Abstract
Purpose
Cigarette smoking is associated with increased incidence of pancreatic cancer. However, few studies have prospectively evaluated the association of smoking with patient survival.
Patients and Methods
We analyzed survival by smoking status among 1,037 patients from two large US prospective cohort studies diagnosed from 1986 to 2013. Among 485 patients from four prospective US cohorts, we also evaluated survival by prediagnostic circulating levels of cotinine, a metabolite of nicotine that is proportional to tobacco smoke exposure. On the basis of prediagnosis cotinine levels, we classified patients as nonsmokers (< 3.1 ng/mL), light smokers (3.1-20.9 ng/mL), or heavy smokers (≥ 21.0 ng/mL). We estimated hazard ratios (HRs) for death by using Cox proportional hazards models, with adjustment for age, sex, race/ethnicity, body mass index, diabetes status, diagnosis year, and cancer stage.
Results
The multivariable-adjusted HR for death was 1.37 (95% CI, 1.11 to 1.69) comparing current smokers with never smokers (P = .003). A statistically significant negative trend in survival was observed for increasing pack-years of smoking (Ptrend = .008), with HR for death of 1.49 (95% CI, 1.05 to 2.10) for > 60 pack-years of smoking versus never smoking. Survival among former smokers was similar to that for never smokers, regardless of time since quitting. Heavy smokers defined by prediagnostic circulating cotinine levels had a multivariable-adjusted HR for death of 1.76 (95% CI, 1.23 to 2.51) compared with nonsmokers. Among patients with circulating cotinine levels measured within 5 years before diagnosis, heavy smokers had a multivariable-adjusted HR for death of 2.47 (95% CI, 1.24 to 4.92) compared with nonsmokers.
Conclusion
Cigarette smoking was associated with a reduction in survival among patients with pancreatic cancer.
INTRODUCTION
Pancreatic cancer is the third leading cause of cancer-related death in the United States, and most patients survive less than 12 months after diagnosis.1 Aside from disease stage, few prognostic factors have been well characterized.2 Cigarette smoking is a consistent risk factor for pancreatic cancer, which may contribute to development of approximately 20% of pancreatic cancer cases.3 In a pooled analysis of 12 prospective cohorts and one case-control study, current cigarette smokers had an 80% increased risk of pancreatic cancer compared with never smokers, and the risk increased with smoking intensity, duration, and cumulative smoking dose.4 Nevertheless, few studies have assessed cigarette smoking and survival among patients with pancreatic cancer.
Tobacco products contain nicotine, and the predominant laboratory method for defining active cigarette smoking is by measuring plasma cotinine, the major circulating metabolite of nicotine. Cotinine has an in vivo half-life of approximately 20 hours and is typically detectable for up to 1 week after using tobacco. Studies that compare nonsmokers and smokers have consistently demonstrated that cotinine in the urine, saliva, or plasma can distinguish active smokers from nonsmokers.5-9 In addition, cotinine has been shown to be more sensitive and specific than carbon monoxide monitoring for measuring smoking status.10 Among 16,156 participants from the National Health and Nutrition Examination Survey (NHANES), the optimal serum cotinine cut point had a high degree of sensitivity and specificity for discriminating adult smokers from nonsmokers.11
In this study, we prospectively evaluated the association of cigarette smoking with overall survival (OS) among patients diagnosed with pancreatic cancer from two large prospective US cohort studies. We next sought to examine survival in relation to plasma cotinine levels among patients from four large US prospective cohort studies with plasma samples collected before diagnosis of cancer.
PATIENTS AND METHODS
Study Population
We assessed the association of cigarette smoking with survival among patients with pancreatic cancer from the Health Professionals Follow-Up Study (HPFS) and Nurses’ Health Study (NHS). HPFS began in 1986 when 51,529 men age 40 to 75 years working in health professions returned a mailed questionnaire on health-related behaviors and medical history.12 NHS was initiated in 1976 when 121,700 female registered nurses age 30 to 55 years returned a mailed questionnaire describing demographics, lifestyle choices, and medical history.13 Participants have updated their exposures and medical history through biennial follow-up questionnaires. We examined the association between plasma cotinine and survival among patients with pancreatic cancer who had banked prediagnostic blood from HPFS, NHS, and two other prospective cohorts, the Physicians’ Health Study I (PHS I) and the Women’s Health Initiative (WHI) Observational Study. PHS I is a completed clinical trial that was initiated in 1982 of aspirin and β-carotene among 22,071 male physicians age 40 to 84 years. After completing the randomized components, participants were followed as an observational cohort.14 WHI consists of 93,676 postmenopausal women age 50 to 79 years enrolled between 1994 and 1998 at 40 US clinical centers.15 Participants completed a baseline clinic visit and annual mailed questionnaires. This study was approved by the Human Research Committee at the Brigham and Women’s Hospital, Boston, MA, and all participants provided informed consent.
We identified 1,037 incident patients with pancreatic cancer from HPFS and NHS with available smoking status and 485 patients with measured plasma cotinine from HPFS, NHS, PHS I, and WHI (Appendix Table A1, online only).16 Incident cases of pancreatic cancer were identified by self-report or during follow-up of a participant’s death. Deaths were ascertained from next-of-kin or the US Postal Service and by searching the National Death Index; this method has been shown to capture more than 98% of deaths.17 Diagnoses were confirmed by review of medical records, death certificates, and/or tumor registry data. Patients with nonadenocarcinoma histology or unclear survival time were excluded.
Assessment of Cigarette Smoking
For HPFS and NHS participants, former and current smokers were asked on the baseline questionnaire to indicate the average number of cigarettes they smoked per day, the age at which they began smoking and, among former smokers, the age at which they stopped smoking. Information on smoking status and the number of cigarettes smoked was updated biennially. The primary exposure of cigarette smoking was obtained from a questionnaire within 2 or 4 years before cancer diagnosis. Never smokers were defined as participants who reported never smoking, current smokers were defined as participants who reported active smoking on the questionnaire before cancer diagnosis, and former smokers were defined as participants who smoked in the past but did not report active smoking on the questionnaire before cancer diagnosis.
On each questionnaire, current smokers were further classified as smoking 1 to 4, 5 to 14, 15 to 24, 25 to 34, 35 to 44, or 45 or more cigarettes per day. To examine the influence of packs per day among current smokers, categories of current smoking were collapsed into 1 to 14, 15 to 24, and 25 or more cigarettes per day. By using data on intensity and duration of smoking, we estimated cumulative lifetime exposure to cigarettes as pack-years, with 1 pack-year of exposure equivalent to 7,300 cigarettes (1 year × 365 days per year × 1 pack per day × 20 cigarettes per pack). To examine the influence of pack-years among current smokers, categories of 1 to 30, 31 to 60, and 61 or more pack-years of smoking were generated.
Assessment of Plasma Cotinine Levels
EDTA tubes were used to collect blood samples from 18,225 men in HPFS from 1993 to 1995, 14,916 men in PHS I from 1982 to 1984, and 93,676 women in WHI from 1994 to 1998; heparin tubes were used to collect blood samples from 32,826 women in NHS from 1989 to 1990. Details on blood draw procedures, transportation, and storage of plasma samples in these cohorts have been described in detail.16 As previously described, plasma cotinine was measured by targeted liquid chromatography-mass spectrometry at the Broad Institute of the Massachusetts Institute of Technology and Harvard University (Cambridge, MA).16 Three heparin quality control (QC) plasma pools (57 total QC samples) and three EDTA QC plasma pools (128 total QC samples) were randomly interspersed among participant samples. The mean coefficient of variance for cotinine across QC plasma pools was 10.1%, indicating good reproducibility.
Assessment of Covariates
Individual characteristics and habits were obtained either from the same questionnaires assessing smoking exposure or from the questionnaires before blood collection in the plasma cotinine analyses. In all cohorts, data were available for age, sex, race/ethnicity, weight, height, diabetes status, and alcohol intake. Date of diagnosis and pancreatic cancer stage at diagnosis were obtained from medical record review. Cancer stage was classified as local disease amenable to surgical resection, locally advanced disease with extrapancreatic extension rendering it unresectable but without distant metastases, distant metastatic disease, or unknown.
Statistical Analyses
The association of cigarette smoking with OS was examined in HPFS and NHS patients by using Cox proportional hazards regression to calculate pooled hazard ratios (HRs) and 95% CIs. Survival time was calculated from the date of cancer diagnosis to the date of death or last follow-up if a participant was still alive. Proportionality of hazards assumption was satisfied by evaluating a time-dependent variable, which was the cross-product of smoking and time (P = .51).
In multivariable models, we adjusted for potential confounders, including age at diagnosis, cohort (which also adjusted for sex), race/ethnicity, body mass index (BMI), diabetes status, year of diagnosis, cancer stage, and alcohol intake.18,19 Survival curves were investigated for patients in each category adjusted for covariates by using direct adjusted survival estimation.20,21 This method uses Cox proportional hazards regression to estimate probabilities of survival at each follow-up time point for each individual and averages them to obtain an OS estimate. To consider overall comorbidity, we adjusted for a continuous propensity score derived by regressing smoking on comorbidities and lifestyle factors with the potential to limit survival,22 including physical activity, calorie intake, and history of high cholesterol, stroke, hypertension, or heart disease (angina pectoris, coronary bypass, angioplasty, stent, myocardial infarction).
In a prior study of NHANES participants from 1999 to 2004,11 the optimal serum cotinine level for discriminating adult smokers from nonsmokers was 3.08 ng/mL (sensitivity, 96.3%; specificity, 97.4%). We validated this cut point among 480 patients with self-reported smoking status in our four cohorts, with sensitivity of 94.8% and specificity of 95.3%. We then classified our patients with pancreatic cancer who had prediagnostic cotinine levels < 3.1 ng/mL as cotinine-defined nonsmokers. Patients with cotinine levels ≥ 3.1 ng/mL were classified as current smokers, with light and heavy smokers defined as below (3.1 to 20.9 ng/mL) or above (≥ 21.0 ng/mL) the median cotinine level among smokers by using pooled levels from the four cohorts. In multivariable models, we adjusted for the above potential confounders as well as time between blood collection and cancer diagnosis. Statistical analyses were performed by using SAS 9.4, and all P values are two-sided.
RESULTS
Baseline characteristics of 1,037 patients with pancreatic cancer by smoking status are described in Table 1. Among those with known disease stage, 19.4% had localized disease, 15.3% had locally advanced disease, and 65.3% had metastatic disease. Median survival by cancer stage was 19 months for those with localized disease, 9 months for those with locally advanced disease, and 3 months for those with metastatic disease. At the end of follow-up, 1,020 patients (98.4%) had died.
Table 1.
Characteristics of Patients With Pancreatic Cancer According to Smoking History in Two Prospective Cohorts

Patients who currently smoked cigarettes had a reduced survival compared with never smokers (Table 2; Fig 1). The multivariable-adjusted HR for death was 1.37 (95% CI, 1.11 to 1.69) comparing current smokers with never smokers (P = .003). Results were similar across cohorts; the HR for death was 1.40 (95% CI, 0.95 to 2.06) in HPFS and 1.39 (95% CI, 1.07 to 1.80) in NHS. We considered whether current smoking may predominantly impact survival among patients who undergo surgery because of a potential increase in perioperative mortality. However, after excluding patients with localized disease, our results were not materially altered (HR, 1.43; 95% CI, 1.14 to 1.79).
Table 2.
HRs for Death Among Patients With Pancreatic Cancer From Two Prospective Cohorts According to Smoking History

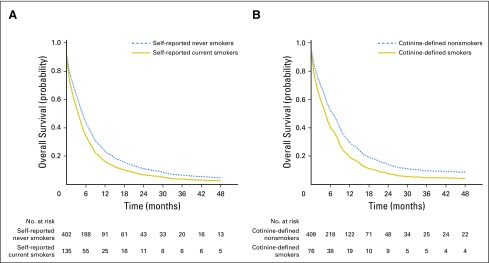
Fig 1.
Overall survival curves by (A) self-reported or (B) plasma cotinine-defined smoking status among patients with pancreatic cancer.
We considered whether the association of current smoking with survival was modified by BMI or diabetes status. No statistically significant interaction was identified (all Pinteraction ≥ .44). The HR for death was 1.47 (95% CI, 1.09 to 1.98) in patients with BMI ≥ 25 kg/m2 and 1.23 (95% CI, 0.91 to 1.66) in patients with BMI < 25 kg/m2. Furthermore, the HR for death was 1.56 (95% CI, 0.87 to 2.82) in patients with long-term diabetes and 1.31 (95% CI, 1.03 to 1.66) in patients without diabetes. We considered whether patients who currently smoked cigarettes had a greater degree of comorbid illness, which might have led to worse survival. After adjustment for a propensity score to account for differences in comorbidity, our results also remained largely unchanged (HR, 1.31; 95% CI, 1.04 to 1.65).
We next examined the association between survival and cumulative cigarette exposure measured in pack-years. We observed a statistically significant negative trend in survival with increasing pack-years of smoking (Ptrend = .008) (Table 3). The multivariable-adjusted HRs for death were 1.25 (95% CI, 0.75 to 2.06) for patients with 1 to 30 pack-years of smoking, 1.32 (95% CI, 1.00 to 1.75) for 31 to 60 pack-years, and 1.49 (95% CI, 1.05 to 2.10) for more than 60 pack-years. Median OS was 5 months among nonsmokers and 3 months among current smokers with more than 60 pack-years of smoking. A clear relationship between the number of cigarettes smoked per day and survival was not evident (Appendix Table A2, online only).
Table 3.
HRs for Death Among Patients With Pancreatic Cancer from Two Prospective Cohorts According to Pack-Years of Smoking
In contrast to current smokers, former smokers did not have an increase in their hazards for death (Table 2). Compared with never smokers, former smokers had a multivariable-adjusted HR for death of 0.99 (95% CI, 0.86 to 1.14; P = .90). A relationship was not observed between patient survival and time since quitting smoking among former smokers (< 5 years, 5 to < 10 years, ≥ 10 years; Appendix Table A3, online only). Compared with never smokers, the multivariable-adjusted HRs for death were 0.90 (95% CI, 0.66 to 1.22) for former smokers who discontinued smoking within the past 5 years, 1.15 (95% CI, 0.85 to 1.56) for those who discontinued smoking within the past 5 to 10 years, and 1.00 (95% CI, 0.86 to 1.16) for those who discontinued smoking more than 10 years ago.
We also evaluated the association of prediagnostic plasma cotinine levels and patient survival in patients with pancreatic cancer from HPFS, NHS, PHS I, and WHI. Cotinine is the primary breakdown product from nicotine metabolism and is a widely used marker for tobacco exposure.5-9 By using prediagnostic circulating cotinine levels to classify patients’ tobacco use, cotinine-defined heavy smokers had a multivariable-adjusted HR for death of 1.76 (95% CI, 1.23 to 2.51) compared with nonsmokers (Table 4; Fig 1). Median OS was 7 months among nonsmokers and 4 months among cotinine-defined heavy smokers. In a sensitivity analysis that excluded 17 patients who quit smoking between the time of blood collection and their cancer diagnosis, the HR for death was 2.06 (95% CI, 1.38 to 3.06) for cotinine-defined heavy smokers. A stronger association of cotinine and patient survival was seen among patients with blood collected within 5 years of their cancer diagnosis, with an HR for death of 2.47 (95% CI, 1.24 to 4.92; Table 5).
Table 4.
HRs for Death Among Patients With Pancreatic Cancer From Four Prospective Cohorts According to Prediagnostic Plasma Levels of Cotinine
Table 5.
HRs for Death Among Patients With Pancreatic Cancer From Four Prospective Cohorts According to Plasma Levels of Cotinine by Time Since Blood Collection
DISCUSSION
In this large prospective study, patients with pancreatic cancer who smoked cigarettes near the time of diagnosis had an approximately 40% increased hazard for death compared with those who never smoked. This reduction in survival remained statistically significant after adjustment for potential confounding factors, including a propensity score that provided an aggregate measure of comorbid illness. Greater pack-years of smoking portended a worse prognosis among current smokers, but former smokers experienced outcomes similar to those of never smokers, suggesting a potential beneficial effect of smoking cessation. In a partially overlapping patient population, higher prediagnostic plasma cotinine levels were also associated with reduced survival, with particularly strong associations observed among cotinine-defined heavy smokers. Taken together, these data implicate cigarette smoking as an adverse prognostic factor in patients with pancreatic cancer and lend continued support to efforts directed at smoking cessation.
A detrimental effect of smoking on survival has been previously reported for patients with other malignancies,23-29 including those etiologically associated with tobacco use, such as head and neck squamous cell carcinoma24,27,28 and lung cancer.25,29 Although cigarette smoking is an established risk factor for pancreatic cancer, few studies have investigated its association with patient survival. Among 648 patients from two Italian hospital–based studies, those who currently smoked had an HR for death of 1.42 (95% CI, 1.16 to 1.73) compared with never smokers.30 In a prospective cohort study of 348 Korean men with pancreatic cancer who reported smoking status on a baseline questionnaire, a nonsignificant increase in mortality was observed for current smokers with an HR for death of 1.20 (95% CI, 0.84 to 1.72).23 Additional hospital-based studies that evaluated multiple potential prognostic factors have not identified a clear association between cigarette smoking and pancreatic cancer survival.31,32
A number of mechanisms have been identified by which smoking appears to affect cancer progression.33 Nicotine and other carcinogenic components of tobacco smoke have direct growth-promoting effects on tumor cells, alter cross-talk between tumor and stromal cells within the tumor microenvironment, and increase infiltration of myeloid-derived suppressor cells.34-36 The impact of cigarette smoking on these or other pathways may underlie the reduced survival that we observed. Individuals who smoke are also more likely to have other comorbidities, such as cardiovascular disease, which might adversely affect their survival. However, our results were largely unchanged after considering a propensity score that provided an aggregate measure of comorbid illness. Furthermore, our results remained unchanged after exclusion of patients with localized disease, thus limiting the potential of increased perioperative morbidity and mortality among smokers to explain our results.
We observed a reduction in survival among current smokers who were enrolled in two large prospective cohort studies. An important strength of the prospective cohort design is its ability to fully capture the spectrum of patients with pancreatic cancer in terms of disease aggressiveness and stage of disease, because individuals are enrolled before their diagnosis and are not identified at selected tertiary care centers. Notably, survival times and stage distribution for patients in HPFS and NHS were highly similar to those of patients with pancreatic cancer captured by the National Cancer Database.37 Cohort studies also prospectively collect data on numerous exposures, including cigarette smoking and other factors that may affect survival. Thus, these studies limit issues with misclassification and recall bias and allow for rigorous adjustment for comorbidities.
After identifying a reduction in survival for patients who reported current smoking, we confirmed these findings by examining prediagnostic plasma cotinine levels and survival among patients from four prospective cohorts. Plasma cotinine is the major circulating metabolite of nicotine, and its levels provide an objective assessment of exposure to both personal and second-hand tobacco smoke. Higher plasma cotinine levels were also associated with reduced patient survival, with a greater reduction in survival time for cotinine-defined heavy smokers.
Limitations of our study also require consideration. We used overall mortality as the outcome rather than pancreatic cancer–specific mortality. Nevertheless, < 5% of patients with pancreatic cancer are cured of their disease, such that overall mortality is a good surrogate for cancer-specific mortality. Treatment information was not available for patients with pancreatic cancer in our cohorts. However, chemotherapy and radiotherapy have had only a modest impact on patient survival and are unlikely to have varied by prediagnosis smoking status or plasma cotinine level.2 We cannot rule out that our findings may have been influenced in part by residual confounding. Nonetheless, we considered multiple possible confounding covariates and adjusted for a propensity score of comorbid illness without observing changes in our results. Finally, our study population consisted primarily of white participants, and further studies in nonwhite participants are warranted.
In conclusion, self-reported current cigarette smoking was associated with a statistically significant reduction in survival among patients with pancreatic cancer. Furthermore, reduced survival was also observed for patients with high levels of plasma cotinine, the major circulating metabolite of nicotine. In contrast, no reduction in survival was identified for former smokers, suggesting a potential benefit to smoking cessation and an opportunity to improve survival for patients with pancreatic cancer.
ACKNOWLEDGMENT
We thank the participants and staff of the Health Professionals Follow-Up Study, Nurses’ Health Study, Physicians’ Health Study I, and Women’s Health Initiative for their valuable contributions and the cancer registries of the following states for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY.
Appendix
Table A1.
Number of Patients With Pancreatic Cancer by Cohort With Self-Reported Smoking Status and Plasma Cotinine Level
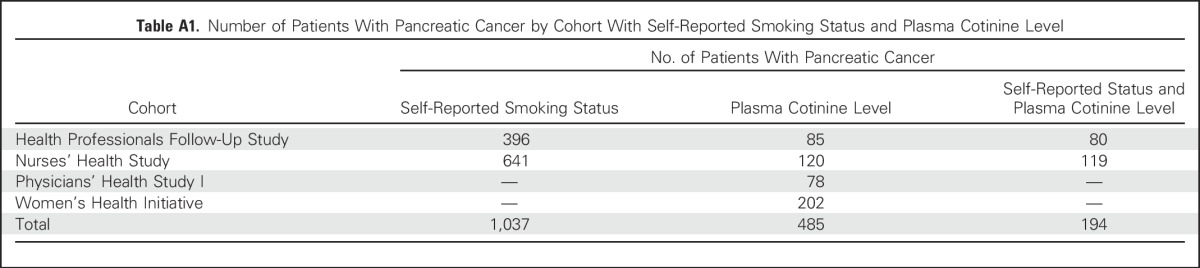
Table A2.
HRs for Death Among Patients With Pancreatic Cancer From Two Prospective Cohorts According to Smoking Intensity

Table A3.
HRs for Death Among Patients With Pancreatic Cancer from Two Prospective Cohorts According to Time Since Quitting Smoking

Footnotes
HPFS is supported by National Institutes of Health (NIH) Grant No. UM1 CA167552. NHS is supported by NIH Grants No. UM1 CA186107, P01 CA87969, and R01 CA49449. PHS is supported by NIH Grants No. CA 34944, CA 40360, HL 26490, and HL 34595. The WHI program is funded by the NIH through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–32119, 32122, 42107–26, 42129–32, and 44221.
Supported by National Cancer Institute (NCI) Grant No. R35 CA197735 (S.O.), by the Robert T. and Judith B. Hale Fund for Pancreatic Cancer, the Perry S. Levy Fund for Gastrointestinal Cancer Research, and by the Lustgarten Foundation, the Pancreatic Cancer Action Network, the Noble Effort Fund, the Peter R. Leavitt Family Fund, the Wexler Family Fund, and Promises for Purple (B.M.W.).
The authors assume full responsibility for analyses and interpretation of these data.
Listen to the podcast by Dr McRee at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Chen Yuan, Brian M. Wolpin
Administrative support: JoAnn E. Manson,
Collection and assembly of data: Chen Yuan, Clary B. Clish, Edward L. Giovannucci, Meir J. Stampfer, Howard D. Sesso, Barbara B. Cochrane, JoAnn E. Manson, Charles S. Fuchs, Brian M. Wolpin
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cigarette Smoking and Pancreatic Cancer Survival
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Chen Yuan
No relationship to disclose
Vicente Morales-Oyarvide
No relationship to disclose
Ana Babic
Stock or Other Ownership: Biogen Idec
Clary B. Clish
No relationship to disclose
Peter Kraft
Consulting or Advisory Role: Merck Research Laboratory
Ying Bao
No relationship to disclose
Zhi Rong Qian
No relationship to disclose
Douglas A. Rubinson
Consulting or Advisory Role: Celgene
Kimmie Ng
Honoraria: Tarrex Biopharma
Consulting or Advisory Role: Genentech, Eli Lilly
Research Funding: Pharmavite (Inst), Genentech (Inst), Gilead Sciences, Celgene, Trovagene
Edward L. Giovannucci
No relationship to disclose
Shuji Ogino
No relationship to disclose
Meir J. Stampfer
Stock or Other Ownership: Elysium Health
Consulting or Advisory Role: Elysium Health
John Michael Gaziano
No relationship to disclose
Howard D. Sesso
No relationship to disclose
Barbara B. Cochrane
No relationship to disclose
JoAnn E. Manson
No relationship to disclose
Charles S. Fuchs
Consulting or Advisory Role: Genentech, Eli Lilly, Sanofi, Bayer AG, Celgene, Merck, Bristol-Myers Squibb, Entrinsic Health Solutions, Five Prime Therapeutics, Agios
Brian M. Wolpin
No relationship to disclose
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 3.Iodice S, Gandini S, Maisonneuve P, et al. Tobacco and the risk of pancreatic cancer: A review and meta-analysis. Langenbecks Arch Surg. 2008;393:535–545. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 4.Lynch SM, Vrieling A, Lubin JH, et al. Cigarette smoking and pancreatic cancer: A pooled analysis from the pancreatic cancer cohort consortium. Am J Epidemiol. 2009;170:403–413. doi: 10.1093/aje/kwp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis MJ, Tunstall-Pedoe H, Feyerabend C, et al. Comparison of tests used to distinguish smokers from nonsmokers. Am J Public Health. 1987;77:1435–1438. doi: 10.2105/ajph.77.11.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pérez-Stable EJ, Benowitz NL, Marín G. Is serum cotinine a better measure of cigarette smoking than self-report? Prev Med. 1995;24:171–179. doi: 10.1006/pmed.1995.1031. [DOI] [PubMed] [Google Scholar]
- 7.Lewis SJ, Cherry NM, McL Niven R, et al. Cotinine levels and self-reported smoking status in patients attending a bronchoscopy clinic. Biomarkers. 2003;8:218–228. doi: 10.1080/1354750031000120125. [DOI] [PubMed] [Google Scholar]
- 8.Abrams DB, Follick MJ, Biener L, et al. Saliva cotinine as a measure of smoking status in field settings. Am J Public Health. 1987;77:846–848. doi: 10.2105/ajph.77.7.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glasgow RE, Mullooly JP, Vogt TM, et al. Biochemical validation of smoking status: Pros, cons, and data from four low-intensity intervention trials. Addict Behav. 1993;18:511–527. doi: 10.1016/0306-4603(93)90068-k. [DOI] [PubMed] [Google Scholar]
- 10.Murray RP, Connett JE, Lauger GG, et al. Error in smoking measures: Effects of intervention on relations of cotinine and carbon monoxide to self-reported smoking. Am J Public Health. 1993;83:1251–1257. doi: 10.2105/ajph.83.9.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol. 2009;169:236–248. doi: 10.1093/aje/kwn301. [DOI] [PubMed] [Google Scholar]
- 12.Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 13.Colditz GA, Hankinson SE. The Nurses’ Health Study: Lifestyle and health among women. Nat Rev Cancer. 2005;5:388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 14.Manson JE, Grobbee DE, Stampfer MJ, et al. Aspirin in the primary prevention of angina pectoris in a randomized trial of United States physicians. Am J Med. 1990;89:772–776. doi: 10.1016/0002-9343(90)90220-8. [DOI] [PubMed] [Google Scholar]
- 15.Langer RD, White E, Lewis CE, et al. The Women’s Health Initiative Observational Study: Baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 16.Mayers JR, Wu C, Clish CB, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20:1193–1198. doi: 10.1038/nm.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140:1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 18.Yuan C, Bao Y, Wu C, et al. Prediagnostic body mass index and pancreatic cancer survival. J Clin Oncol. 2013;31:4229–4234. doi: 10.1200/JCO.2013.51.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan C, Rubinson DA, Qian ZR, et al. Survival among patients with pancreatic cancer and long-standing or recent-onset diabetes mellitus. J Clin Oncol. 2015;33:29–35. doi: 10.1200/JCO.2014.57.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghali WA, Quan H, Brant R, et al. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286:1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- 21.Makuch RW. Adjusted survival curve estimation using covariates. J Chronic Dis. 1982;35:437–443. doi: 10.1016/0021-9681(82)90058-3. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 23.Park SM, Lim MK, Shin SA, et al. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol. 2006;24:5017–5024. doi: 10.1200/JCO.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- 24.Duffy SA, Ronis DL, McLean S, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009;27:1969–1975. doi: 10.1200/JCO.2008.18.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujisawa T, Iizasa T, Saitoh Y, et al. Smoking before surgery predicts poor long-term survival in patients with stage I non-small-cell lung carcinomas. J Clin Oncol. 1999;17:2086–2091. doi: 10.1200/JCO.1999.17.7.2086. [DOI] [PubMed] [Google Scholar]
- 26.Talamini R, Polesel J, Spina M, et al. The impact of tobacco smoking and alcohol drinking on survival of patients with non-Hodgkin lymphoma. Int J Cancer. 2008;122:1624–1629. doi: 10.1002/ijc.23205. [DOI] [PubMed] [Google Scholar]
- 27.Dikshit RP, Boffetta P, Bouchardy C, et al. Lifestyle habits as prognostic factors in survival of laryngeal and hypopharyngeal cancer: A multicentric European study. Int J Cancer. 2005;117:992–995. doi: 10.1002/ijc.21244. [DOI] [PubMed] [Google Scholar]
- 28.Kawakita D, Hosono S, Ito H, et al. Impact of smoking status on clinical outcome in oral cavity cancer patients. Oral Oncol. 2012;48:186–191. doi: 10.1016/j.oraloncology.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Bryant A, Cerfolio RJ. Differences in epidemiology, histology, and survival between cigarette smokers and never-smokers who develop non-small cell lung cancer. Chest. 2007;132:185–192. doi: 10.1378/chest.07-0442. [DOI] [PubMed] [Google Scholar]
- 30.Pelucchi C, Galeone C, Polesel J, et al. Smoking and body mass index and survival in pancreatic cancer patients. Pancreas. 2014;43:47–52. doi: 10.1097/MPA.0b013e3182a7c74b. [DOI] [PubMed] [Google Scholar]
- 31.Olson SH, Chou JF, Ludwig E, et al. Allergies, obesity, other risk factors and survival from pancreatic cancer. Int J Cancer. 2010;127:2412–2419. doi: 10.1002/ijc.25240. [DOI] [PubMed] [Google Scholar]
- 32.Dandona M, Linehan D, Hawkins W, et al. Influence of obesity and other risk factors on survival outcomes in patients undergoing pancreaticoduodenectomy for pancreatic cancer. Pancreas. 2011;40:931–937. doi: 10.1097/MPA.0b013e318215a9b1. [DOI] [PubMed] [Google Scholar]
- 33.Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res. 2014;12:14–23. doi: 10.1158/1541-7786.MCR-13-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delitto D, Zhang D, Han S, et al. Nicotine reduces survival via augmentation of paracrine HGF-MET signaling in the pancreatic cancer microenvironment. Clin Cancer Res. 2016;22:1787–1799. doi: 10.1158/1078-0432.CCR-15-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hermann PC, Sancho P, Cañamero M, et al: Nicotine promotes initiation and progression of KRAS-induced pancreatic cancer via Gata6-dependent dedifferentiation of acinar cells in mice. Gastroenterology 147:1119-1133.e4, 2014 [DOI] [PubMed]
- 36.Kumar S, Torres MP, Kaur S, et al. Smoking accelerates pancreatic cancer progression by promoting differentiation of MDSCs and inducing HB-EGF expression in macrophages. Oncogene. 2015;34:2052–2060. doi: 10.1038/onc.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bilimoria KY, Bentrem DJ, Ko CY, et al: Validation of the 6th edition AJCC Pancreatic Cancer Staging System: Report from the National Cancer Database. Cancer 110:738-744, 2007 [DOI] [PubMed] [Google Scholar]