Abstract
Purpose
This analysis of patients in the Children’s Oncology Group A3973 study evaluated the impact of extent of primary tumor resection on local progression and survival and assessed concordance between clinical and central imaging review–based assessments of resection extent.
Patients and Methods
The analytic cohort (n = 220) included patients who had both central surgery review and resection of the primary tumor site. For this analysis, resection categories of < 90% and ≥ 90% were used, with data on resection extent derived from operating surgeons’ assessments (all patients), as well as blinded central imaging review of computed tomography scans for a subset of 84 patients; assessment results were compared for concordance. Treatment outcomes included event-free survival (EFS), overall survival (OS), and cumulative incidence of local progression (CILP).
Results
Surgeon-assessed extent of resection was ≥ 90% in 154 (70%) patients and < 90% in 66 (30%). Five-year EFS, OS, and CILP (± SE) were 43.5% ± 3.7%, 54.9% ± 3.7%, and 11.9% ± 2.2%, respectively. EFS was higher with ≥ 90% resection (45.9% ± 4.3%) than with < 90% resection (37.9% ± 7.2%; P = .04). Lower CILP (P = .01) was associated with ≥ 90% resection (8.5% ± 2.3%) compared with < 90% resection (19.8% ± 5.0%). On multivariable analysis, ≥ 90% resection was associated with longer EFS after adjustment for MYCN amplification or diploidy but had no significant effect on OS. Concordance between surgeons’ assessments of resection extent and central image–guided review was low, with agreement of 63% (< 90% v ≥ 90%; simple κ = −0.0301).
Conclusion
Despite discordance between clinical assessment of resection extent and assessment via central imaging review, a surgeon-assessed resection extent ≥ 90% was associated with significantly better EFS and lower CILP. Improving OS, however, remains a challenge in this disease. These findings support continued attempts at ≥ 90% resection of the primary tumor in high-risk neuroblastoma.
INTRODUCTION
The role of primary tumor resection, including regional lymphadenectomy, in patients with high-risk neuroblastoma remains controversial.1-13 The uncertainty results from a lack of randomized trials assessing the impact of surgical variables. Indeed, the effect of primary tumor resection has not been included as a specific aim in any reported cooperative group studies until a recent analysis by the International Society of Pediatric Oncology Europe–Neuroblastoma (SIOPEN).14 This lack of level 1 data regarding resection is understandable, given the historically poor outcome in these patients (5-year survival ≤ 30%) and the reluctance to leave resectable masses in place. Furthermore, complete resection can be difficult to attain in this disease because of primary tumor encasement of nerves and blood vessels and frequent regional lymph node involvement. In addition, metastases to bone, bone marrow, distant lymph nodes, and other sites are unaffected by primary tumor resection. Although the greatest impact of resection should be on local control, only one cooperative group report has specifically analyzed whether completeness of resection improves local tumor control.1 Furthermore, most studies examining the impact of resection on outcomes have relied on clinical assessments by operating surgeons rather than central review of postoperative imaging. Concordance between these two assessment modes is suboptimal, with only a 66% correlation in one study.15
Recently, antibody therapy has been shown to improve event-free survival (EFS) and overall survival (OS) in high-risk neuroblastoma.16,17 Because antibody therapy seems most effective against bone marrow and not soft tissue masses, its use might unmask a positive effect of resection on outcome. Alternatively, improved survival might obscure any clinically apparent effect of resection. The primary goal of this study was to determine the correlation between the extent of primary tumor resection and EFS, OS, and cumulative incidence of local progression (CILP) in patients enrolled in the Children’s Oncology Group (COG) A3973 study of high-risk neuroblastoma (NCT#00004188).18 We also evaluated concordance between the extent of resection determined clinically and that established by central review of postoperative imaging.
PATIENTS AND METHODS
Selection of the Analytic Cohort
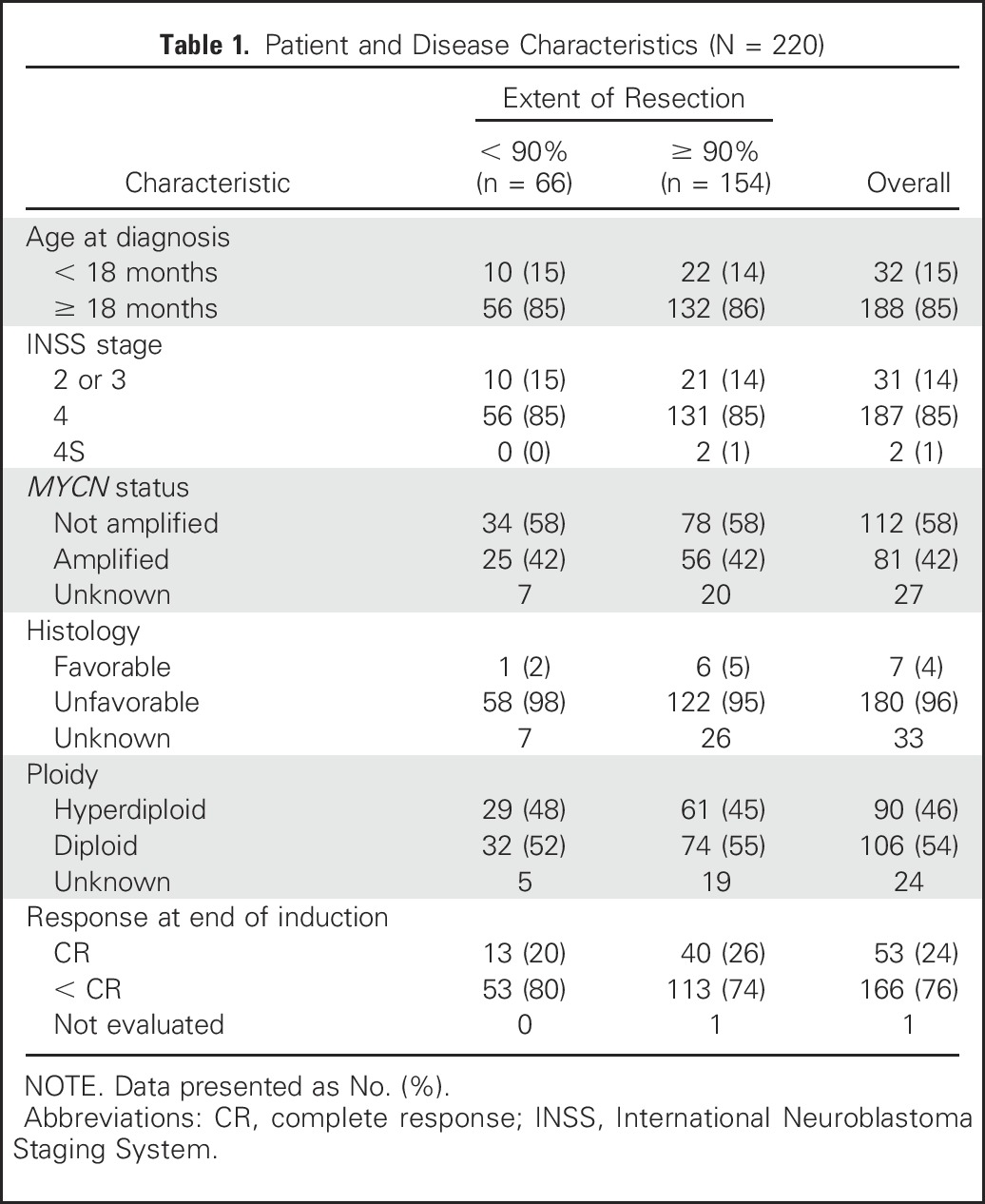
A summary of the A3973 protocol and its findings are included in the Appendix (online only). Of 486 eligible patients, 242 had surgery central review. Twenty-one had surgery on a nonprimary site, and one patient died on the day of enrollment; these 22 patients (9%) were excluded from analysis, yielding an analytic cohort of 220 patients (Table 1).
Table 1.
Patient and Disease Characteristics (N = 220)
For local control of disease, patients received surgery and radiation therapy. Completeness of resection was determined initially by the individual operating surgeon and noted in the surgical checklist as complete (no visible/palpable disease), near complete (> 90%), significant (> 50%), or limited (< 50%). For this analysis, resection categories of < 90% and ≥ 90% were used. For a subset of patients (n = 84; 38%), central reviews of pre- and postoperative computed tomography scans were used to determine resection extent by cross-sectional primary tumor measurements and compared with the resection extent as determined by the operating surgeon and recorded in the surgical checklist. Surgical complications were tabulated in a subset of 110 patients.
Statistical Considerations
EFS time was calculated from study enrollment until first occurrence of relapse, progressive disease, secondary malignancy, death from any cause, or until last contact if no event occurred. For OS, the time to event was calculated from study enrollment until death from any cause or until last patient contact. For CILP, time was calculated from study enrollment until first occurrence of the event of interest (progressive disease at the primary resection site) or a competing risk (relapse or progressive disease at a nonprimary site, secondary malignancy, or death from any cause), or until last patient contact if no event or competing risk occurred. For EFS and OS, Kaplan-Meier survival probabilities were computed and subgroups were compared using the log-rank test.19 CILP estimates were computed and compared between subgroups using Gray’s test.20 Point estimates of EFS, OS, and CILP are reported ± SE. Multivariable Cox proportional hazards models of EFS and OS were constructed to determine the risk of an event for the < 90% versus ≥ 90% resection groups (hazard ratio [HR]).21 The Cox models tested resection extent with adjustment for other biologic or clinical prognostic factors, including the use of backward selection to obtain the most parsimonious model. Similarly, multivariable proportional hazards models of CILP were constructed, testing resection extent with adjustment for other biologic or clinical prognostic factors in the most parsimonious model using the methods of Fine and Gray.22 Concordance between the operating surgeon’s assessment and central review image-aided assessment of resection extent was calculated using Cohen’s simple κ statistic (< 90% v ≥ 90%),23 with κ values < 0 indicating no agreement, 0 to 0.20 as slight, 0.21 to 0.40 as fair, 0.41 to 0.60 as moderate, 0.61 to 0.80 as substantial, and 0.81 to 1 as almost perfect agreement.24 Fisher’s exact test was used to evaluate the association between the resection extent and occurrence of complications. A Wilcoxon rank sum test was used to analyze associations between resection extent and estimated blood loss volume for patients with major intraoperative hemorrhage. Analyses were performed to compare < 90% versus ≥ 90% resection as assessed by the surgeons and as determined by imaging central review. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc, Cary, NC) and R (https://www.r-project.org/). P < .05 was considered statistically significant.
RESULTS
Patient Characteristics
Our analytic cohort included 220 patients, 188 (85%) of whom presented at age ≥ 18 months. On the basis of International Neuroblastoma Staging System (INSS) criteria, the disease was stage 4 in 187 (85%) patients, stage 4S in two (1%), and either stage 2 or 3 in 31 (14%) patients. The primary site was adrenal/abdominal in 192 (87%), thoracic in 11 (5%), nonthoracic/mediastinal/adrenal in seven, and unknown in 10 patients. MYCN was amplified in 81 (42%) of 193 patients with known MYCN status, and histology was unfavorable in 180 (96%) of the 187 patients with available data. Of 196 patients with known DNA ploidy, 106 (54%) had diploid tumors and 90 (46%) had hyperdiploid tumors. Among 219 patients evaluated for induction response, complete response was achieved in 53 (24%) and response was incomplete in 166 (76%). For each characteristic, the proportion of patients was evenly balanced between < 90% and ≥ 90% resection (Table 1).
Extent of Resection and Survival
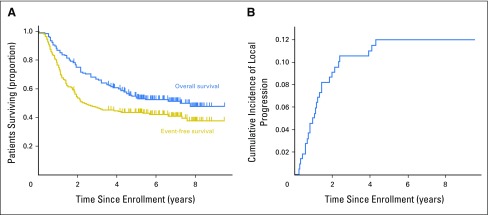
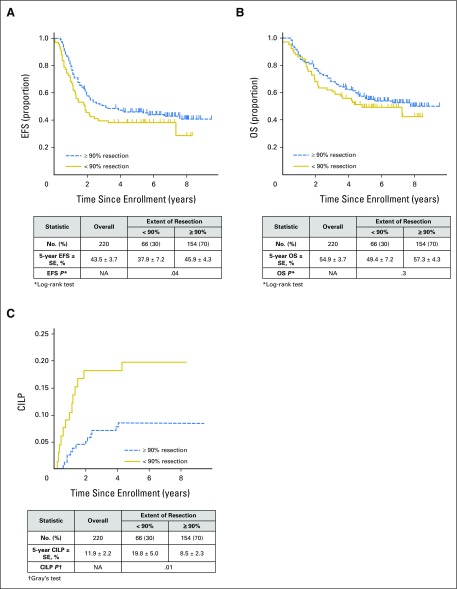
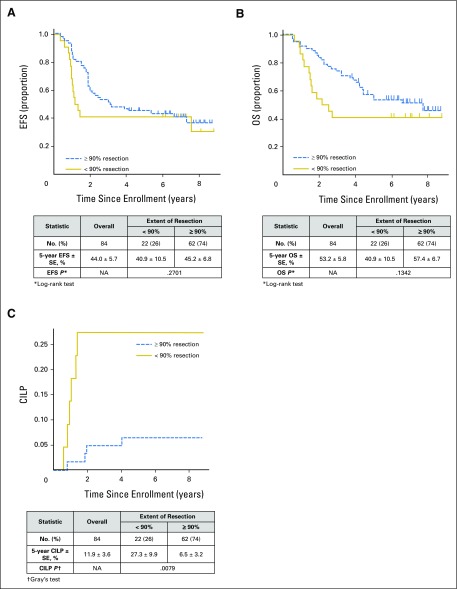
Resection extent, as determined by local surgeons’ assessment, was ≥ 90% in 154 (70%) patients, and < 90% in 66 (30%) patients. Five-year EFS, OS, and CILP were 43.5% ± 3.7%, 54.9% ± 3.7%, and 11.9% ± 2.2%, respectively (Fig 1). EFS (HR = 1.4 [95% CI, 0.9 to 2.0]; P = .04 [log-rank test]) was significantly higher with ≥ 90% resection (45.9% ± 4.3%) versus < 90% resection (37.9% ± 4.3%), but observed differences in OS were not statistically significant (HR = 1.2 [95% CI, 0.8 to 1.9]; P = .3; 57.3% ± 4.3% v 49.4% ± 7.2%, respectively; Figs 2A and 2B). Lower CILP (P = .01) was observed for ≥ 90% resection (8.5% ± 2.3%) compared with < 90% resection (19.8% ± 5.0%; HR = 2.6 [95% CI, 1.2 to 5.5]; P = .02; Fig 2C). Differences in EFS, OS, and CILP for < 90% versus ≥ 90% resection, as determined by imaging central review, were of similar or greater magnitude (Fig 3), but the smaller sample size (n = 84) limited statistical power. Lower CILP, however, remained strongly associated with ≥ 90% resection. Comparing adrenal/abdominal primary site (n = 192) versus thoracic (n = 11), we found no differences between groups with respect to EFS, OS, or CILP (Appendix Table A1, online only).
Fig 1.
(A) Event-free survival and overall survival, and (B) cumulative incidence of local progression in 220 patients from Children’s Oncology Group high-risk neuroblastoma study A3973 whose data underwent surgical central review.
Fig 2.
Survival curves for the 220 patients from the Children’s Oncology Group A3973 high-risk neuroblastoma study whose data underwent surgical central review of extent of primary tumor resection (< 90%: n = 66; ≥ 90%: n = 154). (A) Event-free survival (EFS), P = .04; (B) overall survival (OS), P = .3; and (C) cumulative incidence of local progression (CILP), P = .014. NA, not applicable.
Fig 3.
Survival curves for the subset of 84 patients from the Children’s Oncology Group A3973 high-risk neuroblastoma study whose data underwent central, cross-sectional image review of pre- and postoperative computed tomography scans to determine extent of resection, by extent of primary tumor resection (< 90%: n = 22; ≥ 90%: n = 62). (A) Event-free survival (EFS), P = .3; (B) overall survival (OS), P = .1; and (C) cumulative incidence of local progression (CILP), P = .008. NA, not applicable.
In multivariable Cox models, resection extent remained statistically significant for EFS after adjustment for MYCN amplification or diploidy, but resection extent was not significant for OS after adjustment for another biologic/clinical factor (Table 2). In the final parsimonious model for EFS, INSS stage 4 was independently significant. For OS, INSS stage 4 and MYCN amplification were independently significant (Table 2). However, in multivariable proportional hazards regression of CILP, extent of resection was the only independently statistically significant factor.
Table 2.
Proportional Hazards Models of EFS, OS, and CILP Testing Resection Extent (< 90% v ≥ 90% [reference level]), Alone and With Adjustment for Other Biologic/Clinical Factors (N = 220)

Extent of Resection and Response to Induction Therapy
There was no association detected between resection extent (< 90% v ≥ 90%) and response to induction therapy (complete response v less than complete response P = .3). Results were similar according to the imaging central review of resection extent.
Comparison of Approaches to Assessment of Extent of Resection
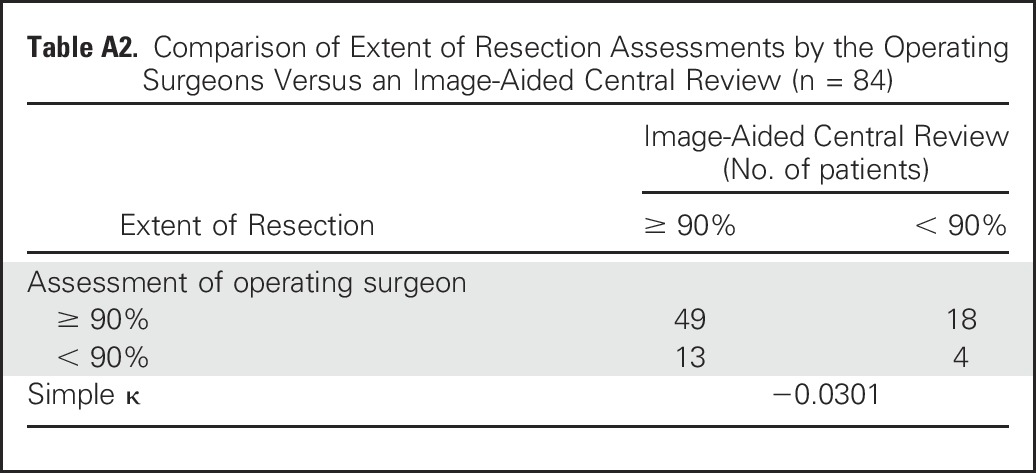
There was limited concordance between the operating surgeon’s assessment and central review image-aided assessment of resection extent. Considering the two categories (< 90% or ≥ 90%), simple κ = −0.0301 indicates no agreement, with concordance for 63% of patients (Appendix Table A2, online only).
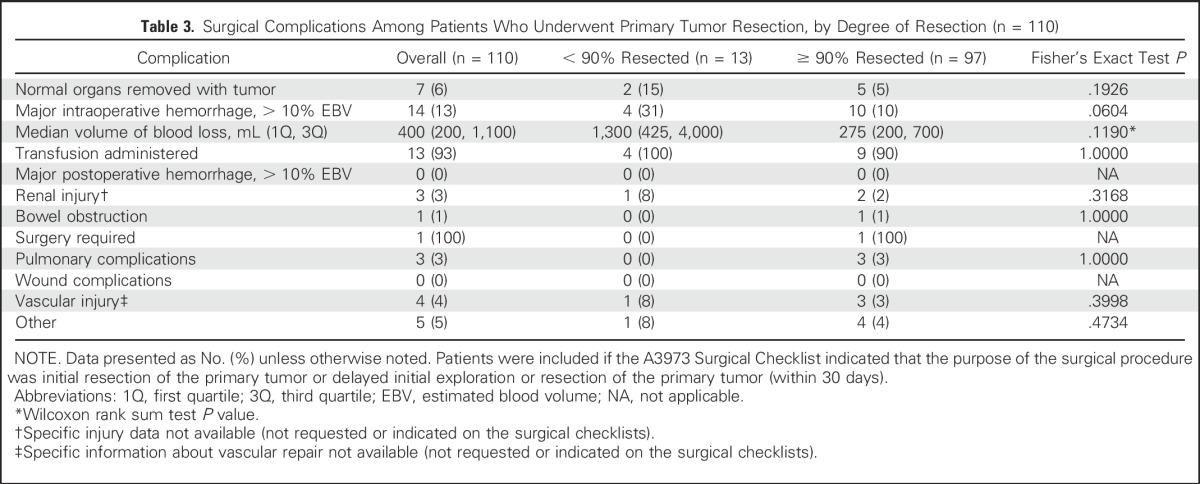
Extent of Resection and Complications
The most frequently occurring complication was major intraoperative hemorrhage in 14 (13%) patients (Table 3). Specific information about type of renal injury or vascular injury repair (eg, patch vein repairs, use of synthetic vascular grafts) was not listed on the surgical checklists. The number of normal organs removed was not different for the < 90% versus ≥ 90% resection groups. There were no occurrences of major postoperative hemorrhages or wound complications. No significant difference in the proportion of patients with a complication was detected between the < 90% versus ≥ 90% resection. Among those patients with major intraoperative hemorrhage, no difference was detected in estimated blood loss volume or proportion of patients receiving a transfusion when comparing < 90% versus ≥ 90% resection (Table 3). There were no reports of major chyle leaks.
Table 3.
Surgical Complications Among Patients Who Underwent Primary Tumor Resection, by Degree of Resection (n = 110)
Operative Mortality
There were no intraoperative deaths in this study. Two deaths occurred within 30 days of surgery and both were in the < 90% resection group.
DISCUSSION
On the basis of our analysis of patients with high-risk neuroblastoma enrolled in the COG A3973 trial, achieving a surgeon-assessed resection extent of ≥ 90% seems to have a beneficial therapeutic role in terms of decreasing the cumulative incidence of local relapse and possibly a positive impact on EFS. However, resection of ≥ 90% of the primary tumor ultimately had little effect on OS. The proportions of patients in this analytic cohort, according to the risk factors in use when the A3973 study was designed, seem similar to the overall A3973 study cohort, in which 86% of patients were ≥ 18 months of age at diagnosis, 86% had INSS stage 4 disease, 44% had MYCN amplification, and 96% had tumors with unfavorable histology. The EFS and OS curves for this patient cohort are similar to those of the overall A3973 high-risk neuroblastoma study cohort.18 Therefore, this surgical central review study cohort is considered sufficiently representative of patients with high-risk neuroblastoma from this treatment era. However, our analysis is limited by a modest sample size (n = 220), which did not provide sufficient power to detect, as statistically significant, the observed 8% difference in 5-year OS.
To understand the role of primary site resection in high-risk neuroblastoma, the first consideration is determining a realistic and feasible definition of surgical completeness. However, the nature of neuroblastoma is such that large-volume primary tumors, lymph node involvement, and widely disseminated metastasis are common in newly diagnosed patients. Neuroblastoma frequently spreads to the regional lymphatic system, where metastases may grow to enormous size, resulting in the huge masses seen in many patients at diagnosis. Although the combination of a large primary tumor and conglomerate regional nodal metastases can be approached with resection, these tumors are typically in close proximity to major visceral blood vessels, the pancreas, spleen, and kidneys, and the spine. Furthermore, vessel and nerve encasement can preclude a complete, microscopically clear resection. Often, the best resection possible is a resection removing all visible and palpable neuroblastoma. Therefore, because of the biology of high-risk neuroblastoma, margins are invariably positive, requiring added locoregional radiotherapy for tumor control. Because the radiotherapy dose needed to sterilize macroscopic tumor deposits is higher than that needed for microscopic residual tumor, only a gross total resection may affect radiotherapy dose selection. Rational analysis of the role of resection now depends on the ability to determine extent of resection.
Completeness of resection is ideally determined by central, nonbiased review and comparison of pre- and postoperative imaging studies (computed tomography scan, magnetic resonance imaging, [125I]metaiodobenzylguanidine scan). Given the expense and complexity of coordinating such assessments in studies involving hundreds of institutions, the most commonly used surrogate is the operating surgeon’s report. However, in a combined study from two pediatric centers, the resection extent determined by imaging studies was different from the surgical evaluation in at least 33% of patients.15 In the current study, the discordance between the operating surgeon’s assessment and imaging-aided assessment was also rather high (37%). The reason for the lack of agreement is difficult to assess. In some cases, a lack of neuroblastoma surgical experience may affect the preoperative determination of the extent of primary tumor involvement, which then affects the surgeon’s determination of the extent of primary tumor resection.
Despite the anatomic difficulties presented by many tumors, 70% of patients in our cohort underwent a surgeon-assessed resection of ≥ 90%, which was associated with a reduced cumulative incidence of local progression. Interestingly, the survival analysis on the basis of the resection extent determined via central imaging review also showed that ≥ 90% resection correlated with improved local control (Fig 3). Therefore, the most compelling reason to perform ≥ 90% resection is its positive impact on local control. Indeed, near-complete resection was the only covariate that had an independent effect on local progression-free survival (PFS) in multivariate analysis, which is consistent with findings from several single-institution studies.7,15,25 Our study used competing risk analysis to assess the effect of ≥ 90% resection and, to our knowledge, is the first to establish an independent effect in multivariate analysis as well. Surgery is a focal intervention, and it is logical to suppose that its greatest influence would be on the primary site, as we observed.
A number of publications have questioned the role of resection in high-risk neuroblastoma.1,9-13 Simon et al1 reported data from the German NB97 trial, completed in 2003. The investigators analyzed OS, EFS, and local control in patients with stage-4 disease, but no correlation with the extent of surgery was found for these outcomes. It is important to recognize that the NB97 protocol differed greatly from COG A3973. The principal question in the German study was a comparison between patients undergoing myeloablative chemotherapy with autologous stem-cell rescue versus maintenance chemotherapy. In comparison, all patients in the COG A3973 study underwent stem-cell transplantation. Of crucial importance is the fact that German patients with residual local disease were treated with 40 Gy to the primary site, whereas all patients on COG A3973, regardless of resection extent, received 21.6 Gy. This differential of almost 20 Gy in the analysis by Simon et al1 may explain the equivalent survival of patients with residual disease after surgery. However, a significant increase in radiation-associated late effects, including secondary malignancies, has been associated with doses > 31 Gy in patients with neuroblastoma.26 Although it may be possible to improve local control in patients with residual disease by administering radiation doses in the 40-Gy range, this management strategy would substantially worsen the toxicity profile. Consistent with the German findings, a single-institution Italian study, reported by De Ioris et al,10 also found no correlation between more radical surgery and OS, PFS, or local PFS in a retrospective study of 58 patients. However, their study comprised two separate, different treatment protocols. Treatment details and outcomes stratified for each group were not provided in the published report. One group, consisting of 48% of patients reported, did not receive radiotherapy to the primary site, as was administered in A3973. Both of these factors confound the analysis. Another study, by Yeung et al,13 also found no association between more extensive surgery and OS or EFS. However, this report included only 34 patients and, of these, only four had a < 90% resection. The 5-year OS and EFS rates were 51% and 55%, respectively. Thus, it is quite possible that these relatively good outcomes may be related to the ≥ 90% resection achieved in 88% of patients.
Mullassery et al12 performed a systematic literature review of neuroblastoma surgery comparing patients undergoing gross total resection to subtotal resection. A significant limitation of this analysis, however, is the retrospective nature of the studies included; no randomized trials were included, because none have been published to date. Furthermore, these were post-hoc analyses, and surgery was not considered in the initial specific aims of the studies. Finally, only two cooperative group studies were included. Although the authors concluded that more extensive surgery had no effect on OS, the odds ratio for survival among patients in whom gross total resection was achieved was 1.36 (95% CI, 0.96 to 1.91). Given the heterogeneity observed, this comes remarkably close to favoring the more extensive surgery arm. In addition, this analysis did show improvement in disease-free survival with more complete resection (odds ratio, 1.55; 95% CI, 1.12 to 2.14), as we found in COG A3973.
Englum et al11 retrospectively analyzed a cohort of 104 children referred to a major center for stem-cell transplantation who underwent surgeries at 14 different referring institutions. Patients who underwent > 90% resection had improved EFS and OS.11 In a 2004 report, Adkins et al8 noted that, although not statistically significant, EFS improved from 19% to 26% with more extensive resection in CCG 3891. Finally, Holmes et al,14 in a report published in abstract form that included almost 1,500 high-risk patients from the SIOPEN I study, showed that ≥ 90% resection was positively correlated with both EFS and OS.
In our univariate analysis, ≥ 90% resection was associated with improved EFS. This effect remained after controlling for MYCN amplification and diploidy but not when INSS stage 4 was added to the model, implying a predominant effect of metastases on outcome. This presumed impact of metastatic disease is consistent with the OS curves, in which ≥ 90% resection was not associated with a better outcome. Metastases to bone and bone marrow remain the major impediment to improving outcomes in high-risk neuroblastoma and are unaffected by primary tumor resection. It is possible that better control of metastases, particularly the control of bone marrow metastases with anti-disialoganglioside (GD2) antibody therapy, may further clarify the role of removing large soft tissue deposits in neuroblastoma therapy. Nevertheless, cure in high-risk neuroblastoma depends on achieving a complete response at primary as well as metastatic sites. Our analysis indicates that primary-site local control is best achieved by ≥ 90% resection. Finally, as the rate of major complications did not differ between resection groups, we conclude that ≥ 90% resection is both feasible and safe in most patients with high-risk neuroblastoma.
In summary, our analysis demonstrates that ≥ 90% resection of the primary tumor in the treatment of high-risk neuroblastoma has a positive effect on outcomes in a representative cohort from the COG A3973 trial. These data indicate a strong association between more complete resection and better local control; indeed, resection of ≥ 90% was the only independent predictor of reduced cumulative incidence of local progression in multivariate analysis. In addition, the complication rate was not increased in patients who underwent more extensive surgery. In multivariate analysis, ≥ 90% resection was not correlated with EFS or OS. We found poor concordance between the assessment of resection extent by the operating surgeons and assessment via central imaging review. Our findings support continued attempts at ≥ 90% resection of the primary tumor in this cohort. The role of unbiased review of postsurgical imaging in predicting patient outcome warrants further investigation.
Appendix
Summary of the COG A3973 Protocol and Its Findings
COG A3973 was a prospective, randomized trial comparing peripheral blood stem cell transplantations purged of tumor cells with nonpurged peripheral blood stem cell transplantations in the treatment of patients with high-risk neuroblastoma (N = 486).18 Overall 5-year event-free survival was 40% (95% CI, 33% to 46%) and 36% (95% CI, 30% to 42%) for purged and nonpurged regimens (P = .77), respectively. Five-year overall survival rates were 50% (95% CI, 43% to 56%) and 51% (95% CI, 44% to 57%) in the purged and nonpurged groups, respectively (P = .81). Extent of surgery, surgical complications, and impact of the extent of surgery on outcome were secondary prospective aims in the study. By protocol, patients underwent an attempt at definitive resection of the primary tumor and involved regional lymph node echelons after the fifth cycle of induction chemotherapy. There was no recommendation to remove grossly normal lymphatic tissue.
Table A1.
EFS, OS, and CILP by Primary Site
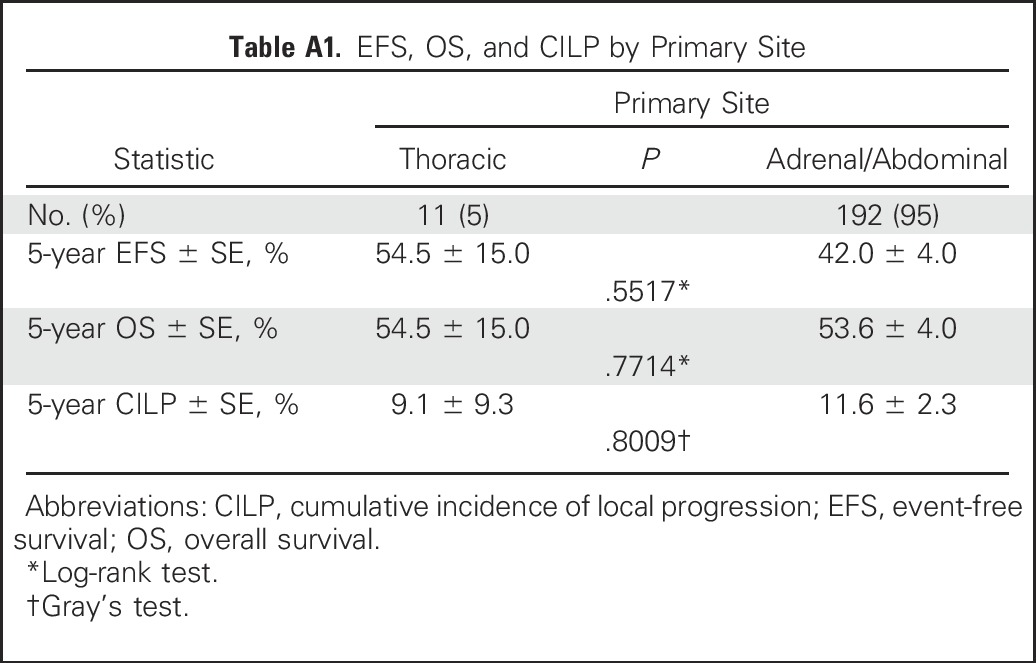
Table A2.
Comparison of Extent of Resection Assessments by the Operating Surgeons Versus an Image-Aided Central Review (n = 84)
Footnotes
Written on behalf of the Children’s Oncology Group, Monrovia, CA.
Supported by National Cancer Institute Grants No. U10 CA29139, U10 CA98413, and U10-CA098543 to the Children’s Oncology Group; and National Institutes of Health Grant No. P30 CA008748 to Memorial Sloan Kettering Cancer Center (M.P.L.).
Presented in part at the 2014 Advances in Neuroblastoma Research Congress, Cologne, Germany, May 13-16, 2014.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Clinical trial information: NCT00004188.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel von Allmen, Andrew M. Davidoff, Wendy B. London, Susan G. Kreissman, Geetika Khanna, Nancy Rosen, Julie R. Park, Michael P. La Quaglia
Administrative support: Wendy B. London, Julie R. Park
Provision of study materials or patients: Daniel von Allmen, Daphne A. Haas-Kogan, Geetika Khanna, Michael P. La Quaglia
Collection and assembly of data: Daniel von Allmen, Wendy B. London, Susan G. Kreissman, Nancy Rosen, Julie R. Park, Michael P. La Quaglia
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Impact of Extent of Resection on Local Control and Survival in Patients From the COG A3973 Study With High-Risk Neuroblastoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Daniel von Allmen
No relationship to disclose
Andrew M. Davidoff
Patents, Royalties, Other Intellectual Property: uniQure, BioMarin, uniQure (Inst), BioMarin (Inst)
Wendy B. London
No relationship to disclose
Collin Van Ryn
No relationship to disclose
Daphne A. Haas-Kogan
Leadership: Cellworks
Stock or Other Ownership: Pfizer, Accuray
Research Funding: Novartis (Inst)
Susan G. Kreissman
Employment: Quintiles (I)
Stock or Other Ownership: Quintiles (I), Johnson & Johnson
Geetika Khanna
No relationship to disclose
Nancy Rosen
No relationship to disclose
Julie R. Park
No relationship to disclose
Michael P. La Quaglia
No relationship to disclose
REFERENCES
- 1.Simon T, Häberle B, Hero B, et al. Role of surgery in the treatment of patients with stage 4 neuroblastoma age 18 months or older at diagnosis. J Clin Oncol. 2013;31:752–758. doi: 10.1200/JCO.2012.45.9339. [DOI] [PubMed] [Google Scholar]
- 2.Chamberlain RS, Quinones R, Dinndorf P, et al. Complete surgical resection combined with aggressive adjuvant chemotherapy and bone marrow transplantation prolongs survival in children with advanced neuroblastoma. Ann Surg Oncol. 1995;2:93–100. doi: 10.1007/BF02303622. [DOI] [PubMed] [Google Scholar]
- 3.Haase GM, O’Leary MC, Ramsay NK, et al: Aggressive surgery combined with intensive chemotherapy improves survival in poor-risk neuroblastoma. J Pediatr Surg 26:1119-1123, 1991; discussion 1123-1124 [DOI] [PubMed] [Google Scholar]
- 4.Kaneko M, Ohakawa H, Iwakawa M. Is extensive surgery required for treatment of advanced neuroblastoma? J Pediatr Surg. 1997;32:1616–1619. doi: 10.1016/s0022-3468(97)90466-8. [DOI] [PubMed] [Google Scholar]
- 5.Kiely EM. Radical surgery for abdominal neuroblastoma. Semin Surg Oncol. 1993;9:489–492. doi: 10.1002/ssu.2980090606. [DOI] [PubMed] [Google Scholar]
- 6.Koh CC, Sheu JC, Liang DC, et al. Complete surgical resection plus chemotherapy prolongs survival in children with stage 4 neuroblastoma. Pediatr Surg Int. 2005;21:69–72. doi: 10.1007/s00383-004-1353-x. [DOI] [PubMed] [Google Scholar]
- 7.La Quaglia MP, Kushner BH, Su W, et al: The impact of gross total resection on local control and survival in high-risk neuroblastoma. J Pediatr Surg 39:412-417, 2004; discussion 412-417 [DOI] [PubMed] [Google Scholar]
- 8.Adkins ES, Sawin R, Gerbing RB, et al. Efficacy of complete resection for high-risk neuroblastoma: A Children’s Cancer Group study. J Pediatr Surg. 2004;39:931–936. doi: 10.1016/j.jpedsurg.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 9.von Schweinitz D, Hero B, Berthold F. The impact of surgical radicality on outcome in childhood neuroblastoma. Eur J Pediatr Surg. 2002;12:402–409. doi: 10.1055/s-2002-36952. [DOI] [PubMed] [Google Scholar]
- 10.De Ioris MA, Crocoli A, Contoli B, et al. Local control in metastatic neuroblastoma in children over 1 year of age. BMC Cancer. 2015;15:79. doi: 10.1186/s12885-015-1082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englum BR, Rialon KL, Speicher PJ, et al. Value of surgical resection in children with high-risk neuroblastoma. Pediatr Blood Cancer. 2015;62:1529–1535. doi: 10.1002/pbc.25504. [DOI] [PubMed] [Google Scholar]
- 12.Mullassery D, Farrelly P, Losty PD. Does aggressive surgical resection improve survival in advanced stage 3 and 4 neuroblastoma? A systematic review and meta-analysis. Pediatr Hematol Oncol. 2014;31:703–716. doi: 10.3109/08880018.2014.947009. [DOI] [PubMed] [Google Scholar]
- 13.Yeung F, Chung PH, Tam PK, et al. Is complete resection of high-risk stage IV neuroblastoma associated with better survival? J Pediatr Surg. 2015;50:2107–2111. doi: 10.1016/j.jpedsurg.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 14.Holmes K, Sarnacki S, Poetschger U, et al: Influence of surgical excision on survival of patients with high risk neuroblastoma: Report from Study 1 of SIOP Europe (SIOPEN). Presented at Advances in Neuroblastoma Research (ANR) 2014, Cologne, Germany, May 13-16, 2014 [Google Scholar]
- 15.von Allmen D, Grupp S, Diller L, et al: Aggressive surgical therapy and radiotherapy for patients with high-risk neuroblastoma treated with rapid sequence tandem transplant. J Pediatr Surg 40:936-941, 2005; discussion 941 [DOI] [PubMed] [Google Scholar]
- 16.Yu AL, Gilman AL, Ozkaynak MF, et al. Children’s Oncology Group Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu AL, Gilman AL, Ozkaynak MF, et al: Update of outcome for high-risk neuroblastoma treated on a randomized trial of chimeric anti-GD2 antibody (ch14.18) + GM-CSF/IL2 immunotherapy in 1st response: A Children’s Oncology Group study. Presented at Advances in Neuroblastoma Research (ANR) 2014, Cologne, Germany, May 13-16, 2014 [Google Scholar]
- 18.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008. doi: 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan EL, Meier P: Nonparametric-estimation from incomplete observations. J Am Stat Assoc 53:457-481, 1958 [Google Scholar]
- 20.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 21.Cox DR, Oakes D. Analysis of Survival Data. Boca Raton, FL, Chapman and Hall/CRC, 1984. [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 23.Cohen J. Weighted kappa: Nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–220. doi: 10.1037/h0026256. [DOI] [PubMed] [Google Scholar]
- 24.Landis JR, Koch G.G. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 25.Kaneko M, Iwakawa M, Ikebukuro K, et al. Complete resection is not required in patients with neuroblastoma under 1 year of age. J Pediatr Surg. 1998;33:1690–1694. doi: 10.1016/s0022-3468(98)90611-x. [DOI] [PubMed] [Google Scholar]
- 26.Ducassou A, Gambart M, Munzer C, et al. Neuroblastoma study group and radiotherapy group of the French Society of Children with Cancer (SFCE) Long-term side effects of radiotherapy for pediatric localized neuroblastoma: Results from clinical trials NB90 and NB94. Strahlenther Onkol. 2015;191:604–612. doi: 10.1007/s00066-015-0837-z. [DOI] [PubMed] [Google Scholar]