Abstract
Epithelial cells at mucosal surfaces are integral components of innate and adaptive immunity. IL-25 is reportedly produced by epithelial cells and likely plays vital roles in regulating type-2 immune responses. However, little is known regarding the mechanisms that control production and extracellular releases of IL-25. We hypothesized that proteases from the multiple allergens may induce IL-25 production in airway epithelial cells. In this study, we found that IL-25 is constitutively produced and detectable in cytoplasm of resting normal human bronchial epithelial (NHBE) cells. When exposed to airborne allergens such as house dust mite (HDM), stored IL-25 was released rapidly to the extracellular space. IL-25 release was not accompanied by cell death, suggesting involvement of active secretory mechanism(s). HDM also enhanced IL-25 mRNA transcription, which was dependent on their protease activities. Furthermore, activation of NHBE cells with authentic proteases, such as trypsin and papain, or with a peptide agonist for protease-activated receptor 2 was sufficient to enhance IL-25 mRNA transcription and protein. Protease-driven increase in mRNA transcription and allergen-driven extracellular release of IL-25 protein was also observed in primary nasal epithelial cells from healthy individuals. These findings suggest that IL-25 production by airway epithelial cells is regulated by the transcription and protein release levels and that allergen proteases likely play pivotal roles in both biological processes.
Keywords: airway epithelial cells, allergen, PAR-2, protease, IL-25
Clinical Relevance
A better understanding of the protease-activated receptor pathways and their relationships with IL-25 immunobiology may allow us to develop novel therapeutic and preventive strategies for allergic airway diseases.
In addition to being a physical barrier between the airway and the immune system, epithelial cells are now thought to play vital roles in innate and adaptive immune responses (1). Epithelial cells produce chemokines and cytokines that recruit and enhance survival of dendritic cells and interact directly with dendritic cells through membrane-associated chemokines (2–4). Epithelial cells also express soluble and cell-surface molecules that regulate recruitment, differentiation, proliferation, and function of T and B cells (2, 5). In particular, newly discovered epithelial-derived cytokines, such as thymic stromal lymphopoietin, IL-25, and IL-33, may play key roles in the development and regulation of T helper 2 (Th2) cytokine-dependent immune responses (6).
There is increasing evidence to suggest the roles for IL-25 in the type-2 immune response and in the pathogenesis of asthma and allergic diseases. IL-25 (also known as IL-17E) is a member of a family of six structurally related but functionally distinct proteins. IL-17A and IL-17F appear to play important roles in neutrophilic inflammation and autoimmunity. In contrast, IL-25 is exceptional in promoting eosinophilic inflammation and a type-2 immune response (7). This has been well demonstrated in mouse models: exogenous administration of IL-25 (8, 9) or transgenic expression (10, 11) induces Th2-type inflammation. IL-25 is increased in animal bronchial challenge models (12), and its inhibition reduces the associated Th2-type inflammation (13, 14). Thus, IL-25 appears to be sufficient for the development of Th2-type airway inflammation. However, little is known regarding the factors that control the production of IL-25. In vitro, airway epithelial cells (AECs) express IL-25 mRNA when they are activated by the allergens Aspergillus oryzae and ragweed (13–15).
The Th2-type immune response is typically associated with immunity to multicellular helminthes (16). These multicellular organisms secrete proteases (17), and the innate immune system appears to have evolved to recognize the Toll-like receptor (TLR) ligands and proteases produced by them (18). Certain allergens are also proteases (e.g., Amb a from ragweed pollen; Bla g from cockroach; Asp f 5, f 6, and f 11 from Aspergillus; and Der p 1, p 3, p 9 from house dust mite [HDM]) (1). The protease activities produced by HDM, Aspergillus, or ragweed promoted a Th2 response (19, 20). HDM proteases can activate protease-activated receptors (PARs), leading to allergic inflammation (21–23). Recently, a prototypic cysteine protease, papain, promoted Th2-type sensitization to bystander antigens after it was administered subcutaneously into mice (24).
In this study we investigated the mechanisms of IL-25 production by AECs by using natural allergens and authentic proteases. The mRNA transcription and extracellular release of IL-25 protein did not correlate when AECs were exposed to various allergen extracts and TLR ligands. Indeed, IL-25 protein was constitutively produced by AECs, and it was released quickly to extracellular milieu when the cells were exposed to HDM. Furthermore, HDM and prototypic proteases enhanced IL-25 mRNA transcription by the mechanisms dependent on PAR-2. These finding suggest that IL-25 production by AECs is regulated by the distinct processes involving mRNA transcription and protein release and that allergen proteases likely play important roles in both events. IL-25 may serve as an epithelial danger signal that is mobilized rapidly in response to airborne proteases.
Materials and Methods
Detailed methods are available in the online supplement.
Reagents
Pam3CysSerLys4 (Pam3CSK4, San Diego, CA) and polyinosinic-polycytidylic acid [poly(I:C)] were from InvivoGen. ATP peroxidate oxidized sodium salt, Pepstatin A, trypsin from bovine pancreas, Actinomycin D, trans-epoxysuccinyl-l-leucylamide (4-guanidino) butane (E-64), 4-amidinophenylmethanesulfonyl fluoride (APMSF), and nonidet P-40 (NP-40, La Jolla, CA) were from Sigma-Aldrich (St. Louis, MO). Papain and suramin were from Carica papaya from Calbiochem (Charlottesville, VA). Recombinant Der p1 and Der p2 were from Indoor Biotechnologies. The PAR-1 agonist peptide TFLLRN-NH2 and the PAR-2 agonist peptide SLIGKV-NH2 were from AnaSpec (Fremont, CA). Antihuman IL-25 was from Millipore (Billerica, MA). Anti-human TLR2 was from eBioscience (San Diego, CA). Small interfering RNA (siRNA) for PAR-1, PAR-2, TLR2, TLR4 and a control siRNA were obtained from Invitrogen (Grand Island, NY). Crude allergen from Alternaria Alternata (Alternaria), Candida albicans (Candida), oriental cockroach (cockroach), HDM, and short ragweed (ragweed) were purchased from Greer Laboratories (Lenoir, NC).
Cell Culture, Treatment, and Transfection
Normal human bronchial epithelial (NHBE) cells were obtained from Lonza (Walkersville, MD) and were then transfected with the catalytic component of telomerase, the human catalytic subunit of the telomerase reverse transcriptase gene, which was kindly provided by Professor H. Kita (Mayo Clinic, Rochester, MN). Cell culture supernatants and cell lysates were collected and used for IL-25 protein ELISA and IL-25 mRNA real-time RT-PCR (see below). To transfect NHBE cells, Lipofectamine 2000 reagent (Invitrogen) was used and performed following the manufacturer’s instructions. Reproducibility of certain observations was examined in primary human nasal epithelial cells (PNECs). Inferior turbinates were harvested from patients without allergy or infection during septoplasty. All of the patients gave written informed consent, and the study was approved by the Institutional Review Board of Shiga University of Medical Science.
RNA Isolation and Real-Time RT-PCR
Total RNA was purified with PureLink RNA Mini Kit (Invitrogen). cDNAs were synthesized from 1 μg of purified RNA samples using a Tanscriptor High Fidelity cDNA Synthesis Kit (Roche, Basel, Switzerland). Amplification and detection of specific products were performed using the The LightCycler 480 Real-Time PCR System (Roche). Transcription was normalized to the glyceraldehyde 3-phosphate dehydrogenase transcription in each sample and expressed as relative expression compared with the parallel NHBE cells cultured in the absence of stimuli.
Cytokine Production and Release by NHBE Cells
Cell-free supernatants were collected and analyzed for IL-8, IL-33 (R&D Systems, Minneapolis, MN), or IL-25 (Uscn Life Science Inc., Wuhan, China) by ELISA following the procedure recommended by the manufacturers. Total cellular IL-25 was subjected to five freeze/thaw cycles, or cells were treated with 0.5% NP-40. The sensitivity limits of the IL-8, IL-25, and IL-33 assays were 31.2, 7.8 and 23.4 pg/ml, respectively.
Detection of IL-25 by Immunohistochemistry and Confocal Microscopy
NHBE cells were cultured on Lab-Tek 2 chamber slides (Fisher, Rochester, NY). The slides were fixed and permeabilized by Cytofix/Cytoperm reagents (BD Pharmingen, San Diego, CA).
Cell Viability Assays
Viability of NHBE cells was examined with the Live/Dead Cellular Viability/Cytotoxicity kit (Invitrogen), which uses calcein acetoxymethyl and ethidium homodimer-1 dyes to detect active esterase and compromised membrane integrity, respectively.
Statistical Analysis
All data are reported as the mean ± SEM from the indicated number of samples. Two-sided differences between two samples were analyzed with the Mann-Whitney U test. Values of P < 0.05 were considered significant.
Results
IL-25 mRNA Transcription and Extracellular Protein Release by AECs Is Induced by Various Allergens and TLR Agonists
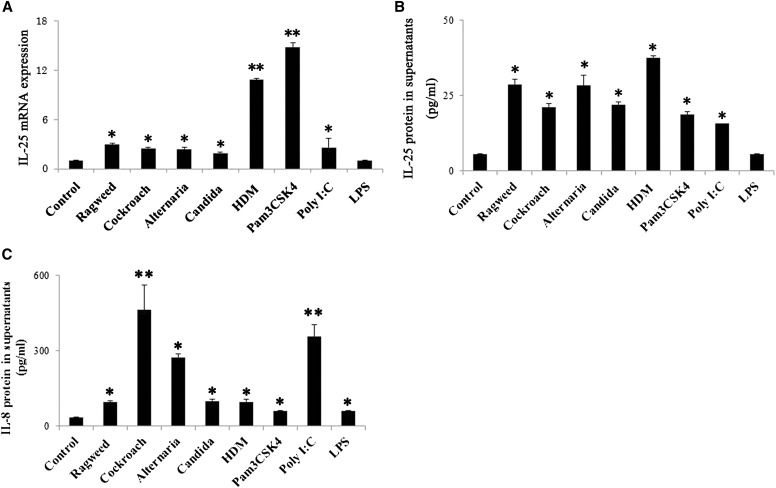
IL-25 is implicated in type-2 immune responses and in the pathogenesis of allergic diseases. To investigate the mechanisms of IL-25 production by AECs, we investigated the effects of environmental allergens and TLR ligands on IL-25 production. NHBE cells were exposed in vitro to the natural allergens, including ragweed, cockroach, Alternaria, Candida, and HDM, as well as to Pam3CSK4 (TLR2 ligand), poly(I:C) (TLR3 ligand), and LPS (TLR4 ligand). The levels of IL-25 transcription within the cells and IL-25 protein in the cell-free supernatants were analyzed at 4 and 8 hours, respectively. IL-25 mRNA was constitutively expressed and significantly up-regulated by stimulation with ragweed, cockroach, Alternaria, Candida, HDM, Pam3CSK4, or poly(I:C) as compared with medium alone (Figure 1A). Particularly, HDM and Pam3CSK4 potently enhanced IL-25 mRNA as compared with other allergens. LPS did not show any significant effects. When the levels of IL-25 protein in the cell-free supernatants were analyzed, significant increase in extracellular IL-25 was observed with various allergens and with Pam3CSK4 and poly(I:C) (Figure 1B); the amounts of IL-25 protein were roughly comparable among all the allergens tested. LPS did not show any significant effects on the IL-25 protein levels, and when the NHBE cells was incubated with the natural allergens or TLR ligands at 2 hours, IL-33 protein in supernatants was significantly detected by stimulation with various allergens except for Candida (see Figure E1 in the online supplement). In contrast, IL-8 protein in the supernatants was strongly induced by poly(I:C) and by cockroach and Alternaria (Figure 1C). Thus, IL-25 mRNA transcription and extracellular release of IL-25 protein may not necessarily correlate and the distinctive mechanisms may operate for production of IL-25 and IL-8 proteins.
Figure 1.
IL-25 mRNA transcription and extracellular protein release from airway epithelial cells. (A–C) Normal human bronchial epithelial (NHBE) cells were incubated with medium alone (control), ragweed (100 μg/ml), cockroach (400 μg/ml), Alternaria (400 μg/ml), Candida (400 μg/ml), house dust mite (HDM) (100 μg/ml), Pam3CSK4 (2 μg/ml), poly(I:C) (10 μg/ml), or LPS (1 μg/ml) for 4 hours (for mRNA) or 8 hours (for protein). (A) IL-25 mRNA expression was analyzed. (B and C) The IL-8 and IL-25 protein levels in the cell-free supernatants were analyzed by ELISA. *P < 0.05 and **P < 0.01 compared with medium alone (n = 5).
IL-25 Protein Is Detectable in Resting AECs
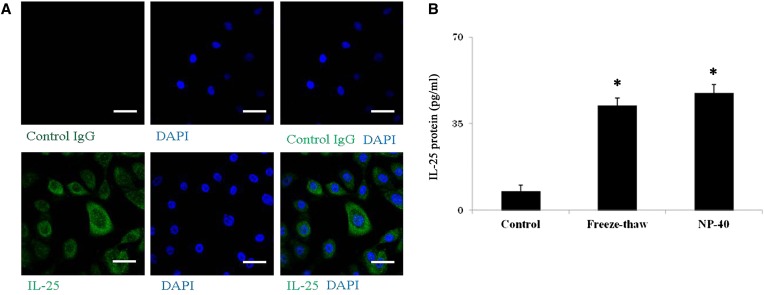
Based on the information above, we sought to investigate the mechanisms involved in the extracellular release of IL-25 protein and mRNA transcription. In a previous study, we found that an IL-1–family cytokine, IL-33, is constitutively produced and stored in nuclei of NHBE cells. Extracts of certain allergens, but not LPS, stimulated extracellular release of this stored IL-33 (25). Therefore, we suspected that IL-25 might be regulated similarly to IL-33. By staining NHBE cells with anti–IL-25 antibody and 4′-6-diamidino-2-phenylindole, IL-25 protein was clearly detected in the cytoplasm of resting NHBE cells (Figure 2A). By ELISA, IL-25 protein was detectable in cell lysates of resting NHBE cells treated with freeze-thaw cycles or with the detergent NP-40 (Figure 2B). IL-8 protein was not detectable in the lysates of resting NHBE cells (data not shown).
Figure 2.
IL-25 is stored in airway epithelial cells and released by cell necrosis. (A) NHBE cells were cultured, fixed, permeabilized, and stained with control IgG (upper panels) or rabbit antihuman IL-25 (lower panels) followed by FITC-conjugated goat antirabbit IgG. After mounting and staining with the nuclear staining dye 4′-6-diamidino-2-phenylindole (DAPI), cells were visualized under confocal microscopy. The left panels show FITC images with control IgG or anti–IL-25, the middle panels show nuclear staining with DAPI, and the right panels show overlays of the left and middle panels. Results are representative of four independent experiments. Scale bars: 40 μm. (B) NHBE cells were treated with five freeze/thaw cycles or 1% nonidet P-40 (NP-40). Cell-free supernatants were analyzed for their IL-25 protein levels. *P < 0.05 compared with medium alone (n = 5).
IL-25 Is Actively and Rapidly Released without Apparent Cellular Injury
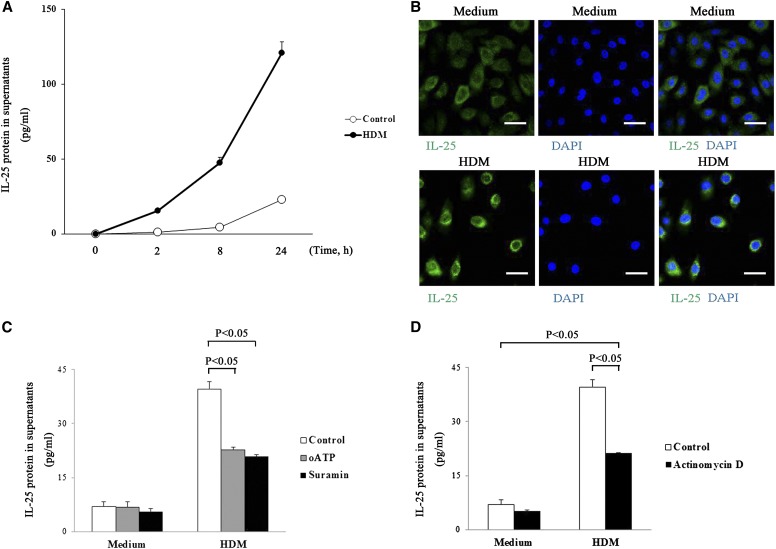
We examined whether this preformed IL-25 is released extracellularly by AECs. Because HDM potently stimulated mRNA transcription and extracellular protein release of IL-25 (Figure 1), we used HDM for the remainder of the experiments. When NHBE cells were exposed to HDM (100 μg/ml), IL-25 was rapidly released extracellular at 2 hours and was produced time dependently for up to 24 hours after stimulation (Figure 3A). In addition, when the cells were exposed to HDM, IL-25 protein was detected predominantly in the cytoplasm (Figure 3B). Endogenous ATP and engagement of purinergic receptors plays a pivotal role in IL-33 release by NHBE cells (25). Extracellular release of IL-25 protein was also partially but significantly inhibited by global antagonists for purinergic receptors, including oxidized ATP and suramin (Figure 3C). To investigate the source of IL-25, whether it is preformed or newly synthesized, NHBE cells were pretreated with actinomycin D for 30 minutes and stimulated with HDM for 2 hours. The levels of extracellular IL-25 were significantly inhibited by actinomycin D, but extracellular IL-25 was not abolished (Figure 3D), suggesting involvement of preformed and newly synthesized pools.
Figure 3.
IL-25 is rapidly released without transcription. (A) NHBE cells were incubated with medium alone (control) or HDM (100 μg/ml) for up to 24 hours. (B) NHBE cells were stimulated with medium (upper panels) or HDM (lower panels) for 8 hours. Cells were stained and analyzed in Figure 2. The left panels show FITC images with anti–IL-25, the middle panels show nuclear staining with DAPI, and the right panels show overlays of the left and middle panels. Scale bars: 40 μm. (C) After pretreatment with organic anion-transporting polypeptide (oATP) (100 μM), Suramin (300 μM), or medium alone for 30 minutes at 37°C, NHBE cells were incubated with HDM (100 μg/ml) for 2 hours. P < 0.05 compared after HDM exposure without oATP or Suramin (n = 5). (D) After pretreatment with or without Actinomycin D (1 μg/ml) for 30 minutes, NHBE cells were incubated with medium or HDM (100 μg/ml) for 2 hours. Cell-free supernatants were analyzed for their IL-25 protein levels (n = 5).
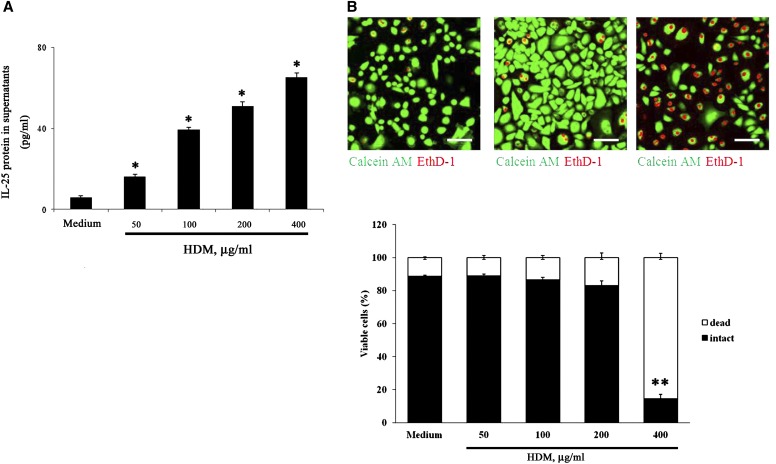
Extracellular release of “alarmins” or damage-associated molecular patterns such as IL-33 and HMGB-1 is generally mediated by necrotic cell death (26, 27). To address whether cellular injury is responsible for extracellular release of IL-25 induced by HDM, we performed dose–response experiments followed by measurement of extracellular IL-25 and analyses of membrane integrity by EthD-1, a membrane-impermeable nucleic acid dye. NHBE cells were incubated with various concentrations of HDM for 24 hours. HDM increased extracellular IL-25 in a concentration-dependent manner up to 400 μg/ml (Figure 4A). In contrast, no differences were observed in the membrane permeability between the cells incubated with medium alone or those incubated with 200 μg/ml or less HDM (Figure 4B). Dead cells were significantly increased by incubation with 400 μg/ml HDM. Approximately 90% of NHBE cells were alive with HDM up to 200 μg/ml, as judged by conversion of calcein acetoxymethyl to fluorescent calcein (Figure 4B). Altogether, these findings suggest that IL-25 is released extracellularly without apparent cellular injury or by necrosis, depending on the doses of stimuli.
Figure 4.
IL-25 is released without apparent cellular injury. (A) NHBE cells were incubated with medium alone (control) or HDM (50–400 μg/ml) for 8 hours. IL-25 protein levels in the cell-free supernatants were analyzed by ELISA. *P < 0.05 and **P < 0.01 compared with medium alone (n = 5). (B) NHBE cells were incubated with medium (left panel), 100 μg/ml HDM (middle panel), or 400 μg/ml HDM (right panel) for 24 hours and then incubated with calcein acetoxymethyl (AM) and ethidium homodimer-1 (EthD-1) for 30 minutes. The upper panels show the fluorescence images using confocal microscopy. The lower panels show intact (calcein AM positive and EthD-1 negative) and damaged (EthD-1 positive) cells in five randomly selected fields that were counted and are expressed as the percentage of intact or damaged cells per total cell number. Error bars represents the mean ± the SEM of three independent experiments. *P < 0.05 and **P < 0.01 compared with medium alone. Scale bars: 100 μm.
Protease Activities in HDM Enhance IL-25 mRNA Transcription by AECs
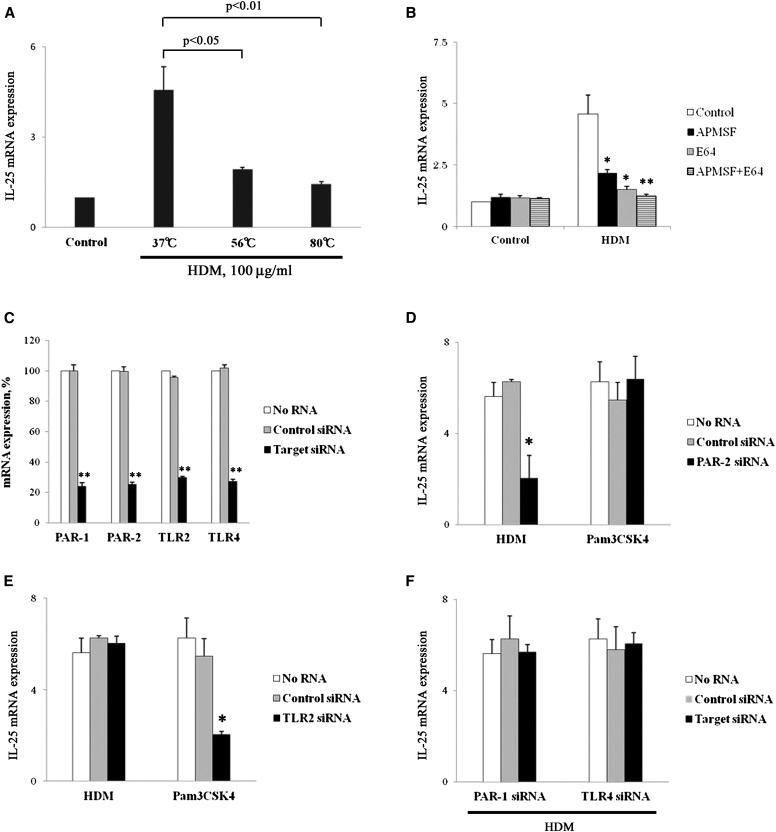
Because HDM-mediated extracellular release of IL-25 is partially inhibited by actinomycin D, we hypothesized that HDM and its protease activities regulates IL-25 mRNA transcription. We first examined the effects of heat treatment on the HDM-induced mRNA transcription. HDM (100 μg/ml) treated at 37°C for 30 minutes significantly enhanced expression of IL-25 mRNA (Figure 5A). Heat treatment (30 min at 56 or 80°C) significantly inhibited the activity of the HDM to increase IL-25 mRNA. Second, we directly examined whether protease activities in the HDM is involved in the increased IL-25 mRNA expression by using protease inhibitors. The HDM was preincubated with an aspartate protease inhibitor (Pepstatin A), a serine protease inhibitor (APMSF), a cysteine protease inhibitor (E-64), or their combination before being added to NHBE cells. APMSF and E-64 significantly inhibited IL-25 mRNA and protein expression induced by HDM (Figure 5B; Figure E2). A combination of E-64 and APMSF showed inhibition slightly larger than E-64 or APMSF alone. In contrast, pretreatment of Pam3CSK4 with AMPSF or E-64 showed no effects on the IL-25 mRNA expression (data not shown). Third, to examine the cellular receptor(s) involved in the NHBE response to HDM, we used a gene knock-down approach. NHBE cells were transfected with siRNAs specific for PAR-1, PAR-2, TLR2, or TLR4 or a control siRNA. Transfection with these specific siRNAs, but not the control siRNA, significantly suppressed the target mRNA expression by greater than 80% (Figure 5C). These knock-down NHBE cells were then stimulated with HDM or Pam3CSK4. The HDM-induced IL-25 mRNA expression was significantly inhibited in the PAR-2 siRNA knock-down cells (Figure 5D). In contrast, HDM-induced IL-25 mRNA expression was not affected in the PAR-1, TLR2, or TLR4 siRNA knock-down cells (Figures 5E and 5F). Pam3CSK4-induced IL-25 mRNA expression was significantly inhibited in the TLR2 siRNA knock-down cells but not in the PAR-2 siRNA knock-down cells (Figures 5E and 5F). Moreover, IL-25 protein staining of the PAR-2 siRNA knock-down cells did not change in comparison with the resting NHBE cells (data not shown) and was not obviously affected in the exposure of HDM (Figure E3). Altogether, these findings suggest that serine and cysteine protease activities in HDM, which interact with epithelial cell PAR-2, are likely involved to enhance transcription of IL-25 mRNA.
Figure 5.
Protease activities in HDM enhance IL-25 mRNA transcription by airway epithelial cells. (A) HDM were heated at 37, 56, or 80°C for 30 minutes. NHBE cells were incubated with preheated HDM for 4 hours. IL-25 mRNA expression is expressed as a ratio to the cells incubated with medium only. The results were P < 0.05 and P < 0.01 compared with HDM heated at 37°C (n = 5). (B) Before incubation with NHBE cells, HDM was pretreated along with their protease inhibitors (Pepstain A, APMSF, and E-64, respectively) at 37°C for 30 minutes. IL-25 mRNA was analyzed at 4 hours. *P < 0.05 and **P < 0.01 compared with no protease inhibitors (n = 5). (C) NHBE cells were transfected with small interfering RNA (siRNA) against protease-activated receptor (PAR)-1, PAR-2, Toll-like receptor (TLR)2, TLR4, or control siRNA for 48 hours. Expression of the mRNA of the target molecules was examined by real-time RT-PCR. Data are expressed as a ratio to the mock-transfected cells without siRNA, which were used as 100%. **P < 0.01 compared with control cells without siRNA (n = 5). (D–F) Transfected NHBE cells were stimulated with HDM or Pam3CSK4 for 4 hours. IL-25 mRNA expression was expressed as a ratio to the cells incubated without siRNA. *P < 0.05 compared with no siRNA (n = 5).
Activation of PAR-2 Is Sufficient to Increase IL-25 mRNA and Protein Expression
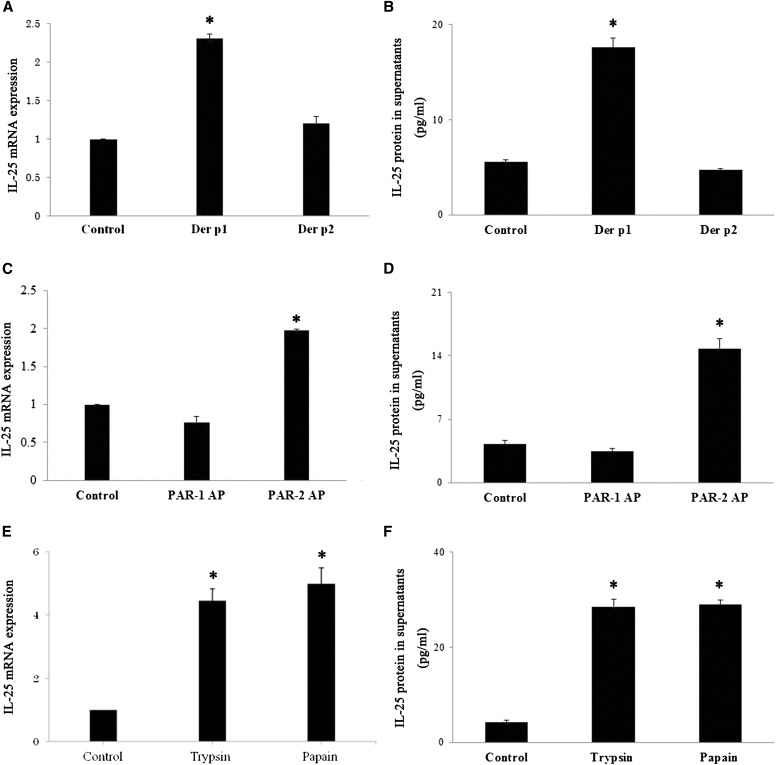
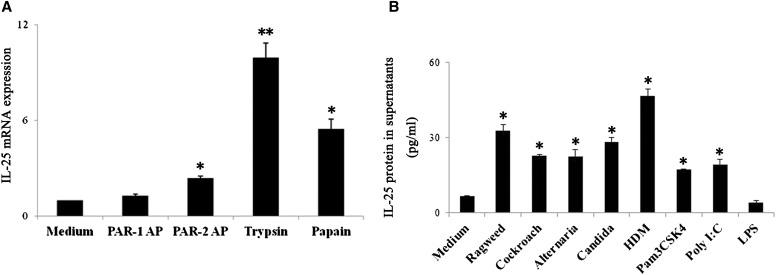
HDM possess endogenous proteases, including cysteine protease (Der p1) and serine proteases (Der p3, -6, and -9). Dep p2 is not a protease, but it has structural homology with the lipid-recognition protein MD-2 and activates TLR4 (28). We found that recombinant Der p1 and natural Der p1, but not Der p2, induce the expression of IL-25 mRNA and protein (Figures 6A and 6B). To investigate whether increases in IL-25 mRNA and protein expression stimulated with HDM is unique to HDM protease(s) or can be induced by proteases in common, we exposed NHBE cells to the prototypic proteases trypsin and papain. Trypsin (a serine protease) and papain (a cysteine protease) are used to model the exogenous proteases naturally found in various airborne allergens (e.g., mites, fungi, and cockroaches) (29, 30). After incubation with trypsin or papain, mRNA and protein for IL-25 were up-regulated in NHBE cells (Figures 6C and 6D). We next examined whether the stimulatory effects of these proteases are due to their protease activities. When trypsin was pretreated with APMSF or papain was pretreated with E-64, the IL-25 mRNA expression was inhibited, suggesting that the stimulatory effects of these proteases are mediated by protease-specific activities (Figure E4). Trypsin cleaves the extracellular N-terminus of PAR-2 molecules between the R36 and S37 residues to expose a tethered “neo-ligand” (i.e., S37LIGKV-) that binds intramolecularly to PAR-2 and triggers receptor activation (31). We found that a synthetic peptide, SLIGKV-NH2 (denoted PAR-2 AP), that corresponds to the sequence of the tethered “neo-ligand” but not to the PAR-1–activating peptide TFLLRN-NH2, stimulates IL-25 mRNA and protein expression by NHBE cells (Figures 6E and 6F). These results demonstrate that the stimulation of serine or cysteine proteases may mediate IL-25 secretion by PAR-2. Finally, to examine the physiological significance of these observations, we examined the reproducibility of certain findings in PNECs. Incubation of PNECs with trypsin, papain, or the PAR-2 AP SLIGKV-NH2 significantly increased IL-25 mRNA expression, whereas the PAR-1 AP TFLLRN-NH2 did not show significant effects (Figure 7A). Furthermore, ragweed, cockroach, Alternaria, HDM, Pam3CSK4, and poly(I:C) significantly enhanced extracellular release of IL-25 protein (Figure 7B).
Figure 6.
Activation of PAR-2 is sufficient to increase IL-25 mRNA expression. (A and B) NHBE cells were incubated with 10 μg/ml Der p1 or 10 μg/ml Der p2 for 4 hours (for mRNA) or 8 hours (for protein) (n = 5). (C and D) NHBE cells were incubated with PAR-1–activating TFLLRN-NH2 peptide (PAR-1 AP) (100 μM) or PAR-2–activating SLIGKV-NH2 peptide (PAR-2 AP) (100 μM) for 4 hours (for mRNA) or 8 hours (for protein) (n = 5). (E and F) NHBE cells were incubated with trypsin (10 nM) or papain (10 μM) for 4 hours (for mRNA) or 8 hours (for protein) (n = 5). IL-25 mRNA was analyzed by real-time RT-PCR. The IL-25 transcription was normalized to the glyceraldehyde 3-phosphate dehydrogenase transcription in each sample and expressed as a ratio to the NHBE cells cultured in the absence of proteases. The IL-25 protein levels in the supernatants were analyzed by ELISA. *P < 0.05 compared with medium alone.
Figure 7.
IL-25 mRNA transcription and extracellular protein release from primary nasal epithelial cells (PNECs). (A) PNECs were stimulated with medium alone (control), PAR-1–activating TFLLRN-NH2 peptide (PAR-1 AP) (100 μM), PAR-2–activating SLIGKV-NH2 peptide (PAR-2 AP) (100 μM), trypsin (10 nM), or papain (10 nM) for 4 hours. IL-25 mRNA was analyzed by real-time RT-PCR. (B) PNECs were incubated with medium alone (control), ragweed (100 μg/ml), cockroach (400 μg/ml), Alternaria (400 μg/ml), Candida (400 μg/ml), HDM (100 μg/ml), Pam3CSK4 (2 μg/ml), poly(I:C) (10 μg/ml), or LPS (1 μg/ml) for 8 hours. The IL-25 protein levels in the supernatants were analyzed using ELISA. *P < 0.05 and **P < 0.01 compared with cells with medium alone (n = 4).
Discussion
IL-25 enhances Th2-cell immune responses by inducing Th2-cell cytokines, such as IL-4, IL-5, and IL-13, and by promoting IgE production and tissue eosinophilia, contributing to the host defense against nematodes and allergic disorders (32). Several allergens, such as ragweed and fungal protease, can induce IL-25 mRNA expression in lung epithelial cells (14). However, the environmental factors that control the expression of IL-25 are largely unknown. Our study provides a series of evidence for potent effects of allergen protease(s) to drive IL-25 mRNA in AECs.
This conclusion is based on several observations: 1) HDM enhances IL-25 mRNA expression in NHBE; 2) HDM-mediated IL-25 induction is highly heat labile and inhibited by protease inhibitors or by knocking down epithelial PAR-2; 3) a HDM-derived protease, Dep p 1, but not Der p 2, stimulated IL-25 expression; and 4) authentic proteases (trypsin and papain) and PAR-2 agonistic peptide induced IL-25 mRNA expression in NHBE cells and in PNECs. Many allergens relevant to human diseases, such as fungi, mite, cockroach, and pollens, have protease activities (29); these protease activities of allergens potently induce Th2-type immune responses in several experimental animal models (19, 20). Our results provide a mechanistic understanding for these protease-related observations when AECs are exposed to environmental allergens.
Although proteases likely play important roles in regulating IL-25 mRNA expression, IL-25 protein is constitutively expressed and stored within the cytoplasm of AECs without prior exposure to allergens or proteases (Figure 2). IL-25 protein was released extracellularly without apparent cellular damage or by cellular injury depending on the concentrations of stimuli (Figure 4). Blocking of mRNA transcription suggests extracellular IL-25 is derived from the preformed pool and from a newly synthesized source (Figure 3). In vivo cell necrosis generally triggers an acute inflammatory and immune response by releasing damage-associated molecular patterns (26). Danger signals, which alert epithelial cells, comprise many proteins and molecules, potently contributing to an enhanced Th2-type immune response to inhaled allergens. For example, the epithelial-derived cytokine IL-33 is typically known as an “alarmin” because potential cytotoxic effects of allergen extracts might release stored IL-33 extracellularly (27). Our findings in this study suggest that IL-25 may also belong to the category of “alarmin” because it is preformed and released by cellular damage. In addition, similarly to IL-33 release (25), the ATP and purinergic receptor pathway may play pivotal roles in extracellular release of IL-25 even without cellular injury (Figure 3). Therefore, allergen exposure may cause AECs to secrete several inflammatory cytokines, including IL-25 and IL-33, and induce a Th2-type immune response and subsequent development of allergic diseases. Further studies are necessary to elucidate precisely the molecular mechanisms involved in damage-dependent and -independent mechanisms for extracellular release of these “alarmin” molecules.
HDM allergen induces asthma via TLR-4 triggering of airway structural cells (33). A nonenzymatic mite allergen, Der p 2, likely stimulates an innate immune response through TLR4 by molecular mimicry of MD-2, a lipid-recognition protein (28). Furthermore, β-glucan moieties, but not proteases, TLR2 and TLR4 in HDM extract mediated CCL20 production by AECs (34). Therefore, several biologically active molecules and their receptors may be involved in the recognition and initiation of immune responses to HDM. In this study, we found that PAR-2 exerts a critical role when NHBE cells respond HDM and induce IL-25 mRNA expression (Figure 5). Previously, Cho and colleagues (35) showed that HDM activates epithelial cells through PAR-2. Recently, chitinase from Streptomyces griseus stimulated the intracellular calcium response and IL-8 production in human bronchial epithelial cells through PAR-2 (36). Thus, at mucosal surfaces, human PAR-2 may monitor the activities of certain exogenous proteases and may play roles in regulating inflammation and potentially healing or homeostatic processes. In mice, in vivo airway administration of a PAR-2 agonist peptide enhanced the Th2-type sensitization to ovalbumin, an innocuous antigen (37). Similarly, Th2-type airway inflammation and airway hyperreactivity were attenuated in mice deficient in PAR-2 and enhanced in mice overexpressing PAR-2 (38). In humans, patients with asthma show increased expression of PAR-2 on their AECs (39, 40). Therefore, further studies on the roles of PAR-2 in response to environmental proteases and to endogenous enzymes and in the production of epithelial-derived immunoregulatory factors, such as IL-25 and IL-33, may provide important information about the interactions between the airway immune system and environmental factors.
AECs have been shown to express functional TLRs, notably TLR2, -3, -4, -5, and -6 (4, 41). Although TLRs share signal transduction pathways for activation of the transcription factors NF-κB and IRF-3 (42), Pam3CSK4 (a TLR2 ligand) and poly(I:C) (a TLR3 ligand) induced IL-25 mRNA expression in NHBE cells (Figure 1). The failure of the other TLR ligands tested, including TLR4 ligand, to induce IL-25 expression could be explained by low levels of receptor expression. On the other hand, although LPS (a TLR4 ligand) did not induce IL-25 mRNA expression or extracellular protein release, LPS potentially stimulated IL-8 production by NHBE cells. The specific differences in the signal transduction pathways and/or adaptor proteins in various TLRs may dictate the biological activities of TLRs and downstream immunologic outcomes. Previous reports looking at the interaction between TLR2 stimulation and allergic response have contradictory data. TLR2 ligands administered during the sensitization period led to enhancement of Th2-mediated allergic inflammation (43, 44). Conversely, synthetic lipopeptides administered immediately before airway allergen challenge inhibit Th2-type responses and IgE production (45). Recent studies also showed that TLR2 stimulation inhibits established allergic airway inflammation, an effect mediated by an augmented Th1 response (46). In this study, Pam3CSK4 activated expression and extracellular release of IL-25, which is a Th2-promoting cytokine. Further studies are required to examine the potential roles of TLR2 and their ligands in allergic immune responses.
In conclusion, we propose a model in which perturbation of PAR-2 on AECs by proteases from HDM and perhaps other allergens enhances mRNA transcription and extracellular protein secretion of IL-25. The IL-25 response to allergens may be quick because the protein is partially preformed and stored. IL-25 can then stimulate target cells, leading to the subsequent development and/or exacerbation of Th2-type inflammatory responses. The PARs may be non-TLRs that form an interface between innate and adaptive immune responses. A better understanding of the PAR pathways and their relationships with IL-25 immunobiology may allow us to develop novel therapeutic and preventive strategies for allergic airway diseases.
Footnotes
This work was supported in part by the grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Grant-in-Aid for Young Scientists [B]).
Originally Published in Press as DOI: 10.1165/rcmb.2012-0304OC on April 12, 2013.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 2.Schleimer RP, Kato A, Kern R, Kuperman D, Avila PC. Epithelium: at the interface of innate and adaptive immune responses. J Allergy Clin Immunol. 2007;120:1279–1284. doi: 10.1016/j.jaci.2007.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol. 2003;28:648–654. doi: 10.1165/rcmb.2002-0095OC. [DOI] [PubMed] [Google Scholar]
- 4.Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 5.Kato A, Truong-Tran AQ, Scott LA, Matsumoto K, Schleimer RP. Airway epithelial cells produce B cell-activating factor of TNF family by an IFN-beta-dependent mechanism. J Immunol. 2006;177:7164–7172. doi: 10.4049/jimmunol.177.10.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong C. Regulation and pro-inflammatory function of interleukin-17 family cytokines. Immunol Rev. 2008;226:80–86. doi: 10.1111/j.1600-065X.2008.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 9.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, et al. New IL-17 family members promote Th1 or Th2 responses in lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 10.Pan G, French D, Mao W, Maruoka M, Risser P, Lee J, Foster J, Aggarwal S, Nicholes K, Guillet S, et al. Forced expression of murine IL-17E induces growth retardation retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J Immunol. 2001;167:6559–6567. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- 11.Kim MR, Manoukian R, Yeh R, Silbiger SM, Danilenko DM, Scully S, Sun J, DeRose ML, Stolina M, Chang D, et al. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–2340. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- 12.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, et al. IL-25 enhances allergic airway inflammation by amplifying a Th2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118:606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 13.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 14.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu HS, Angkasekwinai P, Chang SH, Chung Y, Dong C. Protease allergens induce the expression of IL-25 via Erk and p38 MAPK pathway. J Korean Med Sci. 2010;25:829–834. doi: 10.3346/jkms.2010.25.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anthony RM, Rutitzky LI, Jr, Urban JF, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 18.Gottar M, Gobert V, Matskevich AA, Reichhart JM, Wang C, Butt TM, Belvin M, Hoffmann JA, Ferrandon D. Dual detection of fungal infections in Drosophila via recognition of glucans and sensing of virulence factors. Cell. 2006;127:1425–1437. doi: 10.1016/j.cell.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comoy EE, Pestel J, Duez C, Stewart GA, Vendeville C, Fournier C, Finkelman F, Capron A, Thyphronitis G. The house dust mite allergen, Dermatophagoides pteronyssinus, promotes type 2 responses by modulating the balance between IL-4 and IFN-gamma. J Immunnol. 1998;160:2456–2462. [PubMed] [Google Scholar]
- 20.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 21.Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol. 2001;167:1014–1021. doi: 10.4049/jimmunol.167.2.1014. [DOI] [PubMed] [Google Scholar]
- 22.Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PT, Stewart GA. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. 2002;169:4572–4578. doi: 10.4049/jimmunol.169.8.4572. [DOI] [PubMed] [Google Scholar]
- 23.Adam E, Hansen KK, Astudillo OF, Coulon L, Bex F, Duhant X, Jaumotte E, Hollenberg MD, Jacquet A. The house dust mite allergen Der p1, unlike Der p3, stimulates the expression of interleukin-8 in human airway epithelial cells via a proteinase-activated receptor-2-independent mechanism. J Biol Chem. 2006;281:6910–6923. doi: 10.1074/jbc.M507140200. [DOI] [PubMed] [Google Scholar]
- 24.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouzaki H, Iijima K, Kobayashi T, O'Grady SM, Kita H. The danger signal, extracellular ATP, is a sensor for an airborne allergen and triggers IL-33 release and innate Th2-type responses. J Immunol. 2011;186:4375–4387. doi: 10.4049/jimmunol.1003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamkanfi M, Dixit VM. IL-33 raises alarm. Immunity. 2009;31:5–7. doi: 10.1016/j.immuni.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, Thorne PS, Wills-Karp M, Gioannini TL, Weiss JP, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457:585–588. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 30.McGlade JP, Gorman S, Lenzo JC, Tan JW, Watanabe T, Finlay-Jones JJ, Thomas WR, Hart PH. Effect of both ultraviolet B irradiation and histamine receptor function on allergic responses to an inhaled antigen. J Immunol. 2007;178:2794–2802. doi: 10.4049/jimmunol.178.5.2794. [DOI] [PubMed] [Google Scholar]
- 31.King C, Brennan S, Thompson PJ, Stewart GA. Dust mite proteolytic allergens induce cytokine release from cultured airway epithelium. J Immunol. 1998;161:3645–3651. [PubMed] [Google Scholar]
- 32.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nathan AT, Peterson EA, Chakir J, Wills-Karp M. Innate immune responses of airway epithelium to house dust mite are mediated through beta-glucan-dependent pathways. J Allergy Clin Immunol. 2009;123:612–618. doi: 10.1016/j.jaci.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho HJ, Choi JY, Yang YM, Hong JH, Kim CH, Gee HK, Lee HJ, Shin DM, Yoon JH. House dust mite extract activates apical Cl(-) channels through protease-activated receptor 2 in human airway epithelia. J Cell Biochem. 2010;109:1254–1263. doi: 10.1002/jcb.22511. [DOI] [PubMed] [Google Scholar]
- 36.Hong JH, Hong JY, Park B, Lee SI, Seo JT, Kim KE, Sohn MH, Shin DM. Chitinase activates protease-activated receptor-2 in human airway epithelial cells. Am J Respir Cell Mol Biol. 2008;39:530–535. doi: 10.1165/rcmb.2007-0410OC. [DOI] [PubMed] [Google Scholar]
- 37.Ebeling C, Lam T, Gordon JR, Hollenberg MD, Vliagoftis H. Proteinase-activated receptor-2 promotes allergic sensitization to an inhaled antigen through a TNF-mediated pathway. J Immunol. 2007;179:2910–2917. doi: 10.4049/jimmunol.179.5.2910. [DOI] [PubMed] [Google Scholar]
- 38.Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, Gater PR, Geppetti P, Bertrand C, Stevens ME. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 39.Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, Thompson PJ. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 40.Roche N, Stirling RG, Lim S, Oliver BG, Oates T, Jazrawi E, Caramori G, Chung KF. Effect of acute and chronic inflammatory stimuli on expression of protease-activated receptors 1 and 2 in alveolar macrophages. J Allergy Clin Immunol. 2003;111:367–373. doi: 10.1067/mai.2003.6. [DOI] [PubMed] [Google Scholar]
- 41.Homma T, Kato A, Hashimoto A, Batchelor J, Yoshikawa M, Imai S, Wakiguchi H, Saito H, Matsumoto K. Corticosteroid and cytokines synergistically enhance Toll-like receptor 2 expression in respiratory epithelial cells. Am J Respir Cell Mol Biol. 2004;31:463–469. doi: 10.1165/rcmb.2004-0161OC. [DOI] [PubMed] [Google Scholar]
- 42.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 43.Chisholm D, Libet L, Hayashi T, Horner AA. Airway peptidoglycan and immunostimulatory DNA exposures have divergent effects on the development of airway allergen hypersensitivities. J Allergy Clin Immunol. 2004;113:448–454. doi: 10.1016/j.jaci.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Redecke V, Häcker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 45.Akdis CA, Kussebi F, Pulendran B, Akdis M, Lauener RP, Schmidt-Weber CB, Klunker S, Isitmangil G, Hansjee N, Wynn TA, et al. Inhibition of T helper 2-type responses, IgE production and eosinophilia by synthetic lipopeptides. Eur J Immunol. 2003;33:2717–2726. doi: 10.1002/eji.200323329. [DOI] [PubMed] [Google Scholar]
- 46.Patel M, Xu D, Kewin P, Choo-Kang B, McSharry C, Thomson NC, Liew FY. TLR2 agonist ameliorates established allergic airway inflammation by promoting Th1 response and not via regulatory T cells. J Immunol. 2005;174:7558–7563. doi: 10.4049/jimmunol.174.12.7558. [DOI] [PubMed] [Google Scholar]