Abstract
Purpose
T cells genetically modified to express chimeric antigen receptors (CARs) targeting CD19 (CAR-19) have potent activity against acute lymphoblastic leukemia, but fewer results supporting treatment of lymphoma with CAR-19 T cells have been published. Patients with lymphoma that is chemotherapy refractory or relapsed after autologous stem-cell transplantation have a grim prognosis, and new treatments for these patients are clearly needed. Chemotherapy administered before adoptive T-cell transfer has been shown to enhance the antimalignancy activity of adoptively transferred T cells.
Patients and Methods
We treated 22 patients with advanced-stage lymphoma in a clinical trial of CAR-19 T cells preceded by low-dose chemotherapy. Nineteen patients had diffuse large B-cell lymphoma, two patients had follicular lymphoma, and one patient had mantle cell lymphoma. Patients received a single dose of CAR-19 T cells 2 days after a low-dose chemotherapy conditioning regimen of cyclophosphamide plus fludarabine.
Results
The overall remission rate was 73% with 55% complete remissions and 18% partial remissions. Eleven of 12 complete remissions are ongoing. Fifty-five percent of patients had grade 3 or 4 neurologic toxicities that completely resolved. The low-dose chemotherapy conditioning regimen depleted blood lymphocytes and increased serum interleukin-15 (IL-15). Patients who achieved a remission had a median peak blood CAR+ cell level of 98/μL and those who did not achieve a remission had a median peak blood CAR+ cell level of 15/μL (P = .027). High serum IL-15 levels were associated with high peak blood CAR+ cell levels (P = .001) and remissions of lymphoma (P < .001).
Conclusion
CAR-19 T cells preceded by low-dose chemotherapy induced remission of advanced-stage lymphoma, and high serum IL-15 levels were associated with the effectiveness of this treatment regimen. CAR-19 T cells will likely become an important treatment for patients with relapsed lymphoma.
INTRODUCTION
Chimeric antigen receptors (CARs) are artificial proteins that incorporate an antigen recognition domain and T-cell signaling domains.1-8 T cells genetically modified to express a CAR recognize and kill malignant cells expressing the antigen targeted by the CAR.9-11 T cells expressing CARs that target the B-cell antigen CD19 (CAR-19) have potent activity against acute lymphoid leukemia (ALL).12-16 Lymphoma is much more common than ALL,17,18 but compared with ALL, fewer cases of effective treatment of lymphoma with CAR-19 T cells have been published.19-22 Diffuse large B-cell lymphoma (DLBCL) that is either refractory to chemotherapy17,18,23-25 or relapsed after autologous stem-cell transplantation (ASCT)26,27 carries a grim prognosis. New therapies are needed for advanced-stage B-cell lymphomas. CAR T cells cause adverse events including but not limited to hypotension, cardiac toxicity, and neurologic toxicities. These toxicities are thought to be caused by cytokines directly released by CAR T cells or released by other cells in response to cytokines produced by CAR T cells.12,13,15,21,28
Recipient leukocyte depletion by radiation therapy or chemotherapy enhanced the antitumor activity of adoptively transferred T cells in multiple murine models,29-31 and most clinical trials of adoptive T-cell therapy include chemotherapy before T-cell infusions.12,14-16,20,21 In mice, one way that recipient lymphocyte depletion enhances the antitumor activity of adoptively transferred T cells is by increasing serum levels of cytokines such as interleukin-15 (IL-15).30 IL-15 is a glycoprotein in the 4-α-helix bundle family of cytokines.32-34 IL-15 is primarily produced by dendritic cells, monocytes, and macrophages,32,34 and it induces T-cell proliferation and enhances T-cell function.32-34
We previously treated B-cell malignancies with CAR-19 T cells preceded by treatment with fludarabine and high-dose cyclophosphamide.21 This high-dose chemotherapy regimen combined with an infusion of CAR-19 T cells resulted in many long-term remissions of B-cell malignancies, but it also caused significant toxicity.20,21 We hypothesized that administering a low-dose chemotherapy regimen before infusion of CAR-19 T cells would promote their antilymphoma activity and would cause less hematologic toxicity than high-dose chemotherapy. Use of low-dose chemotherapy would also allow a clear determination of the antilymphoma activity of CAR-19 T cells because the low-dose chemotherapy has limited direct antilymphoma activity.
PATIENTS AND METHODS
Clinical Trial and Patient Information
All enrolled patients gave informed consent. The protocol was approved by the Institutional Review Board of the National Cancer Institute. CD19 expression by malignancies was confirmed by either flow cytometry or immunohistochemistry.
Preparation of Anti-CD19 CAR T Cells and Ex Vivo Assays
The CAR used in this work was encoded by a gamma-retroviral vector and contained an anti-CD19 single-chain variable fragment derived from a murine monoclonal antibody, hinge and transmembrane regions from human CD28, the CD28 costimulatory domain, and the CD3ζ T-cell activation domain.35 Anti-CD19 CAR T cells were cultured for 6 to 10 days by adding the anti-CD3 monoclonal antibody OKT3 directly to whole (unsorted) peripheral blood mononuclear cells suspended in culture medium containing IL-2 and transducing the cells as described in the Data Supplement.20,36 CAR T-cell doses were administered as CD3+CAR+ cells per kilogram of body weight (Table 1). The percentage of CAR+ T cells was determined by flow cytometry and was used to calculate the number of cells to infuse. Flow cytometry, immunohistochemistry, cytokine assays, and quantitative polymerase chain reaction are described in the Data Supplement.11,20,35
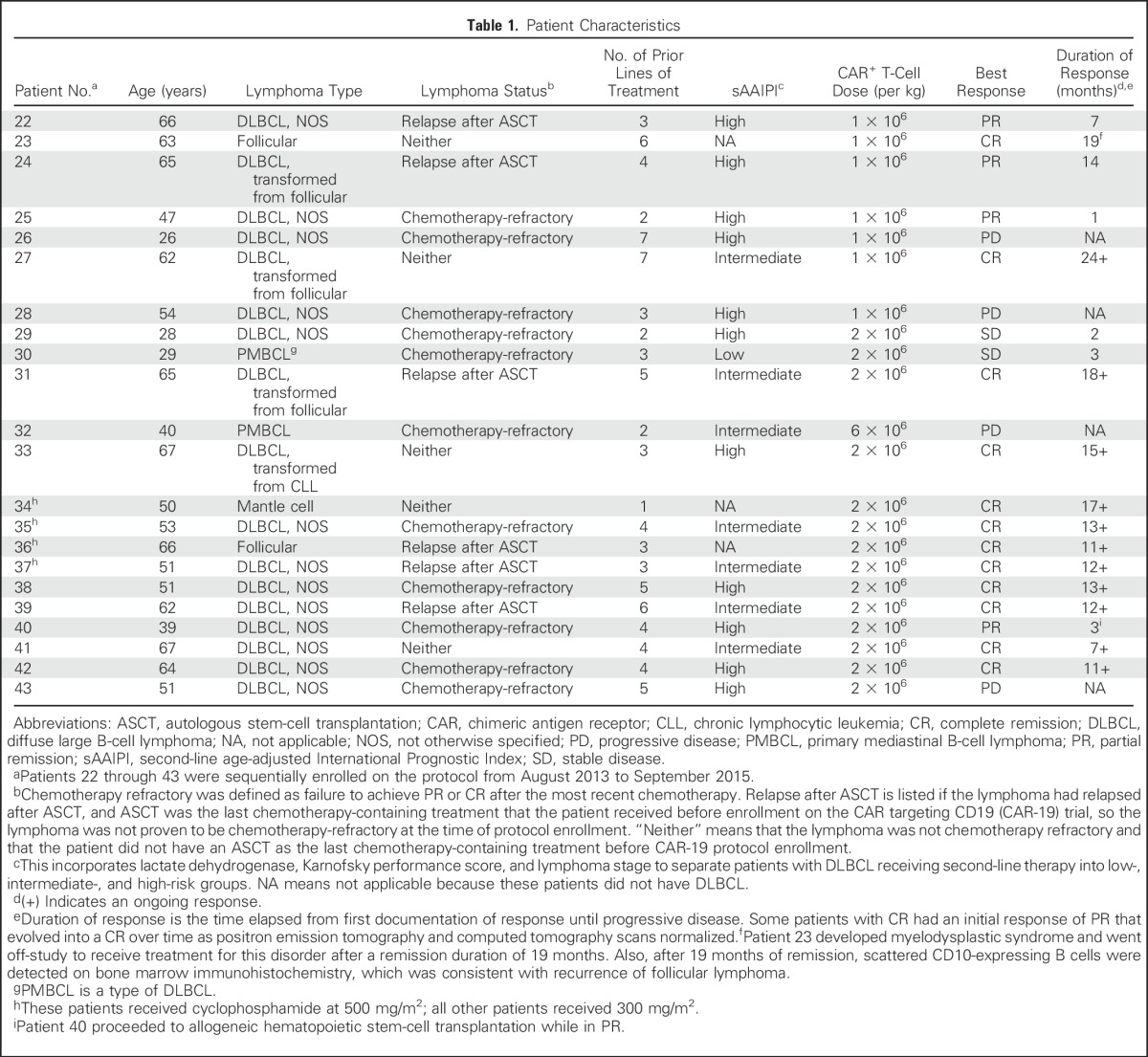
Table 1.
Patient Characteristics
Anti-CD19 CAR Treatment Plan
A fludarabine and cyclophosphamide chemotherapy regimen37 was administered before CAR-19 T-cell infusions to enhance the activity of adoptively transferred T cells.29-31 Both chemotherapy agents were given intravenously once per day for 3 days on the same days (Fig 1A). Treatment responses were defined according to standard international criteria.38
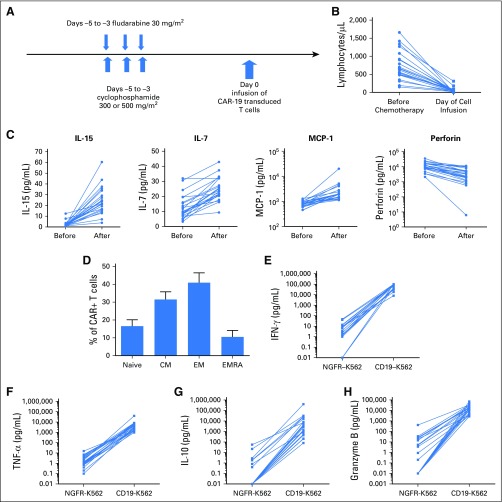
Fig 1.
Low-dose chemotherapy depletes lymphocytes and modulates serum proteins. (A) Schematic of the clinical protocol. Fludarabine and cyclophosphamide were administered on days –5 to –3. Fludarabine dose was 30 mg/m2 per day for all patients. Cyclophosphamide dose was 300 mg/m2 for 18 patients and 500 mg/m2 for four patients. A single dose of chimeric antigen receptor (CAR) targeting CD19 (CAR-19) T cells was administered on day 0. (B) The blood lymphocyte counts for each patient are shown before and after chemotherapy. Each patient’s lymphocyte counts are connected by a line (P < .001 for the paired comparison of before and after chemotherapy). The after time point was the day of CAR T-cell infusion. The median blood lymphocyte count just before the start of chemotherapy was 635/μL (range, 150 to 1,660/μL). The median blood lymphocyte count on the day of CAR T-cell infusion was 40/μL (range, 0 to 310/μL). The normal range for blood lymphocytes was 1,320 to 3,570/μL. (C) Interleukin-15 (IL-15), IL-7, and monocyte chemoattractant protein-1 (MCP-1) all increased after chemotherapy, and perforin decreased. The protein levels before and after chemotherapy for each patient are connected by a line. Chemotherapy samples for the after time point were all drawn on the day of CAR T-cell infusion. For all four proteins, P < .001 when paired before and after chemotherapy levels for each patient were compared. (D) CAR+ T cells from the time of infusion were stained for C-C chemokine receptor-7 (CCR7) and CD45RA to identify T cells with phenotypes of these subsets: naïve (CCR7+CD45RA+), central memory (CM; CCR7+CD45RA–), effector memory (EM; CCR7–CD45RA–), and effector memory RA subsets (EMRA; CCR7–CD45RA+). Plots were gated on CD3+CAR+ lymphocytes. Means and standard error of the mean are shown. (E-H) Anti-CD19 CAR T cells secreted a variety of proteins in an antigen-specific manner. Graphs show the levels of proteins in culture supernatants after CAR-19 T cells from the time of infusion were cultured overnight with either CD19–NGFR-K562 cells or CD19+CD19-K562 cells. Results for each patient are connected by a line. CD19-specific production of interferon-γ (IFN-γ), granzyme B, IL-10, and tumor necrosis factor alpha (TNF-α) occurred.
RESULTS
Patient Characteristics
Nineteen of the 22 treated patients had one of the various types of DLBCL, two patients had follicular lymphoma, and one patient had mantle cell lymphoma (Table 1). Eleven of 19 patients with DLBCL had chemotherapy-refractory lymphoma. Five other patients with DLBCL had lymphoma that had relapsed 10 months or less after ASCT as their last treatment prior to protocol enrollment. Eleven patients with DLBCL were high risk by second-line, age-adjusted International Prognostic Index.39 The median number of unique lymphoma therapies received before protocol enrollment was four (range, one to seven). Therapies received before protocol enrollment and lymphoma chromosome rearrangements are provided in the Data Supplement.
Chemotherapy Regimen Depleted Lymphocytes and Modulated Multiple Serum Proteins
The chemotherapy depleted recipient lymphocytes (Fig 1B; Data Supplement). We compared levels of 41 serum proteins before and after the low-dose chemotherapy regimen (Data Supplement). Serum IL-15 levels increased in all 22 patients after the chemotherapy regimen (Figure 1C). Serum perforin decreased after chemotherapy administration.
Infused CAR-19 T-Cell Characteristics
A median of 97.6% (range, 90.6% to 99.5%) of the infused cells were CD3+; a median of 74.4% (range, 39.4% to 93.6%) of the infused CD3+ cells expressed CAR-19. A median of 43.4% of CD3+CAR+ cells expressed CD4 (range, 1.9% to 82.1%), and 54% of CD3+CAR+ cells expressed CD8 (range, 13.4% to 92.1%). C-C chemokine receptor-7 (CCR7) and CD45RA were used to divide T cells into four different subsets (Fig 1D). Naïve and central memory T cells have greater proliferative capacity than effector memory and effector memory-RA T cells.40 Infused CAR-19 T cells included T cells with the phenotypes of all four of these T-cell subsets, including a substantial fraction of cells with the phenotype of central memory T cells.40 The CAR T cells secreted a variety of proteins in an antigen-specific manner (Fig 1E-H; Data Supplement).
CAR-19 T Cells Induced Remissions of Lymphoma
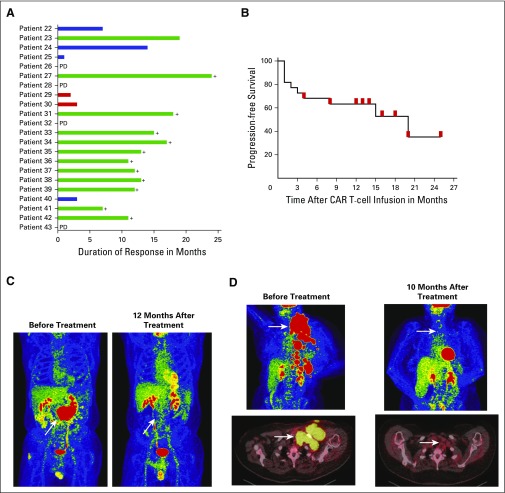
For all 22 patients treated, there was a 73% remission rate with 55% complete remissions (CRs) and 18% partial remissions (PRs). Among patients with DLBCL, the overall remission rate was 68% with 47% CRs and 21% PRs. The duration of responses currently ranges from 7 months to 24 months (Fig 2A). Twelve of 22 patients achieved CRs, 11 of 12 CRs are ongoing, and the current median duration of all CRs is 12.5 months (Table 1). No patient with an ongoing remission received any further antilymphoma therapy after CAR T-cell infusion. In contrast to CRs, no PRs or outcomes of stable disease (SD) are ongoing. Patient 40 underwent allogeneic hematopoietic stem-cell transplantation after achieving a PR (Table 1). The 12-month progression-free survival of all patients was 63.3% (Fig 2B), and overall survival is provided in the Data Supplement. Figure 2C-D shows two examples of positron emission tomography/computed tomography scans from patients who achieved CR of chemotherapy-refractory DLBCL.
Fig 2.
Chimeric antigen receptor (CAR) targeting CD19 (CAR-19) T cells eradicated large masses of chemotherapy-refractory lymphoma. (A) Graphical representation of the types of antilymphoma responses and the durations of responses. (B) Progression-free survival starting at the day of cell infusion and ending at the day of disease progression is shown for all patients. Red marks indicate censored patients with ongoing complete remissions (CRs) at the time of last follow-up with one exception: the red mark at 4 months after CAR T-cell infusion indicates the time point when patient 40 underwent allogeneic stem-cell transplantation while in partial remission. Two patients were censored at the 13-month time point and three patients were censored at the 14-month time point, but there is only one red mark on the graph for each of these time points. (C) Patient 35 had received four types of lymphoma therapy, and his diffuse large B-cell lymphoma was chemotherapy refractory at the time of protocol enrollment. After CAR-19 T-cell infusion, his lymphoma entered an ongoing CR. (D) At the time of protocol enrollment, patient 38 had received five types of prior lymphoma therapy, and her diffuse large B-cell lymphoma was refractory to chemotherapy. After CAR-19 T-cell infusion, the lymphoma entered an ongoing CR. For (C) and (D), the white arrows indicate sites of lymphoma. Residual red-colored areas in the after-treatment images are normal findings in the brain, heart, kidneys, and bladder. PD, progressive disease.
Toxicities of CAR-19 T Cells Preceded by Low-Dose Chemotherapy
All toxicities greater than grade 1 are listed in Table 2. The most prominent toxicities in this trial were neurologic; 55% of patients had grade 3 or 4 neurologic toxicities. In contrast, only four (18%) of 22 patients had grade 3 or 4 hypotension; three patients required brief courses of vasopressor drugs to treat it. One patient required mechanical ventilation for severe dyspnea; another patient required mechanical ventilation for airway control during severe neurologic toxicity. Common grade 3 and 4 neurologic toxicities included dysphasia, confusion, and tremor. All acute toxicities resolved completely, and no patients died as a result of toxicity. Three patients received specific immunosuppressive drugs: patient 32 received the corticosteroid dexamethasone for severe neurologic toxicity, and patients 36 and 40 received the IL-6 receptor antagonist tocilizumab. Aside from those three patients, all other patients received only supportive treatment without any immunosuppressive drugs, so most patients on this trial had acute CAR T-cell toxicities that resolved spontaneously within a limited period of time.
Table 2.
All Grade 2, 3, and 4 Adverse Events in the First Month After Anti-CD19 CAR T-Cell Infusion
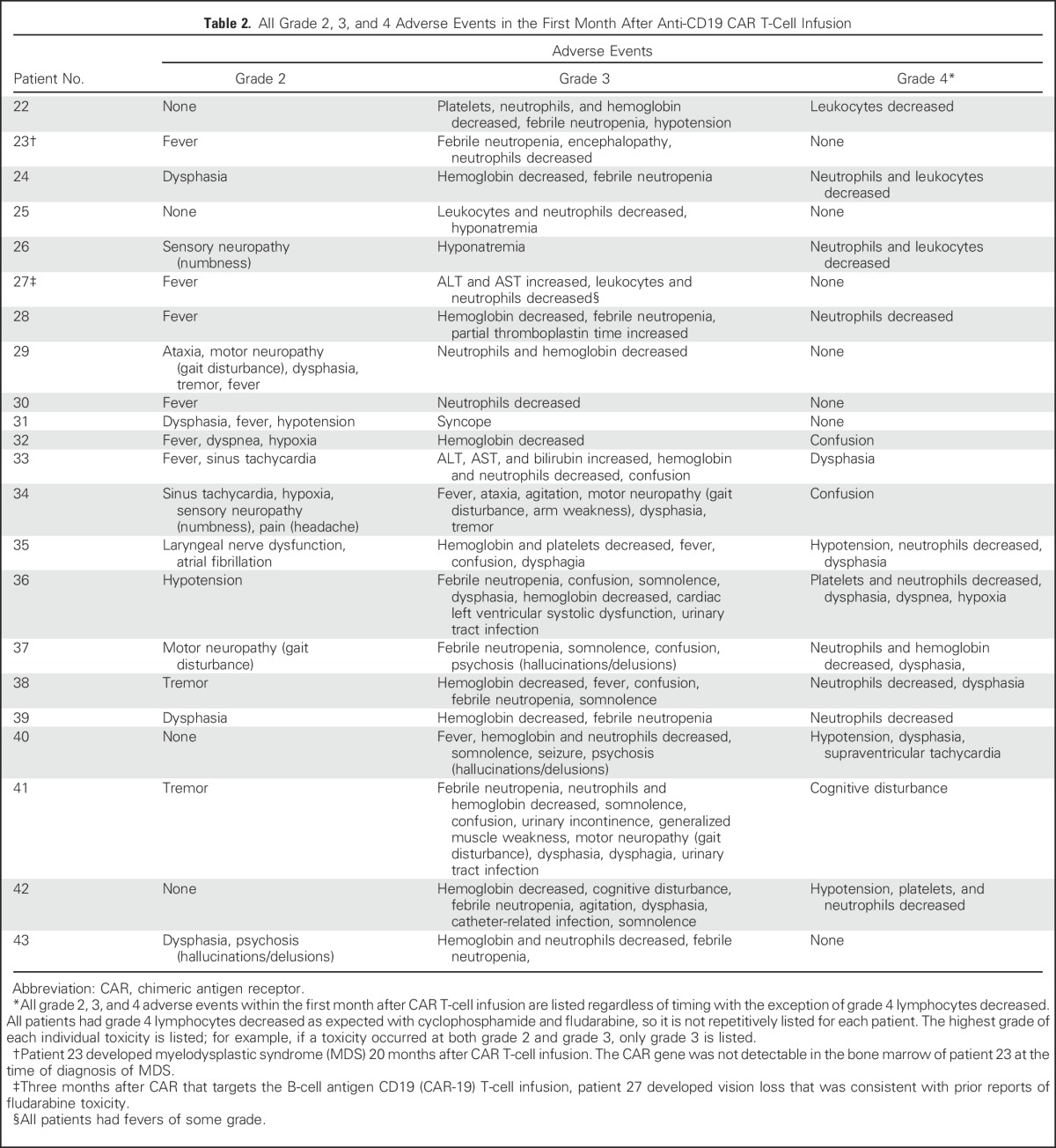
Two patients had notable delayed adverse events. Patient 23 developed myelodysplastic syndrome (MDS) 20 months after CAR T-cell infusion, but the CAR-19 gene was not detectable in the patient’s bone marrow at the time of MDS diagnosis. MDS is common in lymphoma patients with histories of chemotherapy and ASCT, so the extensive treatment received by patient 23 before protocol enrollment likely contributed to his MDS.41 Patient 27 developed vision loss 3 months after CAR T-cell infusion. Although the etiology of this vision loss is not definitively known, the clinical course and electroretinography were consistent with fludarabine toxicity.42
Depletion of normal B cells is an expected toxicity of anti-CD19 CAR T cells.8,12,19,20 Because of extensive prior treatment, B-cell counts were already low in most patients at the time of protocol enrollment. The median blood B-cell count at enrollment was 1/μL (range, 0 to 123/μL). Only patient 23 had a normal pretreatment B-cell count. Six patients in CR have recovered normal blood B-cell counts, and five remain in CR (Data Supplement). This demonstrates that patients can remain in CR after recovery of normal B-cell counts.
Higher Numbers of Blood CAR+ Cells Were Present in Patients With Lymphoma Remission
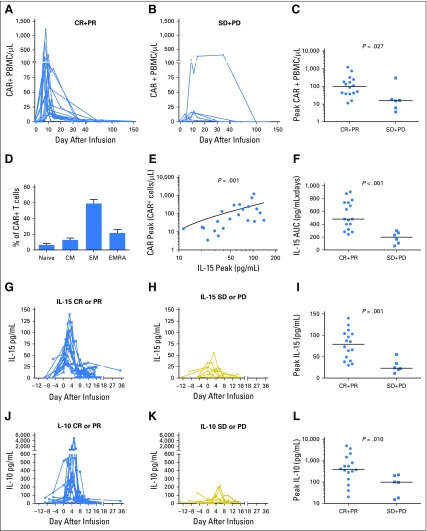
The number of blood CAR+ cells peaked at a median of 8.5 days (range, 6 to 35 days) after infusion (Fig 3A-B). CAR+ cell numbers dropped rapidly after peaking. Blood CAR+ cell numbers decreased to 0 or 1/μL by 3 months after infusion in all patients. Peak blood CAR+ cell numbers were higher in patients who achieved lymphoma responses of CR or PR compared with those who achieved SD or progressive disease (Fig 3A-C). There was no statistically significant difference in the peak number of blood CAR+ T cells in patients who received infusions of CAR-19 T cells at 1 × 106/kg versus 2 × 106/kg. CAR+ T cells collected from the blood of patients after infusion primarily had phenotypes of effector memory or effector memory-RA T cells (Fig 3D); in comparison, CAR-19 T cells at the time of infusion included more T cells with phenotypes of naïve and central memory T cells (Fig 1D). There was an association between the percentage of central memory T cells among the infused CAR T cells and peak blood CAR T-cell levels (P < .001; Spearman r = 0.7; Data Supplement).
Fig 3.
Clinical remissions of lymphoma were associated with high peak blood levels of chimeric antigen receptor–positive (CAR+) cells and interleukin-15 (IL-15). The absolute number of CAR+ peripheral blood mononuclear cells (PBMCs) in patients who achieved clinical antilymphoma responses of either (A) complete remission (CR) or partial remission (PR) or (B) stable disease (SD) or progressive disease (PD) were quantified by polymerase chain reaction. (C) Patients who achieved remission (CR + PR) had higher levels of CAR+ PBMCs than patients who did not (SD + PD; P = .027). Horizontal lines represent the medians in panels C, F, I, and L. (D) CAR+ T cells from patient blood samples collected between 4 and 15 days after CAR T-cell infusion were stained for C-C chemokine receptor-7 (CCR7) and CD45RA. The graph shows the fraction of T cells with phenotypes of four different T-cell subsets: naïve (CCR7+CD45RA+), central memory (CM; CCR7+CD45RA–), effector memory (EM; CCR7–CD45RA–), and effector memory-RA (EMRA; CCR7–CD45RA+) subsets. Plots were gated on CD3+CAR+ cells. For each T-cell subset, mean and standard error of the mean are shown. (E) The peak level of blood CAR+ cells correlated with the peak level of IL-15 (Spearman correlation r = 0.7; P = .001). (F) Patients who achieved remission of lymphoma (CR + PR) had higher serum IL-15 area-under-the-curve (AUC) levels from day –5 to day 14 than patients who did not (SD + PD; P < .001). Serum levels of IL-15 for (G) all patients who achieved remission (CR + PR) or (H) patients who did not (SD + PD) are shown. Day 0 is the day of CAR T-cell infusion. (I) Patients who achieved remission (CR + PR) after CAR T-cell infusion had higher peak serum IL-15 levels than patients who did not (P = .001). The serum levels of IL-10 for (J) all patients who achieved remission (CR + PR) or (K) patients who did not (SD + PD) are shown. (L) Patients who achieved remission (CR + PR) had higher peak serum levels of IL-10 than patients who did not achieve remission (SD + PD; P = .010). A total of 41 serum proteins were assessed (panels I and L), and the results of the two proteins with the most impressive differences are shown. Results for all 41 serum proteins are provided in the Data Supplement. Statistical correction for multiple comparisons was not performed.
Peak Blood CAR+ Cell Numbers and Lymphoma Remission Were Associated With High Serum IL-15 Levels
The low-dose chemotherapy conditioning regimen administered before CAR-19 T-cell infusions caused an increase in serum IL-15 (Fig 1C). Because IL-15 is known to induce T-cell proliferation,32-34 we reasoned that IL-15 might be associated with peak blood levels of CAR-19 T cells after infusion. We measured serum IL-15 levels at several time points before and after CAR-19 T-cell infusion, and the median day of peak IL-15 levels was day 2 after CAR T-cell infusion. We found a correlation between peak serum IL-15 levels and peak blood CAR+ cell numbers (Fig 3E).
We assessed serum levels of 41 different proteins in patients who achieved a remission (CR or PR) and patients who did not achieve a remission (SD or progressive disease; Data Supplement). IL-15 was the cytokine associated most closely with remissions. Patients who achieved a remission of lymphoma had higher IL-15 area-under-the-curve levels from day –5 to day 14 and higher peak serum IL-15 levels than patients who did not achieve a remission (Fig 3F-I). On the day of CAR T-cell infusion, the median serum IL-15 level of patients who achieved a remission was higher than that of patients who did not achieve a remission (30 v 16 pg/mL; P = .010; Data Supplement). This suggests that the environment that CAR T cells enter upon infusion is a determinant of treatment outcomes.
IL-10 is a cytokine that is known to have immunosuppressive properties43; however, IL-10 can also promote T-cell antitumor activity.44,45 Peak serum IL-10 levels were significantly higher among patients who achieved a remission of lymphoma compared with those who did not achieve a remission (Fig 3J-L). Aside from IL-15, IL-10 was the cytokine with the clearest difference in peak serum levels when comparing patients who did or did not achieve a remission.
Grade 3 and 4 Neurologic Toxicities Were Associated With High Blood CAR+ Cell Levels, and CAR-19 T Cells Were Detected in Cerebrospinal Fluid
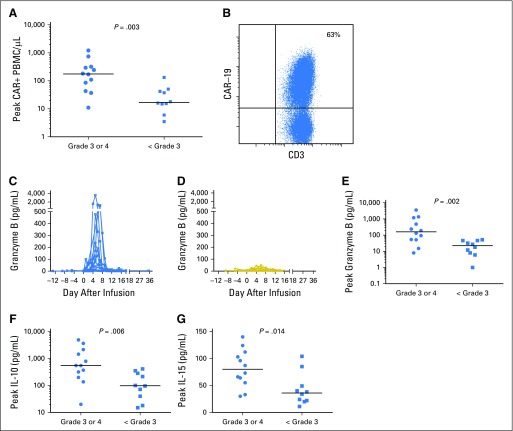
Patients who experienced grade 3 or 4 neurologic toxicity had higher levels of blood CAR+ cells compared with patients who had only < grade 3 neurologic toxicities (Fig 4A). We performed lumbar punctures to collect cerebrospinal fluid (CSF) from 11 patients with neurologic toxicity. The median number of white blood cells in the CSF of these patients was 22/μL (range, 1 to 215/μL). Flow cytometry was performed on the CSF of nine of the 11 patients who underwent CSF collection, and CAR-19 T cells were detected in the CSF of all nine patients (Fig 4B; Data Supplement).
Fig 4.
Neurologic toxicity was associated with high peak blood levels of chimeric antigen receptor–positive (CAR+) cells and increased levels of certain serum proteins. (A) The number of blood CAR+ cells in patients with grade 3 or 4 neurologic toxicities was higher than that in patients with only < grade 3 neurologic toxicities (P = .003). (B) Flow cytometry revealed CAR targeting CD19 (CAR-19) T cells in the cerebrospinal fluid (CSF) in all assessed patients with neurologic toxicity. The flow cytometry result of CSF from patient 31 from 9 days after CAR-19 T-cell infusion is shown as a representative example. The plot is gated on CSF mononuclear cells. Granzyme B serum levels of (C) patients with grade 3 or 4 neurologic toxicities and (D) those with only < grade 3 neurologic toxicities are shown. Patients with grade 3 or 4 neurologic toxicities had higher peak serum levels of (E) granzyme B (P = .002), (F) interleukin-10 (IL-10; P = .006), and (G) IL-15 (P = 0.014) than patients with only < grade 3 neurologic toxicities. A total of 41 serum proteins were assessed (panels E, F, and G), and the results of the three proteins with the most impressive differences are shown. Results for all 41 serum proteins are provided in the Data Supplement. Statistical correction for multiple comparisons was not performed.
Serum Proteins Are Associated With Neurologic Toxicities
We assessed serum levels of 41 proteins in patients with grade 3 or 4 neurologic toxicities and patients with only < grade 3 neurologic toxicities (Data Supplement). Peak serum granzyme B levels were higher in patients with grade 3 or 4 neurologic toxicities compared those who had only < grade 3 neurologic toxicities (Fig 4C-E). In addition, peak levels of serum IL-10 and IL-15 were higher in patients with grade 3 or 4 neurologic toxicities compared with those who had only < grade 3 neurologic toxicities (Fig 4F-G).
DISCUSSION
An infusion of CAR-19 T cells preceded by a low-dose conditioning chemotherapy regimen of cyclophosphamide and fludarabine induced remissions of advanced-stage lymphoma. The low-dose chemotherapy regimen used in this study could not directly induce the remissions of lymphoma reported here because of the documented resistance of these lymphomas to antilymphoma chemotherapy regimens (Data Supplement). Importantly, our CAR T-cell production process was simple and robust. No patient failed to receive treatment because of cell production issues.
We recently treated patients who had had an allogeneic hematopoietic stem-cell transplantation with human leukocyte antigen–matched allogeneic T cells expressing the same CAR-19 used with autologous T cells in this study.36,46 We did not administer chemotherapy before infusions of the allogeneic CAR-19 T cells. We administered allogeneic CAR+ T cells at up to 8.2 × 106/kg with acceptable levels of toxicity.46 In stark contrast to this report of patients treated with autologous CAR-19 T cells at 1 to 2 × 106/kg, neurologic toxicity in the trial of allogeneic anti-CD19 CAR T cells without chemotherapy was rare and mild.36,46 These results suggest that lowering or eliminating the conditioning chemotherapy administered before CAR T-cell infusions might lessen the severity of toxicity; however, lowering chemotherapy intensity might also lead to lower remission rates.46
We compared hematologic toxicities in patients who received CAR-19 T cells after our current low-dose chemotherapy regimen and patients who received our previously reported high-dose chemotherapy regimen, which included a total dose of cyclophosphamide of 60 to 120 mg/kg and a total dose of fludarabine of 125 mg/m2.21 Two of 22 patients treated with the new low-dose chemotherapy required platelet transfusions; 10 of 15 patients receiving high-dose chemotherapy required platelet transfusions (P < .001). The median length of time that patients had severe neutropenia with an absolute neutrophil count of less than 500/μL was 0 days (range, 0 to 6 days) with the low-dose chemotherapy versus 5 days (range, 0 to 16 days) with the previously used high-dose chemotherapy (P < .001). In this study, we increased the daily cyclophosphamide dose from 300 mg/m2 to 500 mg/m2 in four patients in an attempt to increase the CR rate. Because all four of these patients experienced substantial toxicity (Table 2), we reduced the cyclophosphamide dose back to 300 mg/m2; however, because we had a small number of patients treated with the 500 mg/m2 dose of cyclophosphamide, we cannot draw firm conclusions.
Our data indicate that IL-15 is associated with the efficacy of CAR-19 T cells. In agreement with prior work,47 IL-15 was one of the serum proteins most prominently increased after the low-dose chemotherapy regimen. Serum IL-15 levels were strongly associated with peak levels of CAR+ cells, and blood IL-15 levels were higher in patients who achieved a remission of lymphoma than in patients who did not achieve a remission. IL-15 causes T-cell (including CAR T-cell) proliferation and activation,32-34,48-50 so the mechanism of IL-15 improving lymphoma treatment outcomes by increasing activated CAR-19 T-cell levels is quite plausible. Other investigators have performed preclinical experiments that demonstrated the ability of IL-15 to enhance adoptive T-cell therapies in mice.9,48,50-53 To the best of our knowledge, our work is the first to show an association in humans between serum IL-15 levels and remission of lymphoma after adoptive T-cell therapy. Clinical trials that evaluate the ability of IL-15 agonists to enhance CAR T-cell antimalignancy activity should be considered to formally assess the role of IL-15 in T-cell therapy.32,33 It must be kept in mind that IL-15 might worsen toxicity in clinical trials combining CAR T cells and IL-15.
We undertook experiments to better understand the important problem of neurologic toxicity with CAR-19 T-cell therapies.12,14-16,21 Neurologic toxicity was closely associated with high peak blood CAR+ cell levels, and by using flow cytometry, we found CAR T cells in the CSF of every patient with neurologic toxicity. Interestingly, peak serum granzyme B levels were closely associated with neurologic toxicity (Fig 4C-E). High granzyme B levels were associated with grade 3 or 4 neurologic toxicity but not with a remission of lymphoma (Data Supplement). Granzyme B has been previously linked to neuronal toxicity.54 Notably, high serum IL-10 and IL-15 levels were associated with both remission of lymphoma and neurologic toxicity. Other investigators have previously shown an association of interferon-γ and IL-6 with neurologic toxicity.16 We also found that serum levels of interferon-γ were higher in patients with grade 3 or 4 neurologic toxicities than in those with only < grade 3 neurologic toxicities (P = .04; Data Supplement), but this difference was not as consistent as the differences for granzyme B, IL-10, and IL-15. We observed a trend that was not statistically significant for higher peak serum IL-6 levels in patients with grade 3 or 4 neurologic toxicity (Data Supplement). Development of new approaches to prevent or treat neurologic toxicity caused by CAR T cells is an important need.
Patients with DLBCL that is refractory to chemotherapy or relapsed less than 12 months after ASCT generally survive less than 9 months.23,25-27 Our results from treating advanced-stage lymphoma with CAR-19 T cells compare favorably to established treatment regimens.17,18,23-25 Induction of CRs in patients with large masses of chemotherapy-refractory lymphoma is especially notable (Fig 2). Our results should encourage further research aimed at developing CAR T-cell therapies with less toxicity and higher remission rates.
ACKNOWLEDGMENT
We thank the staff of the Surgery Branch of the National Cancer Institute (NCI) cell production facility, Surgery Branch NCI Immunotherapy Fellows, and the staffs of the three Northwest Nursing Units and the Intensive Care Unit of the National Institutes of Health Clinical Center, and the National Gene Vector Biorepository for help monitoring replication-competent retroviruses.
Footnotes
Supported by the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health, and by NCI Cooperative Research and Development Agreements with Kite Pharma for development of anti-CD19 CAR T-cell therapies (J.N.K. and S.A.R.).
Clinical trial information: NCT00924326.
AUTHOR CONTRIBUTIONS
Conception and design: James N. Kochenderfer
Collection and assembly of data: James N. Kochenderfer, Robert P.T. Somerville, Tangying Lu, Victoria Shi, John Rossi, Stephanie L. Goff, Christopher A. Klebanoff, Udai S. Kammula, Marika Sherman, Arianne Perez, Constance M. Yuan, Tatyana Feldman, Jonathan W. Friedberg, Mark J. Roschewski, Steven A. Feldman, Lori McIntyre, Mary Ann Toomey, Steven A. Rosenberg
Data analysis and interpretation: James N. Kochenderfer, Tangying Lu, Victoria Shi, Adrian Bot, John Rossi, Allen Xue, Stephanie L. Goff, James C. Yang, Richard M. Sherry, Christopher A. Klebanoff, Arianne Perez, Steven A. Rosenberg
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
James N. Kochenderfer
Research Funding: Bluebird Bio, Kite Pharma
Patents, Royalties, Other Intellectual Property: Three patents pending on chimeric antigen receptor T-cell technology
Travel, Accommodations, Expenses: Kite Pharma
Robert P.T. Somerville
No relationship to disclose
Tanying Lu
No relationship to disclose
Victoria Shi
No relationship to disclose
Adrian Bot
Employment: Kite Pharma
Leadership: Kite Pharma
Stock or Other Ownership: Kite Pharma
John Rossi
Employment: Kite Pharma
Stock or Other Ownership: Kite Pharma, Amgen
Allen Xue
Employment: Kite Pharma
Stock or Other Ownership: Kite Pharma
Stephanie L. Goff
No relationship to disclose
James C. Yang
No relationship to disclose
Richard M. Sherry
No relationship to disclose
Christopher A. Klebanoff
Stock or Other Ownership: Kite Pharma
Consulting or Advisory Role: Cell Design Labs
Udai S. Kammula
No relationship to disclose
Marika Sherman
Employment: Kite Pharma, Amgen
Stock or Other Ownership: Kite Pharma, Amgen
Arianne Perez
Employment: Kite Pharma
Stock or Other Ownership: Kite Pharma
Patents, Royalties, Other Intellectual Property: Two patents in the cell therapy field while at Kite Pharma
Constance M. Yuan
No relationship to disclose
Tatyana Feldman
Honoraria: Celgene, Seattle Genetics, AbbVie, Pharmacyclics
Consulting or Advisory Role: Celgene
Speakers’ Bureau: Celgene, Seattle Genetics, AbbVie, Pharmacyclics
Research Funding: Seattle Genetics (Inst), Millennium Pharmaceuticals (Inst), Eisai (Inst), Roche (Inst)
Travel, Accommodations, Expenses: Celgene, Seattle Genetics, AbbVie
Jonathan W. Friedberg
Consulting or Advisory Role: Bayer
Research Funding: Seattle Genetics (Inst), Kite Pharma (Inst), Dynavax Technologies (Inst)
Mark J. Roschewski
No relationship to disclose
Steven A. Feldman
No relationship to disclose
Lori McIntyre
No relationship to disclose
Mary Ann Toomey
No relationship to disclose
Steven A. Rosenberg
Research Funding: Kite Pharma
Patents, Royalties, Other Intellectual Property: Multiple patents in cell and gene therapy
REFERENCES
- 1.Sadelain M: CAR therapy: The CD19 paradigm. J Clin Invest 125:3392-3400, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dotti G, Gottschalk S, Savoldo B, et al. : Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev 257:107-126, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin L, Cruz CR, Bollard CM: Adoptive T-cell therapies for refractory/relapsed leukemia and lymphoma: Current strategies and recent advances. Ther Adv Hematol 6:295-307, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kebriaei P, Singh H, Huls MH, et al. : Phase I trials using Sleeping Beauty to generate CD19-specific CAR T cells. J Clin Invest 126:3363-3376, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochenderfer JN, Rosenberg SA: Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol 10:267-276, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cruz CR, Micklethwaite KP, Savoldo B, et al. : Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: A phase 1 study. Blood 122:2965-2973, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savoldo B, Ramos CA, Liu E, et al. : CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 121:1822-1826, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter DL, Levine BL, Kalos M, et al. : Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365:725-733, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brentjens RJ, Latouche JB, Santos E, et al. : Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med 9:279-286, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Cooper LJ, Topp MS, Serrano LM, et al. : T-cell clones can be rendered specific for CD19: Toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood 101:1637-1644, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Carpenter RO, Evbuomwan MO, Pittaluga S, et al. : B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin Cancer Res 19:2048-2060, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maude SL, Frey N, Shaw PA, et al. : Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:1507-1517, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. : T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 385:517-528, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brentjens RJ, Davila ML, Riviere I, et al. : CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5:177ra38, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davila ML, Riviere I, Wang X, et al. : Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6:224ra25, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turtle CJ, Hanafi LA, Berger C, et al. : CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 126:2123-2138, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedberg JW: Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program 2011:498-505, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Martelli M, Ferreri AJM, Agostinelli C, et al. : Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol 87:146-171, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Kochenderfer JN, Wilson WH, Janik JE, et al. : Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116:4099-4102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochenderfer JN, Dudley ME, Feldman SA, et al. : B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119:2709-2720, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kochenderfer JN, Dudley ME, Kassim SH, et al. : Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 33:540-549, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turtle CJ, Hanafi LA, Berger C, et al. : Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 8:355ra116, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elstrom RL, Martin P, Ostrow K, et al. : Response to second-line therapy defines the potential for cure in patients with recurrent diffuse large B-cell lymphoma: Implications for the development of novel therapeutic strategies. Clin Lymphoma Myeloma Leuk 10:192-196, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Moore S, Kayani I, Peggs K, et al. : Mini-BEAM is effective as a bridge to transplantation in patients with refractory or relapsed Hodgkin lymphoma who have failed to respond to previous lines of salvage chemotherapy but not in patients with salvage-refractory DLBCL. Br J Haematol 157:543-552, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Crump M, Neelapu SS, Farooq U, et al: Outcomes in refractory aggressive diffuse large b-cell lymphoma (DLBCL): Results from the international SCHOLAR-1 study. J Clin Oncol 34, 2016 (suppl; abstr 7516) [Google Scholar]
- 26.Nagle SJ, Woo K, Schuster SJ, et al. : Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol 88:890-894, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Vose JM, Bierman PJ, Anderson JR, et al. : Progressive disease after high-dose therapy and autologous transplantation for lymphoid malignancy: Clinical course and patient follow-up. Blood 80:2142-2148, 1992 [PubMed] [Google Scholar]
- 28.Brudno JN, Kochenderfer JN: Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 127:3321-3330, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.North RJ: Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med 155:1063-1074, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. : Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med 202:907-912, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kochenderfer JN, Yu Z, Frasheri D, et al. : Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 116:3875-3886, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waldmann TA: Interleukin-15 in the treatment of cancer. Expert Rev Clin Immunol 10:1689-1701, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilipow K, Roberto A, Roederer M, et al. : IL15 and T-cell stemness in T-cell-based cancer immunotherapy. Cancer Res 75:5187-5193, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishra A, Sullivan L, Caligiuri MA: Molecular pathways: Interleukin-15 signaling in health and in cancer. Clin Cancer Res 20:2044-2050, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kochenderfer JN, Feldman SA, Zhao Y, et al. : Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J Immunother 32:689-702, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kochenderfer JN, Dudley ME, Carpenter RO, et al. : Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation. Blood 122:4129-4139, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Brien SM, Kantarjian HM, Cortes J, et al. : Results of the fludarabine and cyclophosphamide combination regimen in chronic lymphocytic leukemia. J Clin Oncol 19:1414-1420, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Cheson BD, Pfistner B, Juweid ME, et al. : Revised response criteria for malignant lymphoma. J Clin Oncol 25:579-586, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Hamlin PA, Zelenetz AD, Kewalramani T, et al. : Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood 102:1989-1996, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Sallusto F, Lenig D, Förster R, et al. : Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401:708-712, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Friedberg JW, Neuberg D, Stone RM, et al. : Outcome in patients with myelodysplastic syndrome after autologous bone marrow transplantation for non-Hodgkin’s lymphoma. J Clin Oncol 17:3128-3135, 1999 [DOI] [PubMed] [Google Scholar]
- 42.Bishop RJ, Ding X, Heller CK, III, et al. : Rapid vision loss associated with fludarabine administration. Retina 30:1272-1277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oft M: IL-10: Master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res 2:194-199, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Fujii S, Shimizu K, Shimizu T, et al. : Interleukin-10 promotes the maintenance of antitumor CD8(+) T-cell effector function in situ. Blood 98:2143-2151, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Emmerich J, Mumm JB, Chan IH, et al. : IL-10 directly activates and expands tumor-resident CD8(+) T cells without de novo infiltration from secondary lymphoid organs. Cancer Res 72:3570-3581, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Brudno JN, Somerville RP, Shi V, et al. : Allogeneic T cells that express an anti-CD19 chimeric antigen receptor induce remissions of B-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol 34:1112-1121, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudley ME, Yang JC, Sherry R, et al. : Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol 26:5233-5239, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y, Zhang M, Ramos CA, et al. : Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123:3750-3759, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoyos V, Savoldo B, Quintarelli C, et al: Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 24:1160-1170, 2010 [DOI] [PMC free article] [PubMed]
- 50.Klebanoff CA, Finkelstein SE, Surman DR, et al. : IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA 101:1969-1974, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoyos V, Savoldo B, Quintarelli C, et al. : Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 24:1160-1170, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roychowdhury S, May KF, Jr, Tzou KS, et al. : Failed adoptive immunotherapy with tumor-specific T cells: Reversal with low-dose interleukin 15 but not low-dose interleukin 2. Cancer Res 64:8062-8067, 2004 [DOI] [PubMed] [Google Scholar]
- 53.Miller JS, Rooney CM, Curtsinger J, et al. : Expansion and homing of adoptively transferred human natural killer cells in immunodeficient mice varies with product preparation and in vivo cytokine administration: Implications for clinical therapy. Biol Blood Marrow Transplant 20:1252-1257, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang T, Lee MH, Choi E, et al. : Granzyme B-induced neurotoxicity is mediated via activation of PAR-1 receptor and Kv1.3 channel. PLoS One 7:e43950, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]