Abstract
Lung tissue remodeling in chronic obstructive pulmonary disease (COPD) is characterized by airway wall thickening and/or emphysema. Although the bronchial and alveolar compartments are functionally independent entities, we recently showed comparable alterations in matrix composition comprised of decreased elastin content and increased collagen and hyaluronan contents of alveolar and small airway walls. Out of several animal models tested, surfactant protein C (SPC)-TNF-α mice showed remodeling in alveolar and airway walls similar to what we observed in patients with COPD. Epithelial cells are able to undergo a phenotypic shift, gaining mesenchymal properties, a process in which c-Jun N-terminal kinase (JNK) signaling is involved. Therefore, we hypothesized that TNF-α induces JNK-dependent epithelial plasticity, which contributes to lung matrix remodeling. To this end, the ability of TNF-α to induce a phenotypic shift was assessed in A549, BEAS2B, and primary bronchial epithelial cells, and phenotypic markers were studied in SPC–TNF-α mice. Phenotypic markers of mesenchymal cells were elevated both in vitro and in vivo, as shown by the expression of vimentin, plasminogen activator inhibitor-1, collagen, and matrix metalloproteinases. Concurrently, the expression of the epithelial markers, E-cadherin and keratin 7 and 18, was attenuated. A pharmacological inhibitor of JNK attenuated this phenotypic shift in vitro, demonstrating involvement of JNK signaling in this process. Interestingly, activation of JNK signaling was also clearly present in lungs of SPC–TNF-α mice and patients with COPD. Together, these data show a role for TNF-α in the induction of a phenotypic shift in vitro, resulting in increased collagen production and the expression of elastin-degrading matrix metalloproteinases, and provide evidence for involvement of the TNF-α–JNK axis in extracellular matrix remodeling.
Keywords: c-Jun N-terminal kinase, TNF-α, matrix remodeling, lung
Clinical Relevance
The findings described in this study show a role for TNF-α in the induction of a phenotypic shift in epithelial cells in vitro, and provide evidence for involvement of the TNFα–JNK axis in extracellular matrix remodeling as observed in patients with chronic obstructive pulmonary disease.
Airflow limitation in patients with chronic obstructive pulmonary disease (COPD) is associated with remodeling of the lung. The greatest decline in forced expiratory volume in 1 second, as seen in mild to moderate COPD, is attributed largely to alterations in the small airways, including airway wall thickening. The contribution of emphysema to airflow obstruction increases as the disease progresses (1, 2). Although the bronchial and alveolar compartments may be considered functionally independent entities that are affected sequentially in the progression of the disease, we recently showed that the matrix of small airway walls and alveolar walls from patients with COPD displays very similar features of remodeling (3). Moreover, in patients with mild to moderate disease, the composition of the extracellular matrix of both compartments was affected in a similar way as observed in patients with very severe disease. In particular, it was demonstrated that the elastin content of alveolar and small airway walls was decreased, whereas an increased collagen and hyaluronan content was found in both compartments. Strong correlations between the matrix alterations in alveolar and small airway walls were present, suggesting that the alveolar and small airway walls remodel as one unit, and that similar underlying mechanisms are possibly involved.
Different experimental mouse models of COPD, using several etiological factors, are employed to study disease pathology in general and remodeling specifically. Recently, we characterized extracellular matrix components in different murine models of COPD: mice chronically exposed to cigarette smoke; mice chronically exposed to LPS; and surfactant protein C (SPC)–TNF-α mice, which overexpress TNF-α in alveolar type II cells. Our data showed an increased collagen and hyaluronan content in both alveolar and small airway walls, which was similar in all three models. Interestingly, elastin was only decreased in both compartments in the SPC–TNF-α mice. These data indicate that matrix remodeling in SPC–TNF-α mice is most comparable to the remodeling observed in patients with COPD, when compared with the chronic smoke and chronic LPS models. Furthermore, evidence was shown for increased expression of the elastin-degrading enzymes, matrix metalloproteinase (MMP) 2 and MMP9 (4).
Epithelial cells have an important role in orchestrating the response to inhaled toxins and pathogens. Interestingly, it was shown, using an epithelial-specific collagen-1 knockout mouse in the bleomycin model of pulmonary fibrosis, that the epithelium plays an important role in collagen deposition (5). One possible mechanism by which epithelial cells could contribute to enhanced matrix deposition and breakdown of elastin by MMPs is through a phenotypic shift, whereby they lose epithelial properties and gain characteristics and functions of mesenchymal cells, including production of MMPs and of extracellular matrix components (6). To study this process, which is also referred to as epithelial-to-mesenchymal transition (EMT), in vitro stimulation of cultured epithelial cells with transforming growth factor (TGF)-β1 is commonly used. We have shown a role for c-Jun N-terminal kinase (JNK) 1 in TGF-β1–induced EMT in lung epithelial cells, as the induction of this phenotypic shift was blunted in JNK1 knockout cells (7). Furthermore, our group recently demonstrated that cigarette smoke extract also has the ability to induce such a phenotypic shift, in which hypoxia inducible factor (HIF1α) signaling was implicated (8).
TNF-α is known to be involved in extracellular matrix remodeling, and has been shown to suppress elastin precursor levels in lung fibroblast, and to promote elastin breakdown through enhanced release of elastolytic enzymes, including MMP2 and MMP9. A role for TNF-α in tissue remodeling is further evident from a study that demonstrated that TNF-α receptor knockout mice were protected from cigarette smoke–induced connective tissue breakdown (9, 10). In addition, it was recently shown that, in alveolar epithelial cells, TNF-α can induce JNK signaling (11) and that JNK is involved in TNF-α–induced MMP12 expression (12).
Based on the above, we hypothesized that TNF-α induces JNK-dependent epithelial plasticity, which contributes to matrix remodeling. To this end, changes in the expression of epithelial and mesenchymal markers were investigated in SPC–TNF-α mice in vivo and in TNF-α–stimulated alveolar and bronchial epithelial cell (BEC) lines and human primary BECs (pBECs) in vitro. Furthermore, the role of JNK was studied in vitro, and the presence and localization of phosphorylated JNK was studied in SPC–TNF-α mice and patients with COPD.
Materials and Methods
Cell Culture
A549 and BEAS2B cell lines (ATCC, Manassas, VA) were cultured as described previously (13, 14). At confluency, cells were starved and stimulated twice with 10 ng/ml TNF-α with a 24-hour interval (R&D, Minneapolis, MN). For JNK inhibition, cells were preincubated for 30 minutes with 10 μM SP600125 (Sigma, St. Louis, MO).
pBECs were kindly provided by the Primary Lung Culture (PLUC) facility of the Maastricht University Medical Center (Maastricht, the Netherlands). Isolation procedures and culture conditions can be found in the online supplement.
Quantitative PCR
RNA was isolated from cells using the High Pure RNA Isolation Kit (Roche, Indianapolis, IN) or mouse whole-lung tissue using the RNeasy kit (Qiagen, Alameda, CA) and reverse transcribed using Transcriptor First Strand cDNA Synthesis Kit (Roche). PCR reactions were performed on an ABI 7900HT thermal cycler (Applied Biosystems, Foster City, CA) using SYBR green dye (Applied Biosystems) and primer sequences listed in the supplemental Materials and Methods. Relative mRNA expression of genes was corrected for Rpl13a, which was found to be the most stable housekeeper using geNorm (15).
Western Blotting
Protein lysates and whole-lung lysates, obtained using RIPA buffer and treatment with ultrasonic waves, were separated (20 μg) on 12% Criterion XT Bis-Tris gels (Bio-Rad, Hercules, CA) and transferred onto a nitrocellulose membrane (Protran, Kent, UK). Blocking in 5% milk was followed by incubation with primary monoclonal antibodies raised against E-cadherin (no. 3195, 1:2,000 dilution), vimentin (no. 5741, 1:5,000 dilution), or glyceraldehyde phosphate dehydrogenase (no. 2118, 1:10,000 dilution) (all from Cell Signaling, Danvers, MA) and peroxidase-conjugated secondary antibody (Vector, Burlingame, CA), which was detected with SuperSignal West Pico Chemiluminescent Substrate (ThermoFisher, Waltham, MA) in an LAS-3000 Luminescent Image analyzer (Fujifilm, Tokyo, Japan). All data were corrected for protein loading by glyceraldehyde phosphate dehydrogenase.
Luciferase Reporter Assay
Transient transfections were performed using X-tremeGENE HP (Roche) according to the manufacturer’s instructions, with 0.875 μg hypoxia-responsive element (16) promotor luciferase and 0.125 μg simian vacuolating–β-galactosidase plasmids to correct for transfection efficiency. Luciferase (Promega, Madison, WI) and β-galactosidase (Tropix, Applied Biosystems, Foster City, CA) activities were measured according to the manufacturers’ instructions.
Collagen Assay
Collagen content of cell-free medium was determined by the addition of Sirius Red staining reagent (Klinipath, Duiven, the Netherlands) in saturated picric acid (Klinipath). The precipitate was dissolved in 0.5 M NaOH. Extinction was measured at 540 nm and concentrations were calculated using a standard curve.
SPC–TNF-α Mice
The left lung of SP-C–TNF-α mice (17) was fixed by tracheal infusion of 4% paraformaldehyde under a constant pressure of 20 cm H2O above the highest point of the lung according to American Thoracic Society/European Respiratory Society guidelines. The lung lobes were embedded in paraffin and cut into 4-μm transverse sections, which were randomly selected. Housing conditions are described in the online supplement.
Phosphorylated c-Jun N-terminal Kinase Staining
Phosphorylated c-Jun N-terminal Kinase (pJNK) was detected using antibodies against rabbit pJNK (Invitrogen, Carlsbad, CA). pJNK staining was analyzed in a double-blinded fashion by a researcher and a pathologist. The detailed procedure is described in the online supplement.
Statistical Analyses
In a case of more than two groups, between-group comparisons were analyzed using the Kruskal-Wallis test. The differences between two groups were analyzed using the Mann-Whitney U test (SPSS20; IBM Corporation, Armonk, NY). Data were expressed as mean (±SD or SEM). A P value less than 0.05 was considered statistically significant.
Results
Increased Expression of Mesenchymal Markers and Attenuated Expression of Epithelial Markers in SPC–TNF-α Mice
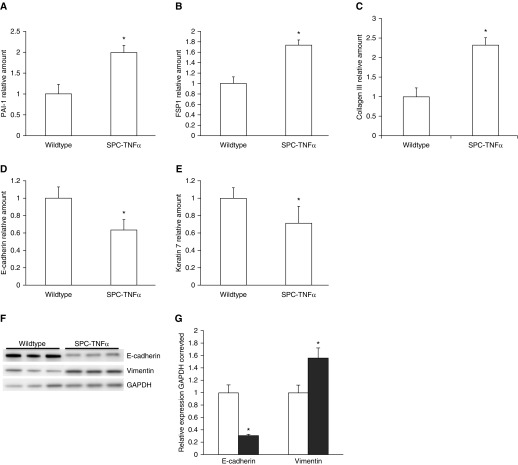
Previous research showed matrix remodeling in alveolar and airway walls of SPC–TNF-α mice. Here, we investigated whether increased mesenchymal marker expression was associated with alterations in matrix composition, and if there was also a concurrent decrease of epithelial marker expression. In Figure 1, it is demonstrated that the mRNA levels of the mesenchymal markers, plasminogen activator inhibitor (PAI-1), fibroblast specific protein (FSP1), and collagen III (Figure 1A–1C), were increased in mice with the TNF-α transgene compared with littermate controls. In addition, the mRNA levels of the epithelial markers, E-cadherin and keratin 7, were decreased in these mice (Figure 1D and 1E). To confirm these results at the protein level, Western blots were performed on whole-cell lysates of lung tissue. As shown in Figures 1F and 1G, protein levels of the mesenchymal marker, vimentin, were increased, whereas levels of the epithelial protein E-cadherin were significantly decreased in SPC–TNF-α mice.
Figure 1.
Increased expression of mesenchymal markers and attenuated expression of epithelial markers in surfactant protein C (SPC)–TNF-α mice. Lung tissue from 12-month-old SPC–TNF-α (n = 7) and wild-type littermates (n = 7) was used to measure mRNA expression of the mesenchymal markers (A) plasminogen activator inhibitor (PAI-1), (B) fibroblast specific protein (FSP1), and (C) collagen III, and the epithelial markers (D) E-cadherin and (E) keratin 7. Protein levels of E-cadherin and vimentin in SPC–TNF-α mice and wild-type littermates, as detected by Western blotting of three representative mice per group (F). Quantification of Western blot data (n = 7) shown as mean (±SEM) (G). *P < 0.05 compared with wild-type mice (in open bars). GAPDH, glyceraldehyde phosphate dehydrogenase.
TNF-α Induced a Phenotypic Shift in A549 and BEAS2B Cells
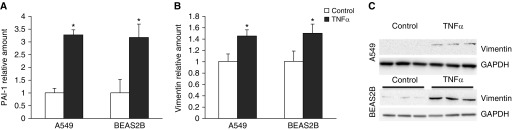
Because whole-lung lysates of SPC–TNF-α mice were analyzed, it cannot be concluded that the increased mesenchymal marker expression occurred in epithelial cells, but the concurrent loss of epithelial marker expression suggests that the epithelial cell phenotype was altered. To determine if lung epithelial cells undergo a phenotypic shift under the influence of TNF-α, A549 and BEAS2B cells were stimulated with TNF-α for 48 hours and mesenchymal markers were analyzed at the mRNA level by quantitative PCR and at the protein level by Western blotting. Significantly increased levels of PAI-1 (Figure 2A) and vimentin (Figure 2B) mRNA and protein (Figure 2C) were observed after TNF-α stimulation in both A549 and BEAS2B cells.
Figure 2.
TNF-α–induced mesenchymal marker expression in A549 and BEAS2B cells. A549 and BEAS2B cells were stimulated with 10 ng/ml TNF-α twice, with a 24-hour interval. mRNA expression of mesenchymal markers PAI-1 (A) and vimentin (B) was measured using quantitative PCR (qPCR). Data are shown as mean (±SD). *P < 0.05 compared with control. (C) Protein expression of vimentin in A549 cells and BEAS2B cells analyzed by Western blotting.
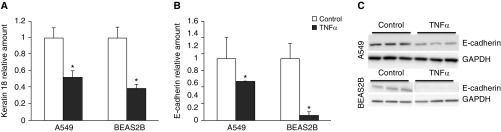
In addition to the increased expression of mesenchymal markers, the effect of TNF-α stimulation in vitro on epithelial markers was studied. Results shown in Figure 3 demonstrate that mRNA levels of keratin 18 (Figure 3A) and E-cadherin (Figure 3B) were decreased in both A549 and BEAS2B cells after stimulation with TNF-α. Furthermore, protein levels of E-cadherin were also significantly decreased in both cell lines after 48 hours of stimulation with TNF-α (Figure 3C).
Figure 3.
TNF-α decreased expression of epithelial markers in A549 and BEAS2B cells. A549 and BEAS2B cells were stimulated with 10 ng/ml TNF-α twice, with a 24-hour interval. mRNA expression of epithelial markers keratin 18 (A) and E-cadherin (B) was measured using qPCR. Data are shown as mean (±SD). *P < 0.05 compared with control. (C) Protein expression of E-cadherin in A549 and BEAS2B cells analyzed by Western blotting.
We previously found evidence of increased MMP2 and MMP9 expression and collagen content in SPC–TNF-α mice. To investigate whether the changes in mesenchymal and epithelial marker expression after TNF-α stimulation in vitro resulted in enhanced expression of MMPs and collagen production, mRNA levels of MMPs and collagen content in the medium were measured. As shown in Figure E1 in the online supplement, mRNA expression of MMP2 and MMP9 was markedly increased after TNF-α stimulation (Figures E1A and E1B). In addition, collagen levels in the medium measured using the sircoll assay were also increased due to TNF-α stimulation (Figure E1C). These data indicate that TNF-α induces functional changes in A549 and BEAS2B cells that may be related to the matrix alterations observed in the transgenic mice.
Hypoxia Signaling Was Not Involved in the TNF-α–Induced Phenotypic Shift
Our previous investigation showed that a similar transition of alveolar cells and BECs toward a mesenchymal phenotype was induced by cigarette smoke extract, and that this was HIF1α dependent. Therefore, we examined whether the phenotypic shift induced by TNF-α was also dependent on HIF1 signaling. However, TNF-α stimulation was found not to induce hypoxia-responsive element luciferase activity after 24 hours of stimulation, as shown in Figure E2A, or after 4, 8, and 48 hours (data not shown). In addition, HIF1α and carbonic anhydrase 9 mRNA levels were not affected by TNF-α stimulation for 48 hours (Figures E2B and E2C). To fully exclude a role for HIF1α signaling in the phenotypic shift induced by TNF-α, this shift was examined in A549 and BEAS2B cells in which HIF1α was stably down-regulated using short hairpin RNA, which resulted in a knockdown of 85% of HIF1α mRNA expression (data not shown). In agreement with the lack of effect of TNF-α on HIF1α signaling, neither the induction of mesenchymal gene expression nor the attenuation of epithelial gene expression by TNF-α was affected by the knockdown of HIF1α (data not shown). These data indicate that hypoxia-induced signaling may not have a role in the TNF-α–induced phenotypic shift observed in this study.
JNK Inhibition Prevented the Phenotypic Shift in Response to TNF-α
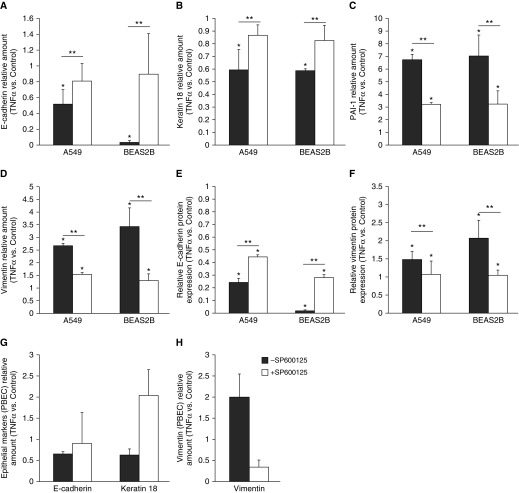
Evidence is provided for the role of JNK in TGF-β1–induced EMT in lung epithelial cells. To examine whether JNK signaling is also involved in the TNF-α–induced expression changes in epithelial and mesenchymal markers, A549 and BEAS2B cells were preincubated with the JNK inhibitor, SP00125, for 30 minutes before each TNF-α stimulation. Testing the specificity of SP600125 in in vitro assays with some 16 other purified protein kinases showed 300-fold selectivity of inhibition of the extracellular signal–regulated kinases and p38 mitogen-activated protein kinases (the closest kinase relatives of the JNKs) (18). JNK inhibition did not affect basal expression of the markers investigated (data not shown). Interestingly, inhibition of JNK completely prevented the decrease in mRNA expression of E-cadherin and keratin 18, and the decrease in E-cadherin protein expression as observed after TNF-α stimulation (Figures 4A, 4B, and 4E). Furthermore, JNK inhibition significantly attenuated the induction of the mesenchymal markers, PAI-1 and vimentin, observed after TNF-α stimulation at the mRNA level (Figures 4C and 4D) and vimentin at the protein level (Figure 4F). In addition, these data were confirmed in pBECs, as TNF-α–attenuated the mRNA expression of the epithelial markers, E-cadherin and keratin 18, and increased mRNA expression of the mesenchymal marker, vimentin. Inhibition of JNK signaling was also able to prevent the induction of mesenchymal gene expression after TNF-α stimulation (Figures 4G and 4H).
Figure 4.
c-Jun N-terminal kinase (JNK) inhibition prevented a phenotypic shift in response to TNF-α. JNK inhibition was performed using preincubation for 30 minutes with the pharmacological inhibitor SP600125 (10 μM) before each stimulation. After preincubation, cells were stimulated twice, with a 24-hour interval, for 48 hours with 10 ng/ml TNF-α. mRNA expression of epithelial-to-mesenchymal transition markers E-cadherin (A), keratin 18 (B), PAI-1 (C), and vimentin (D) was measured using qPCR. Quantification of protein expression of E-cadherin (E) and vimentin (F) in A549 cells and BEAS2B cells analyzed by Western blotting. Data are shown as mean (±SD). *P < 0.05 compared with control, **P < 0.05 comparing with and without inhibitor. mRNA expression of epithelial markers E-cadherin and keratin 18 (G), and mesenchymal marker vimentin (H), in primary bronchial epithelial cells (pBECs) of one representative donor after stimulation with 10 ng/ml TNF-α with or without preincubation for 30 minutes with SP600125 (1 μM). Data from one representative donor are shown.
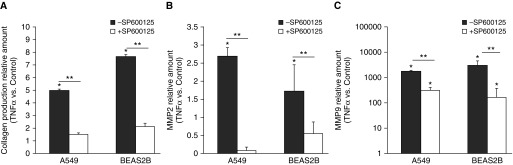
Next, it was examined whether the prevention of the phenotypic shift by inhibition of JNK also limited the functional changes observed after TNF-α stimulation. As shown in Figure 5A, collagen production was significantly increased by TNF-α stimulation. However, in cells in which JNK signaling was inhibited, TNF-α–induced collagen production was almost completely blunted in both cell lines. In addition, the increased expression of MMP2 and MMP9 after TNF-α stimulation in both A549 and BEAS2B cells was attenuated by blocking JNK signaling (Figures 5B and 5C). Again, there were no basal effects of JNK inhibition (data not shown). These data indicate the involvement of JNK signaling in the functional transition of epithelial cells toward a mesenchymal phenotype induced by TNF-α.
Figure 5.
JNK inhibition prevented collagen production and matrix metalloproteinase (MMP) expression in response to TNF-α. JNK inhibition was performed using preincubation for 30 minutes with the pharmacological inhibitor SP600125 (10 μM). Thereafter, cells were stimulated twice, with a 24-hour interval, for 48 hours with 10 ng/ml TNF-α. (A) The production of collagen was measured in the medium using the sircoll assay. mRNA expression of MMP2 (B) and MMP9 (C) was measured using qPCR. Data are shown as mean (±SD). *P < 0.05 compared with control, **P < 0.05 between conditions.
pJNK Was Increased in the Lung of SPC–TNF-α Mice
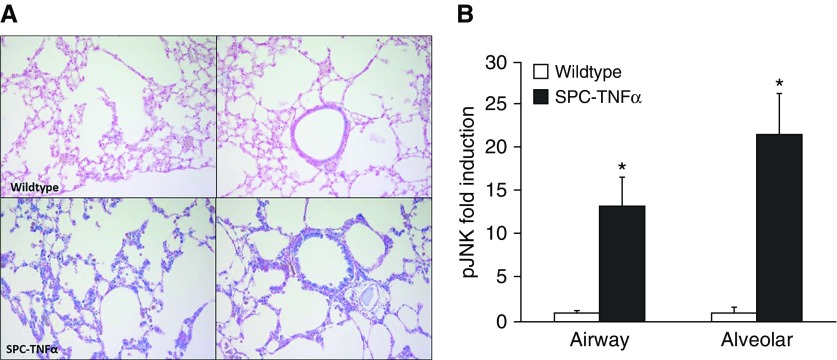
Because we demonstrated a role for JNK signaling in the functional transition of epithelial cells toward a mesenchymal phenotype in vitro, we next assessed the activation of JNK in lung tissue of SPC–TNF-α mice in which matrix remodeling was characterized. The amount and localization of JNK phosphorylation was immunohistochemically determined. Nuclear staining of pJNK (in blue) was quantitatively analyzed in alveolar and airway walls by two independent observers. As shown in Figure 6, the percentage of epithelial cells positively stained for pJNK was increased significantly in both alveolar cells (13-fold) and airway epithelial cells (21-fold).
Figure 6.
Phospho (p) JNK was increased in lungs of SPC–TNF-α mice. (A) Photomicrographs of pJNK staining (pJNK, blue; counterstain, red/pink) in the lungs of one representative mouse from each condition (original magnification, ×40). (B) Fold induction of pJNK-positive–stained cells in alveolar and airway walls. Data are shown as mean (±SEM). *P < 0.05 compared with control.
Phosphorylation of JNK Was Increased in Patients with COPD
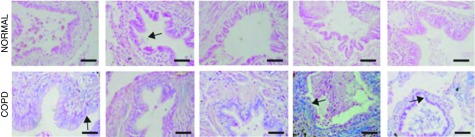
To investigate if JNK was also activated in patients with COPD, lung tissue in which matrix remodeling was previously studied (refer to Ref. 3) was stained for phosphorylation of JNK. In Figure 7, it was demonstrated in patients with COPD that pJNK was readily apparent in bronchioles of patients with COPD in areas of fibrotic remodeling compared with control tissues (arrows).
Figure 7.
pJNK was increased in lungs of patients with chronic obstructive pulmonary disease (COPD). pJNK (blue) in airways from patients with COPD or healthy airways of five representative subjects. Arrows indicate localization of pJNK in epithelial cells. Scale bars, 100 μm.
Discussion
In this study, it was shown, both in vivo in SPC–TNF-α mice as well as in vitro using TNF-α–stimulated alveolar cell and BEC lines and primary bronchial cells, that expression of phenotypic markers of mesenchymal cells was elevated, and those of epithelial cells attenuated. JNK signaling was shown to be involved in the phenotypic change induced by TNF-α in alveolar cells and BECs, and the percentage of pJNK-positive cells was increased in SPC–TNF-α mice in which extracellular matrix alterations were characterized (3).
In previously published research, we showed that the lungs of SPC–TNF-α mice display comparable extracellular matrix remodeling to patients with COPD (3). In the current study, it was shown that expression of the mesenchymal markers, PAI-1, FSP1, and collagen III, was increased in the lungs of these mice, and could underlie the reported changes in matrix composition. Because epithelial markers, E-cadherin and cytokeratin 7, were decreased, it is suggested that epithelial cells lost some of their characteristics, and possibly underwent a phenotypic shift toward a mesenchymal cell type. The in vitro experiments in this study indeed show the induction of such a phenotypic shift in response to TNF-α by increased mesenchymal marker expression, concurrent with decreased epithelial marker levels in A549, BEAS2B, and pBECs. This is in line with several other studies showing an increase in expression of mesenchymal markers and a decrease of epithelial markers after stimulation with the combination of TNF-α and TGFβ, IL-1β, or a cytomix (19–21). Here, we show that a phenotypic shift can occur in alveolar cells and BECs through TNF-α only. To investigate if the reported alterations in vivo are due to epithelial plasticity, studies in transgenic mice with labeled epithelial cells would be interesting. However, providing proof for the occurrence of a transition from an epithelial to a mesenchymal cell in vivo remains difficult, as lineage tracing in transgenic mouse models provides controversial evidence (22).
In addition to the gain of the classical mesenchymal markers, TNF-α increased the release of collagen into the medium and expression of MMP2 and MMP9. The increased release of collagen into the medium due to TNF-α stimulation could underlie the increased collagen content in both alveolar and small airway walls of SPC–TNF-α mice, as well as in patients with COPD, as we recently published (3, 4). Evidence for the involvement of epithelial cells in fibrotic processes is also demonstrated by other work, which shows limitation of bleomycin-induced fibrosis in epithelial-specific collagen I knockout mice (5). Furthermore, because MMP2 and MMP9 expression increased after TNF-α stimulation of epithelial cells, and because MMPs were recently associated with the breakdown of elastin in SPC–TNF-α mice and patients with COPD, TNF-α could possibly contribute to the reported degradation of elastin (3, 23). This is supported by evidence of research that shows that suppression of tropoelastin in TNF-α stimulated fibroblasts directly, and breakdown of elastin through increased release of elastolytic enzymes (9). In addition, a role for TNF-α in tissue remodeling is evident from a study demonstrating protection of TNF-α receptor knockout mice to cigarette smoke–induced connective tissue breakdown (10). TNF-α–neutralizing drugs are used in the treatment of some inflammatory diseases, including rheumatoid arthritis. We have performed a small-scale study on the effects of infliximab in patients with cachexia and COPD, which showed no effects on local inflammation and very minor effects on systemic inflammation (24). In addition, other studies indicated no effect of infliximab on the systemic inflammatory profile in COPD, nor was clinical efficacy proven (25, 26). Research into signaling targets more downstream of TNF-α, such as the one described here, is therefore very relevant.
We have shown that hypoxia signaling is involved in the induction of a phenotypic shift of epithelial cells by cigarette smoke extract, especially with respect to the mesenchymal transition (8). In the current study, in the context of TNF-α–induced EMT, no role for hypoxia signaling could be demonstrated. It is known that, in patients with COPD who quit smoking, inflammation and remodeling are ongoing. It is therefore likely that, not cigarette smoking per se, but inflammatory mediators, such as TNF-α, are responsible for the ongoing remodeling in vivo.
Evidence shows a role for JNK in TGF-β1–induced EMT in lung epithelial cells, as the effects of TGF-β on EMT markers were blunted using JNK knockout cells [13]. Furthermore, TNF-α was able to induce JNK signaling (11), and JNK was involved in TNF-α–induced MMP12 expression in A549 cells (12). In addition, activation of JNK by stress stimuli and cytokines can phosphorylate transcription factors, including c-Jun, and promote activator protein (AP-1)–mediated transcription. AP-1 activation, which consists of dimerization of Fos and Jun, has been shown in EMT processes, most notably by TGF-β, and was found to regulate the transcription of, for instance, E-cadherin and vimentin (27). Furthermore, it was reported that overexpression of JNK induced cell migration and invasion, and a morphologic change associated with EMT via enhanced AP-1 transcription factors in human breast cancer cells (28). Here, we show that the inhibition of JNK by the pharmacological inhibitor, SP600125, completely prevented the decreased expression of epithelial markers. In addition, the increase in mesenchymal markers was significantly, but not completely, attenuated by JNK inhibition, which implies that other mechanisms are also relevant in prompting the TNF-α–induced phenotypic shift. For instance, NF-κB has been shown to be involved in the induction of EMT of A549 cells costimulated with TGF-β and TNF-α (20). Moreover, in breast cancer cells, TNF-α up-regulated Twist-related protein via NF-κB signaling (29).
In SPC–TNF-α mice, phosphorylation of JNK was found to be increased in both alveolar and airway walls. Because inhibition of JNK signaling in vitro blunted the TNF-α–induced collagen production and MMP expression, this indicates that the increased amounts of collagen and decreased elastin content in SPC–TNF-α mice could be due to JNK activation. In addition, in the current study, it is shown that patients with COPD display increased numbers of positive cells for phosphorylated JNK in epithelial cells. Because TNF-α is found to be increased in sputum of patients with stable COPD, this could be associated with the increased activation of JNK in these patients (30). The data from SPC–TNF-α mice in this study are in line with a study showing that mice lacking JNK1 are protected against TGF-β–induced pulmonary fibrosis and fibrotic gene expression (31, 32). This is also supported by another study showing that JNK knockout mice were found to have an increased pulmonary elastin content compared with wild-type mice, and showed increased elastogenesis, but had impaired alveolar septation during lung development (33). In addition, the JNK inhibitor, SP600125, partly prevented bleomycin-induced hydroxyproline and α smooth muscle actin levels in the lung tissue of rats, and thalidomide, an immunomodulatory drug able to suppress the generation of TNF-α, prevented the induction of JNK phosphorylation and hydroxyproline and α smooth muscle actin levels even more (34). Furthermore, neutrophil elastase–instilled mice showed emphysema and lung epithelial cell apoptosis, which was JNK and protein kinase C signaling dependent (35).
In conclusion, in this study, it was shown that expression of mesenchymal markers was elevated and epithelial markers were attenuated in vivo in SPC–TNF-α mice and in vitro in alveolar cells and BECs treated with TNF-α. Involvement of JNK signaling in this process was found in vitro, and activation of JNK signaling was furthermore shown in SPC–TNF-α mice. Together, these data show a role for TNF-α in the induction of a phenotypic shift in vitro, and provide evidence for involvement of the TNF-α–JNK axis in extracellular matrix remodeling.
Acknowledgments
Acknowledgments
The authors thank Fabienne Wijshof and Mike Eggen (Department of Respiratory Medicine, Maastricht University Medical Center, Maastricht, the Netherlands) for their technical support in carrying out the experiments; and Dawn Boyer, Dawn Phillips, Justine Lucas, and Dawn Nines (Experimental Gerontology Section, Translational Gerontology Branch, National Institute on Aging, National Institutes of Health, Baltimore, MD) for the outstanding husbandry work.
Footnotes
This work was supported by funding for the Surfactant Protein C–TNF-α study provided by the Intramural Research Program of the National Institute on Aging/National Institutes of Health.
Author Contributions: I.M.J.E. performed and analyzed the experiments and performed the statistical analysis, and drafted the manuscript; N.L.R. participated in the study design, interpretation of data, and manuscript preparation; C.v.d.W. participated in the performance of the experiments; S.W.A. validated and participated in semiquantitative immunohistochemical analysis; E.M.M. and R.d.C. participated in the Surfactant Protein C–TNF-α studies and provided the tissues; J.L.v.d.V. and Y.M.J.-H. participated in the study design; E.F.M.W. participated in the design of the study, provided general supervision, and drafted the manuscript; M.A.D. participated in the study design, interpretation of data, and manuscript preparation; all authors read and approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0195OC on November 22, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nakano Y, Müller NL, King GG, Niimi A, Kalloger SE, Mishima M, Paré PD. Quantitative assessment of airway remodeling using high-resolution CT. Chest. 2002;122(6) suppl:271S–275S. [PubMed] [Google Scholar]
- 2.Bhatt SP. Hidden in plain sight: lung impairment in ischemic heart disease. Am J Respir Crit Care Med. 2016;194:528–530. doi: 10.1164/rccm.201607-1521ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eurlings IM, Dentener MA, Cleutjens JP, Peutz CJ, Rohde GG, Wouters EF, Reynaert NL. Similar matrix alterations in alveolar and small airway walls of COPD patients. BMC Pulm Med. 2014;14:90. doi: 10.1186/1471-2466-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eurlings IM, Dentener MA, Mercken EM, de Cabo R, Bracke KR, Vernooy JH, Wouters EF, Reynaert NL. A comparative study of matrix remodeling in chronic models for COPD; mechanistic insights into the role of TNF-α. Am J Physiol Lung Cell Mol Physiol. 2014;307:L557–L565. doi: 10.1152/ajplung.00116.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Wheeler SE, Velikoff M, Kleaveland KR, LaFemina MJ, Frank JA, Chapman HA, Christensen PJ, Kim KK. Activated alveolar epithelial cells initiate fibrosis through secretion of mesenchymal proteins. Am J Pathol. 2013;183:1559–1570. doi: 10.1016/j.ajpath.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R, Neilson EG. Epithelial–mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcorn JF, Guala AS, van der Velden J, McElhinney B, Irvin CG, Davis RJ, Janssen-Heininger YM. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-β1. J Cell Sci. 2008;121:1036–1045. doi: 10.1242/jcs.019455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eurlings IM, Reynaert NL, van den Beucken T, Gosker HR, de Theije CC, Verhamme FM, Bracke KR, Wouters EF, Dentener MA. Cigarette smoke extract induces a phenotypic shift in epithelial cells; involvement of HIF1α in mesenchymal transition. PLoS One. 2014;9:e107757. doi: 10.1371/journal.pone.0107757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sproul EP, Argraves WS. A cytokine axis regulates elastin formation and degradation. Matrix Biol. 2013;32:86–94. doi: 10.1016/j.matbio.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Churg A, Dai J, Tai H, Xie C, Wright JL. Tumor necrosis factor-α is central to acute cigarette smoke-induced inflammation and connective tissue breakdown. Am J Respir Crit Care Med. 2002;166:849–854. doi: 10.1164/rccm.200202-097OC. [DOI] [PubMed] [Google Scholar]
- 11.Shieh JM, Tsai YJ, Tsou CJ, Wu WB. CXCL1 regulation in human pulmonary epithelial cells by tumor necrosis factor. Cell Physiol Biochem. 2014;34:1373–1384. doi: 10.1159/000366344. [DOI] [PubMed] [Google Scholar]
- 12.Yu Y, Chiba Y, Sakai H, Misawa M. Possible involvements of nuclear factor-κB and activator protein-1 in the tumor necrosis factor-α–induced upregulation of matrix metalloproteinase-12 in human alveolar epithelial A549 cell line. J Pharmacol Sci. 2010;112:83–88. doi: 10.1254/jphs.09268fp. [DOI] [PubMed] [Google Scholar]
- 13.Sakamoto O, Iwama A, Amitani R, Takehara T, Yamaguchi N, Yamamoto T, Masuyama K, Yamanaka T, Ando M, Suda T. Role of macrophage-stimulating protein and its receptor, RON tyrosine kinase, in ciliary motility. J Clin Invest. 1997;99:701–709. doi: 10.1172/JCI119214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, Parks WP. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 15.Mestdagh P, Van Vlierberghe P, De Weer A, Muth D, Westermann F, Speleman F, Vandesompele J. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009;10:R64. doi: 10.1186/gb-2009-10-6-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata T, Giaccia AJ, Brown JM. Development of a hypoxia-responsive vector for tumor-specific gene therapy. Gene Ther. 2000;7:493–498. doi: 10.1038/sj.gt.3301124. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki Y, Araki K, Vesin C, Garcia I, Kapanci Y, Whitsett JA, Piguet PF, Vassalli P. Expression of a tumor necrosis factor-α transgene in murine lung causes lymphocytic and fibrosing alveolitis: a mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995;96:250–259. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogoyevitch MA, Boehm I, Oakley A, Ketterman AJ, Barr RK. Targeting the JNK MAPK cascade for inhibition: basic science and therapeutic potential. Biochim Biophys Acta. 2004;1697:89–101. doi: 10.1016/j.bbapap.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Kamitani S, Yamauchi Y, Kawasaki S, Takami K, Takizawa H, Nagase T, Kohyama T. Simultaneous stimulation with TGF-β1 and TNF-α induces epithelial mesenchymal transition in bronchial epithelial cells. Int Arch Allergy Immunol. 2011;155:119–128. doi: 10.1159/000318854. [DOI] [PubMed] [Google Scholar]
- 20.Kumar M, Allison DF, Baranova NN, Wamsley JJ, Katz AJ, Bekiranov S, Jones DR, Mayo MW. NF-κB regulates mesenchymal transition for the induction of non–small cell lung cancer initiating cells. PLoS One. 2013;8:e68597. doi: 10.1371/journal.pone.0068597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito T, Yoshida K, Matsumoto K, Saeki K, Tanaka Y, Ong SM, Sasaki N, Nishimura R, Nakagawa T. Inflammatory cytokines induce a reduction in E-cadherin expression and morphological changes in MDCK cells. Res Vet Sci. 2014;96:288–291. doi: 10.1016/j.rvsc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Xu XF, Dai HP. Type 2 epithelial mesenchymal transition in vivo: truth or pitfalls? Chin Med J (Engl) 2012;125:3312–3317. [PubMed] [Google Scholar]
- 23.Skjøt-Arkil H, Clausen RE, Nguyen QH, Wang Y, Zheng Q, Martinez FJ, Hogaboam CM, Han M, Klickstein LB, Larsen MR, et al. Measurement of MMP-9 and -12 degraded elastin (ELM) provides unique information on lung tissue degradation. BMC Pulm Med. 2012;12:34. doi: 10.1186/1471-2466-12-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dentener MA, Creutzberg EC, Pennings HJ, Rijkers GT, Mercken E, Wouters EF. Effect of infliximab on local and systemic inflammation in chronic obstructive pulmonary disease: a pilot study. Respiration. 2008;76:275–282. doi: 10.1159/000117386. [DOI] [PubMed] [Google Scholar]
- 25.Rennard SI, Fogarty C, Kelsen S, Long W, Ramsdell J, Allison J, Mahler D, Saadeh C, Siler T, Snell P, et al. COPD Investigators. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:926–934. doi: 10.1164/rccm.200607-995OC. [DOI] [PubMed] [Google Scholar]
- 26.Loza MJ, Watt R, Baribaud F, Barnathan ES, Rennard SI. Systemic inflammatory profile and response to anti-tumor necrosis factor therapy in chronic obstructive pulmonary disease. Respir Res. 2012;13:12. doi: 10.1186/1465-9921-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao J, Yan Q, Wang J, Liu S, Yang X. Epithelial-to-mesenchymal transition induced by TGF-β1 is mediated by AP1-dependent EpCAM expression in MCF-7 cells. J Cell Physiol. 2015;230:775–782. doi: 10.1002/jcp.24802. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Kuiatse I, Lee AV, Pan J, Giuliano A, Cui X. Sustained c-Jun-NH2-kinase activity promotes epithelial–mesenchymal transition, invasion, and survival of breast cancer cells by regulating extracellular signal–regulated kinase activation. Mol Cancer Res. 2010;8:266–277. doi: 10.1158/1541-7786.MCR-09-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL, Chao CH, Yamaguchi H, Yang NK, Ding Q, et al. Epithelial–mesenchymal transition induced by TNF-α requires NF-κB–mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72:1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-alpha in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 31.Alcorn JF, van der Velden J, Brown AL, McElhinney B, Irvin CG, Janssen-Heininger YM. c-Jun N-terminal kinase 1 is required for the development of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2009;40:422–432. doi: 10.1165/rcmb.2008-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Velden JL, Hoffman SM, Alcorn JF, Tully JE, Chapman DG, Lahue KG, Guala AS, Lundblad LK, Aliyeva M, Daphtary N, et al. Absence of c-Jun NH2-terminal kinase 1 protects against house dust mite–induced pulmonary remodeling but not airway hyperresponsiveness and inflammation. Am J Physiol Lung Cell Mol Physiol. 2014;306:L866–L875. doi: 10.1152/ajplung.00153.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S, Parameswaran H, Young SM, Varisco BM. JNK suppresses pulmonary fibroblast elastogenesis during alveolar development. Respir Res. 2014;15:34. doi: 10.1186/1465-9921-15-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Qian L, Nan H, Cui M, Hao X, Du Y. Function of the transforming growth factor-β1/c-Jun N-terminal kinase signaling pathway in the action of thalidomide on a rat model of pulmonary fibrosis. Exp Ther Med. 2014;7:669–674. doi: 10.3892/etm.2013.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou HH, Cheng SL, Chung KP, Wei SC, Tsao PN, Lu HH, Wang HC, Yu CJ. PlGF mediates neutrophil elastase-induced airway epithelial cell apoptosis and emphysema. Respir Res. 2014;15:106. doi: 10.1186/s12931-014-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]