Abstract
Molecular characterization of the severe acute respiratory syndrome coronavirus has revealed genetic diversity among isolates. The spike (S) glycoprotein, the major target for vaccine and immune therapy, shows up to 17 substitutions in its 1,255-aa sequence; however, the biologic significance of these changes is unknown. Here, the functional effects of S mutations have been determined by analyzing their affinity for a viral receptor, human angiotensin-converting enzyme 2 (hACE-2), and their sensitivity to Ab neutralization with viral pseudotypes. Although minor differences among eight strains transmitted during human outbreaks in early 2003 were found, substantial functional changes were detected in S derived from a case in late 2003 from Guangdong province [S(GD03T0013)] and from two palm civets, S(SZ3) and S(SZ16). S(GD03T0013) depended less on the hACE-2 receptor and was markedly resistant to Ab inhibition. Unexpectedly, Abs that neutralized most human S glycoproteins enhanced entry mediated by the civet virus S glycoproteins. The mechanism of enhancement involved the interaction of Abs with conformational epitopes in the hACE-2-binding domain. Finally, improved immunogens and mAbs that minimize this complication have been defined. These data show that the entry of severe acute respiratory syndrome coronaviruses can be enhanced by Abs, and they underscore the need to address the evolving diversity of this newly emerged virus for vaccines and immune therapies.
Keywords: angiotensin-converting enzyme 2, enhancement, immunoglobulin G, pseudovirus
Recent outbreaks of severe acute respiratory syndrome (SARS) have been linked to a sole etiologic agent, a recently identified human coronavirus (CoV), SARS-CoV (1-3). SARS-CoV is not closely related to any subgroup of known CoVs, although recent studies suggest it most resembles group II CoVs (4). SARS-CoV genetically and structurally is a typical CoV. Its genome encodes four major structural proteins: the nuclear, membrane, small envelope, and spike (S) proteins. The S protein is believed to bind to a cellular receptor and subsequently mediate viral entry. Recent studies indicate that human angiotensin-converting enzyme 2 (hACE-2) protein is its major cellular receptor (5). S mediates viral entry, and membrane fusion is pH-dependent (6, 7). SARS-CoV S is a major target of vaccine and antiviral drug development.
SARS-CoV infection has caused several infectious outbreaks in humans, with high mortality in elderly populations. Several experimental vaccines have been developed and tested in a murine viral replication model (8-10). These studies showed that S-based vaccines protected against SARS-CoV challenge in this animal model. Protection depends on humoral immunity, indicating the central role of neutralizing Ab in the development of SARS-CoV vaccines (8).
Although the natural reservoir of SARS-CoV is currently unknown, it is likely transmitted from animal hosts to humans. Several SARS-CoV-like viruses were isolated from farm-raised raccoons and civet cats in markets where SARS-CoV infection originally started (11). Recent epidemiological studies also found some degree of variation in the S protein from early to late outbreak phases (12), suggesting potential selective pressure against the virus in humans. To fully evaluate the neutralization potential of S-based DNA vaccine-generated immune serum against different subtypes of SARS-CoV, we performed neutralization assays with purified immune IgG elicited by several vaccine candidates by using lentiviral vectors pseudotyped with different S proteins (6, 7).
Materials and Methods
Plasmids and Cell Lines. The human renal adenocarcinoma cell line 786-O was purchased from American Type Culture Collection and maintained in DMEM plus 10% FBS. The gene for SARS-CoV S (Urbani strain) was synthesized by using human-preferred codons as described in ref. 6. To synthesize the cDNAs encoding S from various strains, the QuikChange XL kit (Stratagene) was used to introduce divergent amino acids into the parental S(Urbani) strain predicted from the translated sequence of S(BJ01) (GenBank accession no. AY278488), S(FRA) (GenBank accession no. AY310120), S(GD01) (GenBank accession no. AY278489), S(GZ02) (GenBank accession no. AY390556), S(HGZ8L1-A) (GenBank accession no. AY394981), S(Tor2) (GenBank accession no. AY274119), S(ZS-A) (GenBank accession no. AY394997), S(GD03T0013) (GenBank accession no. AY525636), S(SZ3) (GenBank accession no. AY304486) and S(SZ16) (GenBank accession no. AY304488) according to the manufacturer's protocol. A stop codon was introduced at amino acid 1153 of S(Urbani) also by using the QuikChange XL kit to make the relevant S(1153) truncations. All cDNAs were cloned into CMV/R vector (6) as a SalI/BamHI fragment, and expression was confirmed by Western blot analysis by using rabbit anti-S polyclonal Ab (catalog no. IMG-541, Imgenex, Sorrento Valley, CA).
Production of Pseudotyped Lentivirus. Recombinant lentiviruses expressing a luciferase reporter gene were produced as described in refs. 13 and 14. Briefly, 5 × 106 293T cells were transfected overnight by using calcium phosphate reagent (Invitrogen) with the following plasmids: 7 μg of pCMVΔR8.2, 7 μg of pHR′CMV-Luc, and 400 ng CMV/R-SARS-S. Supernatants were harvested 48 h after transfection, filtered through a 0.45-μm syringe filter, aliquotted, and used immediately or frozen at -80°C. p24 levels were measured from different viral stocks by using the Beckman Coulter HIV-1 p24 Antigen Assay kit to estimate the viral titer.
Vaccination and Purification of Immune IgG. Five female BALB/c mice (6-8 weeks old) (Charles River Laboratories) per group were immunized three times at weeks 0, 3, and 6 with 25 μg of plasmid DNA (in 200 μl of PBS, pH 7.4) expressing the corresponding immunogen. Ten days after the last immunization, sera were collected, combined, and subsequently purified by using the Nab Spin Purification kit (Pierce) according to the manufacturer's protocol. Animal experiments were approved by the Vaccine Research Center Animal Care and Use Committee and conform to all federal and National Institutes of Health guidelines. Human mAbs against S(Frankfurt) derived from Epstein-Barr virus-transformed B lymphocytes were isolated and purified as described in ref. 15.
Neutralization and Inhibition Assays. Cells (786-O; 30,000 cells per well) were plated onto a 48-well dish the day before infection. The various pseudoviruses, normalized for the amount of p24 added, were mixed with purified mouse IgG, human mAbs, or recombinant hACE-2 ectodomain (amino acids 1-740) (R & D Systems) at the indicated concentrations, and incubated for 5-10 min before addition to 786-O cells. Cells were infected for 14-16 h and collected for luciferase assay by using luciferase assay reagent (Promega) 48 h later.
Biochemical and Immunoprecipitation Analyses. Cells (293T) were transfected with 10 μg of plasmid DNA overnight by using calcium phosphate reagent (Invitrogen) and replenished with fresh media. After 48 h, transfected cells were harvested, washed with PBS, and resuspended in lysis buffer (50 mM Hepes/150 mM NaCl/1% Nonidet P-40, pH 7.0/protease inhibitor mixture) on ice for 45 min. Cell lysates were cleared by centrifugation at 13,000 rpm (Biofuge, Sorvall) for 10 min at 4°C. We incubated 25 μg of cell lysate with 5 μg of mouse immune IgG or 2 μg of human mAbs for 1 h at room temperature, followed by incubation with 25 μl of agarose protein G (Pierce) for an additional 1 h. Immunoprecipitates were washed three times with lysis buffer, resuspended in SDS-loading buffer (Quality Biologicals, Gaithersburg, MD), and separated on 4-15% gradient SDS/PAGE (Bio-Rad), followed by Western blot analysis.
Results
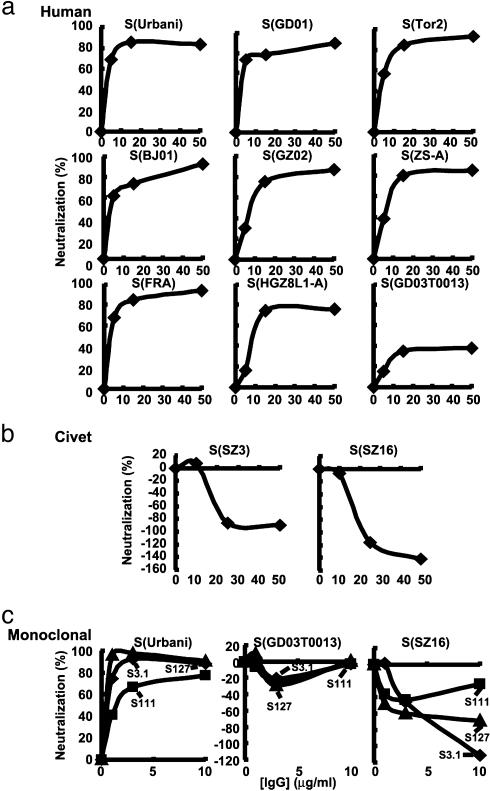
Inhibition of Alternative S Glycoprotein Entry by Anti-S(Urbani) IgG. The entry of SARS-CoV into its target cells is mediated by the S glycoprotein, which depends on an aminopeptidase receptor, hACE-2 (5). Pseudotyping with retroviral and lentiviral vectors has been used to analyze the mechanism and the specificity of entry, which faithfully models infection of different cell types by virus (6, 7, 16, 17). S-dependent entry occurs through pH-sensitive endocytosis (6, 7, 16) and can be inhibited by antisera that protect against pulmonary viral replication in mice (8, 18). To analyze the sensitivity of alternative SARS-CoV S glycoproteins to antibody neutralization, S sequences from alternative human isolates and two palm civet strains were synthesized as codon-modified cDNAs and inserted into expression vectors as described in ref. 8. All pseudoviruses derived from these strains showed incorporation of their S proteins into virions (Fig. 5, which is published as supporting information on the PNAS web site). Each pseudovirus was incubated independently with immune IgG purified from mice vaccinated with S(Urbani) that inhibited entry of this prototype strain (8, 18). Similar patterns of inhibition were seen with pseudoviruses from all early 2003 human isolates, which showed minor differences in neutralization by this polyclonal IgG (Fig. 1a). In contrast, the S(GD03T0013) pseudotype was qualitatively different, e.g., markedly resistant to Ab inhibition (Fig. 1a Bottom Right). Unexpectedly, this immune IgG enhanced entry of two pseudoviruses derived from palm civet S glycoproteins (Fig. 1b).
Fig. 1.
Analysis of different S strains for sensitivity to Ab neutralization. (a) Lentivirus vectors prepared as described in refs. 6 and 8 were pseudotyped with the indicated SARS-CoV S glycoproteins from human isolates. These pseudoviruses were incubated with purified IgG from mice vaccinated with a DNA expression vector encoding S(Urbani) or negative control sera from mock-immunized mice. The inhibition by the negative control at each data point was subtracted from that of immune IgG. The percentage of inhibition assessed by luciferase reporter gene expression was calculated by the reduction in luciferase activity relative to values achieved in the absence of sera. Inhibition seen with control IgG was <10% in general compared with samples lacking IgG. (b) Ab-dependent enhancement of S entry in pseudoviruses from two palm civet pseudoviruses. (c) Pseudovirus from Urbani [S (Urbani)], resistant human [S(GD03T0013)], and palm civets [S(SZ3) and S(SZ16)] were incubated with human neutralizing mAbs S3.1, S127, and S111, which were derived from EBV-transformed B lymphocytes (15).
Inhibition of Entry by Human mAbs to S. To determine whether Abs that enhance civet S entry can be detected in humans, we examined neutralizing mAbs derived from Epstein-Barr virus transformation of memory B cells from a recovered patient (15). Several mAbs with similar specificities were defined. These Abs inhibited S(Urbani) entry (Fig. 1c Left), representative of the eight early 2003 human isolates (Fig. 1a). Although they strongly inhibited S(Urbani) pseudotype entry, minimal effects were seen on the S(GD03T0013) pseudotype, and several mAbs enhanced palm civet S pseudovirus entry (Fig. 1c Center and Right), similar to the polyclonal anti-S(Urbani) IgG. This finding confirmed that enhancing Abs can be detected in humans and further suggested that it is mediated by a specific epitope on S.
Differential Inhibition of S Strains Correlates with hACE-2 Binding. The affinity of these viruses for the hACE-2 receptor was assessed by using inhibition with soluble recombinant hACE-2. Entry by S(Urbani) pseudovirus was substantially inhibited by hACE-2 (Fig. 2a Left). In contrast, S(GD03T0013) entry was much less sensitive to hACE-2 inhibition and similar to the negative control, hACE (Fig. 2a Right). This similar inhibition to the negative control at high protein concentrations suggests that GD03T0013 depends much less on hACE-2 for entry. To determine whether the palm civet and GD03T0013 pseudoviruses could be neutralized by vaccination with the homologous S, mice were injected with a DNA vaccine encoding full-length S from these respective isolates. Neither immune IgG could strongly inhibit the homologous S, even after repeated immunizations (Fig. 2 b and c). Although a small degree of inhibition of S (GD03T0013) pseudovirus was seen at low concentrations, it was not dose-dependent, suggesting possible concentration-dependent effects on inhibition and enhancement in this strain. The Abs induced by S(SZ3) or S(GD03T0013) also effectively neutralized S(Urbani) pseudovirus entry, similar to immune IgG from S(Urbani)-vaccinated animals (Figs. 1a and 2 b and c), implying that these differences are intrinsic to the neutralization sensitivity of the S from each strain.
Fig. 2.
Differential sensitivity of S strains to ACE-2 inhibition and relative resistance of GD03T0013 and SZ3 to homologous neutralization. (a) The indicated pseudoviruses were incubated with increasing amounts of soluble recombinant hACE-2 or hACE (negative control, purified by similar methods) and entry was assessed by using the luciferase reporter gene as described for Fig. 1. (b) Purified IgG from animals immunized with S(GD03T0013) DNA expression vectors inhibits S(Urbani) but does not neutralize S(GD03T0013), S(SZ3), or S(SZ16) pseudoviruses. The pseudotyped viruses are shown according to their species origin (human or civet), shown above each graph. (c) Purified IgG from mice immunized with full-length S(SZ3) DNA expression vectors confers neutralization to S(Urbani) and partial inhibition of S(GD03T0013) but does not neutralize S(SZ3).
The ACE-2-Binding Domain Mediates Ab-Dependent Enhancement of Civet S Entry. The genetic determinants of enhancement and neutralization were defined by expressing full-length recombinant S protein from either S(Urbani), S(SZ3), or relevant chimeras (Fig. 3a). A total of 17 amino acid differences were observed in various S proteins, but those in a central region, between amino acids 248 and 501, have been implicated in binding to the ACE-2 receptor (19, 20). Because of the differential sensitivity to ACE-2 inhibition, these regions, with only 5 amino acid differences between them, were analyzed in chimeric S by using the entry assay. This region from S(Urbani) mediated more efficient entry into 786-O renal epithelial cells (Fig. 3b Left). Importantly, insertion of this region from S(Urbani) into S(SZ3) rendered the chimeric pseudovirus sensitive to neutralization (Fig. 3b Right, SU). Conversely, introduction of the hACE-2 receptor-binding region from S(SZ3) into S(Urbani) switched its sensitivity from neutralization to Ab-dependent enhancement (Fig. 3b Right, US). The five amino acid differences in this region therefore were responsible for both the differential ACE-2 affinity and the sensitivity to Abs. The interaction of S immune IgG with native S(Urbani) compared with S(SZ3) was nearly 20-fold higher by immunoprecipitation (Fig. 3c Right), compared with the total amount of protein detectable in lysates by Western blot analysis (Fig. 3c Left), which suggests that these lower-affinity interactions with native conformations of SZ3 may contribute to enhancement, consistent also with the finding that the hACE-2 receptor-binding region determines this effect.
Fig. 3.
Definition of genetic determinants of S glycoprotein inhibition and enhancement and specific biochemical interaction of Abs with native S(Urbani) and S(SZ3). (a) Schematic diagram of sensitive S(Urbani), resistant palm civet S(SZ3), and chimeric glycoproteins SU and US as shown. (b) (Left) Gene transfer efficiency of pseudoviruses containing the specified wild-type and chimeric S in 786-O cells. Entry of S(Urbani) pseudovirus into 786-O cells is 100-fold more efficient than S(SZ3) pseudovirus. The dashed line indicates the background levels of gene transfer in the absence of S, SU, or US, as defined in a. (Right) Inhibition or enhancement of the indicated S pseudotypes with immune IgG to S(Urbani) shows the dependence on the hACE-2-binding domain. (c) (Right) Interaction of S(Urbani) and S(SZ3) with purified IgG from mice immunized with S(Urbani) full length (FL) gene was determined by immunoprecipitation (IP). (Left) The total amount of S protein used in immunoprecipitation was determined by Western blotting (Input).
Development of Alternative Vaccine Candidates That Do Not Enhance Civet S-Dependent Entry. We next sought to determine whether it was possible to modify the S immunogen to avoid Ab-dependent enhancement of entry of the palm civet S pseudoviruses. Because soluble S proteins reacted differentially by immunoprecipitation analysis, a series of transmembrane-deleted immunogens was prepared. One of these mutants, truncated at amino acid 1153, induced neutralizing Abs to human isolates that failed to cause Ab enhancement of entry (Fig. 4a). Biochemical analysis revealed that this immune IgG reacted poorly with native S(SZ3) by immunoprecipitation but reacted strongly with S(Urbani) (Fig. 4b), consistent with the patterns of neutralization and enhancement. This finding demonstrated that some immunogens can elicit neutralizing Abs for human isolates that do not enhance civet pseudovirus entry. To determine whether mAbs could be identified that also failed to enhance entry, additional mAbs were analyzed. Although most mAbs showed enhancement, some, such as S110, were identified that failed to enhance entry while mediating potent neutralization (Fig. 4c). Taken together, these data further document that Ab reactivity with conformational determinants on the extracellular portion of S is responsible for neutralization, and alterations in the hACE-2-binding region that reduce binding facilitate entry stimulated by Ab.
Fig. 4.
Identification of immunogens and mAbs that circumvent Ab-dependent enhancement of entry and biochemical analysis of the mechanism of enhancement. (a) Neutralization profile of purified IgG from mice vaccinated with a DNA expression vector encoding a secreted form of S(Urbani) terminated at amino acid 1153, S(1153), compared against the indicated human or civet pseudoviruses. (b) (Right) Biochemical interaction of purified IgG from S(1153) DNA-immunized mice with native, expressed S(Urbani) and S(SZ3) proteins by immunoprecipitation (IP) with S(1153) IgG. (Left) The total amount of S protein in the same volume of cell lysates was assessed by Western blotting (Input). Mice were immunized with a S(Urbani) expression vector terminated at amino acid 1153. (c) mAb S110 inhibits S(Urbani) and does not enhance civet pseudovirus entry. S110 or an isotype control Ab (control) was incubated with the indicated pseudoviruses, and inhibition was assessed as described for Fig. 1. The species origin of the S for pseudovirus is shown above each panel (human or civet) in a and c.
Discussion
Immune protection against SARS-CoV infection has been conferred by vaccination directed toward the S glycoprotein (8, 9), and this effect is mediated by humoral immunity. The evolving molecular heterogeneity of SARS-CoV (11, 12, 21, 22) has raised concerns about the breadth and efficacy of protection with specific vaccine strains and the possible development of immune escape. In this study, functional differences between different human and animal SARS-CoV S glycoproteins have been characterized. We find that some S variants were resistant to neutralization, whereas others, specifically those isolated from palm civets, showed enhanced entry in the presence of certain Abs. S derived from the human outbreaks in early 2003 showed similar sensitivity to Ab neutralization, in contrast to the GD03T0013 virus, which showed reduced sensitivity to neutralization. This virus and the palm civet virus S protein that showed Ab-dependent enhancement were markedly less dependent on hACE-2 for entry, and the differential response to Abs mapped to the hACE-2-binding domain (Fig. 3b). It has recently been suggested that adaptation of this virus to humans may have involved increased affinity of SARS-CoV S for this receptor (5), and it therefore appears the Ab neutralization and enhancement correlates with adaptation to this receptor. Alternative or auxillary receptors for SARS-CoV(GD03T0013) and SARS-like-CoV(SZ3 or SZ16) could exist, suggested by recent observations that members of the DC-SIGN family serve as attachment factors (6, 23) for SARS-CoV and by inhibition of S(Urbani) entry by heparin-like molecules (data not shown). Low-affinity binding of anti-S(Urbani) with S(SZ3), S(SZ16), or S(GD03T0013) to hACE-2 could lead to better entry through such a secondary receptor.
It is also interesting that the viruses with lower affinity for hACE-2 were more difficult to neutralize, even with antisera from animals immunized with homologous S. This finding suggests that the animal SARS-CoVs have evolved to resist Ab neutralization, whereas the majority of human strains, those with higher affinity for hACE-2, have not evolved to escape this immune selection, a possibility that could arise if the virus undergoes further selection and transmission. The development of vaccination strategies that may prevent such transmissions through independent mechanisms, for example, through cellular immunity, may therefore contribute to vaccine efficacy.
To date, Ab-dependent enhancement has not been observed with any human SARS-CoV strain, which may allay concerns that such vaccines might enhance viral infection; however, it will be important to assess such vaccines in relevant animal models as they become available. Because it is technically difficult to grow the civet viruses and because they have not been successfully propagated in animals, it is unknown whether the Abs that enhance infection will exacerbate viral replication and/or disease in vivo. We have shown previously that the pseudotype neutralization assay correlates well with the replication assay for inhibitory Abs (6). Additional studies that address this question further when the relevant viral strains can be readily cultured are necessary. We have also found that Ab enhancement of civet virus S entry is less effective with partially purified pseudoviruses than with virus taken directly from cell supernatants (data not shown), suggesting that secreted cellular components, for example, glycosaminoglycans, might potentiate this effect. Although of lower magnitude, similar effects were seen compared with the purified pseudoviruses. Insight into the mechanism of enhancement facilitates an understanding of disease pathogenesis and avoids complications during vaccine development. Such knowledge also provides a model for the study of Ab-dependent enhancement observed in other viruses, such as dengue fever (24) or respiratory syncytial virus (25), whose mechanism is not fully understood. At the same time, the resistance of some S strains to Ab neutralization raises concerns about the ability of SARS-CoV vaccines to contain the spectrum of isolates in nature and highlights the need to develop approaches that control these genetically diverse viruses.
Supplementary Material
Acknowledgments
We thank Ati Tislerics and Tina Suhana for manuscript preparation, Toni Miller and Brenda Hartman for preparation of figures, and members of the G.J.N. laboratory for helpful advice and discussions. This work was funded by the National Institutes of Health.
Author contributions: G.J.N. and Z.-y.Y. designed research; Z.-y.Y., H.C.W., W.-p.K., and K.L. performed research; E.T. and A.L. contributed new reagents/analytic tools; E.T. and A.L. analyzed data; and G.J.N. and Z.-y.Y. wrote the paper.
Abbreviations: hACE-2, human angiotensin-converting enzyme 2; S, spike; SARS, severe acute respiratory syndrome; CoV, coronavirus.
References
- 1.Drosten, C., Gunther, S., Preiser, W., van der Werf, S., Brodt, H. R., Becker, S., Rabenau, H., Panning, M., Kolesnikova, L., Fouchier, R. A., et al. (2003) N. Engl. J. Med. 348, 1967-1976. [DOI] [PubMed] [Google Scholar]
- 2.Marra, M. A., Jones, S. J., Astell, C. R., Holt, R. A., Brooks-Wilson, A., Butterfield, Y. S., Khattra, J., Asano, J. K., Barber, S. A., Chan, S. Y., et al. (2003) Science 300, 1399-1404. [DOI] [PubMed] [Google Scholar]
- 3.Rota, P. A., Oberste, M. S., Monroe, S. S., Nix, W. A., Campagnoli, R., Icenogle, J. P., Penaranda, S., Bankamp, B., Maher, K., Chen, M. H., et al. (2003) Science 300, 1394-1399. [DOI] [PubMed] [Google Scholar]
- 4.Snijder, E. J., Bredenbeek, P. J., Dobbe, J. C., Thiel, V., Ziebuhr, J., Poon, L. L., Guan, Y., Rozanov, M., Spaan, W. J. & Gorbalenya, A. E. (2003) J. Mol. Biol. 331, 991-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li, W., Moore, M. J., Vasilieva, N., Sui, J., Wong, S. K., Berne, M. A., Somasundaran, M., Sullivan, J. L., Luzuriaga, K., Greenough, T. C., et al. (2003) Nature 426, 450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang, Z.-Y., Huang, Y., Ganesh, L., Leung, K., Kong, W.-P., Schwartz, O., Subbarao, K. & Nabel, G. J. (2004) J. Virol. 78, 5642-5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons, G., Reeves, J. D., Rennekamp, A. J., Amberg, S. M., Piefer, A. J. & Bates, P. (2004) Proc. Natl. Acad. Sci. USA 101, 4240-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang, Z.-Y., Kong, W.-P., Huang, Y., Roberts, A., Murphy, B., Subbarao, K. & Nabel, G. J. (2004) Nature 428, 561-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisht, H., Roberts, A., Vogel, L., Bukreyev, A., Collins, P. L., Murphy, B. R., Subbarao, K. & Moss, B. (2004) Proc. Natl. Acad. Sci. USA 101, 6641-6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchholz, U. J., Bukreyev, A., Yang, L., Lamirande, E. W., Murphy, B. R., Subbarao, K. & Collins, P. L. (2004) Proc. Natl. Acad. Sci. USA 101, 9804-9809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan, Y., Zheng, B. J., He, Y. Q., Liu, X. L., Zhuang, Z. X., Cheung, C. L., Luo, S. W., Li, P. H., Zhang, L. J., Guan, Y. J., et al. (2003) Science 302, 276-278. [DOI] [PubMed] [Google Scholar]
- 12.Chinese SARS Molecular Epidemiology Consortium (2004) Science 303, 1666-1669. [DOI] [PubMed] [Google Scholar]
- 13.Kinsella, T. M. & Nolan, G. P. (1996) Hum. Gene Ther. 7, 1405-1413. [DOI] [PubMed] [Google Scholar]
- 14.Naldini, L., Blomer, U., Gage, F. H., Trono, D. & Verma, I. M. (1996) Proc. Natl. Acad. Sci. USA 93, 11382-11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Traggiai, E., Becker, S., Subbarao, K., Kolesnikova, L., Uematsu, Y., Gismondo, M. R., Murphy, B. R., Rappuoli, R. & Lanzavecchia, A. (2004) Nat. Med. 10, 871-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmann, H., Hattermann, K., Marzi, A., Gramberg, T., Geier, M., Krumbiegel, M., Kuate, S., Uberla, K., Niedrig, M. & Pohlmann, S. (2004) J. Virol. 78, 6134-6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wool-Lewis, R. J. & Bates, P. (1998) J. Virol. 72, 3155-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbarao, K., McAuliffe, J., Vogel, L., Fahle, G., Fischer, S., Tatti, K., Packard, M., Shieh, W.-J., Zaki, S. & Murphy, B. (2004) J. Virol. 78, 3572-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babcock, G. J., Esshaki, D. J., Thomas, W. D., Jr., & Ambrosino, D. M. (2004) J. Virol. 78, 4552-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong, S. K., Li, W., Moore, M. J., Choe, H. & Farzan, M. (2004) J. Biol. Chem. 279, 3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan, Y. J., Wei, C. L., Ee, A. L., Vega, V. B., Thoreau, H., Su, S. T., Chia, J. M., Ng, P., Chiu, K. P., Lim, L., et al. (2003) Lancet 361, 1779-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadler, K., Masignani, V., Eickmann, M., Becker, S., Abrignani, S., Klenk, H. D. & Rappuoli, R. (2003) Nat. Rev. Microbiol. 1, 209-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffers, S. A., Tusell, S. M., Gillim-Ross, L., Hemmila, E. M., Achenbach, J. E., Babcock, G. J., Thomas, W. D., Jr., Thackray, L. B., Young, M. D., Mason, R. J., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 15748-15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halstead, S. B. (1988) Science 239, 476-481. [DOI] [PubMed] [Google Scholar]
- 25.Murphy, B. R., Prince, G. A., Walsh, E. E., Kim, H. W., Parrott, R. H., Hemming, V. G., Rodriguez, W. J. & Chanock, R. M. (1986) J. Clin. Microbiol. 24, 197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.