from the hsp70-GAL4/CyO stock (39). Controls included transgenic strains carrying either hsp26-Ya (40) or UAS-GFP (a kind gift from G. Boulianne, Hospital for Sick Children, Toronto) (41); expression of GFP was induced with hsp70-GAL4.
Transgenic Strains. To express subregions of ovulin, we generated the deletions shown in Fig. 2 and cloned them into pUAST by means of EcoRI/XbaI sites. For deletions that would encode a protein without a signal sequence, we added the ovulin signal sequence (amino acids 1-18) to the N terminus of the deletion protein to allow secretion. The signal sequence was cloned into pUAST by means of EcoRI/NotI sites, and the deletion fragments were cloned by means of NotI/XbaI sites. Restriction enzyme digestion and sequencing confirmed that the deletion fragments inserted in the predicted position near the 3′ end of the UAS elements.
Generation of transgenic lines by microinjection into w1118 embryos was performed by using standard methods (42, 43). Southern blot confirmation of independent single-insertion lines was as in Lung et al. (18). Expression of ovulin of appropriate size was verified by Western blotting (data not shown) for all constructs except first CP (CP1; amino acids 18-48), second CP (CP2; amino acids 49-67), and N-terminal, third CP (CP3N; amino acids 68-117) (see also Fig. 1B). These three constructs encoded peptides that were too small to detect with our Western blot method, so for these constructs, expression was confirmed by RT-PCR (data not shown). Line numbers are designated by subscripts, e.g., line 37.2 that expressed CP3N is designated CP3N(37.2).
Ovulation Assays After Induction of Ovulin. Two independent lines for each construct were tested. Three-day-old virgin female progeny from crosses of the driver line and the UAS lines [e.g., hsp70-Gal4;UAS-ovulin· (· indicates the portion of ovulin to be tested)] experimental flies and their sibling CyO;UAS-ovulin· control flies were placed singly in glass vials with a damp piece of Whatman paper. Vials were placed in a water bath at 25°C, and the temperature was gradually raised to 37°C. Vials remained at 37°C for 1 h. Flies recovered for 1 h at room temperature and thereafter were placed on fresh food (one female per vial) and kept at 23°C ± 2°C until dissection.
Vials containing the heat-shocked (or non-heat-shocked control) females, sorted by transgene, were coded by using a random number table, so the experiment proceeded under double-blind conditions. Single females were placed in a drop of Yamamoto's solution (44) on a slide on ice, and their reproductive tracts were gently dissected.
Ovulation and egg deposition levels were measured after induction of expression (10, 18, 24 and 39 h after heat shock) of each construct. Within each reproductive tract, the number of oocytes at each location (lateral oviduct, common oviduct, uterus, and external opening) were recorded (see Fig. 2), as in the ovulation bioassay described by Heifetz et al. (10). The number of eggs that had been deposited by the females in the 24 h before dissection was also counted. Relative ovulation by hsp70-Gal4;UAS-ovulin· compared with their CyO siblings (who carry the UAS-ovulin· transgene but do not express it) was compared with the same measures for hsp70-Gal4;UASfull length-ovulin (positive control) and to hsp70-Gal4;UAS-GFP (negative control).
Statistical Analysis. Path analysis (45, 46) was performed with spss as in Heifetz et al. (10). Briefly, this method indicates the relative odds of finding an egg at a particular position in the reproductive tract in flies ectopically expressing the ovulin transgene (hsp70-Gal4;UAS-ovulin·) relative to flies that do not express the transgene (non-heat-shocked hsp70-Gal4;UAS-ovulin·; CyO;hsp70-Gal4;UAS-ovulin·). For example, for the Path analysis to determine the functionality of ovulin subregions, two regression equations were estimated. Whether or not there was an egg in the lateral oviduct was examined as a function of ovulin subregions and full-length ovulin (positive control) and/or GFP (negative control). (In one of our experiments we used hsp26-Ya as the negative control.) The number of eggs deposited was examined as a function of ovulin subregions and full-length ovulin (or GFP), as well as whether or not there was an egg in the lateral oviduct. ANOVA (spss 8.0 for windows) was used to measure the differences between pairs of treatments.
Results and Discussion
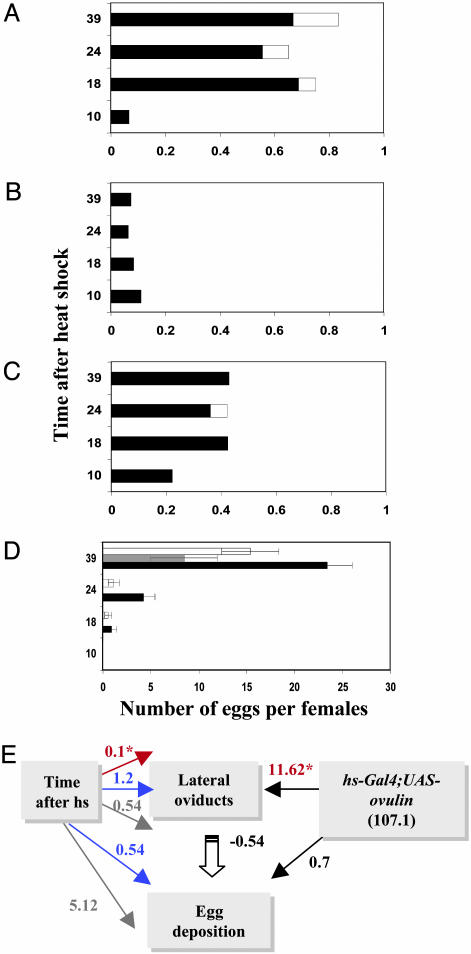
Full-Length Ovulin Can Stimulate Ovulation in Unmated Virgin Females. We tested whether ectopic expression of ovulin can stimulate ovulation in heat-shocked hsp70-Gal4;UAS-ovulin unmated females. The number of eggs in the reproductive tract (lateral oviducts, common oviduct; uterus, and external opening) is elevated in females expressing ovulin (Fig. 3 A and B). By 18 h after heat shock, 67% of the females expressing ovulin had an egg in their reproductive tract vs. 8.3% for control females (Fig. 3B); most eggs (58%) were in the lateral oviducts (averaging 0.69 ± 0.09 eggs per female) (Fig. 3A). By 24 h after heat shock, 17% of the ovulin-expressing females (and none of the control females) had more than one egg in their oviducts, and 12% had released eggs simultaneously into both lateral oviducts, a phenotype also not observed in controls (data not shown). No significant difference was found between the number of eggs in the reproductive tracts of ovulin-expressing females at 18, 24, and 39 h after heat shock (Fig. 3A). To confirm our observations, we compared the effect of heat shock on the ovulation of hsp70-Gal4;UAS-ovulin flies with that of transgenic z1w11e4 flies carrying a non-Acp transgene, hsp26-Ya. Again, at all time points examined, females expressing ovulin had more eggs in their reproductive tracts than females not expressing ovulin (18 h after heat shock: hsp70-Gal4;UAS-ovulin flies vs. hsp26-Ya flies, 0.75 ± 0.1 vs. 0.42 ± 0.087 eggs per female; P < 0.05) (Fig. 3 A and C). Similar results were obtained in comparisons with another control, the non-ovulin-expressing transgenic females, hsp70-Gal4;UAS-GFP (data not shown). The increase in ovulation also indirectly elevated the egg deposition at some time points. Whereas, at 18 h after heat shock, only 18% of all of the ovulating females, control and ovulin-expressing, deposited eggs (see Fig. 3D), by 24 h after heat shock, 37% of ovulin expressing females deposited 4.2 ± 1.2 eggs per female and control hsp26-Ya females deposited 1.12 ± 0.5 per female (P < 0.025) (Fig. 3D). The number of eggs deposited by ovulin-expressing females increased with time after heat shock; however, the average number of eggs deposited per female was very low in comparison with wild-type females mated to wild-type males (e.g., ≈60 eggs per female in our wild-type stocks). The fact that ovulin-expressing females deposited fewer eggs than wild-type mated females supports our previous findings that the main role of ovulin is in releasing oocytes from the ovary (10). Because other factors received by the female during mating, including sex peptide (Acp70A), affect the number of eggs produced, induction of ovulin in unmated females is not expected to elevate egg deposition to postmated levels (14, 15, 20, 21).
Fig. 3.
Full-length ovulin can stimulate ovulation in unmated virgin females. (A-C) The ovulation pattern and rate of hsp70-GAL4;UAS-ovulinfull length-expressing females (A), non-heat-shocked hsp70-GAL4;UAS-ovulinfull length females (B) and hsp26-Ya females (C) at different time points after heat shock (10, 18, 24, and 39 h). The black bars and the white bars represent the average number of eggs found in the lateral oviducts and the external opening of the reproductive tract, respectively. (D) The average number (± SEM) of eggs deposited in the holding vials by hsp70-Gal4;UAS-ovulinfull length-expressing females, (black bars), non-heat-shocked hsp70-Gal4;UAS-ovulinfull length females (gray bar), and hsp26-Ya females (white bars) at different time points after heat shock. For each bar at each time point, n = 50-65 females. Expression of ovulinfull-length in unmated virgin females increased the odds of finding an egg in the lateral oviducts. (E) Path diagram showing the longitudinal model for the combined effects of ovulin and the time after heat shock (10 h, red arrow; 18 h, blue arrow; 24 h, gray arrow) on finding an egg in the lateral oviducts and on egg deposition. Arrows represent cause-and-effect relationships. The number by each arrow is the odds ratio determined by logistic regression. The coefficient for egg deposition was determined by linear regression (spss 8.0 for windows). *, P < 0.001.
To confirm that ectopically expressed ovulin affected ovulation specifically, we performed Path analysis of egg distribution in hsp70-Gal4;UAS-ovulin heat-shocked females and hsp70-Gal4;UAS-ovulin non-heat-shocked females or heat-shocked hsp26-Ya females (negative control) (Fig. 3E). As described in Heifetz et al. (10), such analysis allows the separate quantification of the effect of ovulin expression on each step in the egg-laying process (10). The odds of finding an egg in the lateral oviducts (the probability of finding an egg divided by the probability of not finding an egg) were 11.63 times higher in females expressing ovulin than in non-heat-shocked females that did not express ovulin (P < 0.00001) (Fig. 3E) and were 2.02 times higher in females expressing ovulin than in hsp26-Ya-expressing females (P < 0.01; data not shown). Neither ovulin expression nor the presence of an egg in the lateral oviducts had a significant effect on the number of eggs deposited by the unmated females. Furthermore, the time after heat shock had no effect on the odds of finding an egg in the lateral oviducts. The only significant effect that was seen was at 10 h after heat shock, but this effect was low [the odds of finding an egg in the lateral oviducts at this time was 0.1 (P < 0.008; Fig. 3E)]. Thus, our data indicate that ectopic expression of ovulin in virgin females stimulates, specifically, the release of oocytes by the ovary.
Expression of the Two N-Terminal CPs of Ovulin Does Not Stimulate Ovulation. Because ectopic expression of full-length ovulin is sufficient to induce ovulation, we used the same assay to test the CPs or subregions of ovulin for activity. Ectopic expression of either CP1 (the most N-terminal CP) or CP2 (the next most N-terminal CP) had no effect on ovulation rate. For example, at 24 h after heat shock, the odds of finding an egg in the lateral oviducts were not significantly different in hsp70-Gal4;UAS-CP1-expressing females and in hsp70-Gal4;UAS-GFP-expressing females (CP11.1.1.1e vs. GFP, 1.87 vs. 1.39; CP11.1.3 vs. GFP, 1.6 vs. 1.57; see also Table 1, which is published as supporting information on the PNAS web site). If the processing of ovulin is sequential, it is possible that the first processing events may not generate products that themselves stimulate ovulation; rather, they might be needed to allow for subsequent cleavages to liberate functional product(s). Alternatively, the functions of either of the first two CPs may require other Acps or their CPs, modification of ovulin in the male, or products of genes that females only induce upon mating (47). It is also possible that the first two CPs have some other functions that our bioassay cannot detect.
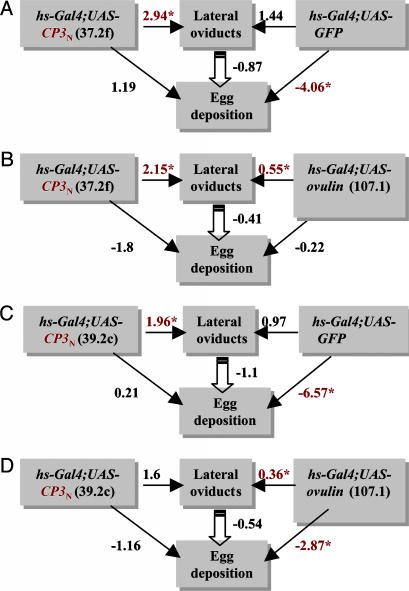
The Final Two C-Terminal CPs of Ovulin Independently Stimulate Ovulation. CP3N and the more C-terminal CP3 (CP3C; amino acids 118-264) each independently stimulated ovulation. The odds of finding an egg in the lateral oviducts are 2.94-fold higher in hsp70-Gal4;UAS-CP3N-expressing females than in their siblings that do not express CP3N (P < 0.0001) (Fig. 4A). The odds of finding an egg in the lateral oviducts also differ between hsp70-Gal4;UAS-CP3N-expressing and hsp70-Gal4;UAS-GFP-expressing females (Fig. 4A). Whereas hsp70-Gal4;UAS-CP3N-expressing females had significantly more eggs in the lateral oviducts than their siblings, GFP-expressing females do not have significantly more eggs in their lateral oviducts than do their siblings that do not express GFP (Fig. 4A). Moreover, CP3N had a greater effect on the lateral oviducts than the full-length ovulin (CP3N(37.2f) vs. ovulin, 2.15 vs. 0.55) (Fig. 4B). The second CP3N line examined showed the same effect on ovulation as the first line (Fig. 4 and Table 2, which is published as supporting information on the PNAS web site) however, in hsp70-Gal4;UAS-CP3N(39.2c)-expressing females the odds of finding an egg in the lateral oviducts are greater than in their siblings that do not express CP3N(39.2c), but this difference was not significant (Fig. 4D).
Fig. 4.
Expression of ovulin CP3N had a higher effect on finding an egg in the lateral oviducts of virgin females than did full-length ovulin. Shown are path diagrams illustrating the longitudinal model for the effects of CP3N(37.2f) vs. GFP (A), CP3N(37.2f) vs. full-length ovulin (line 107.1) (B), CP3N(39.2C) vs. GFP (C), and CP3N(39.2C) vs. full-length ovulin (D) on the presence of an egg in the lateral oviducts and on egg deposition (24 h after heat shock). Arrows represent cause-and-effect relationships. The number by each arrow is the odds ratio determined by logistic regression. The coefficient for egg deposition was determined by linear regression; a negative number indicates fewer eggs deposited. For each line, n = 45-62 females. *, P < 0.001.
Females expressing CP3C (lines 7.1a and 10.1) also had elevated ovulation, which is of particular interest because CP3C contains the regions with sequence similarity to Aplysia egg-laying hormones. The ovulation rate of hsp70-Gal4;UAS-CP3C-expressing females was somewhat higher than hsp70-Gal4;UAS-CP3N-expressing females (CP3C(7.1a) vs. CP3N(37.2f), 50% vs. 31% of the females had an egg in the lateral oviducts; data not shown). As shown above for CP3N, the odds of finding an egg in the lateral oviducts were different between hsp70-Gal4;UAS-CP3C and hsp70-Gal4;UAS-GFP-expressing females. Again, as observed for CP3N(39.2c), CP3C(7.1a) had a greater effect on the lateral oviducts than full-length ovulin (CP3C(7.1a) vs. ovulin, 1.88 vs. 0.34; see Table 1), and CP3C(10.1) had no significant effect on the lateral oviducts.
We conclude that ectopic expression of CP3N and CP3C significantly elevates ovulation rate. Because the transgenic flies we tested expressed only CP3N or CP3C, each of these subregions can stimulate ovulation independently. That two CPs of the same prohormone are active is seen in other cases of processed prohormones, including Aplysia pro-ELH and pro-califin (29, 48, 49). In Aplysia, more potent forms of α-bag cell peptide (which is derived from pro-ELH) are generated upon additional cleavage to α-bag cell peptide and α1-8 and α1-7 forms (50, 51). It is also formally possible that an analogous phenomenon underlies the presence of two active processing products of ovulin or that the two subregions might mediate different functions in the ovulation process that our bioassay cannot distinguish.
Thus, cleavage of ovulin in the mated female can release at least two active products. The two CPs that were active in our assay were only detected within the reproductive tract of a normally mated female. Full-length ovulin was also active in our assay, even though we did not detect cleavage of this molecule upon ectopic expression (data not shown) (35). Although we detected independently active molecules that were liberated by ovulin cleavage, we cannot rule out the possibility that the whole molecule was also active or that cleavage, at levels too low to detect on our Western blots, occurred in target tissues.
The ability of full-length ovulin and of its last two CPs to mediate ovulation is noteworthy. In most cases studied, processing liberates an active product(s) from an inactive prohormone, e.g., as in the case of the ELH of A. californica (28-32). Our results suggest the possibility of regulation associated with the rate of ovulin-processing in the female reproductive tract. Ovulin enters the female reproductive tract during mating, and, by 10 min after the start of mating, its first CP is seen. At 50 min after the start of mating, CP2 and CP3 are observed; at this time CP1 is no longer detected (35). If cleaved products (CP3N and CP3C) are more potent than the full-length form, then it is possible that ovulation is regulated in part by the cleavage of ovulin. For example, full-length ovulin could initiate ovulation at a low rate. Later, when CP3N and CP3C have been generated, the ovulation rate could be increased by their action. This regulation of the increase in the ovulation rate might be helpful in coordinating ovulation with other processes induced by mating, perhaps to ensure the highest possible frequency of fertilization. Alternatively, full-length ovulin and its CPs might simply be functionally redundant. In this case, full-length ovulin ensures that, in the absence of cleavage, oocytes will be ovulated.
The N-Terminal Subregion of Ovulin, Amino Acids 1-138, Can Stimulate Ovulation upon Ectopic Expression. We used the ectopic expression assay to determine whether ovulin ELH and califin C similarity regions were essential for activity. We first deleted ovulin's amino acids 167-264 to generate Acp26Aa2, the truncation allele of ovulin that is functional in vivo (subregion 1-166) (9). This construct includes the entire ovulin ELH-similar region and the entire region with similarity to califin C. We also deleted amino acids 121-264 to generate subregion 1-120, which removes the entire similarity to ELH; amino acids 139-264 to generate subregion 1-138, which removes the uniquely califin C similarity; and amino acids 155-264 to generate subregion 1-154, which removes part of the califin C-similar region (see Fig. 2B). In addition, we generated subregion 118-166 to test whether the ELH/califin C-similar region is sufficient for ovulin function.
In four of these cases (subregions 1-166, 1-120, 1-154, and 118-166), although females produced an ovulin protein of predicted size upon heat shock (data not shown), there was no stimulation of ovulation above background levels (see Table 1). Because this result was negative, we could not determine whether it was due to presence of insufficient overall sequence for function or to improper folding. Amino acids 154 and 166 fall within predicted α-helices; we suggest that disruption of those helices might cause the protein to fold in ways that prevent activity. The latter suggestion is implied by the failure of ectopically expressed subregion 1-166 to stimulate ovulation. This portion of ovulin (encoded by the Acp26Aa2 allele) is sufficient for full function when introduced into the female from the male (9). Perhaps in that situation, it associates with partners that can stabilize it in an active conformation.
Subregion 1-138, which contains the ELH-similar, but not the califin-similar, region, was able to stimulate ovulation. The odds of finding an egg in the lateral oviducts of hsp70-Gal4;UAS-1-138(12.1)-expressing females was 3.08-fold higher than in their siblings that do not express subregion 1-138 (P < 0.001; see Table 1). Whereas hsp70-Gal4;UAS-1-138(12.1)-expressing females had significantly more eggs in the lateral oviducts than their nonexpressing siblings, hsp70-Gal4;UAS-GFP-expressing females were not significantly different from their siblings that do not express GFP (Table 2). Moreover, subregion 1-138 had a greater effect on the lateral oviducts than full-length ovulin (subregion 1-138(12.1) vs. ovulin, 1.98 vs. 0.64; Table 1). As we observed for CP3N and CP3C, in subregion 1-138(10.1a)-expressing females, the odds of finding an egg in the lateral oviducts were greater than in their siblings that did not express subregion 1-138(10.1a), but this difference was not significant (Table 1). Because CP3N alone can stimulate ovulation and subregion 1-138 contains it, we cannot determine whether this result reflects activity of the CP3N part of subregion 1-138 or whether it indicates that CP3C does not require its califin C similarity for function.
Conclusions
We showed here that ectopic expression in females of ovulin, a seminal protein normally provided to females by males, stimulates ovulation by D. melanogaster females. Ovulin is normally processed sequentially upon entry into females (25, 27, 35), and we found that its two final CPs, CP3N and CP3C, each can significantly increase ovulation when ectopically expressed in unmated females.
That ovulin is not processed in the male accessory glands and that accessory gland main cell products are essential for the cleavage of ovulin in the female reproductive tract (35) support the notion that male and female cooperate to ensure a high ovulation rate at the right time. How might such molecular cooperation between the sexes occur? Mating induces expression in females of proteases (47), some of which are primarily expressed in the female reproductive tract (52) and might cleave ovulin. Alternatively, proteases that cleave ovulin could be present in an inactive form in the seminal fluid. Once transferred to the female, such a protease(s) could be activated by factors in the female reproductive tract. Lastly, the protease(s) that cleaves ovulin could be in the male's seminal fluid, but their activity might be inhibited by protease inhibitors until transfer to the female. Our data suggest that cleavage of ovulin may assist in acquiring the capacity to induce the high ovulation rate observed in mated females.
Tsaur et al. (53) report that the N-terminal CP of ovulin is particularly polymorphic within some Drosophila species, and they proposed that this might be due to some functional correlate, such as the action of ovulin on sperm. Our genetic studies do not detect a role for ovulin in sperm storage/competition (35, 54), and ovulin does not associate with sperm (16) or enter the sperm storage organs (M. Bloch-Qazi and M.F.W., unpublished data). Although the ectopic expression studies reported here also do not detect a role for this region of ovulin in ovulation stimulation, it is possible that, in the context of a normal mating rather than of ectopic expression, a function could be found for this region that would explain its unusual evolutionary dynamics.
Identifying the active regions of ovulin is valuable for several reasons. First, finding the regions of the protein that are active could be used biochemically to define ovulin receptors. Second, our results indicate that modulation of ovulin when it enters the female does not terminate its activity. Such modulation of prohormonal Acps could be a function for the proteolytic regulators found in Drosophila seminal fluid (1, 2, 18).
Supplementary Material
Acknowledgments
We thank M. Bloch-Qazi, M. Goldberg, R. MacIntyre, L. McGraw, J. Mueller, K. Ravi Ram, and A. Wong for helpful comments on the manuscript; J. Belote for instruction in embryo injection and helpful discussions; S. Ji for assistance with Western blots; and G. Boulianne for UAS-GFP flies. This work was supported by National Science Foundation Grant 99-04824 and National Institutes of Health Grant HD38921. L.N.V. and H.I.C. received partial support from the Cornell/Howard Hughes Summer Research Scholar Program.
Author contributions: Y.H. and M.F.W. designed research; Y.H., L.N.V., and H.I.C. performed research; Y.H., L.N.V., H.I.C., and M.F.W. analyzed data; and Y.H., L.N.V., and M.F.W. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: UAS, upstream-activated sequence; Acp, accessory gland protein; CP, cleavage product; CP1, first CP (amino acids 18-48); CP2, second CP (amino acids 49-67); CP3N, the more N-terminal third CP (amino acids 68-117); CP3C, the more C-terminal third CP (amino acids 118-264).
References
- 1.Swanson, W. J., Clark, A. G., Waldrip-Dail, H. M., Wolfner, M. F. & Aquadro, C. F. (2001) Proc. Natl. Acad. Sci. USA 98, 7375-7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mueller, J. L., Ripoll, D. R., Aquadro, C. F. & Wolfner, M. F. (2004) Proc. Natl. Acad. Sci. USA 101, 13542-13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfner, M. F. (2002) Heredity 88, 85-93. [DOI] [PubMed] [Google Scholar]
- 4.Kubli, E. (1996) Adv. Dev. Biochem. 4, 99-128. [Google Scholar]
- 5.Eberhard, W. G. (1996) Female Control: Sexual Selection by Cryptic Female Choice (Princeton Univ. Press, Princeton).
- 6.Wolfner, M. F. (1997) Insect Biochem. Mol. Biol. 27, 179-192. [DOI] [PubMed] [Google Scholar]
- 7.Gillott, C. (2003) Annu. Rev. Entomol. 48, 163-184. [DOI] [PubMed] [Google Scholar]
- 8.Chapman, T. & Davies, S. J. (2004) Peptides 25, 1477-1490. [DOI] [PubMed] [Google Scholar]
- 9.Herndon, L. A. & Wolfner, M. F. (1995) Proc. Natl. Acad. Sci. USA 92, 10114-10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heifetz, Y., Lung, O., Frongillo, E. A., Jr., & Wolfner, M. F. (2000) Curr. Biol. 10, 99-102. [DOI] [PubMed] [Google Scholar]
- 11.Chapman, T. (2001) Heredity 87, 511-521. [DOI] [PubMed] [Google Scholar]
- 12.Chen, P. S., Stumm-Zollinger, E., Aigaki, T., Balmer, J., Bienz, M. & Bohlen, P. (1988) Cell 54, 291-298. [DOI] [PubMed] [Google Scholar]
- 13.Aigaki, T., Fleischmann, I., Chen, P. S. & Kubli, E. (1991) Neuron 7, 557-563. [DOI] [PubMed] [Google Scholar]
- 14.Chapman, T., Bangham, J., Vinti, G., Seifried, B., Lung, O., Wolfner, M. F., Smith, H. K. & Partridge, L. (2003) Proc. Natl. Acad. Sci. USA 100, 9923-9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, H. & Kubli, E. (2003) Proc. Natl. Acad. Sci. USA 100, 9929-9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neubaum, D. M. & Wolfner, M. F. (1999) Genetics 153, 845-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bloch-Qazi, M. C. & Wolfner, M. F. (2003) J. Exp. Biol. 206, 3521-3528. [DOI] [PubMed] [Google Scholar]
- 18.Lung, O., Tram, U., Finnerty, C. M., Eipper-Mains, M. A., Kalb, J. M. & Wolfner, M. F. (2002) Genetics 160, 211-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bloch-Qazi, M. C., Heifetz, Y. & Wolfner, M. F. (2003) Dev. Biol. 256, 195-211. [DOI] [PubMed] [Google Scholar]
- 20.Soller, M., Bownes, M. & Kubli, E. (1997) Eur. J. Biochem. 243, 732-738. [DOI] [PubMed] [Google Scholar]
- 21.Soller, M., Bownes, M. & Kubli, E. (1999) Dev. Biol. 208, 337-351. [DOI] [PubMed] [Google Scholar]
- 22.Morris, J. & Lehmann, R. (1999) Curr. Biol. 9, R55-R58. [DOI] [PubMed] [Google Scholar]
- 23.Matova, N. & Cooley, L. (2001) Dev. Biol. 231, 291-320. [DOI] [PubMed] [Google Scholar]
- 24.Heifetz, Y., Yu, J. & Wolfner, M. F. (2001) Dev. Biol. 234, 416-424. [DOI] [PubMed] [Google Scholar]
- 25.Monsma, S. A., Harada, H. A. & Wolfner, M. F. (1990) Dev. Biol. 142, 465-475. [DOI] [PubMed] [Google Scholar]
- 26.Heifetz, Y. & Wolfner, M. F. (2004) Proc. Natl. Acad. Sci. USA 101, 6261-6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monsma, S. A. & Wolfner, M. F. (1988) Genes Dev. 2, 1063-1073. [DOI] [PubMed] [Google Scholar]
- 28.Scheller, R. H., Jackson, J. F., McAllister, L. B., Schwartz, J. H., Kandel, E. R. & Axel, R. (1982) Cell 28, 707-719. [DOI] [PubMed] [Google Scholar]
- 29.Scheller, R. H., Jackson, J. F., McAllister, L. B., Rothman, B. S., Mayeri, E. & Axel, R. (1983) Cell 32, 7-22. [DOI] [PubMed] [Google Scholar]
- 30.Nagle, G. T., Painter, S. D., Blankenship, J. E., Dixon, J. D. & Kurosky, A. (1986) J. Biol. Chem. 261, 7853-7859. [PubMed] [Google Scholar]
- 31.Nagle, G. T., Painter, S. D., Blankenship, J. E. & Kurosky, A. (1988) J. Biol. Chem. 263, 9223-9237. [PubMed] [Google Scholar]
- 32.Nagle, G. T., de Jong-Brink, M., Painter, S. D., Bergamin-Sassen, M. M., Blankenship, J. E. & Kurosky, A. (1990) J. Biol. Chem. 265, 22329-22335. [PubMed] [Google Scholar]
- 33.Paolillo, L., Simonetti, M., Brakch, N., D'Auria, G., Saviano, M., Dettin, M., Rholam, M., Scatturin, A., Di Bello, C. & Cohen, P. (1992) EMBO J. 11, 2399-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siviter, R. J., Coast, G. M., Winther, A. M., Nachman, R. J., Taylor, C. A., Shirras, A. D., Coates, D., Isaac, R. E. & Nassel, D. R. (2000) J. Biol. Chem. 275, 23273-23280. [DOI] [PubMed] [Google Scholar]
- 35.Park, M. & Wolfner, M. F. (1995) Dev. Biol. 171, 694-702. [DOI] [PubMed] [Google Scholar]
- 36.Rothman, B. S., Weir, G. & Dudek, F. E. (1983) Gen. Comp. Endocrinol. 52, 134-141. [DOI] [PubMed] [Google Scholar]
- 37.Rothman, B. S., Hawke, D. H., Brown, R. O., Lee, T. D., Dehghan, A. A., Shively, J. E. & Mayeri, E. (1986) J. Biol. Chem. 261, 1616-1623. [PubMed] [Google Scholar]
- 38.Chapman, T., Liddle, L. F., Kalb, J. M., Wolfner, M. F. & Partridge, L. (1995) Nature 373, 241-244. [DOI] [PubMed] [Google Scholar]
- 39.Brand, A. H. & Perrimon, N. (1993) Development (Cambridge, U.K.) 118, 401-415. [DOI] [PubMed] [Google Scholar]
- 40.Lopez, J. M., Song, K., Hirshfeld, A. B., Lin, H. & Wolfner, M. F. (1994) Dev. Biol. 163, 202-211. [DOI] [PubMed] [Google Scholar]
- 41.Yeh, E., Gustafson, K. & Boulianne, G. L. (1995) Proc. Natl. Acad. Sci. USA 92, 7036-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubin, G. M. & Spradling, A. C. (1982) Science 218, 348-353. [DOI] [PubMed] [Google Scholar]
- 43.Klemenz, R., Weber, U. & Gehring, W. J. (1987) Nucleic Acids Res. 15, 3947-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart, B. A., Atwood, H. L., Renger, J. J., Wang, J. & Wu, C. F. (1994) J. Comp. Physiol. A 175, 179-191. [DOI] [PubMed] [Google Scholar]
- 45.Wright, S. (1968) Genetic and Biometric Foundations (Univ. Chicago Press, Chicago).
- 46.Li, C. C. (1975) Path Analysis-A Primer (Boxwood, Pacific Grove, CA).
- 47.McGraw, L. A., Gibson, G., Clark, A. G. & Wolfner, M. F. (2004) Curr. Biol. 14, 1509-1514. [DOI] [PubMed] [Google Scholar]
- 48.Newcomb, R. & Scheller, R. H. (1987) J. Neurosci. 7, 854-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Heumen, W. R. A., Nagle, G. T. & Kurosky, A. (1995) Cell Tissue Res. 279, 13-24. [DOI] [PubMed] [Google Scholar]
- 50.Sigvardt, K. A., Rothman, B. S., Brown, R. O. & Mayeri, E. (1986) J. Neurosci. 6, 803-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garden, R. W., Shippy, S. A., Li, L., Moroz, T. P. & Sweedler, J. V. (1998) Proc. Natl. Acad. Sci. USA 95, 3972-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swanson, W. J., Wong, A., Wolfner, M. F. & Aquadro, C. F. (2004) Genetics 168, 1457-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsaur, S. C., Ting, C. T. & Wu, C. I. (2001) Mol. Biol. Evol. 18, 22-26. [DOI] [PubMed] [Google Scholar]
- 54.Herndon, L. A. (1997) Ph.D. thesis (Cornell University, Ithaca, NY).
- 55.Mahowald, A. P. & Kambysellis, M. P. (1980) in The Genetics and Biology of Drosophila, eds. Ashburner, M. & Wright T. R. F. (Academic, New York), Vol. 2D, pp. 141-224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.