Abstract
The superior colliculus (SC) is part of a network of brain areas that directs saccadic eye movements, overtly shifting both gaze and attention from position to position, in space. Here, we seek direct evidence that the SC also contributes to the control of covert spatial attention, a process that focuses attention on a region of space different from the point of gaze. While requiring monkeys to keep their gaze fixed, we tested whether microstimulation of a specific location in the SC spatial map would enhance visual performance at the corresponding region of space, a diagnostic measure of covert attention. We find that microstimulation improves performance in a spatially selective manner: thresholds decrease at the location in visual space represented by the stimulated SC site, but not at a control location in the opposite hemifield. Our data provide direct evidence that the SC contributes to the control of covert spatial attention.
Keywords: discrimination, psychophysics
Several lines of evidence suggest that eye movements and covert attention may be mediated by the same neural mechanisms (1–3). For example, a human subject can direct attention to a specific location in space, thereby gaining a measurable advantage in visual performance, even while maintaining fixation at an altogether different location (4). During a saccadic eye movement, however, the subject is unable to direct attention to any location other than the endpoint of that eye movement (5).
In the monkey, electrophysiological experiments have increasingly implicated eye-movement planning structures in the control of covert spatial attention. Following the original observation by Goldberg and Wurtz (6) of attention-related neural activity in the superior colliculus (SC) (7, 8), single-unit recordings have detected attentional effects in other eye movement-related areas of the brain, including the inferior parietal cortex (9–11) and the frontal eye fields (FEF) (12–14). Recent studies by Bisley and Goldberg (15) in the lateral intraparietal area and by Ignashchenkova et al. (16) in the SC were particularly incisive because the neural effects correlated parametrically with variations in the strength and timing of attentional effects in the behavioral data. Electrophysiological evidence, however, is necessarily correlative and cannot demonstrate that neural activity causes behavior (17).
Remarkable studies published recently by Moore and Fallah (18, 19) bridged this gap. Using visual threshold measurements as a behavioral metric of attention, they showed that electrical microstimulation of the FEF improved psychophysical performance by facilitating the deployment of attention to the location of the visual stimulus. The effect was spatially localized to the region of the visual field encoded at the stimulation site and thus could not be attributed to a general increase in arousal or vigilance. This was a landmark study first because of its implications concerning the neural substrate of visuo-spatial attention, but more importantly because it is the only demonstration (of which we are aware) of a transient brain manipulation that actually improves perceptual performance. Previous studies have shown that microstimulation can bias perceptual choices in discrimination tasks (20–22) or serve as a substitute for a stimulus that is not actually present (23). But no manipulation of this sort has improved an animal's ability to discern what is actually present in the sensory world.
To explore this important phenomenon further, we adopted the general approach of Moore and Fallah to test whether electrical stimulation of the SC, like that of the FEF, exerts a causal influence on covert attention. We sought to determine whether a subcortical oculomotor structure such as the SC contributes to the control of covert attention and to confirm the observation of Moore and Fallah that perceptual performance can be improved by local microstimulation. We find that SC stimulation indeed lowers psychophysical thresholds in a spatially selective manner, suggesting that spatial attention is controlled cooperatively by multiple eye movement structures, including the SC.
Methods
We used standard surgical procedures and techniques for physiological recording, microstimulation, and data collection, all of which have been described (24). All experimental procedures and care of the animals were carried out in compliance with guidelines established by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of Stanford University.
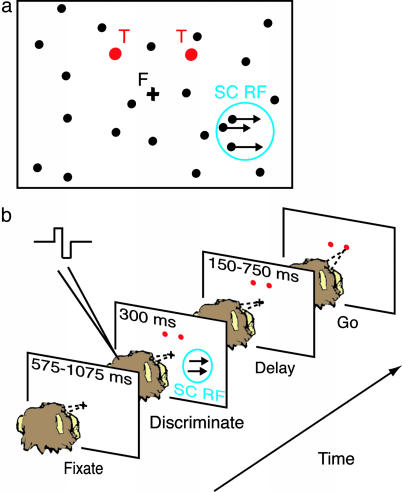
Behavioral Task. Fig. 1 depicts the behavioral task. Monkeys viewed a motion stimulus and made two-alternative forced-choice judgments of its direction of motion. For monkey W, the delay period after stimulus offset was randomized over the range of 350–750 ms. Because monkey S often broke fixation during long delays, we frequently used a shorter range of delays for her (150–400, 250–500, or 350–750 ms). Delay length did not affect the results. Eye position was continuously monitored by means of a scleral search coil (25), sampled at a rate of 1 kHz. The monkey was required to maintain eye position with a 1° radius window around the fixation point until the “go” signal occurred. Inappropriate fixation breaks resulted in aborted trials. Throughout these experiments, the monkeys discriminated between rightward and leftward motion. The monkey indicated its choice by making a saccade to one of two targets flanking the vertical midline. We positioned the saccade targets well away from the coherently moving dots (and thus from the SC response field): when the coherently moving dots were centered above the horizontal midline, the targets were always placed below; when the dots were centered below the horizontal midline, the targets were always placed above. The monkey received a liquid reward for each correct choice.
Fig. 1.
Direction discrimination task used to measure spatial attention. (a) Spatial arrangement of fixation cross (F), coherently moving dots (arrows), flickering distracter dots (black circles), response targets (T, red circles), and SC topographic location (blue circle) for the test condition. The stimulus aperture was square, and the width and height of the stimulus aperture were set to one-half its distance from fixation. The aperture was always centered on the SC response field. Because coherent motion always flowed rightward or leftward in these experiments, the square aperture could not induce artifactual motion signals at its edges (i.e., the barberpole illusion). (b) Sequence of trial events. The monkey was required to fixate throughout the trial. Disappearance of the fixation point cued the monkey to make an eye movement to one of the response targets, corresponding to the perceived direction of motion. Microstimulation overlapped in time with the coherent motion signal (see Methods).
In control blocks, the motion aperture was typically placed at a mirror location across the vertical meridian from the location used in test blocks. We departed from this procedure only when the SC response field was located near the vertical meridian. To ensure adequate distance between test and control aperture locations in these cases, the control aperture was positioned further into the hemifield contralateral to the test aperture by adding 90° of polar angle to the polar coordinates of the test aperture. Thus, the eccentricity of the aperture remained the same as that of the test aperture. Dot density within the stimulus aperture was always 13 dots/deg2 per s. Outside the aperture, the monitor (65° × 48°, viewed at 35 cm) was filled with randomly flickering distracter dots with density 13 dots/deg2 per s (monkey W) or 3–8 dots/deg2 per s (monkey S). Thus, the only visual cues to the aperture location were the coherent motion signal itself (both monkeys) and a modest dot density difference (monkey S only). We provided monkey S with the additional cue because she had extreme difficulty with the task otherwise. Stimuli of varying coherence (usually 12–96%; 300-ms duration) were randomly interleaved, as were trials with and without electrical stimulation. Electrical microstimulation (333 Hz, biphasic current pulses, 150 μs per phase) began 50 ms before the random dots appeared and lasted for 300 ms (monkey W) or 400 ms (monkey S).
Psychometric Functions. Behavioral performance was characterized by computing the best-fitting cumulative Weibull distribution for each data set:
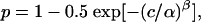 |
[1] |
where p is the proportion of correct decisions computed as a function of motion coherence, c. The fitted parameters α and β are the coherence supporting threshold performance (82% correct) and the slope of the curve, respectively (26, 27). The function generally fitted our data well. For the main data set, even the most stringent criterion (χ2, P < 0.05) would have allowed the fit to be rejected for only 1.4% (4/268) of psychometric functions.
When behavioral data were averaged across multiple sessions, the amount of data was substantially larger, making the χ2 test highly sensitive to the exact position of the data points. To obtain good fits, we generated the smooth curves in Fig. 4 with a modified Weibull function with an additional free parameter, δ, the monkey's asymptotic performance at high coherence.
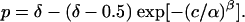 |
[2] |
The additional parameter was necessary to account for the fact that asymptotic performance for the highest coherence was slightly below 100% correct. The fits in Fig. 4 obviously describe the data very well, although one of the four fits could still be rejected statistically because of the very large amount of data (Fig. 4b, dashed trace, χ2, P = 0.033).
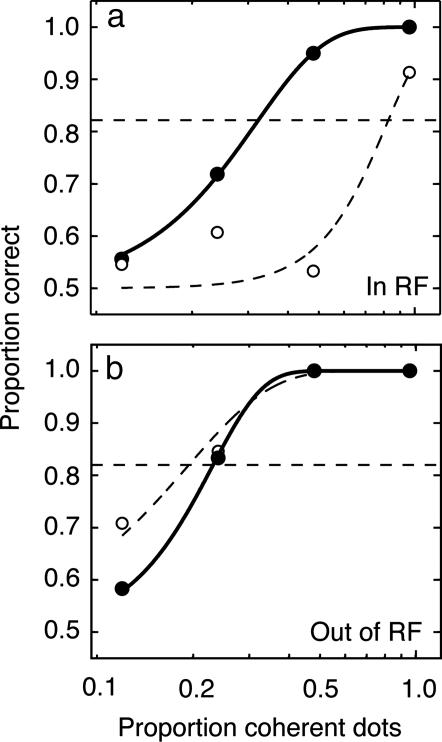
Fig. 4.
Average psychometric function obtained during 64 of our 67 experiments (those during which we measured performance at exactly the same family of four coherences). Each data point is the average proportion correct across 64 experiments. Error bars (often smaller than the symbols, average 0.011) indicate ±1 SEM. Filled and open symbols and solid and dashed curves correspond to trials with and without electrical stimulation, respectively. (a) Average performance when coherent motion was at the location represented by the SC stimulation site. Psychometric functions fit to these average data indicate that threshold is significantly reduced from 51% to 46% coherence (bootstrap test, P < 0.01). (b) Performance when coherent motion was at the control location. Threshold was not reduced (bootstrap test, P > 0.7).
Electrically Evoked Saccades. To elicit saccadic eye movements, we applied trains of microstimulation pulses to the SC (100-ms trains, 333 Hz, biphasic current pulses, 150 μs per phase). The average endpoint of the evoked saccade was always similar to the most responsive region of the visual receptive field (RF) and/or movement field (MF) of neurons at the electrode tip (28–30), as we always confirmed by using passive fixation trials and/or a delayed saccade task
Statistical Tests. All statistical tests were one-tailed unless otherwise specified. We used paired t tests and bootstrap tests. To implement the bootstrap test, we randomly recombined trials with and without microstimulation (main experiment) or with and without distracters (preliminary experiment) at each coherence level and recomputed psychophysical thresholds for the randomly mixed data sets. This procedure was repeated 2,000 times, generating a distribution of threshold differences that would be expected by chance for each data set. The cited P values are the proportion of simulated threshold differences that were greater than or equal to the difference that was observed experimentally.
Analysis of Choice Bias. To analyze choice bias, we plotted psychometric data from each experiment as the percentage of rightward choices (recall that the task always involved a right-left discrimination) versus signed coherence. From logistic curves fitted to such plots, we identified the visual stimulus (direction and coherence) at that yielded rightward and leftward choices with equal frequency. We evaluated choice bias separately for psychometric curves obtained on stimulated and unstimulated trials, and compiled distributions of choice bias for the two trial types across the entire data set. As indicated in the main text, the two distributions were not significantly different.
Results
Behavioral Performance. To test directly the role of the SC in directing spatial attention, we examined the effect of subthreshold electrical microstimulation on the direction discrimination performance of two awake, behaving rhesus monkeys. Monkeys discriminated the direction of coherent motion in a family of stochastic random dot stimuli (31) (Fig. 1). The motion stimulus appeared within a localized aperture; a specified proportion of the random dots moved coherently with a particular direction and speed whereas the remaining dots flickered at random locations and times, creating a masking motion noise. We used a range of stimulus coherences so as to measure psychophysical threshold on the discrimination task. The central question of our experiments is whether microstimulation of the SC causes a spatially selective improvement in coherence thresholds as would be expected from increased spatial attention.
A possible flaw in our experimental design is the relative simplicity of the psychophysical discrimination. If excellent performance can be achieved with little attentional effort, increasing attention by stimulating any specific brain structure may exert little or no effect on psychophysical thresholds. To make the task attentionally demanding, therefore, we filled the remainder of the video monitor with randomly flickering noise dots (distracters), and we presented the visual stimulus for brief intervals (300 ms). To confirm that these manipulations increased task difficulty, we made psychophysical measurements on a monkey who performed the task with and without the distracter dots surrounding the motion aperture. The motion aperture had the same size and location throughout the experiment. Trials incorporating the distracter dots were randomly interleaved among trials lacking the distracters so that we could directly compare performance obtained under the two conditions.
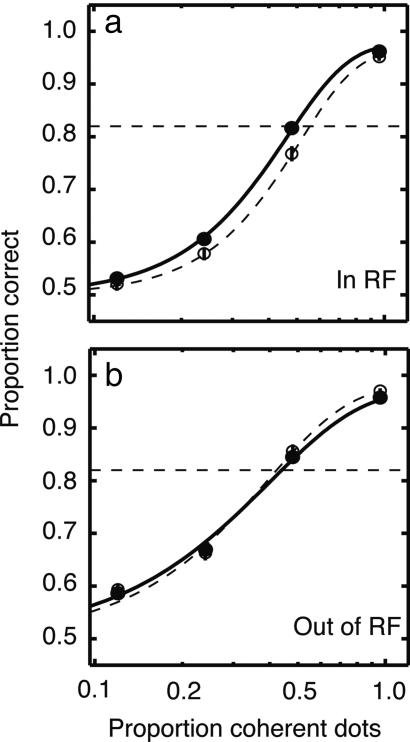
Fig. 2 shows the performance of the monkey in this experiment. Data are displayed as psychometric functions, with the proportion of correct decisions plotted against the strength of the motion signal (i.e., the proportion of coherently moving dots within the stimulus aperture). Performance approached 100% correct for the strongest motion signals, but was near chance (50% correct) for the weakest signals. The filled and open symbols and solid and dashed curves represent trials with and without distracters, respectively. The smooth curves are best fits of a standard psychometric function (see Methods). We took psychophysical threshold to be the proportion of coherently moving dots that supported performance of 82% correct. The distracters made the task substantially more difficult for the monkey, increasing threshold by a factor of 2.2, from 32% to 70% coherence (bootstrap test, P < 0.0005). Thus, we are confident that psychophysical thresholds can be improved in principle by increased spatial attention.
Fig. 2.
Psychometric functions describing direction discrimination performance with distracters (filled circles, solid traces) and without (open circles, dashed traces). The proportion of correct decisions is plotted against the strength of the motion signal. Threshold performance (82% correct, dashed horizontal line) was computed from the fitted curves (see Methods).
Influence of SC Microstimulation on Behavioral Performance. To examine the role of the SC in attention, we performed psychophysical experiments similar to those described above on two rhesus monkeys. Critically, distracters were always present in these experiments to make the task more demanding. While the monkey performed the task, we applied subthreshold microstimulation to the SC on half of the trials, selected randomly. Our database consists of 67 stimulation sites in two animals (42 in monkey W, 25 in monkey S). We began each experiment by placing an electrode in the SC, which contains an orderly, “topographic” map of visual space wherein each SC site represents a localized region in visual space (28–30). Most of our recordings were made in the right SC (65/67 experiments); thus the “test” region fell primarily in the left visual field, with eccentricities ranging between 2° and 35°. This region was identified in each experiment by evoking saccadic eye movements with microstimulation (32). At each site, we also measured the current amplitude that elicited saccades on 50% of the trials (“threshold current”). During psychophysical experiments, we stimulated with 50% of this threshold current, thereby activating the SC locally while always ensuring that we did not move the eyes. Across all psychophysical experiments, average currents used were 9 μA for monkey S (range 3–20 μA) and 41 μA for monkey W (range 3–125 μA). Current thresholds for eliciting saccades were higher in monkey W in part because the electrode was more often positioned more superficially within the SC. However, it was also our strong impression that monkey W resisted the effect of the stimulating current, thereby raising his thresholds (33).
If the SC contributes to the control of attention, we predicted that performance would be improved by microstimulation if, and only if, the location of the motion stimulus corresponded to the topographic location of the SC stimulating electrode. To test this prediction, we alternated blocks of trials in which the motion stimulus indeed corresponded to the location of the stimulating electrode (test condition) with blocks in which the motion stimulus was placed in the opposite hemifield (control condition). Each block consisted of 41 ± 5 trials, and we normally obtained 10 or more blocks for each SC site. Block changes were signaled by four “training” trials at the new location by using the strongest possible motion signal (100% coherence) and a reduced density of distracters. Behavioral data from the training trials were not analyzed.
In 39 of these 67 experiments we also measured multiunit physiological responses during a delayed saccade task in which the saccade target was presented for 600 ms before the animal was cued to make an eye movement. For these 39 experiments, we compiled response histograms and examined the level of sustained activity during the delay period (200–600 ms after target onset). Significant delay period activity was present at 37 of the 39 sites, suggesting that the large majority of our stimulation sites were in the intermediate layers of the SC.
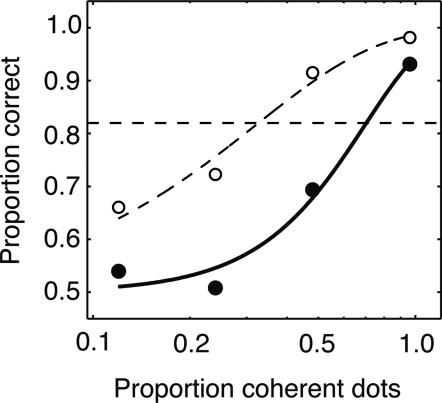
Fig. 3 shows the result of one microstimulation experiment. In this experiment (one of our largest effects), microstimulation improved performance dramatically when the visual stimulus corresponded spatially to the SC stimulation site (Fig. 3a), reducing threshold by a factor of 2.6, from 82% to 32% coherence (bootstrap test, P < 0.01). As predicted, when the visual stimulus was in the hemifield opposite the microstimulation site (Fig. 3b), threshold was not reduced (bootstrap test, P > 0.7). (Note that, in this experiment, psychophysical thresholds differed substantially between the two hemifields on unstimulated trials. We consider this difference further below under the heading Psychophysical Performance on Nonstimulated Trials.)
Fig. 3.
Psychometric functions obtained during study of a single SC site. Filled and open symbols and solid and dashed curves correspond to trials with and without electrical stimulation, respectively. (a) Performance when coherent motion was at the location represented by the SC stimulation site. Psychophysical threshold was significantly reduced from 82% to 32% coherence (bootstrap test, P < 0.01). (b) Performance when coherent motion was at the control location. Threshold was not reduced (bootstrap test, P > 0.7).
The composite psychophysical curves in Fig. 4 depict average performance across 64 of our 67 experiments: for these 64 experiments we measured performance at exactly the same family of four coherences. Each data point gives the average proportion correct across the 64 experiments for a single coherence; the smooth curves were fitted to the average data. Microstimulation improved average performance when the stimulus aperture corresponded spatially to the SC stimulation site (Fig. 4a), reducing threshold from 51% to 46% coherence on average (10% reduction, bootstrap test, P < 0.01). As predicted, this effect was spatially selective: when the stimulus aperture was in the opposite hemifield (Fig. 4b), performance was unaffected on average (bootstrap test, P > 0.7).
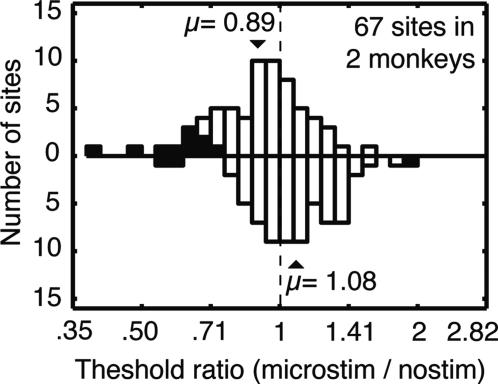
Fig. 5 summarizes the changes in discrimination threshold induced by microstimulation for each site in the entire database of 67 experiments. On average, microstimulation improved performance when the stimulus aperture was positioned at the topographic location of the stimulation site (upward directed bars), reducing thresholds to 89% of the control value on average (paired t test, P < 0.0005). As predicted, this effect was spatially selective: indeed, microstimulation increased thresholds by 8% on average when the stimulus aperture was positioned in the control hemifield (downward bars, paired t test, P < 0.005). Tested individually, the decrease in threshold in the test hemifield was significant for both monkeys, but the impairment in the control hemisphere was significant only for monkey S. (Note that impaired performance was infrequent enough that it does not show up in the population-averaged data in Fig. 4b. The data in Fig. 5 are more revealing because the results are presented for each individual experiment.) The distribution of effects in the test hemifield differed significantly from that in the control hemifield (paired t test, P < 0.00005), and the difference persisted when each monkey was considered individually (P < 0.05 for each). The distribution of effects did not depend on the magnitude of the delay-period activity, nor on the threshold current needed to evoke an eye movement.
Fig. 5.
Frequency histogram showing changes in motion discrimination threshold (as ratios) induced by microstimulation at each of 67 sites in two monkeys. Upward directed bars illustrate data obtained with the coherent motion at the location corresponding to the SC stimulation site. Downward bars illustrate data from the control location. Statistically significant changes in threshold are indicated by filled bars. Arrows indicate average changes for each condition (computed from the log2 threshold change ratios). The dashed vertical line indicates thresholds that are unchanged (ratio 1). Electrical stimulation at this population of SC sites reduced thresholds significantly and selectively.
Considering each experiment individually, the effect of microstimulation on psychophysical threshold was significant for 10 of 67 sites (15%) when the visual stimulus corresponded topographically to the stimulation site (filled bars in Fig. 5, bootstrap test, P < 0.05). For all 10 of these sites, microstimulation improved performance (i.e., lowered threshold). In contrast, significant effects occurred for only 3 of 67 sites (4%) in the control hemifield; these effects were of both signs and were less frequent than the expected rate of false positives. The slopes of the psychometric functions (β, Eq. 1) did not change in either case for either monkey (two-tailed bootstrap test, P > 0.1 for all cases).
Psychophysical Performance on Nonstimulated Trials. Fig. 4 shows that, on nonstimulated trials, the average psychophysical threshold was somewhat higher (51% coherence) in the test hemifield than in the control hemifield (40% coherence), consistent with the individual example illustrated in Fig. 3. Such asymmetries in performance between hemifields are often present in monkey and human observers (e.g., ref. 34; 65/67 of our SC recording sites were in the animals' left visual hemifields) and are not generally worrisome. In the current study, however, this asymmetry is potentially problematic if the lower thresholds in the control hemifield represent “hard” limits to performance that cannot be improved upon irrespective of the level of attention deployed. If this were the case, the spatial specificity of our microstimulation effect could be artifactual because of the hard limit to performance in the control hemifield: performance would be unaffected in the control hemifield, even if the microstimulation effects in the test hemifield resulted from a nonspecific increase in arousal or vigilance.
To address this issue, we analyzed our data set to determine whether the size of the microstimulation effect in fact depended upon the baseline performance (psychophysical threshold) on nonstimulated trials, and whether this dependence could account for the difference in stimulation effects between the two hemifields (i.e., Fig. 5). We used a multiple regression model that included baseline (unstimulated) psychophysical threshold and hemifield as independent regressors:
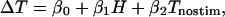 |
[3] |
where ΔT, Tnostim, and H are columns of numbers that contain separate entries for the test and control hemifields for each of the 67 sites in our database. ΔT represents the change in log threshold due to microstimulation, Tnostim represents the log of the psychophysical threshold without microstimulation, and H represents the hemifield containing the coherent motion (a dummy variable, with 1 indicating the test hemifield and 0 indicating the control hemifield). The β values are computed by solving the equation to minimize the total squared error.
If the spatial specificity of the stimulation effect is real, the dependence of the microstimulation effect on hemifield (β1) should be significant even after factoring out the effect of the baseline threshold (β2). Indeed, both regression terms were significant (for β1, slope = -0.16; 95% confidence limits, -0.28 to -0.04; one-tailed P value < 0.01; for β2, slope = -0.27; 95% confidence limits, -0.39 to -0.15; one-tailed P value < 0.00002). The dependence of the stimulation effect on baseline performance (β2) is of course reasonable. If the monkey's attention flags on some blocks of trials, resulting in poorer performance, stimulation of the SC might well exert a larger effect on those blocks. The fact that the hemifield term (β1) is significant, however, shows that our primary conclusion remains firm: SC microstimulation resulted in a spatially selective reduction in psychophysical thresholds in the test hemifield.
Psychophysical Performance with “Artificial Phosphenes.” The results summarized in Figs. 4 and 5 demonstrate that microstimulation of the SC improves psychophysical performance on a visual discrimination task even though the visual stimulus is never the target of an eye movement. The most parsimonious interpretation of our data is that microstimulation of the SC focused visuo-spatial attention on a specific spatial location, thereby improving performance. An alternative interpretation, however, is that SC microstimulation, particularly in the superficial layers, created a visual phosphene (35) that indirectly cued attention to the location of the stimulus. It is impossible, of course, to know what the monkey actually sees during microstimulation, but we tested this hypothesis indirectly by measuring direction discrimination performance with and without artificial phosphenes (Gaussian shaped brightness modulations) of varying sizes and intensities centered at the same location as the coherent motion aperture. We collected a total of 22 pairs of psychometric curves from the two monkeys. On average, this manipulation harmed performance (P < 0.05, two-tailed paired t test), presumably distracting the monkey from the discrimination task. Rarely, the artificial phosphene improved performance modestly in the manner of our microstimulation effects. Such improvements occurred most often when the artificial phosphene was low luminance and precisely coextensive with the stimulus aperture. Even this stimulus, however, failed to improve average performance across nine experiments (paired t test, P > 0.5); rather, on average, it introduced a trend toward worse performance. It therefore seems unlikely that our microstimulation results can be accounted for by phosphenes.
Eye Movements. Because our experiments involved microstimulation of a known oculomotor structure, we examined our data for evidence of an effect of SC microstimulation on two aspects of oculomotor behavior: fixation breaks and choice bias toward one or the other saccade target. For each experiment, we computed the proportion of trials in which the monkey broke fixation during the microstimulation interval or during the 100 ms after it, and we computed the proportion of trials in which the monkey broke fixation during an equivalent interval on unstimulated trials. Across the data set, microstimulation did not influence the frequency of fixation breaks (two-tailed paired t test, P > 0.4). (The difference between the two distributions was approximately normal, so a paired t test was appropriate.) Nor did microstimulation bias the animals' choices toward one or the other saccade target (paired t test, two-tailed, P > 0.3; see Methods). The absence of stimulation effects on oculomotor behavior is unsurprising because we stimulated with subthreshold currents and explicitly avoided placing saccade targets near the stimulated regions of visual space.
Discussion
Our primary finding is that electrical microstimulation of the SC improves psychophysical performance in a specific region of the visual field corresponding to the location of the stimulation site in the SC. The spatial selectivity of the effect shows that improved performance does not result simply from generalized arousal or vigilance. Control experiments indicate that our results are not likely to result indirectly from visual phosphenes, and we found no evidence for stimulation-induced effects on eye movements that might account for our results. The most parsimonious explanation for our data is that microstimulation improved performance by focusing attention on a specific region of visual space, consistent with the hypothesis that SC activity contributes to the control of covert visuo-spatial attention in the absence of eye movements. Thus, our data provide important causal evidence of a role for the SC in the control of attention to complement the correlative evidence provided by electrophysiological recording studies (7, 8, 16).
In addition, our data confirm the remarkable finding of Moore and Fallah (18, 19) that perceptual performance can be improved by transient activation of a specific point in the central nervous system. The size of the effects in our study (11% improvement in threshold) is broadly similar to those in psychophysical studies that measure the effect of covert attention on contrast sensitivity in human subjects [roughly 10% in one study (36), 10–30% in another (37)]. More importantly, our effects are nearly identical in size to those of Moore and Fallah (≈10% improvement in threshold), suggesting that the SC and FEF are equally influential in the control of covert attention. The improvement in performance observed in these two studies is fundamentally different from results of previous electrical stimulation studies in which stimulation created hallucinatory percepts (38) or changed choice behavior without improving the ability to discriminate real-world stimuli (20–23). Rather, the effects we have demonstrated (and those of Moore and Fallah) are more analogous to the effects of deep brain stimulation in Parkinsonian patients whose motor performance improves immediately upon stimulation onset through mechanisms that are still debated (39).
It might be argued that our microstimulation effects do not demonstrate a role for the SC in attention, but rather result from antidromic activation of cortical areas, such as the FEF and lateral intraparietal (LIP), that perform this function. Conversely, however, it might be argued that the results of Moore and Fallah do not demonstrate a role for the FEF in attentional processes, but rather result from orthodromic and/or antidromic activation of other areas, such as SC and LIP, that perform this function. In general, it seems implausible that the biological substrate of any complex cognitive ability will be localized to a single neural structure such as SC, FEF, or LIP; rather, perceptual and cognitive abilities almost certainly arise from interactive networks of neural structures (40–42). Thus, we favor the view that the control of covert spatial attention is distributed among a network of structures, many of which also play known roles in the planning and execution of saccadic eye movements. In this view, stimulation of either the FEF or the SC elicits attentional effects because either is effective in activating a larger network of areas that together control covert attention.
When the attentional control system becomes active, of course, an even broader network of brain areas will be influenced through feedback projections onto cortical and subcortical visual structures. Moore and Armstrong (43) have shown directly that subthreshold microstimulation of the FEF modulates visual responses in extrastriate area V4 in a manner similar to visual attention, and our own preliminary data indicate that SC microstimulation exerts similar effects in extrastriate area MT.
Physiologists have long debated whether the tonic neural activity that exists in the SC, the FEF, and other brain areas during an instructed delay period should be regarded as purely motor preparation activity, consistent with the known role of these structures in the control of eye movements, or whether this activity might also contribute to cognitive functions such as visuo-spatial attention (44–49). It has been difficult to resolve this issue with the purely correlative data provided by electrophysiological recording. To our minds, the causal evidence provided by our microstimulation study (and by ref. 50) tilts the weight of evidence decisively in favor of a more expansive view of the functions of the SC.
Acknowledgments
We thank C. Fiorello and T. Moore for reviews of the manuscript, and J. Powell and J. Brown for expert technical assistance. W.T.N. is an Investigator of the Howard Hughes Medical Institute. This work was supported by National Eye Institute Grant EY 05603 (to W.T.N.). J.R.M. was supported by Ruth L. Kirschstein National Eye Institute National Research Service Award Grant EY 14500.
Author contributions: J.R.M. and W.T.N. designed research; J.R.M. and M.G.P. performed research; J.R.M. analyzed data; and J.R.M. and W.T.N. wrote the paper.
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on May 2, 2000.
Abbreviations: SC, superior colliculus; FEF, frontal eye fields.
See accompanying Biography on page 521.
References
- 1.Ferrier, D. (1890) Cerebral Localisation (Smith, Elder and Co., London).
- 2.Rizzolatti, G., Riggio, L., Dascola, I. & Umilta, C. (1987) Neuropsychologia 25, 31-40. [DOI] [PubMed] [Google Scholar]
- 3.Kowler, E., Anderson, E., Dosher, B. & Blaser, E. (1995) Vision Res. 35, 1897-1916. [DOI] [PubMed] [Google Scholar]
- 4.Sperling, G. & Melchner, M. J. (1978) Science 202, 315-318. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman, J. E. & Subramaniam, B. (1995) Percept. Psychophys. 57, 787-795. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg, M. E. & Wurtz, R. H. (1972) J. Neurophysiol. 35, 560-574. [DOI] [PubMed] [Google Scholar]
- 7.Gattass, R. & Desimone, R. (1996) Rev. Bras. Biol. 56, 257-279. [PubMed] [Google Scholar]
- 8.Kustov, A. A. & Robinson, D. L. (1996) Nature 384, 74-77. [DOI] [PubMed] [Google Scholar]
- 9.Yin, T. C. & Mountcastle, V. B. (1977) Science 197, 1381-1383. [DOI] [PubMed] [Google Scholar]
- 10.Robinson, D. L., Goldberg, M. E. & Stanton, G. B. (1978) J. Neurophysiol. 41, 910-932. [DOI] [PubMed] [Google Scholar]
- 11.Bushnell, M. C., Goldberg, M. E. & Robinson, D. L. (1981) J. Neurophysiol. 46, 755-772. [DOI] [PubMed] [Google Scholar]
- 12.Kodaka, Y., Mikami, A. & Kubota, K. (1997) Neurosci. Res. 28, 291-298. [DOI] [PubMed] [Google Scholar]
- 13.Thompson, K. G. & Schall, J. D. (2000) Vision Res. 40, 1523-1538. [DOI] [PubMed] [Google Scholar]
- 14.Kastner, S., Pinsk, M. A., De Weerd, P., Desimone, R. & Ungerleider, L. G. (1999) Neuron 22, 751-761. [DOI] [PubMed] [Google Scholar]
- 15.Bisley, J. W. & Goldberg, M. E. (2003) Science 299, 81-86. [DOI] [PubMed] [Google Scholar]
- 16.Ignashchenkova, A., Dicke, P. W., Haarmeier, T. & Thier, P. (2004) Nat. Neurosci. 7, 56-64. [DOI] [PubMed] [Google Scholar]
- 17.Desimone, R., Wessinger, M., Thomas, L. & Schneider, W. (1990) Cold Spring Harbor Symp. Quant. Biol. 55, 963-971. [DOI] [PubMed] [Google Scholar]
- 18.Moore, T. & Fallah, M. (2001) Proc. Natl. Acad. Sci. USA 98, 1273-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore, T. & Fallah, M. (2004) J. Neurophysiol. 91, 152-162. [DOI] [PubMed] [Google Scholar]
- 20.Salzman, C. D., Britten, K. H. & Newsome, W. T. (1990) Nature 346, 174-177. [DOI] [PubMed] [Google Scholar]
- 21.DeAngelis, G. C., Cumming, B. G. & Newsome, W. T. (1998) Nature 394, 677-680. [DOI] [PubMed] [Google Scholar]
- 22.Bisley, J. W., Zaksas, D. & Pasternak, T. (2001) J. Neurophysiol. 85, 187-189. [DOI] [PubMed] [Google Scholar]
- 23.Romo, R., Hernandez, A., Zainos, A. & Salinas, E. (1998) Nature 392, 387-390. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz, G. D. & Newsome, W. T. (2001) J. Neurophysiol. 86, 2527-2542. [DOI] [PubMed] [Google Scholar]
- 25.Robinson, D. A. (1963) IEEE Trans. Biomed. Eng. 10, 137-145. [DOI] [PubMed] [Google Scholar]
- 26.Watson, A. B. (1979) Vision Res. 19, 515-522. [DOI] [PubMed] [Google Scholar]
- 27.Quick, R. F. (1974) Kybernetik 16, 65-67. [DOI] [PubMed] [Google Scholar]
- 28.Schiller, P. H. & Koerner, F. (1971) J. Neurophysiol. 34, 920-936. [DOI] [PubMed] [Google Scholar]
- 29.Wurtz, R. H. & Goldberg, M. E. (1972) J. Neurophysiol. 35, 575-586. [DOI] [PubMed] [Google Scholar]
- 30.Schiller, P. H. & Stryker, M. (1972) J. Neurophysiol. 35, 915-924. [DOI] [PubMed] [Google Scholar]
- 31.Britten, K. H., Shadlen, M. N., Newsome, W. T. & Movshon, J. A. (1992) J. Neurosci. 12, 4745-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson, D. A. (1972) Vision Res. 12, 1795-1808. [DOI] [PubMed] [Google Scholar]
- 33.Sparks, D. L. & Mays, L. E. (1983) J. Neurophysiol. 49, 45-63. [DOI] [PubMed] [Google Scholar]
- 34.Newsome, W. T. & Pare, E. B. (1988) J. Neurosci. 8, 2201-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nashold, B. S. (1970) Arch, Ophthalmol, 84, 433-435. [DOI] [PubMed] [Google Scholar]
- 36.Cameron, E. L., Tai, J. C. & Carrasco, M. (2002) Vision Res. 42, 949-967. [DOI] [PubMed] [Google Scholar]
- 37.Carrasco, M., Penpeci-Talgar, C. & Eckstein, M. (2000) Vision Res. 40, 1203-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penfield, W. & Rasmussen, T. (1950) The Cerebral Cortex of Man: A Clinical Study of Localization of Function (Macmillan, New York).
- 39.McIntyre, C. C., Savasta, M., Kerkerian-Le Goff, L. & Vitek J. L. (2004) Clin. Neurophysiol. 115, 1239-1248. [DOI] [PubMed] [Google Scholar]
- 40.Schlag-Rey, M., Schlag, J. & Dassonville, P. (1992) J. Neurophysiol. 67, 1003-1005. [DOI] [PubMed] [Google Scholar]
- 41.Sommer, M. A. & Wurtz, R. H. (2001) J. Neurophysiol. 85, 1673-1685. [DOI] [PubMed] [Google Scholar]
- 42.Sommer, M. A. & Wurtz, R. H. (2002) Science 296, 1480-1482. [DOI] [PubMed] [Google Scholar]
- 43.Moore, T. & Armstrong, K. M. (2003) Nature 421, 370-373. [DOI] [PubMed] [Google Scholar]
- 44.Horwitz, G. D. & Newsome, W. T. (1999) Science 284, 1158-1161. [DOI] [PubMed] [Google Scholar]
- 45.Fecteau, J. H., Bell, A. H. & Munoz, D. P. (2004) J. Neurophysiol. 92, 1728-1737. [DOI] [PubMed] [Google Scholar]
- 46.Carello, C. D. & Krauzlis, R. J. (2004) Neuron 43, 575-583. [DOI] [PubMed] [Google Scholar]
- 47.Andersen, R. A. & Buneo, C. A. (2002) Neurosci. 25, 189-220. [DOI] [PubMed] [Google Scholar]
- 48.Colby, C. L. & Goldberg, M. E. (1999) Annu. Rev. Neurosci. 22, 319-349. [DOI] [PubMed] [Google Scholar]
- 49.Moore, T., Armstrong, K. M. & Fallah, M. (2003) Neuron 40, 671-683. [DOI] [PubMed] [Google Scholar]
- 50.Cavanaugh, J. & Wurtz, R. H. J. Neurosci., in press.