Abstract
Purpose
Blockade of the programmed cell death 1 (PDCD1, PD-1) immune checkpoint pathway can improve clinical outcomes in various malignancies. Evidence suggests that aspirin (a widely used nonsteroidal anti-inflammatory drug) not only prolongs colorectal cancer survival, but can also activate T cell–mediated antitumor immunity and synergize with immunotherapy through inhibition of prostaglandin E2 production. We hypothesized that the survival benefit associated with aspirin might be stronger in colorectal carcinoma with a lower CD274 (PDCD1 ligand 1, PD-L1) expression level that resulted in lower signaling of the immune checkpoint pathway.
Patients and Methods
Using data from 617 patients with rectal and colon cancer in the Nurses’ Health Study and the Health Professionals Follow-Up Study, we examined the association of postdiagnosis aspirin use with patient survival in strata of tumor CD274 expression status measured by immunohistochemistry. We used multivariable Cox proportional hazards regression models to control for potential confounders, including disease stage, microsatellite instability status, CpG island methylator phenotype, long interspersed nucleotide element-1 methylation, cyclooxygenase-2 (PTGS2), and CDX2 expression, and KRAS, BRAF, and PIK3CA mutations.
Results
The association of postdiagnosis aspirin use with colorectal cancer–specific survival differed by CD274 expression status (Pinteraction < .001); compared with aspirin nonusers; multivariable-adjusted hazard ratios for regular aspirin users were 0.16 (95% CI, 0.06 to 0.41) in patients with low CD274 and 1.01 (95% CI, 0.61 to 1.67) in patients with high CD274. This differential association seemed consistent in patients with microsatellite-stable or PIK3CA wild-type disease and in strata of PTGS2 expression, CDX2 expression, tumor-infiltrating lymphocytes, or prediagnosis aspirin use status.
Conclusion
The association of aspirin use with colorectal cancer survival is stronger in patients with CD274-low tumors than CD274-high tumors. Our findings suggest a differential antitumor effect of aspirin according to immune checkpoint status.
INTRODUCTION
Accumulating evidence indicates that the programmed cell death 1 (PDCD1, PD-1) immune checkpoint pathway plays a key role in suppressing T cell–mediated antitumor immune response in the tumor microenvironment.1,2 Studies have shown that inhibitors targeting the CD274 (PDCD1 ligand 1, PD-L1) or PDCD1 (PD-1) protein can improve clinical outcomes in various types of malignancies,1-4 including colorectal carcinoma with high-level microsatellite instability (MSI).5-8 Emerging evidence also suggests that the response to immunotherapy can be affected by tumor molecular alterations and other host factors.8,9 A better understanding of host-tumor interactions in the tumor microenvironment may improve immunotherapy strategies.
Colorectal carcinomas represent a heterogeneous group of neoplasms with varying sets of genetic and epigenetic alterations, influenced by exposures to multiple factors, including medications.10-15 Accumulating evidence indicates that regular use of aspirin, a widely used nonsteroidal anti-inflammatory drug (NSAID), can reduce colorectal cancer incidence and improve clinical outcomes after diagnosis.16-21 In a recent meta-analysis of seven studies, aspirin use after colorectal cancer diagnosis was associated with longer overall survival (pooled hazard ratio [HR] for regular users of aspirin compared with nonusers, 0.84; 95% CI, 0.75 to 0.94).21 Studies have shown that the association of aspirin use with colorectal cancer survival seems more pronounced for patients with tumor PTGS2 (cyclooxygenase-2) overexpression17 or tumor PIK3CA mutation;19,22 however, neither PTGS2 expression nor PIK3CA mutation is a perfect predictor of response to aspirin. Aspirin has been shown to reverse immune evasion of tumor cells through inhibition of prostaglandin E2 (PGE2) production.23-27 Emerging evidence also points to possible synergism of PDCD1 (PD-1) immune checkpoint blockade and prostaglandin inhibition via aspirin for combination immunotherapy strategies.23-25 On the basis of these lines of evidence, it is speculated that suppression of the immune checkpoint may be required to enhance the effects of aspirin on tumors that have activated the immune checkpoint. We therefore hypothesized that aspirin might be less effective for colorectal carcinomas with high-level CD274 (PD-L1) expression than tumors with low-level CD274 expression and that tumor CD274 expression might serve as a biomarker that predicts resistance to aspirin use.
To test this hypothesis, we used integrated data on aspirin use, colorectal tumor characteristics, and patient outcomes within two large prospective cohort studies in the United States and examined the prognostic association of aspirin use in strata of tumor CD274 expression status.
PATIENTS AND METHODS
Study Population and Histopathologic Assessment
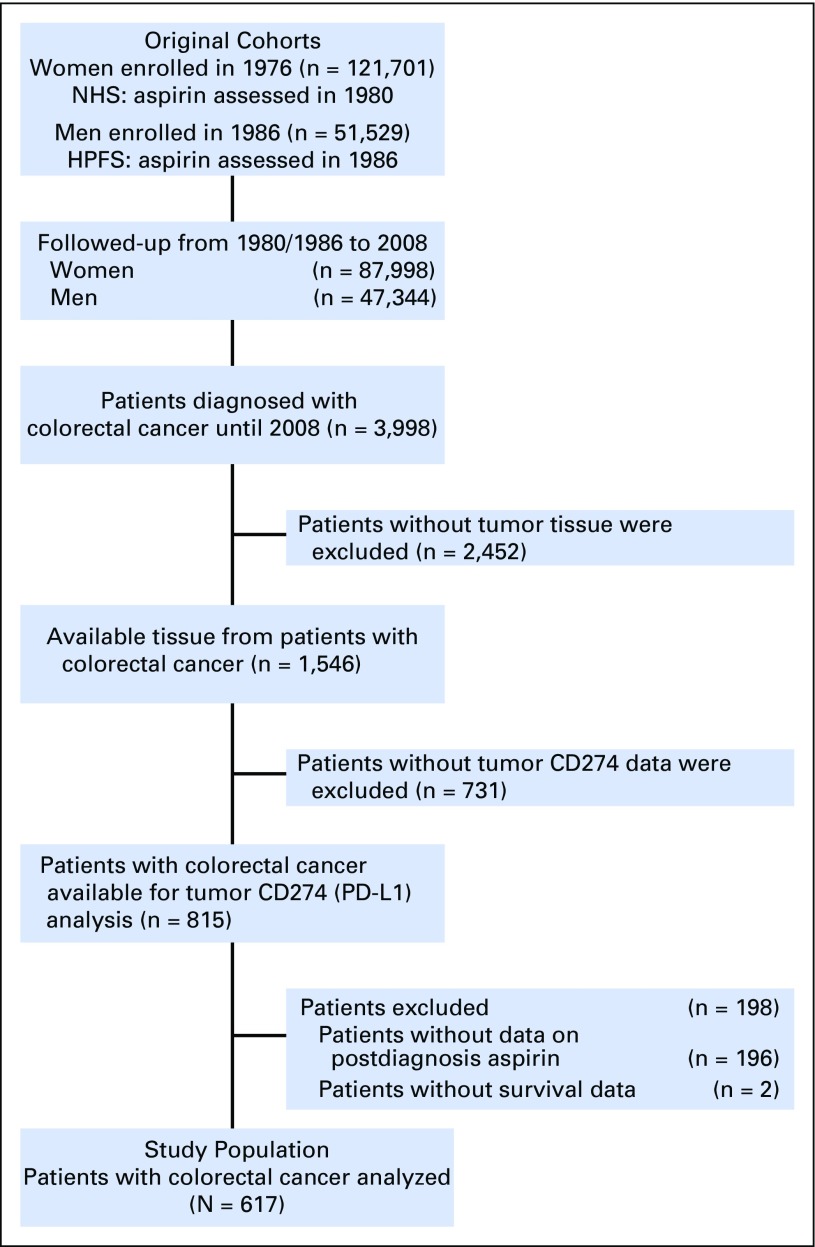
We used two prospective cohort studies in the United States, the Nurses' Health Study (which observed 121,701 women since 1976) and the Health Professionals Follow-up Study (which observed 51,529 men since 1986).28 Study participants had been sent follow-up questionnaires biennially to update information on lifestyle factors, including medication use, and to identify newly diagnosed diseases in themselves and their first-degree relatives. The National Death Index was used to ascertain deaths of study participants and identify patients with unreported fatal colorectal cancer. Study physicians reviewed the medical records of patients with colorectal cancer and recorded disease stage and tumor size and location, as well as cause of death. We collected archival formalin-fixed paraffin-embedded tumor tissue from hospitals throughout the United States where participants diagnosed with colorectal cancer had undergone tumor resection. There were no significant differences in demographic features or exposures between patients with and without available tissue data.19 A single pathologist (S.O.), who was unaware of other data, reviewed hematoxylin and eosin–stained tissue sections and recorded pathologic features, including tumor differentiation and tumor-infiltrating lymphocytes (TIL), as previously described.29 On the basis of available data on postdiagnosis aspirin use and tumor CD274 (PD-L1) expression, we included 617 patients in our current analysis (Fig 1). We included both colon and rectal carcinomas, on the basis of the colorectal continuum model.30 Patients were observed until death or January 1, 2012, whichever came first.
Fig 1.
Flow diagram of the study population in the Nurses' Health Study and the Health Professionals Follow-Up Study. HPFS, Health Professional’s Follow-Up Study; NHS, Nurse’s Health Study; PD-L1, programmed cell death 1 ligand 1.
The current study was designed as a post hoc analysis of the tumor biomarker within the two prospective cohort studies. Written informed consent for enrollment in one of the prospective cohort studies was obtained from all cohort participants, and consent for use of cancer tissue specimens was obtained from all patients included in this study. This study was approved by the institutional review boards at Harvard T.H. Chan School of Public Health and Brigham and Women’s Hospital (Boston, MA).
Assessment of Use of Aspirin and Other NSAIDs
A detailed description about collection of data on use of aspirin and other NSAIDs is provided in the Data Supplement. As previously described,31 regular aspirin users were defined as those who took aspirin (either standard-dose or low-dose, or both) two or more times per week. Nonusers were defined as those who took aspirin fewer than two times per week or used no aspirin.31 We also collected information on regular use (two or more times per week) of other NSAIDs.
Analyses of Tumor Characteristics
A detailed description of analyses of tumor characteristics is provided in the Data Supplement. We constructed tissue microarray for patients with sufficient tissue materials, including up to four cores from each patient with colorectal cancer in one tissue microarray block and performed immunohistochemistry of PTGS2 and CDX2, as previously described.16,32 Immunohistochemistry of CD274 (PD-L1) was performed using anti-CD274 antibody (dilution, 1:50; eBioscience, San Diego, CA), and tumor CD274 expression level (an ordinal scale of 0 to 4) was scored by a single pathologist (Y.M.), as previously described.33 CD274 expression levels in selected tumors (n = 148) were independently examined by a second pathologist (A.dS.), and the concordance between the two pathologists was reasonable with a weighted κ of 0.65 (95% CI, 0.57 to 0.73).33 In the current survival analyses, we categorized CD274 levels with the most balanced subgrouping as low (scale of 0 to 1) versus high (scale of 2 to 4) to maximize statistical power.
MSI status34 and mutation status for KRAS (codons 12, 13, 61, and 146),35 BRAF (codon 600),35 and PIK3CA (exons 9 and 20) were determined as previously described.19 Methylation status of eight CpG island methylator phenotype–specific promoters and long interspersed nucleotide element-1 was analyzed as previously described.35
Statistical Analysis
A detailed description of the statistical analysis and accompanying supplemental data are shown in the Data Supplement. All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC), and all P values were two-sided. Our primary hypothesis testing was an assessment of the interaction (using the Wald test for the cross-product) between postdiagnosis aspirin use (regular use v nonuse) and tumor CD274 expression status (low v high) in the multivariable-adjusted Cox proportional hazards regression model. To account for multiple hypothesis testing inherent in subgroup analyses in molecular pathologic epidemiology research,10,36 we adjusted the two-sided alpha level to .01. All other analyses were secondary, and we used the alpha level of .01. Outcome end points were colorectal cancer–specific survival and overall survival. Survival time was defined as the period from the date of return of the first postdiagnosis questionnaire to death or the end of follow-up for those who had not died. In colorectal cancer–specific survival analysis, deaths from other causes and those with missing data on cause of death were treated as censored events. To reduce a bias resulting from the availability of postdiagnosis questionnaire data, the inverse probability weighting (IPW) method was applied to all survival analyses.37 Cumulative survival probabilities were estimated using the IPW-adjusted Kaplan-Meier method and compared using the weighted log-rank test.38 We initially included the variables described in the Data Supplement and conducted backward elimination with a threshold P of .05 to select variables for the final models. We conducted a single regression model to calculate HRs for aspirin use status in strata of tumor CD274 status, with a reparameterization of the interaction term.35 Results of Kaplan-Meier survival analyses and Cox regression analyses without IPW, which were similar to those with IPW, are shown in the Data Supplement.
RESULTS
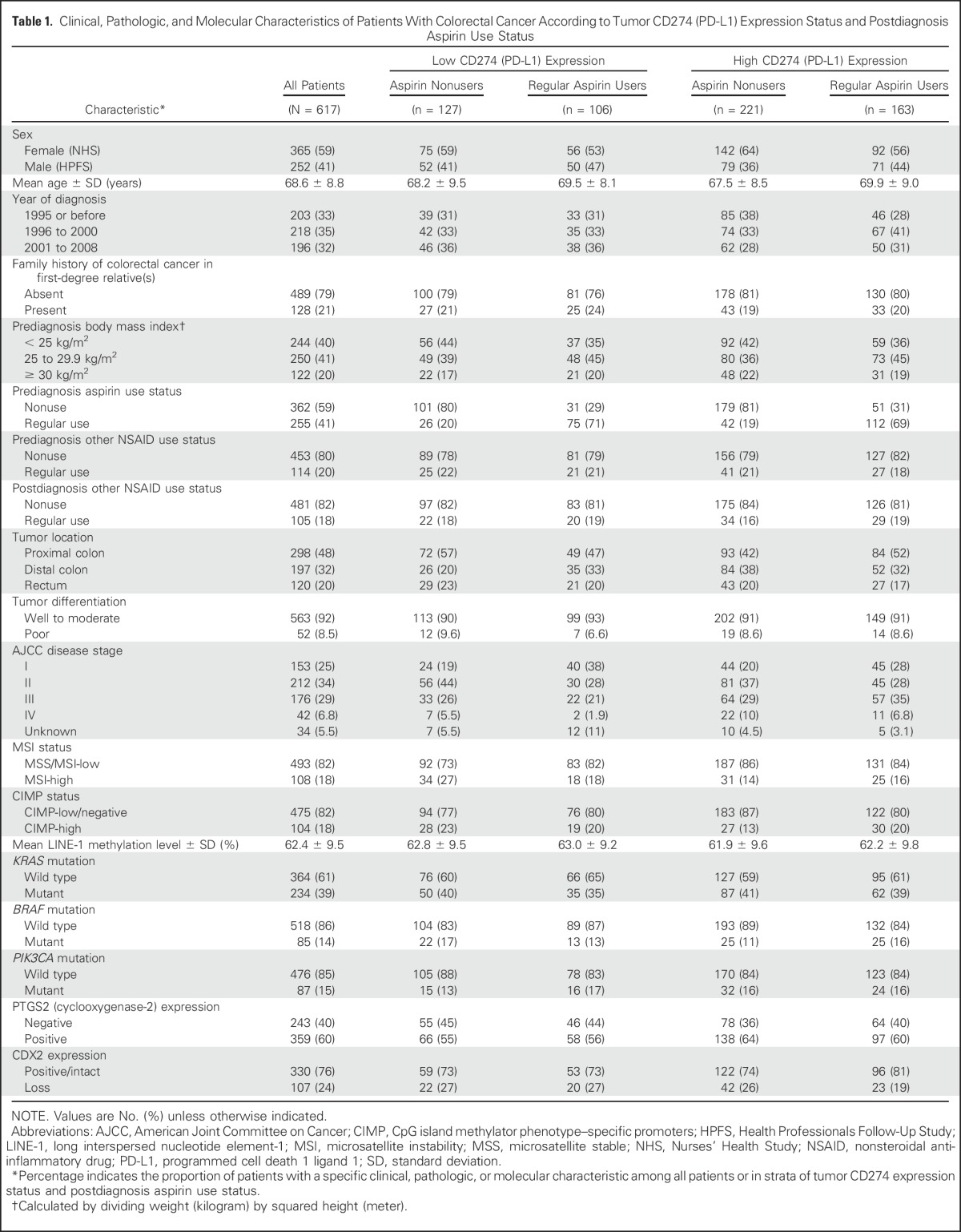
We included 617 patients with colorectal cancer in the two prospective cohort studies (Table 1 and Data Supplement). Prediagnosis regular aspirin users tended to continue this medication after diagnosis. During the median follow-up time of 11.5 years (interquartile range, 7.8 to 15.5 years) for all censored patients, there were 325 all-cause deaths, including 118 colorectal cancer–specific deaths.
Table 1.
Clinical, Pathologic, and Molecular Characteristics of Patients With Colorectal Cancer According to Tumor CD274 (PD-L1) Expression Status and Postdiagnosis Aspirin Use Status
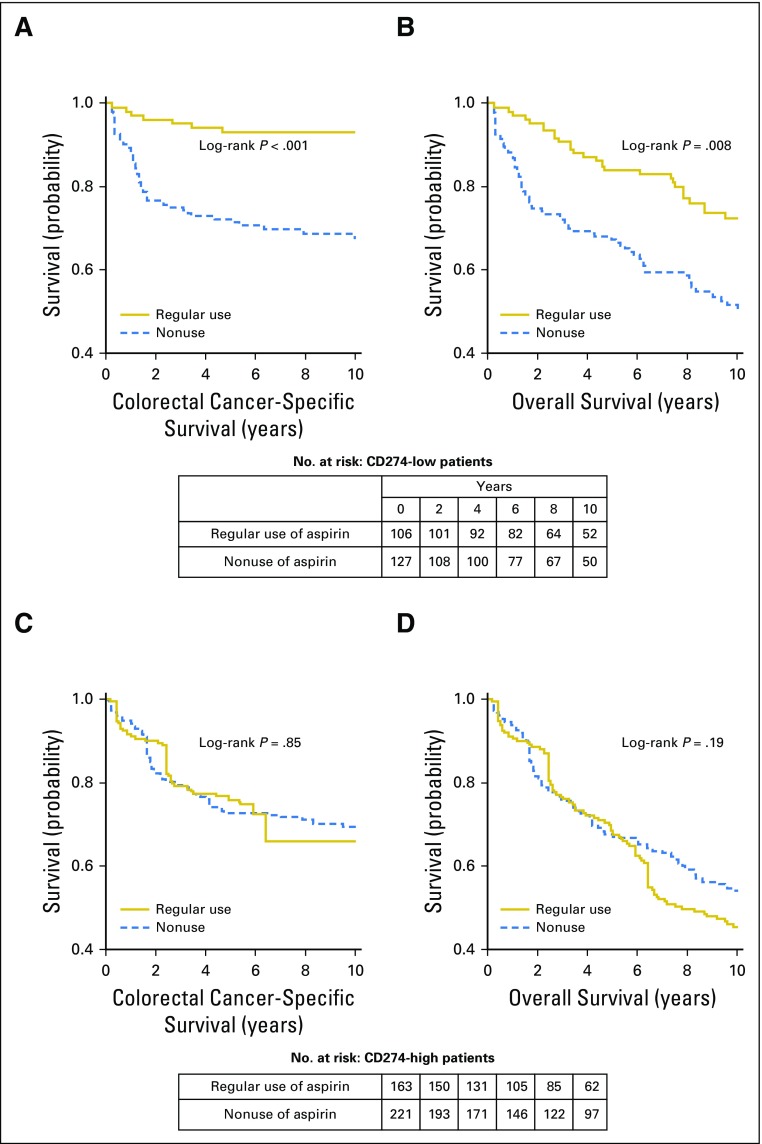
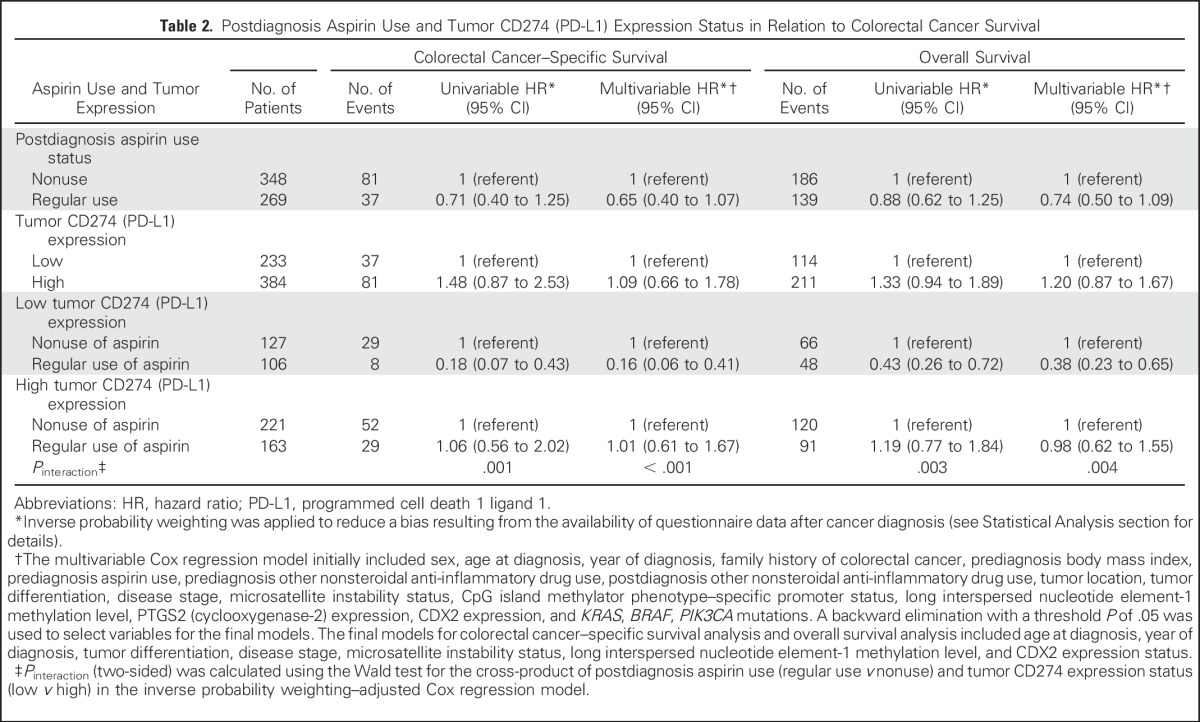
We examined the prognostic association of aspirin use after colorectal cancer diagnosis in strata of tumor CD274 expression status. In Kaplan-Meier survival analysis, postdiagnosis aspirin use was associated with longer colorectal cancer–specific survival in patients with low-level tumor CD274 expression (P < .001), but not in patients with high-level CD274 expression (P = .85; Fig 2). In our primary hypothesis testing using Cox regression analysis, we observed a statistically significant interaction between postdiagnosis aspirin use and tumor CD274 expression status in colorectal cancer–specific survival analysis (Pinteraction < .001) and overall survival analysis (Pinteraction = .004; Table 2). Postdiagnosis aspirin use was associated with longer colorectal cancer–specific survival in patients with low CD274 (multivariable-adjusted HR, 0.16; 95% CI, 0.06 to 0.41), but not in patients with high CD274 (multivariable-adjusted HR, 1.01; 95% CI, 0.61 to 1.67). A similar, although attenuated, differential association of aspirin use with overall survival by tumor CD274 status was observed.
Fig 2.
Inverse probability weighting–adjusted Kaplan-Meier survival curves of patients with colorectal cancer according to postdiagnosis aspirin use status, in strata of tumor CD274 (programmed cell death 1 ligand 1 [PD-L1]) expression status. The P values were calculated using the weighted log-rank test (two-sided). (A) Colorectal cancer–specific survival and (B) overall survival of patients with low tumor CD274 expression level; (C) colorectal cancer–specific survival and (D) overall survival of patients with high tumor CD274 expression level.
Table 2.
Postdiagnosis Aspirin Use and Tumor CD274 (PD-L1) Expression Status in Relation to Colorectal Cancer Survival
We conducted survival analyses in each cohort (Data Supplement) and observed no significant heterogeneity between the two cohorts in terms of the prognostic association of postdiagnosis aspirin use or tumor CD274 expression status (Pheterogeneity > .19 by the Q statistic). Therefore, we pooled the cohorts for further analyses to increase statistical power.
We conducted secondary analyses stratified by stage. Although the differential association of postdiagnosis aspirin use with colorectal cancer survival by tumor CD274 expression status was more apparent in stage III to IV cancer than in stage I to II cancer, statistical power was limited in the subgroup analysis (Data Supplement). We conducted a sensitivity analysis excluding patients with stage IV, and results were similar (Data Supplement). In analysis of 185 patients with available data on chemotherapy (Data Supplement), although statistical power was limited, we observed a trend toward a similar differential prognostic association of postdiagnosis aspirin use by tumor CD274 status after adjusting for chemotherapy use (Pinteraction = .057 for colorectal cancer–specific survival).
As another secondary analysis, we examined the prognostic association of postdiagnosis weekly aspirin dose in strata of tumor CD274 expression status (Data Supplement). Although there seemed to be a differential prognostic association by tumor CD274 status (similar to the data in Table 2), statistical power was limited for determining a dose-response relationship.
We performed an exploratory analysis to examine the survival association of postdiagnosis aspirin use across strata of tumor CD274 expression status and prediagnosis aspirin use status (Data Supplement). Although the differential prognostic association of postdiagnosis aspirin use by CD274 status seemed consistent across strata of prediagnosis aspirin use status, statistical power was limited.
A classification system on the basis of tumor CD274 expression status and TIL, which is called Tumor Immunity in the Microenvironment (TIME) has been proposed.39,40 We examined the prognostic association of postdiagnosis aspirin use in strata of TIME categories (Data Supplement). Although statistical power was limited, the HR for colorectal cancer–specific survival of postdiagnosis aspirin users (v nonusers) seemed to be consistently lower in patients with low CD274 than in patients with high CD274 in both the TIL-negative and TIL-positive strata.
As exploratory analyses, we examined the prognostic association of postdiagnosis aspirin use according to tumor CD274 expression status in strata of MSI,5-8 PIK3CA mutation,19,22 PTGS2 expression,17 and CDX2 expression32,41 status (Data Supplement). The differential prognostic association of aspirin use by CD274 status seemed consistent in microsatellite stable/MSI-low tumors. There were only six colorectal cancer–specific deaths among patients with MSI-high tumors, precluding a robust statistical assessment in this stratum. Although the differential survival association of postdiagnosis aspirin use by CD274 status seemed consistent across the strata of PTGS2 or CDX2 expression, statistical power was limited in these subgroup analyses. There was only one colorectal cancer–specific death among regular aspirin users with PIK3CA-mutant tumors, precluding a statistical assessment in the PIK3CA-mutant stratum.
DISCUSSION
Experimental evidence supports a synergistic effect of aspirin and immune checkpoint blockade on stimulating T cell–mediated antitumor immune response,23 which implies that activated status of immune checkpoint in cells within the tumor microenvironment may confer resistance to aspirin. Hence, we tested the hypothesis that the association of postdiagnosis aspirin use with colorectal cancer survival might be weaker or not present for tumors with high-level CD274 (PD-L1) expression, compared with tumors with low-level CD274 expression, in which we expected to observe a stronger prognostic association of aspirin use. We found such a differential prognostic association with aspirin use by tumor CD274 expression. Our data provide evidence for possible synergism of aspirin-mediated antitumor pathways and CD274-PDCD1 (PD-1) immune checkpoint blockade to improve clinical outcome of colorectal tumors that have activated the immune checkpoint. This study suggests that regular users of aspirin before colorectal cancer diagnosis may benefit from antitumor effects of aspirin after diagnosis if tumor CD274 expression level is low. Alterations in the tumor microenvironment during tumor progression may contribute to differential sensitivity to aspirin exposure before and after cancer diagnosis. Our findings support the potential of the synergism of prostaglandin inhibition and PDCD1 (PD-1) immune checkpoint blockade at a later phase of tumor progression.
Colorectal carcinoma is a heterogeneous disease, and local immune status in the tumor microenvironment has been associated with clinical outcome.42-48 Studies have shown that tumor CD274 expression is associated with tumor molecular features33,49,50 and low density of FOXP3+ cells in colorectal cancer.33 Although blockade of the CD274-PDCD1 (PD-1) immune checkpoint pathway has been shown to effectively activate T cell–mediated antitumor immunity in various tumor types,1-4 only a small subset of patients with colorectal cancer can benefit from immune checkpoint blockade.3,4 Therefore, there is a substantial need to better understand host-tumor interactions that potentially modify the effectiveness of immunotherapy.
PTGS1 (cyclooxygenase-1) and PTGS2 (cyclooxygenase-2) are enzymes that produce prostaglandins, including PGE2. Experimental evidence suggests that local inflammatory status caused by overproduction of PGE2 may be one mechanism that allows tumor cells to evade host immune surveillance through accumulation of myeloid-derived suppressor cells, suppression of dendritic cells, and, more importantly, evasion from T cell–mediated immune attack.23,27,51,52 Evidence also suggests that the innate immune response can be reactivated by reversing the immune evasion via aspirin.23-27 Aspirin may reverse the inhibitory effect of PGE2 on regulatory T cells and dendritic cells26,53,54 and restore balance of inflammatory cytokines,55 thereby enhancing T cell–mediated immune response. Furthermore, emerging evidence suggests that the immunomodulatory effect of prostaglandin production inhibition can synergize with immune checkpoint blockade to suppress growth of various tumors, including colorectal cancer.23-25,51 Our findings supporting the differential effects of aspirin according to immune checkpoint status underscore the potential of dual prostaglandin inhibition and immune checkpoint blockade as a combination immunotherapy strategy to further improve clinical outcome.
Limitations exist in this study. Limited data on cancer treatment represent a weakness, considering improvements in chemotherapy during the past decades. We adjusted multivariable models for year of diagnosis and disease stage and confirmed that these variables had little confounding effects. Among patients with available data on adjuvant chemotherapy, postdiagnosis aspirin use was not associated with chemotherapy use. Among patients with stage II to III disease, 65% of regular users of aspirin received chemotherapy compared with 67% of nonusers (P = .86). In addition, the treatment decision was not made on the basis of tumor CD274 expression status because the CD274 data were not available for treating physicians. However, chemotherapy use is an important variable, and future studies need to address its effects on the potential interactive association of aspirin and tumor CD274 expression with colorectal cancer survival. Our aspirin data depended on questionnaire returns, which might have caused a bias resulting from the availability of postdiagnosis questionnaire data; hence, we applied the IPW method to all survival analyses to adjust for differential questionnaire returns. In addition, our cohort participants were health professionals who were more likely to be accurate in their report of aspirin intake. Limited information on cancer recurrence in the two cohorts was another limitation. Nonetheless, given the long-term follow-up of censored patients, colorectal cancer–specific survival may be regarded as a reasonable surrogate end point for clinical outcomes of colorectal cancer. We should acknowledge a possible bias in assessment of tumor CD274 expression due to intratumor heterogeneity. Our validation study using whole tissue sections of colorectal cancer did not find substantial intratumoral heterogeneity.33 Lack of a standardized evaluation method for tumor CD274 expression is another issue; nonetheless, it is expected that potential misclassification of tumors in terms of CD274 expression more likely would have driven our results toward the null hypothesis.
A major strength of this study is use of a molecular pathologic epidemiology56,57 database on the basis of the two large prospective cohort studies. An integrated analysis incorporating prospectively collected data on aspirin use, clinicopathological features, and tumor molecular markers allowed us to comprehensively examine the interactive prognostic associations of postdiagnosis aspirin use and tumor CD274 expression. Availability of prediagnosis aspirin use data enabled us to adjust for prediagnosis aspirin use status and to evaluate potential effect modification by prediagnosis aspirin status. Importantly, our patients with colorectal cancer were derived from a large number of hospitals throughout the United States (rather than a few hospitals), which increases the generalizability of our findings. However, our results need to be validated in independent datasets.
In conclusion, we have found a stronger association of aspirin use with colorectal cancer survival in tumors with low-level CD274 expression than in tumors with high-level CD274 expression. Our data suggest that overexpression of the CD274 immune checkpoint ligand may attenuate survival benefits associated with aspirin use and that tumor CD274 expression status may serve as a biomarker to select patients for adjuvant aspirin therapy. Given growing popularity of immune checkpoint inhibitors for cancer treatment, our findings, if validated, may have considerable clinical implications for adjuvant aspirin use in the era of immunotherapy.
ACKNOWLEDGMENT
We thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-Up Study for their valuable contributions, as well as the following state cancer registries for their help: AL, AR, AZ, CA, CO, CT, DE, FL, GA, IA, ID, IL, IN, KY, LA, MA, ME, MI, NC, ND, NE, NH, NJ, NY, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, and WY. The authors assume full responsibility for analyses and interpretation of these data.
Footnotes
Supported by National Institutes of Health Grants No. P01 CA87969 (M.J. Stampfer); UM1 CA186107 (M.J. Stampfer); P01 CA55075 (W.C. Willett); UM1 CA167552 (W.C. Willett); P50 CA127003 (C.S.F.); R01 CA137178 and K24 DK098311 (A.T.C.); K07 CA190673 (R.N.); and K07 CA188126 (X.Z.); R01 CA151993, R35 CA197735, and Nodal Award from the Dana-Farber Harvard Cancer Center (S.O.); and by grants from the Project P Fund, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. T.H. was supported by a fellowship grant from the Uehara Memorial Foundation and by a grant from the Mochida Memorial Foundation for Medical and Pharmaceutical Research. K.M. and K.K. were supported by a grant from Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers from Japan Society for the Promotion of Science. L.L. was supported by a scholarship grant from Chinese Scholarship Council and a fellowship grant from Huazhong University of Science and Technology. S.J.R. was supported in part by research funding from Bristol-Myers Squibb, MedImmune, and Merck.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
AUTHOR CONTRIBUTIONS
Conception and design: Tsuyoshi Hamada, Andrew T. Chan, Shuji Ogino
Financial support: Andrew T. Chan, Shuji Ogino
Administrative support: Shuji Ogino
Provision of study materials or patients: Andrew T. Chan, Shuji Ogino
Collection and assembly of data: Tsuyoshi Hamada, Yin Cao, Zhi Rong Qian, Yohei Masugi, Jonathan A. Nowak, Juhong Yang, Mingyang Song, Kosuke Mima, Keisuke Kosumi, Li Liu, Yan Shi, Annacarolina da Silva, Mancang Gu, Wanwan Li, NaNa Keum, Xuehong Zhang, Kana Wu, Jeffrey A. Meyerhardt, Edward L. Giovannucci, Andrew T. Chan, Charles S. Fuchs, Shuji Ogino
Data analysis and interpretation: Tsuyoshi Hamada, Yin Cao, Zhi Rong Qian, Yohei Masugi, Jonathan A. Nowak, Edward L. Giovannucci, Marios Giannakis, Scott J. Rodig, Gordon J. Freeman, Daniel Nevo, Molin Wang, Andrew T. Chan, Charles S. Fuchs, Reiko Nishihara, Shuji Ogino
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Aspirin Use and Colorectal Cancer Survival According to Tumor CD274 (Programmed Cell Death 1 Ligand 1) Expression Status
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Tsuyoshi Hamada
No relationship to disclose
Yin Cao
No relationship to disclose
Zhi Rong Qian
No relationship to disclose
Yohei Masugi
No relationship to disclose
Jonathan A. Nowak
No relationship to disclose
Juhong Yang
No relationship to disclose
Mingyang Song
No relationship to disclose
Kosuke Mima
No relationship to disclose
Keisuke Kosumi
No relationship to disclose
Li Liu
No relationship to disclose
Yan Shi
No relationship to disclose
Annacarolina da Silva
No relationship to disclose
Mancang Gu
No relationship to disclose
Wanwan Li
No relationship to disclose
NaNa Keum
No relationship to disclose
Xuehong Zhang
No relationship to disclose
Kana Wu
No relationship to disclose
Jeffrey A. Meyerhardt
No relationship to disclose
Edward L. Giovannucci
No relationship to disclose
Marios Giannakis
No relationship to disclose
Scott J. Rodig
Honoraria: Perkin Elmer, Bristol-Myers Squibb
Consulting or Advisory Role: AstraZeneca, Perkin Elmer
Research Funding: Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Patent pending for use of anti-galectin1 antibodies for diagnostic use
Travel, Accommodations, Expenses: Bristol Myers Squibb
Gordon J. Freeman
Consulting or Advisory Role: Novartis, Novartis (I), Surface Oncology (I), Genentech, Bristol-Myers Squibb, Bethyl Laboratories, Xios, Quiet, Seattle Genetics,
Research Funding: Novartis (I), Genentech (I)
Patents, Royalties, Other Intellectual Property: Genentech, Genentech (I), Pfizer (I), Bristol-Myers Squibb/Medarex, Amplimmune/AstraZeneca, Merck, EMD Serono, Boehringer Ingelheim, Novartis, Novartis (I)
Daniel Nevo
No relationship to disclose
Molin Wang
No relationship to disclose
Andrew T. Chan
Consulting or Advisory Role: Bayer Schering Pharma, Pfizer, Aralez Pharmaceuticals
Charles S. Fuchs
Consulting or Advisory Role: Genentech, Lilly, Sanofi, Bayer, Celgene, Merck, Bristol-Myers Squibb, Entrinsic Health, Five Prime Therapeutics, Agios
Reiko Nishihara
Honoraria: Chugai Pharmaceutical
Shuji Ogino
No relationship to disclose
REFERENCES
- 1.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov. 2015;5:16–18. doi: 10.1158/2159-8290.CD-14-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li SK, Martin A. Mismatch repair and colon cancer: Mechanisms and therapies explored. Trends Mol Med. 2016;22:274–289. doi: 10.1016/j.molmed.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gentles AJ, Newman AM, Liu CL, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogino S, Chan AT, Fuchs CS, et al. Molecular pathological epidemiology of colorectal neoplasia: An emerging transdisciplinary and interdisciplinary field. Gut. 2011;60:397–411. doi: 10.1136/gut.2010.217182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colussi D, Brandi G, Bazzoli F, et al. Molecular pathways involved in colorectal cancer: Implications for disease behavior and prevention. Int J Mol Sci. 2013;14:16365–16385. doi: 10.3390/ijms140816365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phipps AI, Limburg PJ, Baron JA, et al: Association between molecular subtypes of colorectal cancer and patient survival. Gastroenterology 148:77-87.e72, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi: 10.1038/nrdp.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Useros J, Garcia-Foncillas J. Obesity and colorectal cancer: Molecular features of adipose tissue. J Transl Med. 2016;14:21. doi: 10.1186/s12967-016-0772-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 17.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 19.Liao X, Lochhead P, Nishihara R, et al. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596–1606. doi: 10.1056/NEJMoa1207756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: A study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Wu H, Zhang H, et al. Aspirin use after diagnosis but not prediagnosis improves established colorectal cancer survival: A meta-analysis. Gut. 2015;64:1419–1425. doi: 10.1136/gutjnl-2014-308260. [DOI] [PubMed] [Google Scholar]
- 22.Domingo E, Church DN, Sieber O, et al. Evaluation of PIK3CA mutation as a predictor of benefit from nonsteroidal anti-inflammatory drug therapy in colorectal cancer. J Clin Oncol. 2013;31:4297–4305. doi: 10.1200/JCO.2013.50.0322. [DOI] [PubMed] [Google Scholar]
- 23.Zelenay S, van der Veen AG, Böttcher JP, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, DuBois RN. The role of prostaglandin E(2) in tumor-associated immunosuppression. Trends Mol Med. 2016;22:1–3. doi: 10.1016/j.molmed.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutchinson L. Immunotherapy: Evading immune escape: Synergy of COX and immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2015;12:622. doi: 10.1038/nrclinonc.2015.167. [DOI] [PubMed] [Google Scholar]
- 26.Marzbani E, Inatsuka C, Lu H, et al. The invisible arm of immunity in common cancer chemoprevention agents. Cancer Prev Res (Phila) 2013;6:764–773. doi: 10.1158/1940-6207.CAPR-13-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu AA, Drake V, Huang HS, et al. Reprogramming the tumor microenvironment: Tumor-induced immunosuppressive factors paralyze T cells. OncoImmunology. 2015;4:e1016700. doi: 10.1080/2162402X.2015.1016700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–1105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–6420. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–854. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao Y, Nishihara R, Wu K, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. 2016;2:762–769. doi: 10.1001/jamaoncol.2015.6396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baba Y, Nosho K, Shima K, et al. Relationship of CDX2 loss with molecular features and prognosis in colorectal cancer. Clin Cancer Res. 2009;15:4665–4673. doi: 10.1158/1078-0432.CCR-09-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masugi Y, Nishihara R, Yang J, et al: Tumour CD274 (PD-L1) expression and T cells in colorectal cancer. Gut [epub ahead of print on May 5, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogino S, Nosho K, Kirkner GJ, et al. CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut. 2009;58:90–96. doi: 10.1136/gut.2008.155473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nosho K, Irahara N, Shima K, et al. Comprehensive biostatistical analysis of CpG island methylator phenotype in colorectal cancer using a large population-based sample. PLoS One. 2008;3:e3698. doi: 10.1371/journal.pone.0003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogino S, Nishihara R, VanderWeele TJ, et al. Review article: The role of molecular pathological epidemiology in the study of neoplastic and non-neoplastic diseases in the era of precision medicine. Epidemiology. 2016;27:602–611. doi: 10.1097/EDE.0000000000000471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22:278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 38.Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med. 2005;24:3089–3110. doi: 10.1002/sim.2174. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Chen L. Classification of advanced human cancers based on Tumor Immunity in the MicroEnvironment (TIME) for cancer immunotherapy. JAMA Oncol. 2016;2:1403–1404. doi: 10.1001/jamaoncol.2016.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanmamed MF, Chen L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. 2014;20:256–261. doi: 10.1097/PPO.0000000000000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalerba P, Sahoo D, Paik S, et al. CDX2 as a prognostic biomarker in stage II and stage III colon cancer. N Engl J Med. 2016;374:211–222. doi: 10.1056/NEJMoa1506597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nosho K, Baba Y, Tanaka N, et al. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: Cohort study and literature review. J Pathol. 2010;222:350–366. doi: 10.1002/path.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogino S, Galon J, Fuchs CS, et al. Cancer immunology--analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711–719. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Caro G, Marchesi F, Laghi L, et al. Immune cells: Plastic players along colorectal cancer progression. J Cell Mol Med. 2013;17:1088–1095. doi: 10.1111/jcmm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galon J, Mlecnik B, Bindea G, et al. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rozek LS, Schmit SL, Greenson JK, et al. Tumor-infiltrating lymphocytes, Crohn’s-like lymphoid reaction, and survival from colorectal cancer. J Natl Cancer Inst. 2016;108:djw027. doi: 10.1093/jnci/djw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mlecnik B, Bindea G, Angell HK, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 48. Prizment AE, Vierkant RA, Smyrk TC, et al: Cytotoxic T-cells and granzyme B associated with improved colorectal cancer survival in a prospective cohort of older women. Cancer Epidemiol Biomarkers Prev 10.1158/1055-9965.EPI-16-0641 [epub ahead of print on December 15, 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenbaum MW, Bledsoe JR, Morales-Oyarvide V, et al. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod Pathol. 2016;29:1104–1112. doi: 10.1038/modpathol.2016.95. [DOI] [PubMed] [Google Scholar]
- 50.Inaguma S, Lasota J, Wang Z, et al: Clinicopathologic profile, immunophenotype, and genotype of CD274 (PD-L1)-positive colorectal carcinomas. Mod Pathol 30:278-285, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Markosyan N, Chen EP, Evans RA, et al. Mammary carcinoma cell derived cyclooxygenase 2 suppresses tumor immune surveillance by enhancing intratumoral immune checkpoint activity. Breast Cancer Res. 2013;15:R75. doi: 10.1186/bcr3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Göbel C, Breitenbuecher F, Kalkavan H, et al. Functional expression cloning identifies COX-2 as a suppressor of antigen-specific cancer immunity. Cell Death Dis. 2014;5:e1568. doi: 10.1038/cddis.2014.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Javeed A, Zhang B, Qu Y, et al. The significantly enhanced frequency of functional CD4+CD25+Foxp3+ T regulatory cells in therapeutic dose aspirin-treated mice. Transpl Immunol. 2009;20:253–260. doi: 10.1016/j.trim.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Sharma S, Stolina M, Yang SC, et al. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res. 2003;9:961–968. [PubMed] [Google Scholar]
- 55.Stolina M, Sharma S, Lin Y, et al. Specific inhibition of cyclooxygenase 2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 56.Ogino S, Stampfer M. Lifestyle factors and microsatellite instability in colorectal cancer: The evolving field of molecular pathological epidemiology. J Natl Cancer Inst. 2010;102:365–367. doi: 10.1093/jnci/djq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishihara R, VanderWeele TJ, Shibuya K, et al. Molecular pathological epidemiology gives clues to paradoxical findings. Eur J Epidemiol. 2015;30:1129–1135. doi: 10.1007/s10654-015-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]