Abstract
Purpose
The optimal regimen intensity before allogeneic hematopoietic cell transplantation (HCT) is unknown. We hypothesized that lower treatment-related mortality (TRM) with reduced-intensity conditioning (RIC) would result in improved overall survival (OS) compared with myeloablative conditioning (MAC). To test this hypothesis, we performed a phase III randomized trial comparing MAC with RIC in patients with acute myeloid leukemia or myelodysplastic syndromes.
Patients and Methods
Patients age 18 to 65 years with HCT comorbidity index ≤ 4 and < 5% marrow myeloblasts pre-HCT were randomly assigned to receive MAC (n = 135) or RIC (n = 137) followed by HCT from HLA-matched related or unrelated donors. The primary end point was OS 18 months post–random assignment based on an intent-to-treat analysis. Secondary end points included relapse-free survival (RFS) and TRM.
Results
Planned enrollment was 356 patients; accrual ceased at 272 because of high relapse incidence with RIC versus MAC (48.3%; 95% CI, 39.6% to 56.4% and 13.5%; 95% CI, 8.3% to 19.8%, respectively; P < .001). At 18 months, OS for patients in the RIC arm was 67.7% (95% CI, 59.1% to 74.9%) versus 77.5% (95% CI, 69.4% to 83.7%) for those in the MAC arm (difference, 9.8%; 95% CI, −0.8% to 20.3%; P = .07). TRM with RIC was 4.4% (95% CI, 1.8% to 8.9%) versus 15.8% (95% CI, 10.2% to 22.5%) with MAC (P = .002). RFS with RIC was 47.3% (95% CI, 38.7% to 55.4%) versus 67.8% (95% CI, 59.1% to 75%) with MAC (P < .01).
Conclusion
OS was higher with MAC, but this was not statistically significant. RIC resulted in lower TRM but higher relapse rates compared with MAC, with a statistically significant advantage in RFS with MAC. These data support the use of MAC as the standard of care for fit patients with acute myeloid leukemia or myelodysplastic syndromes.
INTRODUCTION
Hematopoietic cell transplantation (HCT) was originally used in acute myeloid leukemia (AML) to treat patients for marrow aplasia resulting from high-dose radiotherapy and chemotherapy, administered with curative intent. Subsequent studies demonstrated that donor-derived cells exerted a potent immunologic antileukemic effect, termed graft versus leukemia (GVL), which contributed to cure. Although HCT can cure AML, high-intensity preparative regimens (ie, myeloablative conditioning [MAC]) lead to considerable toxicity and treatment-related mortality (TRM). This prompted development of reduced-intensity conditioning (RIC) regimens with less toxicity. RIC relies more on GVL and less on cytotoxic effects for efficacy. RIC was originally developed for older and less fit patients considered intolerant of MAC. However, RIC use has increased dramatically, now accounting for 40% of allogeneic HCT in the United States, including many patients considered candidates for MAC.1
Several groups have described RIC strategies providing donor-cell engraftment and GVL responses.2-5 Retrospective studies comparing MAC with RIC in patients with AML or myelodysplastic syndromes (MDS) have suggested that RIC is associated with increased relapse but reduced TRM, resulting in similar overall survival (OS), despite patients receiving RIC being, on average, older and less fit.6-14 Patients receiving RIC for AML in first or second remission have 5-year survival rates of approximately 40%, considered favorable given the median patient age of 60 years.15 A prospective age-adapted strategy for adults with AML showed no difference in OS between MAC and RIC.16 Age is among many factors considered to select for RIC versus MAC, thus complicating the interpretation of nonrandomized studies. Consequently, the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) conducted a phase III randomized trial comparing RIC and MAC in patients with AML or MDS and < 5% marrow myeloblasts pre-HCT. We hypothesized that RIC would result in an improvement in OS given the lower TRM compared with MAC.
PATIENTS AND METHODS
Study Design
A phase III randomized trial comparing RIC with MAC in patients with AML or MDS was conducted through the BMT CTN. The protocol is available on the BMT CTN Web site (protocol 0901). The institutional review boards of participating centers approved the protocol; all patients signed informed consent. An independent data and safety monitoring board (DSMB) appointed by the National Heart, Lung, and Blood Institute oversaw the trial. Patients were randomly assigned at a one-to-one ratio to either MAC and RIC using permuted blocks of random sizes with stratification by center. Patients and physicians were informed of the random assignment. However, study investigators assigned to evaluate end points were blinded to each participant’s random assignment.
Patients
Participants had a WHO-defined diagnosis of AML or MDS,17 were undergoing a first HCT, and had < 5% marrow myeloblasts pre-HCT.18 Patients were 18 to 65 years of age and had an HLA-A, -B, and -DRB1 (6/6) –matched sibling donor or a ≥ 7/8 HLA-A, -B, -C, and -DRB1–matched unrelated donor and an HCT comorbidity index ≤ 4.19 In AML, a composite definition of high risk included unfavorable risk cytogenetics according to the Eastern Cooperative Oncology Group/SWOG cytogenetic classification schema,20 presence of FLT3 mutation regardless of cytogenetic abnormalities, or three or more complete remissions. High-risk MDS was defined as patients with intermediate-II or high-risk disease per the International Prognostic Scoring System.21
Conditioning Regimens and Immune Suppression
The RIC regimens were fludarabine (120 to 180 mg/m2) with busulfan (≤ 8 mg/kg orally or 6.4 mg/kg intravenously; Flu/Bu2) or melphalan (≤ 150 mg/m2; Flu/Mel). The MAC regimens were busulfan (16 mg/kg orally or 12.8 mg/kg intravenously) with cyclophosphamide (120 mg/kg) or fludarabine (120 to 180 mg/m2; Flu/Bu4) or cyclophosphamide (120 mg/kg) and total-body irradiation (12 to 14.2 Gy). Each center selected one preferred MAC and RIC regimen. Graft-versus-host disease (GVHD) prophylaxis included methotrexate 10 to 15 mg/m2 on day 1 and 5 to 10 mg/m2 on days 3, 6, and 11 administered with cyclosporine or tacrolimus or tacrolimus with sirolimus or cyclosporine with mycophenolate mofetil. Experimental GVHD therapies were allowed provided they included a calcineurin inhibitor and no post-HCT cyclophosphamide or T-cell depletion. Antithymocyte globulin was allowed; however, its use was declared pre–random assignment, and it was administered regardless of conditioning intensity. Stem-cell sources were bone marrow or peripheral-blood stem cells. Supportive care including growth factors and transfusion support was provided per institutional guidelines. In addition to regular clinic visits, patients had study-related visits weekly for the first 12 weeks and at 100 days, 6 months, and 12 months. Bone marrow aspirates or biopsies were performed at 100 days and 18 months and as clinically indicated. Toxicity assessments occurred at days 28, 56, and 100 and at 6, 12, and 18 months.
Outcomes
The primary end point was OS difference at 18 months post–random assignment, compared pointwise and assessed on an intent-to-treat basis. This end point accommodated potential higher early TRM with MAC versus potential higher relapse-related mortality with RIC, resulting in the possibility of nonproportional hazards.
Secondary objectives included relapse-free survival (RFS), TRM, absolute neutrophil count (ANC) and platelet recovery, kinetics of donor-cell engraftment, graft failure, GVHD, grade 3 to 4 toxicities, infections, and quality of life. Failure for RFS was considered a failure if a patient died, experienced relapse, or were treated for relapse.
Acute and chronic GVHD were graded according to the BMT CTN Manual of Procedures. The first day of acute GVHD of a certain grade was used to calculate cumulative incidence curves for that GVHD grade. Additional end points are described in the Appendix (online only).
Primary cause of death was adjudicated using previously described criteria.22 All reviewers who assigned cause of death were blinded to random assignment. When relapse occurred, it was considered the primary cause of death regardless of other events.
Statistical Analysis
The primary null hypothesis, no difference in OS 18 months post–random assignment, was assessed using the difference in Kaplan-Meier estimates. The calculated sample size was 356 (178 per treatment arm). Assuming 3% loss to follow-up 18 months post–random assignment, complete survival information would be available for 346 patients. Even with 3% loss to follow-up, the targeted sample size of 356 was sufficient to maintain type I error of 5% across all planned interim analyses while providing 80% power for a two-sided test to detect an increase in 18-month OS from 45% with MAC to 60% with RIC. This sample size provided sufficient power to detect a 15% increase in 18-month OS between treatment arms.
The DSMB conducted interim analyses for efficacy using an O’Brien-Fleming boundary at 19%, 45%, and 71% information for OS. Because of early closure of accrual, the remaining type I error was spent at final analysis; a nominal two-sided P value of < .049 was considered significant for the primary end point. Demographic and baseline characteristics were described for MAC and RIC using frequencies and medians and ranges. A preplanned secondary analysis included outcomes based on disease (AML v MDS). Kaplan-Meier estimates of 18-month RFS were compared between MAC and RIC. Cumulative incidences of TRM (with relapse as a competing risk), relapse (with death in first post-HCT remission as a competing risk), and acute and chronic GVHD (with death ± relapse as a competing risk) were compared between MAC and RIC using Gray’s test. Cumulative incidences of ANC recovery at 28 days and platelet recovery at 60 days were compared pointwise. Donor chimerism at 100 days and 18 months was categorized as full (> 95%), mixed (5% to 95% donor cells), graft rejection (< 5%), or death before assessment of donor chimerism; these were compared at each time point using the χ2 test. Cox proportional hazards models were used in planned secondary analyses for OS, RFS, TRM, relapse, and GVHD; nonproportional hazards were assessed, but there was minimal evidence of this, so hazard ratios (HRs) are provided as a summary measure, along with adjusted survival estimates using stratification on treatment. Treatment, disease risk, and donor type were forced into all models, and stepwise variable selection was used to identify variables with strong effects for inclusion. Prespecified subgroup analyses of OS were conducted using interaction tests in the Cox model and forest plots for the HRs. Analyses of OS, relapse, TRM, and RFS were conducted by intent to treat. All other end points were assessed in patients completing the assigned treatment (n = 265). Statistical analyses were performed with SAS software (version 9.3; SAS Institute, Cary, NC), and cumulative incidence analyses were performed with R software (version 2.15.1; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Enrollment
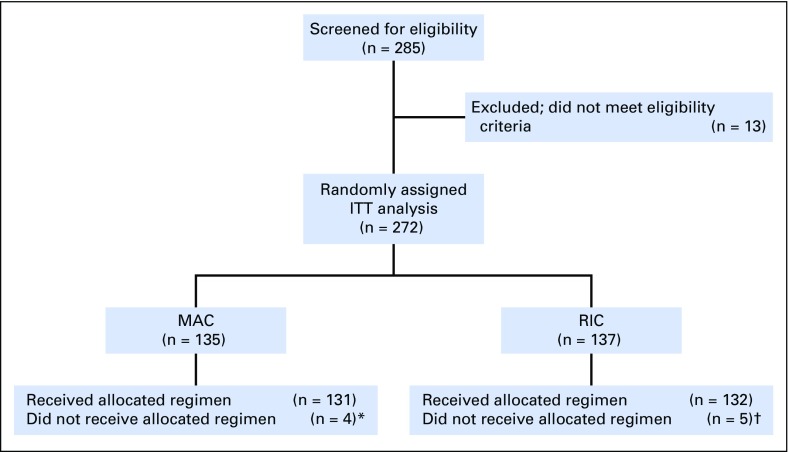
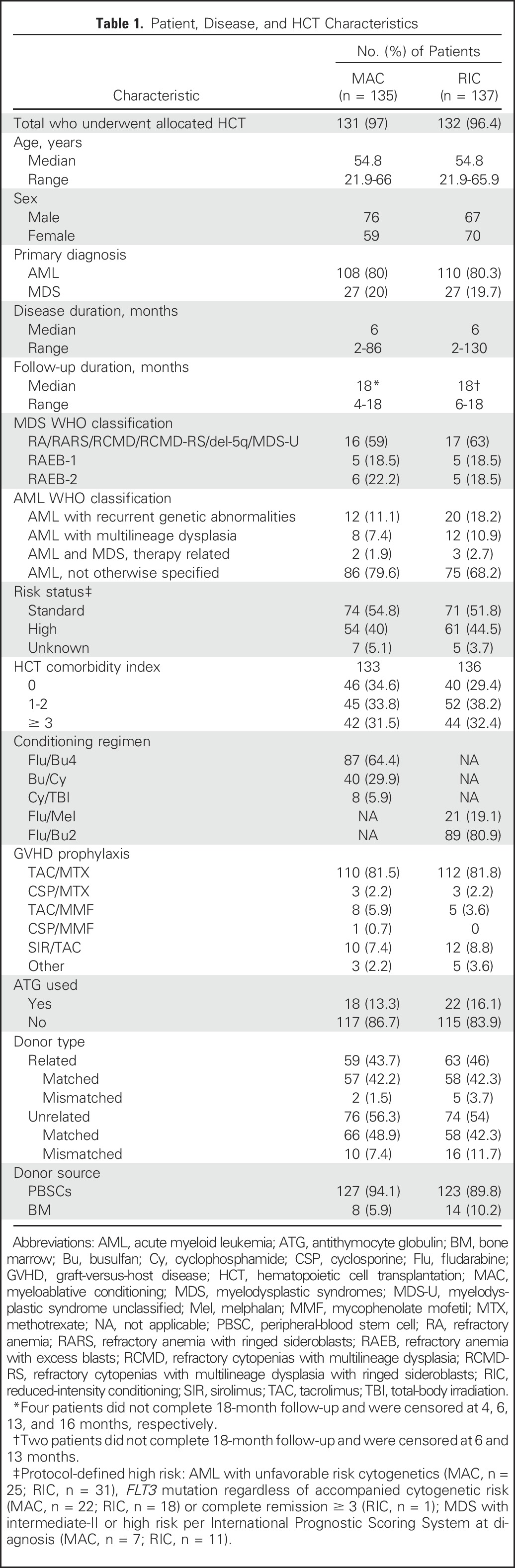
The study opened on June 2, 2011, and closed to accrual on April 10, 2014, as recommended by the DSMB because of a large difference in RFS favoring MAC, although there were no prespecified stopping boundaries for RFS. Overall, 285 patients were screened, and 272 patients were enrolled; 135 were randomly assigned to MAC and 137 to RIC (Fig 1). Enrollment occurred at 32 transplantation centers across the United States. The highest accruing center enrolled 40 patients; 13 centers enrolled less than five patients. Four patients randomly assigned to MAC and five patients randomly assigned to RIC did not receive the assigned HCT. All participants were included in the primary end point analysis irrespective of whether they received allocated HCT. Patient and transplantation characteristics are summarized in Table 1.
Fig 1.
Trial profile. ITT, intent to treat; MAC, myeloablative conditioning; RIC, reduced-intensity conditioning. (*) One patient received an RIC regimen, one patient did not proceed to hematopoietic cell transplantation (HCT) because of physician decision, one patient experienced relapse before conditioning, one patient withdrew consent after random assignment and did not undergo HCT; this latter patient was censored at the time of consent withdrawal. (†) One patient received a MAC regimen, and four patients experienced relapse before HCT and did not undergo HCT.
Table 1.
Patient, Disease, and HCT Characteristics
Engraftment
ANC did not decline below 0.5 × 109/L in five patients (MAC, n = 1; RIC, n = 4). The cumulative incidence of ANC recovery at day 28 was 98.5% with MAC and 97.75% with RIC. The median time to ANC > 0.5 × 109/L was 15 days with MAC and 19 days with RIC (P = .002). The platelet count did not decline below 20,000/µL in 66 patients (MAC, n = 13; RIC, n = 53). The cumulative incidence of platelet recovery (> 20,000/µL) at day 60 was 95.5% with MAC and 96.2% with RIC. The median time to platelet recovery was 16 days with MAC and 13 days with RIC (P = .065). There was one primary graft failure and one secondary graft failure among patients receiving MAC and three primary and four secondary graft failures among patients receiving RIC (P = .17). There were significant differences in complete donor engraftment and mixed donor chimerism at days 28 and 100, but not at day 540 (Appendix Table A1, online only).
GVHD
With death as a competing risk, the cumulative incidence of grade 2 to 4 GVHD at day 100 was 44.7% (95% CI, 36% to 53%) with MAC and 31.6% (95% CI, 23.8% to 39.6%) with RIC (P = .024). The cumulative incidence of grade 3 to 4 GVHD at day 100 was 13.6% (95% CI, 8% to 20%) with MAC and 6.8% (95% CI, 3% to 12%) with RIC (P = .066). The cumulative incidence of chronic GVHD at 18 months post-HCT was 64% (95% CI, 55% to 71.7%) with MAC and 47.6% (95% CI, 38.8 to 58.8) with RIC (P = .019). Among patients who received MAC, there were 40, 33, and 12 who developed mild, moderate, and severe chronic GVHD, respectively. Among patients who received RIC, there were 34, 17, and 12 who developed mild, moderate, and severe chronic GVHD, respectively. When treating both death and relapse as competing risks, the cumulative incidence of grade 2 to 4 GVHD at day 100 was 44.7% (95% CI, 36% to 53%) with MAC and 28.6% (95% CI, 21% to 36%) with RIC (P = .006). The cumulative incidence of grade 3 to 4 GVHD at day 100 was 14.4% (95% CI, 9% to 21%) with MAC and 3.8% (95% CI, 1% to 8%) with RIC (P = .003). The cumulative incidence of chronic GVHD at 18 months post-HCT was 65.6 (95% CI, 57% to 73%) with MAC and 36.9% (95% CI, 28% to 45%) with RIC (P < .01).
OS and RFS
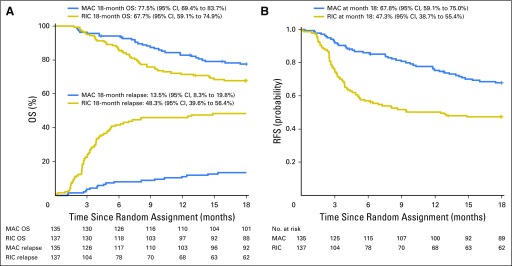
OS at 18 months was 77.5% (95% CI, 69.4% to 83.7%) with MAC and 67.7% (95% CI, 59.1% to 74.9%) with RIC (Fig 2A). The pointwise difference in OS at 18 months did not reach statistical significance (9.8%; 95% CI, −0.8% to 20.3%; P = .07). Subgroup analyses evaluated comparisons between specific conditioning regimens. The most common MAC regimen was Flu/Bu4; the most common RIC regimen was Flu/Bu2. The difference in OS favoring Flu/Bu4 over Flu/Bu2 (10.8%; 95% CI, −2.7% to 24.3%) was not statistically significant. Neither subgroup analyses nor Cox regression showed a significant interaction between specific RIC or MAC regimens with OS, although numbers of patients receiving individual regimens were small, limiting power (Appendix Fig A1, online only).
Fig 2.
(A) Overall survival (OS) and incidence of relapse by treatment arm and (B) relapse-free survival (RFS). MAC, myeloablative conditioning; RIC, reduced-intensity conditioning.
RFS at 18 months was 67.8% (95% CI, 59.1% to 75%) with MAC and 47.3% (95% CI, 38.7% to 55.4%) with RIC (P < .01; Fig 2B). An 18-month pointwise comparison showed a difference in RFS of 20.4% (95% CI, 8.9 to 32) in favor of MAC. Among patients with AML, 18-month RFS was 65.2% (95% CI, 55.3% to 73.4%) with MAC and 45.3% (95% CI, 35.8% to 54.3%) with RIC (P = .003). Among patients with MDS, 18-month RFS was 77.8% (95% CI, 57.1 to 89.3) with MAC and 55.6% (95% CI, 35.2% to 71.8%) with RIC (P = .07).
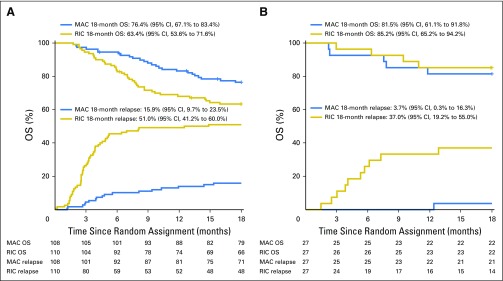
Among patients with AML, 18-month OS was 76.4% (95% CI, 67.1% to 83.4%) with MAC and 63.4% (95% CI, 53.6% to 71.6%) with RIC (P = .035; Fig 3A). Among patients with MDS, 18-month OS was 81.5% (95% CI, 61.1% to 91.8%) with MAC and 85.2% (95% CI, 65.2% to 94.2%) with RIC (P = .175; Fig 3B). There was no statistically significant interaction between disease (AML v MDS) and treatment effects (P = .71).
Fig 3.
Overall survival (OS) and incidence of relapse by treatment arm in patients with (A) acute myeloid leukemia and (B) myelodysplastic syndromes. MAC, myeloablative conditioning; RIC, reduced-intensity conditioning.
Relapse
Eighteen patients randomly assigned to MAC experienced relapse compared with 66 patients randomly assigned to RIC, for a cumulative incidence at 18 months of 13.5% (95% CI, 8.3% to 19.8%) and 48.3% (95% CI, 39.6% to 56.4%), respectively (P < .001; Fig 2A). Among patients with AML, cumulative incidence was 15.9% (95% CI, 9.7% to 23.5%) with MAC and 51% (95% CI, 41.2% to 60%) with RIC (Fig 3A). Among patients with MDS, cumulative incidence was 3.7% (95% CI, 0.3% to 16.3%) with MAC and 37% (95% CI, 19.2% to 55%) with RIC (Fig 3B).
TRM, Toxicities, and Infections
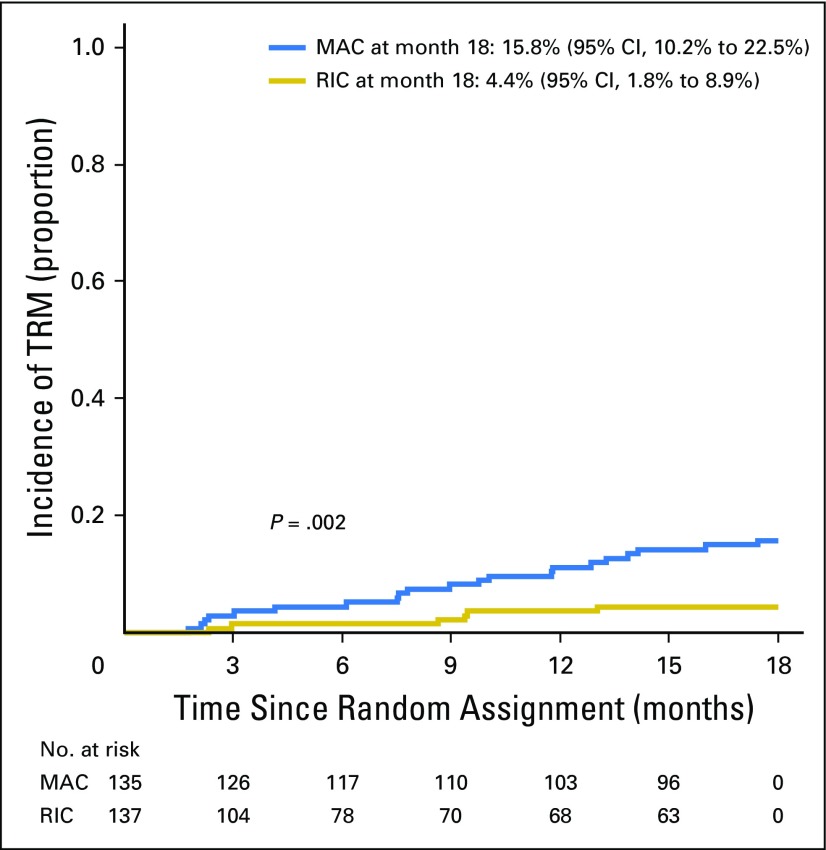
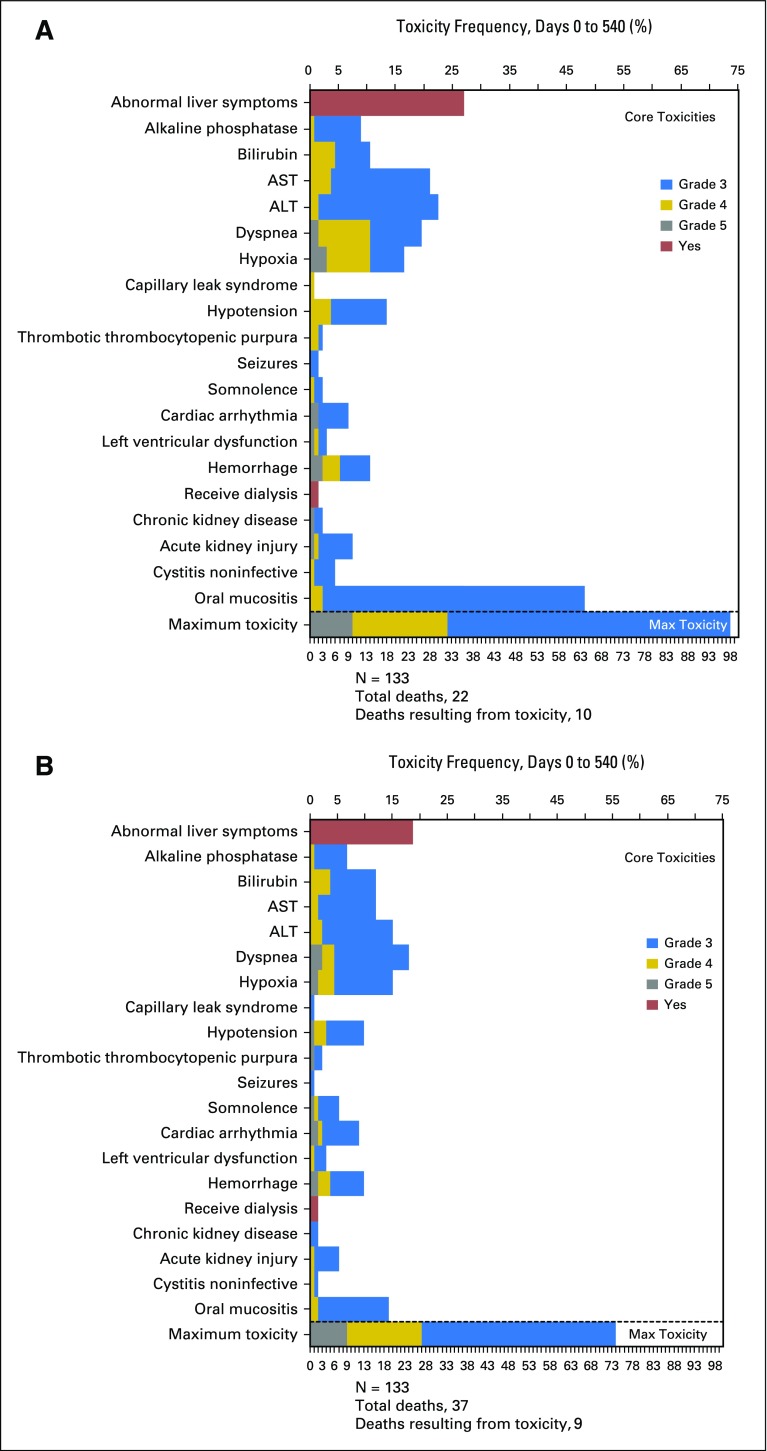
TRM occurred in 22 patients randomly assigned to MAC and eight randomly assigned to RIC. The 18-month post–random assignment incidence was 15.8% (95% CI, 10.2% to 22.5%) and 4.4% (95% CI, 1.8% to 8.9%), respectively (P = .002; Fig 4). The most common adverse events were mucositis and abnormal liver function with MAC versus abnormal liver function and dyspnea with RIC (Appendix Fig A2, online only). Infectious complications were comparable in both treatment arms (Appendix Table A2, online only).
Fig 4.
Incidence of treatment-related mortality (TRM) by treatment arm. MAC, myeloablative conditioning; RIC, reduced-intensity conditioning.
Causes of Death
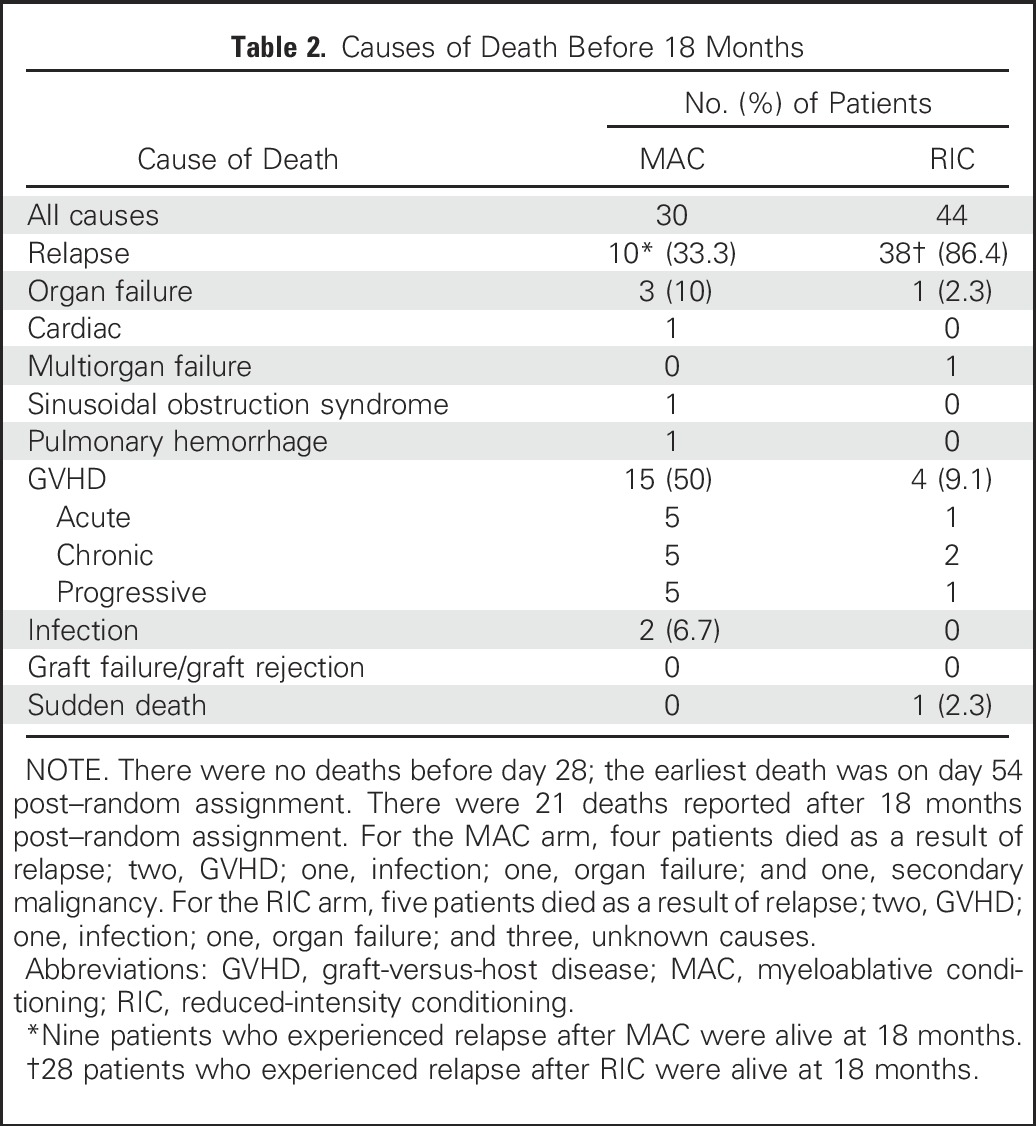
Causes of death are summarized in Table 2. Thirty of 135 patients randomly assigned to MAC died; GVHD was the primary cause of death (50%) followed by relapse (33.3%). Forty-four of 137 patients randomly assigned to RIC died; relapse was the primary cause of death (86.4%).
Table 2.
Causes of Death Before 18 Months
Multivariable Analysis
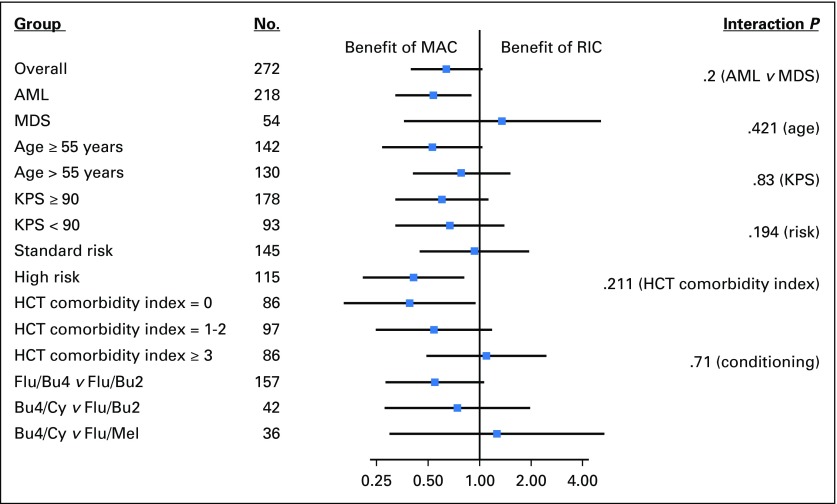
Cox proportional hazards were used to calculate HRs and end point estimates, adjusting for disease risk and donor type. The HR for mortality with MAC versus RIC was 0.64 (95% CI, 0.38 to 0.98; P = .065). The difference in adjusted 18-month OS was 9.2% (P = .085). The HR for treatment failure (relapse or death, inverse of RFS) was 0.47 (95% CI, 0.32 to 0.69; P < .001). The difference in adjusted 18-month RFS was 19.4% (P = 0.001). Relapse remained significantly lower, and acute and chronic GVHD significantly higher, with MAC in multivariable analyses. Multiple covariates were assessed to identify a subset benefiting from MAC or RIC. Among multiple factors examined, diagnosis of AML (interaction P = .2), presence of high-risk disease (interaction P = .194), and HCT comorbidity index of 0 (interaction P = .211) showed a significant OS benefit with MAC (Appendix Fig A1).
DISCUSSION
We hypothesized that a reduction in TRM with RIC would improve OS. TRM was significantly lower among patients in the RIC arm (4.4% v 15.8%), but this difference was less than expected and offset by substantial differences in relapse. OS was inferior in patients receiving RIC, even though the difference did not reach statistical significance. The sample size was reduced as a result of DSMB safety concerns, resulting in decreased power to detect a difference. The difference in OS was significant in patients with AML but not MDS. Additional follow-up is warranted because mortality in patients experiencing relapse who were still alive at the time of data closure is expected to be high. Prior data suggest that patients who experience relapse within 18 months of HCT for AML or MDS have a 2-year OS of only 9% to 14%.23
The DSMB halted accrual because of a presumed benefit for MAC. Preset boundaries for early termination as a result of OS differences were in place for guidance, but stopping rules for relapse or TRM were not included in the trial design, because RIC was expected to result in higher rates of relapse and MAC in higher rates of early TRM. However, the discrepancy in relapse rate exceeded what was anticipated, and the trial was halted because of ethical concerns rather than statistical considerations. An earlier phase III randomized trial compared conditioning intensity in patients with AML in first remission (MAC, n = 96; RIC, n = 99).24 All patients received a total-body irradiation–based regimen (RIC, 8 Gy; MAC, 12 Gy). There were no significant differences in 3-year TRM (13% v 18%), relapse (28% v 26%), or OS (61% v 58%) after RIC versus MAC, respectively. However, the statistical power was limited. This study was also closed before full accrual; however, it was closed because of poor accrual rather than differential outcomes.
The fundamental question of whether to select an MAC or RIC regimen in patients who could tolerate either is answered by our trial. Patients receiving RIC regimens had a substantially higher relapse rate than those receiving MAC but only a modest decrease in TRM. The lower antileukemia efficacy was likely a result of lower cytotoxicity, but lower rates of GVHD and GVHD-associated GVL effects might have contributed.25-27
One challenge is identifying younger, fitter patients who would benefit from RIC without experiencing an excessive relapse risk. Our study targeted patients with < 5% marrow myeloblasts pre-HCT; however, highly sensitive techniques such as flow cytometry or polymerase chain reaction–based assays to screen for minimal identifiable disease were not required.28 The risk for relapse in patients with minimal identifiable disease is particularly high after RIC.29 Furthermore, 42% of the patients had disease considered high risk for post-HCT relapse based on genetic features.30,31
Several compromises were required to assure adequate enrollment, including options for RIC and MAC. Screening questionnaires from participating centers in the BMT CTN indicated that the two most common RIC regimens were Flu/Bu2 and Flu/Mel, and a retrospective comparison indicated no difference in OS between the two regimens.32 Subgroup analysis and interaction tests showed no differential effect on OS based on choice of RIC regimen in our trial; however, only 21 patients received Flu/Mel, thus limiting our ability to specifically evaluate this regimen.
Our trial strongly supports that patients age < 65 years with acceptable HCT comorbidity index scores should receive MAC. Patients who are not candidates for MAC should be considered for novel regimens that exploit enhanced antimyeloid activity through chemotherapy, immunotherapy, or biologically targeted mechanisms. MAC remains the standard of care for patients undergoing HCT for AML or MDS.
ACKNOWLEDGMENT
We thank the members of the Blood and Marrow Transplant Clinical Trials Network, the research nurses, and the patients who participated in this trial. We also thank Bonnie Larson and Helen Crawford for help with manuscript submission.
Appendix
Participating Centers
The 32 transplantation centers (with principal investigator) that enrolled patients in this trial under the auspices of the Blood and Marrow Transplant Clinical Trials Network (protocol 0901) were as follows: Baylor University Medical Center (Edward Agura), Blood & Marrow Transplant Program at Northside Hospital (Lawrence E. Morris), Cleveland Clinic Foundation (Ronald Sobecks), Dana-Farber Cancer Institute/Brigham & Women’s (Edwin Alyea), Duke University Medical Center (Mitch Horwitz), Emory University (Amelia Langston), Florida Hospital Cancer Institute (Shahram Mori), Fred Hutchinson Cancer Research Center (Bart Scott), H. Lee Moffitt Cancer Center (Hugo Fernandez), Jewish Hospital Bone Marrow Transplant Program (Ed Faber), Karmanos Cancer Institute (Joseph Uberti), Mayo Clinic–Phoenix (Mark Litzow), Mayo Clinic–Rochester (James Slack), Medical College of Wisconsin (Marcelo Pasquini), Mount Sinai Medical Center (Adriana Malone), Oregon Health & Science University (Gabrielle Meyers), Roswell Park Cancer Institute (Maureen Ross), Texas Transplant Institute (Carlos Bachier; changed to Seema Naik on March 21, 2014), University of California San Diego Medical Center (Edward Ball), University Hospitals of Cleveland/Case Western (Hillard Lazarus), University of Florida College of Medicine (Shands; John Wingard), University of Kansas Hospital (Sunil Abhyankar), University of Kentucky (Zartash Gul; changed to Gerhard Hildebrandt on March 1, 2016), University of Minnesota (Erica Warlick), University of Nebraska Medical Center (Lori Maness-Harris), University of North Carolina Hospital at Chapel Hill (Thomas Shea), University of Pennsylvania Cancer Center (David L. Porter), University of Rochester Medical Center (Michael Becker), University of Wisconsin Hospital & Clinics (Mark B. Juckett), Utah Blood and Marrow Transplant/University of Utah Medical School (Michael Boyer), Washington University/Barnes Jewish Hospital (Peter Westervelt), and West Virginia University Hospital (Michael Craig).
Outcomes
Neutrophil recovery was defined as the first of 3 consecutive days achieving an absolute neutrophil count > 0.5 × 109/L. Platelet recovery was defined as the first of 3 consecutive days achieving a platelet count > 20,000/µL without requiring platelet transfusions in the preceding 7 days. Bone marrow was the preferred source for chimerism analysis, which was performed, at a minimum, on days 28 and 100 and at 18 months post–hematopoietic cell transplantation. Full donor chimerism was defined as ≥ 95% donor cells in all lineages tested (lymphoid, myeloid, or whole blood). Mixed chimerism was defined as the presence of donor cells at < 95% but ≥ 5% in the peripheral blood or bone marrow in any of the lineages tested. Mixed or full donor chimerism was interpreted as evidence of donor-cell engraftment. Graft rejection was defined as the inability to detect or loss of detection of ≥ 5% donor cells. Primary graft failure was defined by lack of neutrophil recovery by 28 days. Secondary graft failure was defined as initial neutrophil recovery followed by subsequent decline in neutrophil counts to < 0.5 × 109/L unresponsive to growth factor therapy.
Table A1.
Donor Cell Engraftment by Treatment Arm
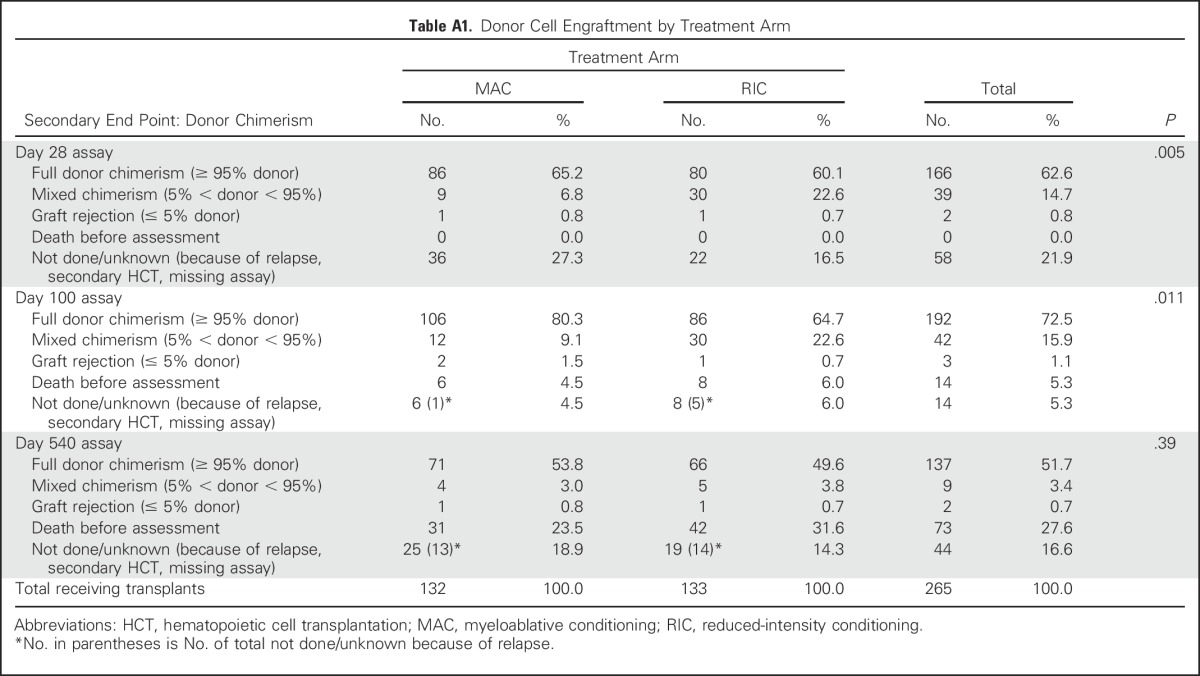
Table A2.
Infection Summary by Treatment Arm
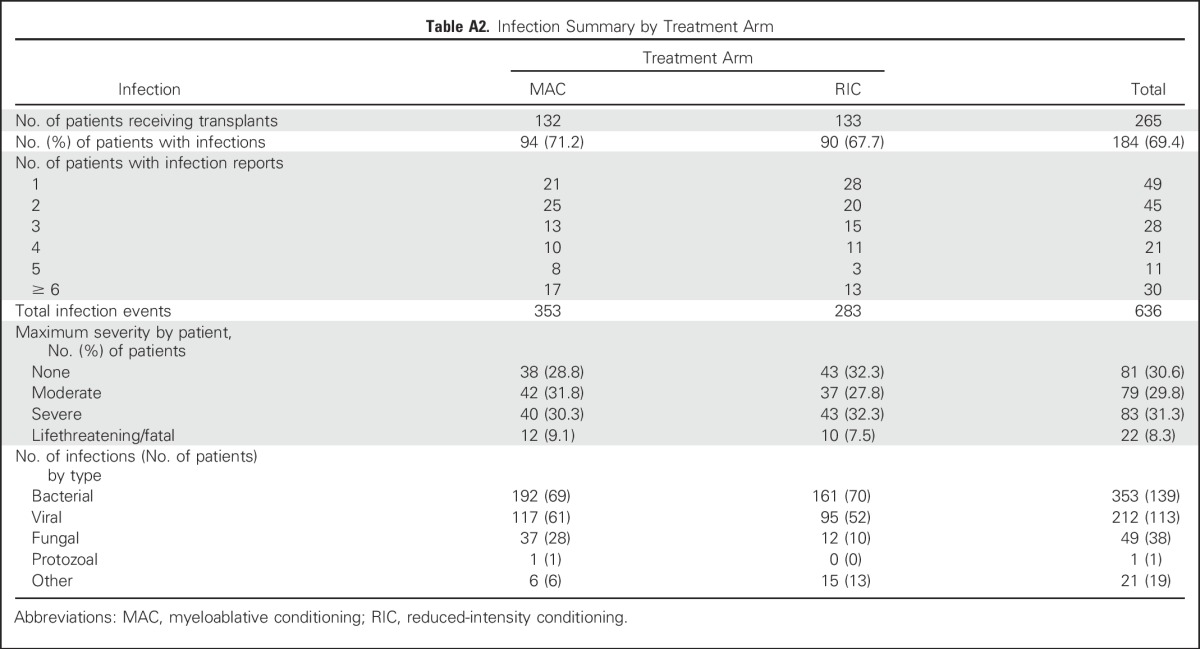
Fig A1.
Forest plot of hazard ratios for overall survival for myeloablative conditioning (MAC) versus reduced-intensity conditioning (RIC) in various subgroup. AML, acute myeloid leukemia; Bu4/Cy, busulfan with cyclophosphamide (MAC); Flu/Bu2, fludarabine with busulfan (RIC); Flu/Bu4, fludarabine with busulfan (MAC); Flu/Mel, fludarabine with melphalan (RIC); HCT, hematopoietic cell transplantation; KPS, Karnofsky performance score; MDS, myelodysplastic syndromes.
Fig A2.
Core and protocol-specific toxicity (grade > 2) by treatment arm: (A) myeloablative conditioning and (B) reduced-intensity conditioning.
Footnotes
Supported by the National Heart, Lung, and Blood Institute (under Grant No. U10HL069294 to the Blood and Marrow Transplant Clinical Trials Network) and National Cancer Institute and by National Institutes of Health Grant No. 4U10HL109322-0po6 (S.M.D.).
Written on behalf of the Blood and Marrow Transplant Clinical Trials Network.
Processed as a Rapid Communication manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical trial information: NCT01339910.
AUTHOR CONTRIBUTIONS
Conception and design: Bart L. Scott, Marcelo C. Pasquini, Brent R. Logan, Steven M. Devine, David L. Porter, Richard T. Maziarz, Erica D. Warlick, Nancy L. Geller, Jennifer Le-Rademacher, Mary M. Horowitz, H. Joachim Deeg, Mitchell E. Horwitz
Provision of study materials or patients: Bart L. Scott, Marcelo C. Pasquini, H. Joachim Deeg, Mitchell E. Horwitz
Collection and assembly of data: Bart L. Scott, Marcelo C. Pasquini, Juan Wu, Richard T. Maziarz, Hugo F. Fernandez, Edwin P. Alyea, Mehdi Hamadani, Asad Bashey, Sergio Giralt, Adam M. Mendizabal, Mitchell E. Horwitz
Data analysis and interpretation: Bart L. Scott, Marcelo C. Pasquini, Brent R. Logan, Juan Wu, Steven M. Devine, David L. Porter, Richard T. Maziarz, Erica D. Warlick, Mehdi Hamadani, Sergio Giralt, Nancy L. Geller, Eric Leifer, Adam M. Mendizabal, Mary M. Horowitz, H. Joachim Deeg, Mitchell E. Horwitz
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Bart L. Scott
No relationship to disclose
Marcelo C. Pasquini
Honoraria: Baxalta, Pfizer
Travel, Accommodations, Expenses: Baxalta, Pfizer, Atara Biotherapeutics
Brent R. Logan
Consulting or Advisory Role: Telesta Therapeutics, Celgene, Rockwell Medical, Puma Biotechnology
Juan Wu
No relationship to disclose
Steven M. Devine
Consulting or Advisory Role: Incyte, Bristol-Myers Squibb
Research Funding: Sanofi
David L. Porter
Employment: Genentech (I)
Stock or Other Ownership: Genentech (I)
Honoraria: Incyte, Astellas Pharma
Consulting or Advisory Role: Novartis, Immunovative Therapies
Research Funding: Novartis
Patents, Royalties, Other Intellectual Property: Patent/royalty rights for CD19-directed CAR T-cell therapy from the University of Pennsylvania and licensed to Novartis
Travel, Accommodations, Expenses: Immunovative Therapies
Richard T. Maziarz
Consulting or Advisory Role: ARIAD Pharmaceuticals, Novartis, Incyte, Athersys
Patents, Royalties, Other Intellectual Property: Athersys
Erica D. Warlick
No relationship to disclose
Hugo F. Fernandez
Employment: Tenet Health Care (I)
Consulting or Advisory Role: Chimerix, Pfizer
Speakers’ Bureau: Sanofi
Edwin P. Alyea
No relationship to disclose
Mehdi Hamadani
Honoraria: Celgene
Consulting or Advisory Role: MedImmune, Celerant, Janssen Oncology
Research Funding: Takeda Pharmaceuticals
Asad Bashey
No relationship to disclose
Sergio Giralt
Honoraria: Celgene, Takeda Pharmaceuticals, Amgen, Jazz Pharmaceuticals, Sanofi
Consulting or Advisory Role: Celgene, Takeda Pharmaceuticals, Sanofi, Jazz Pharmaceuticals, Amgen, Janssen
Research Funding: Celgene (Inst), Takeda Pharmaceuticals
Travel, Accommodations, Expenses: Celgene, Sanofi, Amgen, Jazz Pharmaceuticals
Nancy L. Geller
No relationship to disclose
Eric Leifer
No relationship to disclose
Jennifer Le-Rademacher
No relationship to disclose
Adam M. Mendizabal
No relationship to disclose
Mary M. Horowitz
Research Funding: Novartis, Gamida Cell, Jazz Pharmaceuticals
H. Joachim Deeg
No relationship to disclose
Mitchell E. Horwitz
Honoraria: MolMed, Novartis, Pfizer, Atara Biotherapeutics
Research Funding: Gamida Cell (Inst), Novartis (Inst), Sanofi (Inst), Neovii Biotech (Inst),
REFERENCES
- 1.Pasquini MC, Zhu X: Current uses and outcomes of hematopoietic stem cell transplantation: 2014 CIBMTR summary slides. https://www.cibmtr.org/referencecenter/slidesreports/summaryslides/Pages/index.aspx
- 2.Giralt S, Estey E, Albitar M, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: Harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–4536. [PubMed] [Google Scholar]
- 3.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 4.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: Replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 5.Lowsky R, Takahashi T, Liu YP, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353:1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 6.Aoudjhane M, Labopin M, Gorin NC, et al. Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: A retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT) Leukemia. 2005;19:2304–2312. doi: 10.1038/sj.leu.2403967. [DOI] [PubMed] [Google Scholar]
- 7.Scott BL, Sandmaier BM, Storer B, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: A retrospective analysis. Leukemia. 2006;20:128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 8.Shimoni A, Hardan I, Shem-Tov N, et al. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: The role of dose intensity. Leukemia. 2006;20:322–328. doi: 10.1038/sj.leu.2404037. [DOI] [PubMed] [Google Scholar]
- 9.Martino R, Iacobelli S, Brand R, et al. Retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic hematopoietic stem cell transplantation using HLA-identical sibling donors in myelodysplastic syndromes. Blood. 2006;108:836–846. doi: 10.1182/blood-2005-11-4503. [DOI] [PubMed] [Google Scholar]
- 10.Alyea EP, Kim HT, Ho V, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Flynn CM, Hirsch B, Defor T, et al. Reduced intensity compared with high dose conditioning for allotransplantation in acute myeloid leukemia and myelodysplastic syndrome: A comparative clinical analysis. Am J Hematol. 2007;82:867–872. doi: 10.1002/ajh.20989. [DOI] [PubMed] [Google Scholar]
- 12.Ringdén O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:4570–4577. doi: 10.1200/JCO.2008.20.9692. [DOI] [PubMed] [Google Scholar]
- 13.Luger SM, Ringdén O, Zhang MJ, et al. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2012;47:203–211. doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martino R, Valcárcel D, Brunet S, et al. Comparable non-relapse mortality and survival after HLA-identical sibling blood stem cell transplantation with reduced or conventional-intensity preparative regimens for high-risk myelodysplasia or acute myeloid leukemia in first remission. Bone Marrow Transplant. 2008;41:33–38. doi: 10.1038/sj.bmt.1705879. [DOI] [PubMed] [Google Scholar]
- 15.Gyurkocza B, Storb R, Storer BE, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J Clin Oncol. 2010;28:2859–2867. doi: 10.1200/JCO.2009.27.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lioure B, Béné MC, Pigneux A, et al. Early matched sibling hematopoietic cell transplantation for adult AML in first remission using an age-adapted strategy: Long-term results of a prospective GOELAMS study. Blood. 2012;119:2943–2948. doi: 10.1182/blood-2011-05-352989. [DOI] [PubMed] [Google Scholar]
- 17.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 19.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: Combined FHCRC and MDACC experiences. Blood. 2007;110:4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: A Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 21.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 22.Copelan E, Casper JT, Carter SL, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13:1469–1476. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 23.Mielcarek M, Storer BE, Flowers MED, et al. Outcomes among patients with recurrent high-risk hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13:1160–1168. doi: 10.1016/j.bbmt.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Bornhäuser M, Kienast J, Trenschel R, et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: A prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012;13:1035–1044. doi: 10.1016/S1470-2045(12)70349-2. [DOI] [PubMed] [Google Scholar]
- 25.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 26.Martino R, Caballero MD, Pérez-Simón JA, et al: Evidence for a graft-versus-leukemia effect after allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning in acute myelogenous leukemia and myelodysplastic syndromes. Blood 100:2243-2245, 2002 [Erratum: Blood 112:5259, 2008] [DOI] [PubMed] [Google Scholar]
- 27.Baron F, Maris MB, Sandmaier BM, et al. Graft-versus-tumor effects after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. J Clin Oncol. 2005;23:1993–2003. doi: 10.1200/JCO.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 28.Walter RB, Gyurkocza B, Storer BE, et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2015;29:137–144. doi: 10.1038/leu.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Festuccia M, Deeg HJ, Gooley TA, et al. Minimal identifiable disease and the role of conditioning intensity in hematopoietic cell transplantation for myelodysplastic syndrome and acute myelogenous leukemia evolving from myelodysplastic syndrome. Biol Blood Marrow Transplant. 2016;22:1227–1233. doi: 10.1016/j.bbmt.2016.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armand P, Kim HT, DeAngelo DJ, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant. 2007;13:655–664. doi: 10.1016/j.bbmt.2007.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deeg HJ, Scott BL, Fang M, et al. Five-group cytogenetic risk classification, monosomal karyotype, and outcome after hematopoietic cell transplantation for MDS or acute leukemia evolving from MDS. Blood. 2012;120:1398–1408. doi: 10.1182/blood-2012-04-423046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimoni A, Hardan I, Shem-Tov N, et al. Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: Fludarabine/melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia. 2007;21:2109–2116. doi: 10.1038/sj.leu.2404886. [DOI] [PubMed] [Google Scholar]