Abstract
Purpose:
Aromatase inhibitors (AIs) are an important and effective hormonal adjuvant treatment for early-stage breast cancer. Up to 50% of women stop AIs prematurely, missing a valuable therapeutic intervention.
Patients and Methods:
We used grounded theory methodology to conduct in-depth, semistructured interviews and analyze data among patients with breast cancer diagnosed at age 65 years or older who were receiving an AI. The goal of the interviews was to understand decision making regarding persisting with AIs. Interview transcripts were systematically analyzed to identify emergent categories and relationships.
Results:
Interviews were conducted with 27 women. After completion of primary treatment, women in our sample found themselves “winging it” as they faced substantial struggles with infrequent support during this new phase of the cancer trajectory. Self-management of AI adverse effects occurred in the contexts of older age and early survivorship. “Bearing it” emerged as another important management process regarding the impact of AIs on quality of everyday life. The complex decision to persist with the AI involved weighing the possibility of a cancer-free future against the burden of adverse effects. Women relied on informal networks for support, rather than oncology providers, highlighting the need for practical self-management strategies. The notion of a tipping point in persistence revealed their susceptibility to early discontinuation.
Conclusion:
This study provides insight into potential decisional pathways leading to early discontinuation of AIs among older women with breast cancer. Better support is needed for these women.
INTRODUCTION
With the aging of the US population, breast cancer among women age 65 years or older is expected to increase considerably, from 1,068,000 patient cases in 2010 to 2,858,000 in 2020.1 Currently, the median age at diagnosis is 61 years, with incidence rates for women age 60 years or older on the rise since the mid 2000s.2 According to recent national estimates, women age 60 years or older will account for 131,430 (56%) new cases of invasive breast cancer and 70% of all deaths resulting from breast cancer in the United States annually.2 This disproportionate death rate is of particular concern because older women are usually diagnosed with more-treatable breast cancers than younger women.3 Antihormonal treatments, such as aromatase inhibitors (AIs), decrease recurrence and dramatically improve survival among women with hormone-positive tumors.4-7 Unfortunately, early discontinuation of and nonadherence to these antihormonal treatments are common in women age 65 years or older and directly affect breast cancer outcomes.8-10 Limited evidence suggests early discontinuation of hormonal treatments is associated with older age11,12 and adverse effects13,14 and usually occurs within the first year, but may also occur in subsequent years.15,16 However, underlying reasons for these decisions remain poorly understood.13,17
Because AIs are oral medications that are self-administered in the home setting and started during the transitional survivorship period,18,19 we wanted to understand what factors are associated with persistence and how these medications fit into the broader life context of older breast cancer survivors from the perspectives of the women themselves. However, we found only two studies, both conducted outside the United States, that used qualitative methodologies to investigate adherence from the women’s own perspectives, and neither was solely focused on AIs or women age older than 65 years.20,21 Thus, we explored how survivors of early-stage breast cancer, age 65 years and older, made decisions about persisting with AIs, including specific challenges as well as attempts to manage them.
PATIENTS AND METHODS
Procedures and Participant Recruitment
Qualitative methodology, guided specifically by constructivist grounded theory, was used to explore the processes of persisting with AIs from the perspectives of this sample of older women.22 Eligible women were at least 65 years of age when diagnosed with locoregional (stage I, II, or III) breast cancer, were responsible for taking their own medication, and had started an AI as adjuvant treatment 4 to 36 months before study enrollment. After receiving approval from the University of California Los Angeles Institutional Review Board, we recruited women with flyers in hospitals, community centers, and breast clinics, as well as mailings using cancer registries in southern California, from August 2013 to September 2015. A total of 237 women inquired about the study and were screened for eligibility and interest in the study. Of these, 209 were ineligible to participate, and of the remaining 28 women, 27 agreed to participate. The main reasons for ineligibility were never receiving an AI and a prior history of cancer.
Data Collection and Analysis
After obtaining signed consent for interviews and medical record release, individual, in-person, single-session interviews were conducted (by H.C.P.) using a semistructured interview guide (Table 1). Most interviews took place in participants’ homes (n = 20) based on their preference. The average length of each interview was 87.4 minutes. Interviews were digitally audio recorded and then transcribed verbatim, checked for accuracy, and deidentified by the research team. Additional pertinent information was abstracted from the medical record. Other sources of data included demographic variables obtained by a self-report questionnaire as well as field notes. Three members of the research team systematically and independently coded the data, using initial coding, followed by focused coding to identify emergent categories and theoretic coding to determine relationships among them. Regular meetings among the coders occurred to enhance methodologic rigor and discuss discrepancies, as well as develop categories. Triangulation across analysts facilitated deeper understanding by approaching the data in multiple ways.23 Analytic techniques such as constant comparison, memo writing, diagramming, and mapping were implemented throughout to deepen analysis. The Atlas.ti software program (http://atlasti.com) was used for data management.
Table 1.
Examples of Conversational Interview Questions

RESULTS
Sample Characteristics
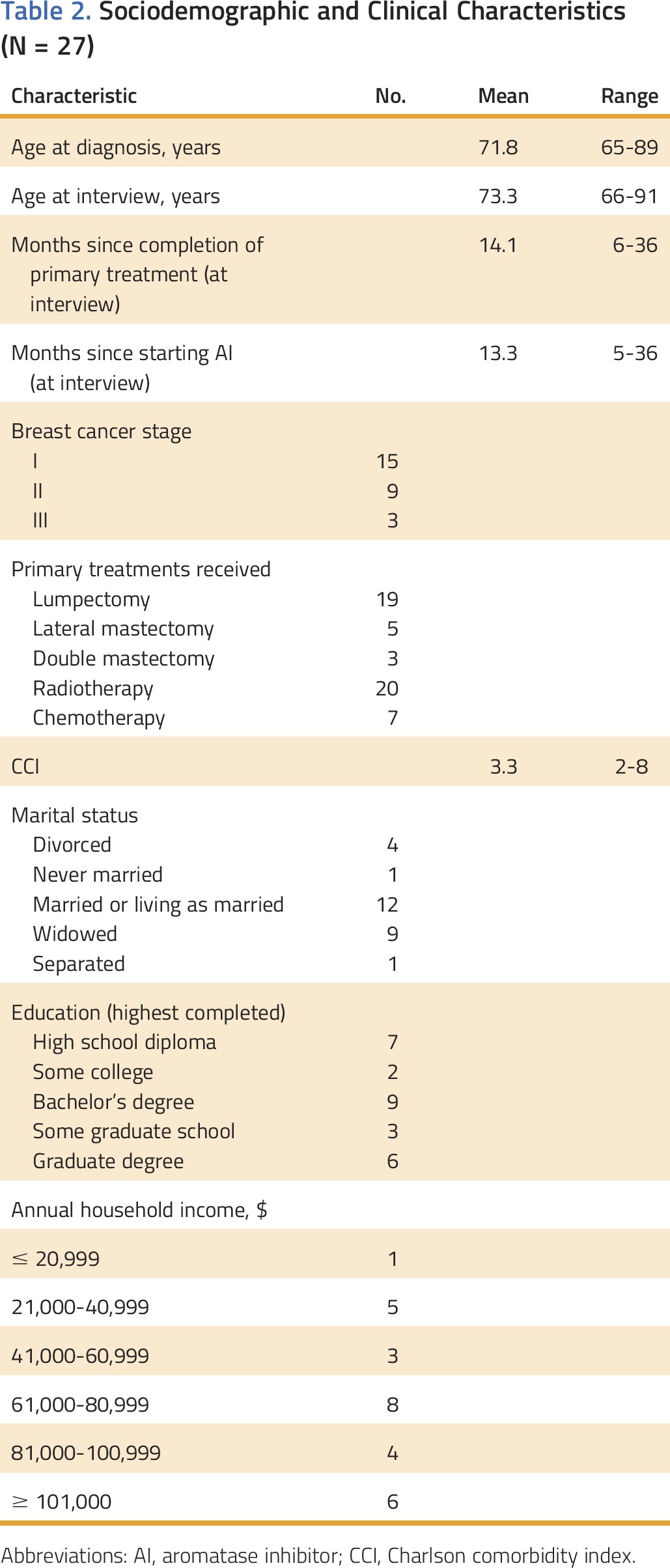
The 27 women were diagnosed on average at 72 years of age and had diverse racial, ethnic, cultural, marital, and socioeconomic backgrounds (Table 2). Medical record review indicated that all participants had at least one other chronic illness, with more than 75% (21 of 27) having three or more chronic illnesses. Most participants (n = 21) received care at an academic medical center or affiliate clinic. Fifteen women had stage I disease, whereas the rest had been diagnosed with stage II or III disease. In terms of primary treatment, the most common surgery was lumpectomy, and more women had undergone radiotherapy than chemotherapy, although these categories were not mutually exclusive. At the time of the interviews, women had completed primary treatment on average 14.1 months earlier and had been receiving an AI for an average of 13.3 months. At the time of the study, participants took one to 13 prescription pills per day (average, 4.6 pills), including the AI. AI-related adverse effects were documented in the medical records of 13 women; however, the vast majority discussed experiencing adverse effects during the interviews.
Table 2.
Sociodemographic and Clinical Characteristics (N = 27)
Overall, the women in this sample described the following key factors in their decision to persist with an AI: being older, proceeding from primary treatment to “winging it” in the next phase of cancer survivorship, and understanding adverse effects. Participants also perceived that persisting with the AI was accompanied by struggling and bearing adverse effects, and they foresaw potential tipping points that could lead to premature discontinuation of the treatment.
Context Matters: Older Age and a New Phase of Treatment
Persisting with the AI occurred in the context of an older life stage and the period of adjustment that follows completion of primary treatment. The older age of the women in our sample was important to their experience with taking the AI. In particular, most of these older women were well versed in managing health conditions and taking oral medications in the home setting. Therefore, before starting the AI, most participants had a well-established home medication routine, such as a daily pillbox. Familiarity with taking medications also meant that these women had prior experience with adverse effects. They understood that recognition and management of these adverse effects were worthwhile endeavors to reap the benefits of a medication. Although one woman occasionally skipped a dose to manage adverse effects, explaining, “Maybe I won’t take that today and see if it helps,” the women generally took the AI medication as prescribed. No participant identified cost as a barrier to receiving the AI.
When the women started the AI, they, along with their friends and family, understood cancer treatment to be over and expected to leave the cancer experience behind them. In fact, it was during their recovery from primary treatment that many women reported that they first learned about the AI, and some felt frustrated by this unexpected extension of treatment. The shift from “receiving” surgery, chemotherapy, and radiotherapy to taking a pill signified a sharp contrast between the AI experience and primary cancer treatments. Also, unlike surgery, chemotherapy, and radiotherapy, the AI represented a treatment that would continue for many years. Women described surprise that such a small, seemingly insignificant pill could hold so much promise.
Navigating this phase while interacting less frequently with the oncology team resulted in new challenges. One participant described how the familiar and reassuring footholds were pulled away just as she was starting the AI. She said, “You feel like you don’t have all the structures we talked about before, so now you’re winging it, and that’s scary” (Table 3). Thus, AI therapy began during a blurry period in which women wondered whether they were still recovering from primary treatment or experiencing new symptoms related to this new medication.
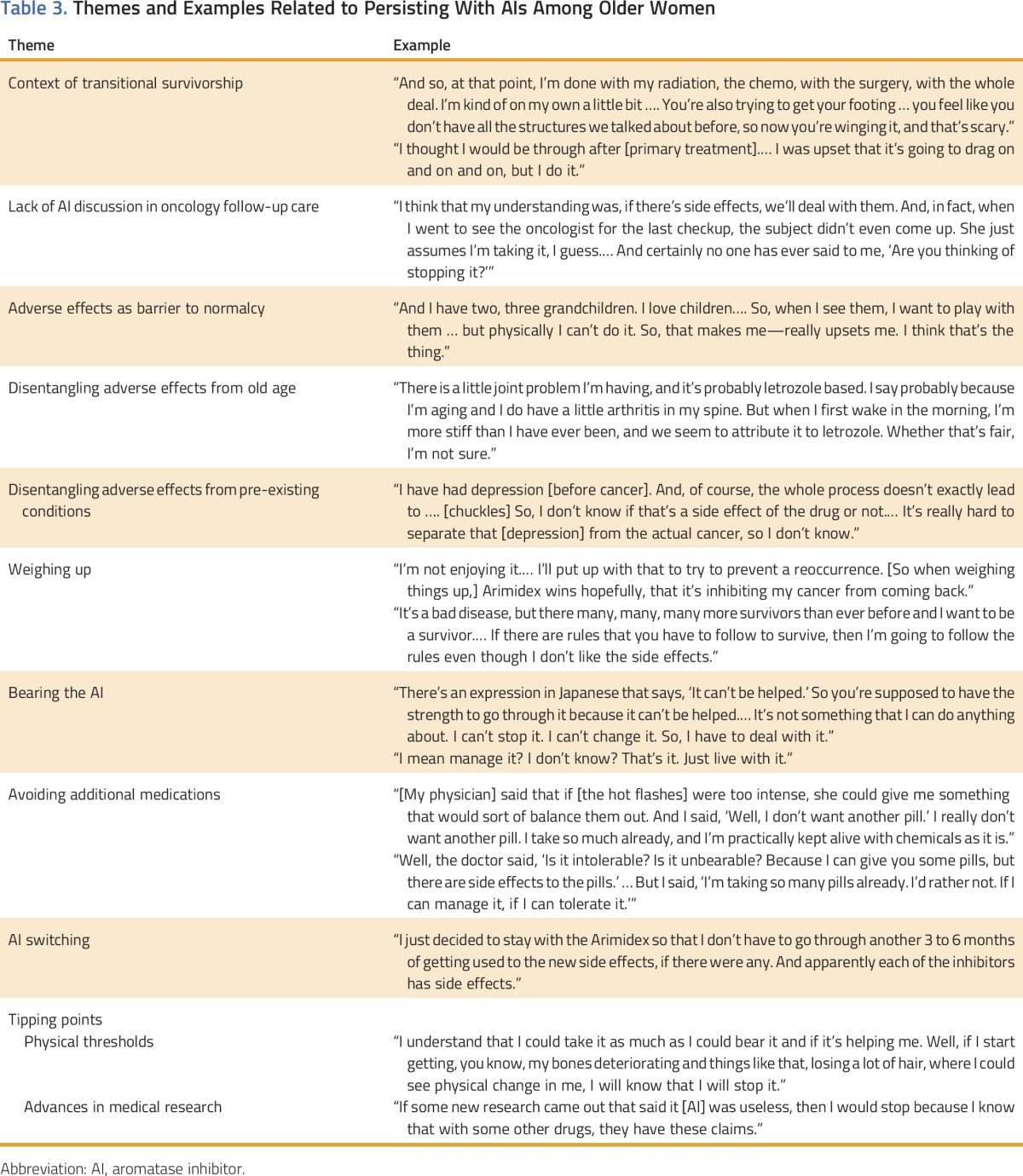
Table 3.
Themes and Examples Related to Persisting With AIs Among Older Women
Inherent in the AI trajectory was the challenge of integrating this tiny pill, so named by several women, into daily life and acknowledging that the AI marked a new phase rather than the end of the cancer experience. This new phase needed to be tolerable in a different way, demanding a marathon rather than sprint mentality and allowing the woman to resume social roles such as wife, mother, grandmother, employee, or volunteer. For women who experienced adverse effects, persisting with the AI brought on unique challenges without the same level of support they had had during primary treatment.
The main motivation for the women’s initiation of and continuation with the AI was the possibility of a cancer-free future. The participants realized that the AI did not guarantee this outcome, and so it was not the promise of a cancer-free future but the possibility of one that motivated them. Compared with the fatal disease that breast cancer was when they were growing up, the possibility of a cancer-free future was reinforced by a changing breast cancer culture in which they saw many women surviving, talking more about it, and going on with their lives. As one woman explained, “You know when I was growing up, it was all hush-hush … you whispered the word ‘cancer’ and now it’s really in our vernacular.” However, despite clear and focused motivation to start the AI, many women experienced burdensome adverse effects, such as hot flashes, joint pain, depression, skin and hair changes, and insomnia.
Disentangling Adverse Effects
For this sample of older women, adverse effects emerged as a noteworthy burden associated with taking the AI. Disentangling whether a physical or psychological change was an adverse effect of the AI, residual from primary treatment, an exacerbation of another medical condition, or simply a part of aging was challenging. The AI worsened some pre-existing conditions, such as joint pain or depression, and so the contribution of the AI was also unclear. As one woman said, “It’s very hard for me to pinpoint what’s causing what because I have all of these different [health conditions].” Interference with quality of life was emphasized as the most distressing aspect of the adverse effects. A participant for whom the adverse effects subsided over time explained, “What is important for me is to go back to my lifestyle, which is very active. And with the side effects, I would have been on that couch.” The women expressed the most frustration when adverse effects acted as a barrier to normalcy and the ability to carry out social roles.
Struggling Despite Persisting
Despite describing good relationships with oncologists, our sample largely did not rely on them to help with adverse effects and did not perceive management of AI adverse effects as part of the oncologist’s role. As women tried to re-establish their lives, many were comforted by the belief that the oncologist now had sicker patients for whom to care. Many women reported that the AI was not mentioned during follow-up oncology appointments, indicating to the women the medication was not important or that the oncologist assumed they were taking it. Instead, women often turned to nononcologist physicians whom they had known for a long time, such as a primary care physician or gynecologist, with concerns about the AI. Although several women were offered additional medications to manage AI-related adverse effects, such as antidepressants to help mitigate hot flashes, they were generally reluctant to add pills that might introduce their own unpredictable adverse effects into their daily routine. Nononcologist physicians were also helpful in offering encouragement and motivation. One woman explained how her primary care physician of many decades helped her understand the purpose and value of the AI when she mentioned wanting to discontinue because of her brittle nails.
Switching to another AI to manage adverse effects was an option that few women mentioned. In general, women were unenthusiastic about new medications with adverse effects that were potentially the same or worse, including alternative AIs. One woman who did try switching drugs ended up switching back to the original drug, whereas another woman considered switching but decided not to go through an adjustment period for another drug (Table 3). Some women incorporated their own supplements and herbs into their routine, but typically, this was something they found out about and initiated on their own and just ran the idea past their physician. The women strongly preferred to use practical lifestyle adjustments, rather than additional medications, to bear or tolerate AI adverse effects.
Bearing AI Adverse Effects
Adverse effects were unpleasant and intrusive but usually not unbearable. Many women acknowledged that adverse effects changed over time. Personal motivations and hopes for the future helped the women to soldier on and persist with the AI despite ongoing struggles. Through weighing the potential benefits of the drug with the discomforts of the adverse effects, women came to the decision to persist and reshifted their focus to make burdensome adverse effects more bearable. Women often discussed their struggles with fellow cancer survivors and shared practical tips that facilitated AI continuation through these informal channels. Tip sharing emerged as an important process that fellow breast cancer survivors used to share information and knowledge, typically to self-manage adverse effects or mitigate the impact of illness and treatment on daily life in the home setting. Through these supportive networks, women valued the process of combining their individual experiences and solutions they had figured out along the way and sharing that collective knowledge.
Tipping Points
Despite bearing the unpleasantness of adverse effects and persisting with the AI at the time of the interview, no participant, when asked, said that she would persist regardless.
Although women were willing to try the AI, many of them were able to articulate a threshold, a potential tipping point, at which they would discontinue or seriously consider discontinuing the AI. These thresholds largely pertained to adverse effects becoming unbearable or even painful, such as in the case of the woman who, considering the years ahead, stated, “He told me I had to take the pill for 5 years. But then, when I started having all these hot flashes, I thought, ugh, maybe not” (Table 3). However, this sample of older women also reflected on a lifetime in which they had witnessed profound changes in scientific knowledge, specifically medical research, and indicated that advances related to AIs in research could also affect their decision to continue or not.
DISCUSSION
To our knowledge, our study is unique in its exploration of the struggles that continuation of AI treatment involved for a group of older women. The interface of older age, cancer survivorship, and starting an AI posed distinct challenges to medication persistence for the women in our sample. Attempts have been made to support survivors as they transition from primary treatment; however, little precedent exists for management of AIs. In the context of gero-oncology, disentangling AI adverse effects from other aspects of older age, such as other medical conditions, slower recovery from primary treatment, and age-related changes themselves, complicates the AI experience for this group of women. Even in cases where women are misattributing their symptoms to adverse effects of the AI, the assumption clearly poses a threat to AI continuation if the AI is culpable from their perspective. When women start receiving AIs, primary therapy is completed, but women are beginning a new treatment, with its own adjustment period and potential adverse effects. The experience of receiving the AI differs from primary treatment, because women are responsible for taking the pill in the home setting for many years, with infrequent interaction with the oncology team. Therefore, the AI must be integrated into a woman’s daily life.
Our research used a lifespan perspective, which highlighted the distinct yet unmet needs of older women as they persisted with AIs. The broader context of older age and related aspects such as comorbidities, polypharmacy, and value of quality of life is essential to the AI experience of older women. Our findings are consistent with other research suggesting that adverse effects account for most of the burden associated with hormonal therapies.12,14,21 However, among older women, the adverse effects of AIs are difficult to disentangle from comorbid conditions or what the women attribute to getting older. This challenge in attribution, coupled with less frequent contact with their oncology team, results in many women “winging it” or persisting with the AI without much-needed professional guidance or support. Given their older age, women in our sample were likely to know other breast cancer survivors and turned to these informal networks for camaraderie and the exchange of practical knowledge. Ultimately, women weighed the possibility of a cancer-free future against the discomfort that they associated with the pill and decided, at least for now, to bear it. This finding is consistent with the decisional balance seen in both older and younger women willing to tolerate the negative impact of antihormonal therapy on daily life to reduce the threat of cancer recurrence and death.20,24 Women engaged in efforts to make the AI experience more tolerable in terms of physical discomfort, as well as in terms of the impact of adverse effects on quality of life and sense of self.
While they persist with an AI, women also struggle and can often verbalize circumstances under which they might discontinue the AI early, thus alerting us to the fluidity between women who are persisting with an AI and those who discontinue prematurely. The notion of a tipping point in their efforts to manage AI adverse effects identified this group as susceptible to discontinuing treatment before the recommended 10 years. Another important finding related to tipping points was the women’s awareness that medical research changes over time. It is noteworthy that this particular age cohort lived through the Women’s Health Initiative, which found that hormone replacement therapy carried more risk than previously thought. Women reflected on this experience as one in which they realized the unfixed nature of medical research. With respect to AIs, women understood that research is ongoing, and many unanswered questions remain. These findings have important clinical implications for AI continuation, especially in light of recent changes to the recommended duration of AI treatment from 5 to 10 years.
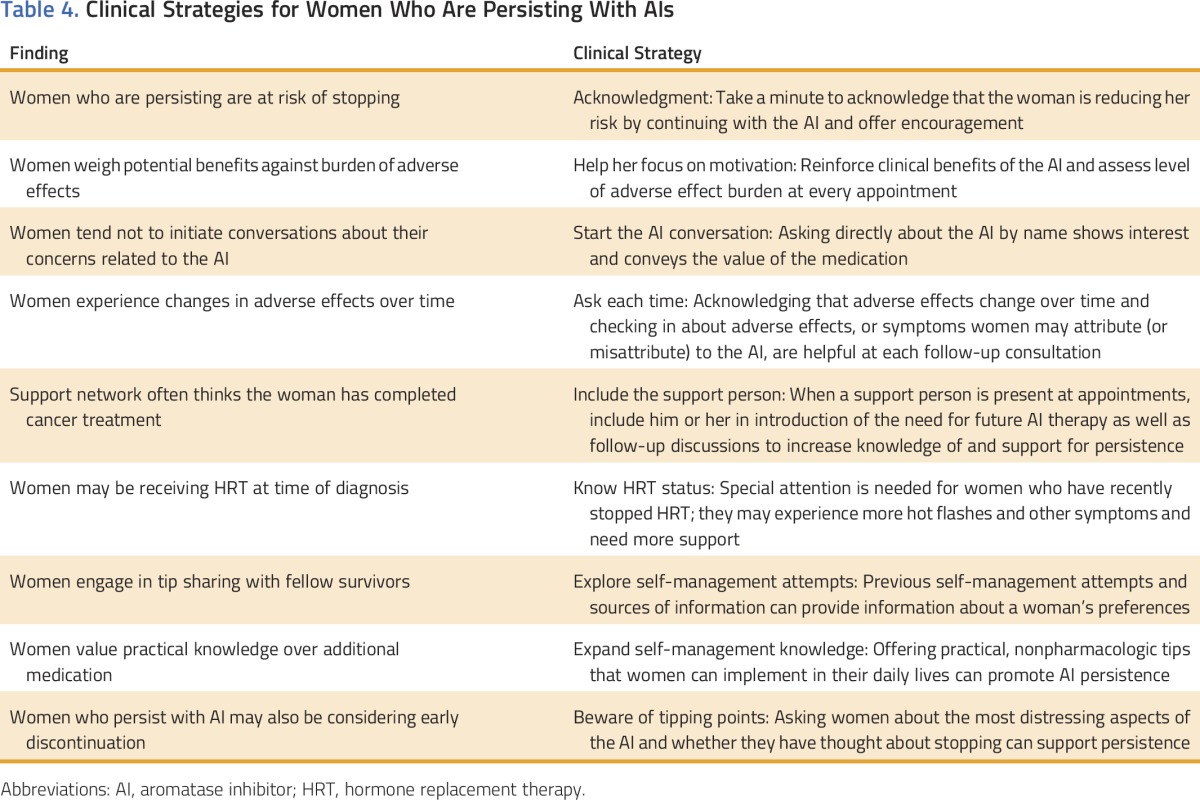
Older breast cancer survivors who are persisting with AI treatment may also be “winging it,” “bearing it,” and at risk for early discontinuation and poorer outcomes. High-quality survivorship care that includes patient monitoring, patient education, adverse effect management, and encouragement may be helpful in ensuring safety and promoting persistence for the prescribed duration.25 According to the American Society of Clinical Oncology guideline, patient–provider communication about AIs should include a discussion of anticipated adverse effects, inquiry about adverse effects, and discussion of possible ways to mitigate them during subsequent appointments.26 Within the limitations of our study, including a small sample limited to one geographic region of the country, our results suggest the need for AI-specific education, support, and practical self-management strategies to improve both medication persistence and quality of life and promote healthy survivorship. On the basis of our observations, we offer some possible strategies to enhance clinical management and adherence as well as inform the development of future interventions for older women receiving AI therapy (Table 4).
Table 4.
Clinical Strategies for Women Who Are Persisting With AIs
Our findings are relevant to both oncology providers as well as providers in other settings who care for this group of women as they navigate complex decisions in the period after primary treatment. From a gero-oncology perspective, our results lay groundwork in understanding older women’s decisional processes related to AIs in the words of the women themselves. It is essential that future research incorporate a gerontology-focused approach in the development, implementation, and evaluation of models of care that will promote continuation and adherence throughout the AI trajectory.
ACKNOWLEDGMENT
Supported by National Cancer Institute Grant No. R21CA167218-01A1. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of an institution or sponsor. We thank Miriam Sleven, RN, MS, OCN, for her input in the preparation of this manuscript.
AUTHOR CONTRIBUTIONS
Conception and design: Patricia A. Ganz, Huibrie C. Pieters
Collection and assembly of data: Huibrie C. Pieters
Data analysis and interpretation: Eden R. Brauer, Huibrie C. Pieters
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
“Winging It”: How Older Breast Cancer Survivors Persist With Aromatase Inhibitor Treatment
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Eden R. Brauer
No relationship to disclose
Patricia A. Ganz
Leadership: Intrinsic LifeSciences (I)
Stock or Other Ownership: Xenon Pharma (I), Intrinsic LifeSciences (I), Silarus Therapeutics (I), Merganser Biotech (I), TEVA Pharmaceuticals Industries, Novartis, Merck, Johnson & Johnson, Pfizer, GlaxoSmithKline, Abbott Laboratories
Consulting or Advisory Role: Keryx (I), Merganser Biotech (I), Silarus Therapeutics (I), InformedDNA, Vifor Pharma (I)
Research Funding: Keryx (I)
Patents, Royalties, Other Intellectual Property: Patent related to iron metabolism and anemia of chronic disease (I), Up-to-Date royalties for section editor on survivorship
Travel, Accommodations, Expenses: Intrinsic LifeSciences (I), Keryx (I)
Huibrie C. Pieters
No relationship to disclose
REFERENCES
- 1.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2012. Bethesda, MD: National Cancer Institute; 2015. [Google Scholar]
- 4.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative Group Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 6.Burstein HJ. Aromatase inhibitor-associated arthralgia syndrome. Breast. 2007;16:223–234. doi: 10.1016/j.breast.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 7.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: Update on adjuvant endocrine therapy for women with hormone receptor–positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: Updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA) Lancet Oncol. 2012;13:e148–e160. doi: 10.1016/S1470-2045(11)70383-7. [DOI] [PubMed] [Google Scholar]
- 9.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30:936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Res Treat. 2012;134:459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor–positive breast cancer. J Clin Oncol. 2008;26:549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 12.He W, Fang F, Varnum C, et al. Predictors of discontinuation of adjuvant hormone therapy in patients with breast cancer. J Clin Oncol. 2015;33:2262–2269. doi: 10.1200/JCO.2014.59.3673. [DOI] [PubMed] [Google Scholar]
- 13.Puts MT, Tu HA, Tourangeau A, et al. Factors influencing adherence to cancer treatment in older adults with cancer: A systematic review. Ann Oncol. 2014;25:564–577. doi: 10.1093/annonc/mdt433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson LJ, Dawson C, Lawrence DH, et al. Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women. Cochrane Database Syst Rev. 2007;(1):CD003370. doi: 10.1002/14651858.CD003370.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Bender CM, Gentry AL, Brufsky AM, et al. Influence of patient and treatment factors on adherence to adjuvant endocrine therapy in breast cancer. Oncol Nurs Forum. 2014;41:274–285. doi: 10.1188/14.ONF.274-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HS, Lee JY, Ah YM, et al. Low adherence to upfront and extended adjuvant letrozole therapy among early breast cancer patients in a clinical practice setting. Oncology. 2014;86:340–349. doi: 10.1159/000360702. [DOI] [PubMed] [Google Scholar]
- 17.Stanton AL, Petrie KJ, Partridge AH. Contributors to nonadherence and nonpersistence with endocrine therapy in breast cancer survivors recruited from an online research registry. Breast Cancer Res Treat. 2014;145:525–534. doi: 10.1007/s10549-014-2961-3. [DOI] [PubMed] [Google Scholar]
- 18.Ganz PA. Survivorship: Adult cancer survivors. Prim Care. 2009;36:721–741. doi: 10.1016/j.pop.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Mullan F. Seasons of survival: Reflections of a physician with cancer. N Engl J Med. 1985;313:270–273. doi: 10.1056/NEJM198507253130421. [DOI] [PubMed] [Google Scholar]
- 20.Harrow A, Dryden R, McCowan C, et al. A hard pill to swallow: A qualitative study of women’s experiences of adjuvant endocrine therapy for breast cancer. BMJ Open. 2014;4:e005285. doi: 10.1136/bmjopen-2014-005285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellegrini I, Sarradon-Eck A, Soussan PB, et al. Women’s perceptions and experience of adjuvant tamoxifen therapy account for their adherence: Breast cancer patients’ point of view. Psychooncology. 2010;19:472–479. doi: 10.1002/pon.1593. [DOI] [PubMed] [Google Scholar]
- 22.Charmaz K. Constructing Grounded Theory (ed 2) Thousand Oaks, CA: Sage Publications; 2014. [Google Scholar]
- 23.Creswell JW. Qualitative Inquiry and Research Design Choosing Among Five Traditions. Thousand Oaks, CA: Sage Publications; 1998. [Google Scholar]
- 24.Fink AK, Gurwitz J, Rakowski W, et al. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor–positive breast cancer. J Clin Oncol. 2004;22:3309–3315. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 25.Levit L, Balogh E, Nass S, et al. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 26.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32:2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]


