Abstract
Proteases are important regulators of pulmonary remodeling and airway inflammation. Recently, we have characterized the enzyme prolyl endopeptidase (PE), a serine peptidase, as a critical protease in the generation of the neutrophil chemoattractant tripeptide Pro-Gly-Pro (PGP) from collagen. However, PE has been characterized as a cytosolic enzyme, and the mechanism mediating PE release extracellularly remains unknown. We examined the role of exosomes derived from airway epithelia as a mechanism for PE release and the potential extracellular signals that regulate the release of these exosomes. We demonstrate a specific regulatory pathway of exosome release from airway epithelia and identify PE as novel exosome cargo. LPS stimulation of airway epithelial cells induces release of PE-containing exosomes, which is significantly attenuated by small interfering RNA depletion of Toll-like receptor 4 (TLR4). These differences were recapitulated upon intratracheal LPS administration in mice competent versus deficient for TLR4 signaling. Finally, sputum samples from subjects with cystic fibrosis colonized with Pseudomonas aeruginosa demonstrate elevated exosome content and increased PE levels. This TLR4-based mechanism highlights the first report of nonstochastic release of exosomes in the lung and couples TLR4 activation with matrikine generation. The increased quantity of these proteolytic exosomes in the airways of subjects with chronic lung disease highlights a new mechanism of injury and inflammation in the pathogenesis of pulmonary disorders.
Keywords: protease, Toll-like receptor 4, exosome, cystic fibrosis, pulmonary
Clinical Relevance
We demonstrate that the activation of Toll-like receptor 4 receptor increases the release of the protease-containing exosomes from the lung. These exosomes are found in the lungs of patients with cystic fibrosis lung disease and may add to the ongoing damage and inflammation observed in chronic lung disorders.
Cell-to-cell communication via secreted soluble mediators is critical to the maintenance of cellular homeostasis in complex metazoans. Much of this intercellular communication is regulated through the classical secretory mechanism, in which proteins containing an endoplasmic reticulum (ER)-signaling sequence are actively released via the ER-to-Golgi pathway (1). Despite the broad range of proteins entering this pathway, a number of actively secreted proteins lack a signal sequence for release and are released via alternative mechanisms, including exosomes (2).
Exosomes are small vesicles (40–100 nm) released from a variety of cells and isolated from all body fluids (3). They are accumulated within multivesicular bodies by the invagination of the lining membrane, thereby incorporating cytosolic proteins for subsequent release (4). Proteins secreted by exosomes can be found either within the exosomes or on the surface of exosomes (5, 6). These vesicles are released by a variety of immune and nonimmune cells (3). Multiple tumor cells also release increased levels of exosomes, suggesting these vesicles may serve as a specific pathway of cell-to-cell communication in cancer, promoting a protooncogenic phenotype in adjacent nontumor tissues (7).
Proteases are found at increased concentrations within the airways of individuals with chronic lung disease (8, 9). Although these enzymes primarily function intracellularly (10), a subset are transported extracellularly, where they have been largely described in regulating ECM remodeling (11). Extracellular proteases have also recently been shown to exhibit unique roles in controlling cellular homeostasis through a variety of mechanisms, including regulation of immune responses, disruption of cell-to-cell contacts, modulation of cell surface receptors for signal transduction, and modulation of cell–ECM interactions (12–15).
The increased extracellular presence of specific proteases in the lung has been linked to inflammatory stimuli and immune cell activation, including the generation of matrix-derived chemoattractants (9, 16, 17). Our laboratory has recently described a serine protease, prolyl endopeptidase (PE), as an extracellular protease critical to the regulation of chronic neutrophilic inflammatory responses in human lung diseases (18). Specifically, this protease cleaves collagen fragments and liberates a three-amino-acid sequence (Pro-Gly-Pro [PGP]), which acts as an important neutrophil chemoattractant (19, 20). The extracellular release of this protease was unexpected because this enzyme harbors no signal sequence for export through the classical secretory pathway, indicating that this protease is released via unconventional secretion.
Previous reports have indicated that engagement of specific extracellular receptors can regulate the release of exosomes from hematopoetic cells (21, 22). However, no specific extracellular ligand/receptor interaction has been identified that induces the release of exosomes from airway epithelia. In this manuscript, we identify a novel population of proteolytic exosomes released from airway epithelial cells upon engagement of the pattern recognition receptor to LPS Toll-like receptor 4 (TLR4). We further describe the presence of these exosomes in clinical samples from patients with cystic fibrosis (CF) lung disease, suggesting that this pathway may be operative in chronic inflammatory lung disorders.
Materials and Methods
Study Approval
All subjects carried the diagnosis of CF based on routine diagnostic criteria (23). Clinical characteristics of the study subjects are described in Table 1. The study protocol was approved by the Institutional Review Board at Emory University and the University of Alabama at Birmingham. Sputum was collected via spontaneous expectoration for subjects with CF and 3% hypertonic saline induction for control subjects without lung disease. Written informed consent was received from participants before inclusion in the study.
Table 1.
Clinical Characteristics of the Study Population
| Subjects with Cystic Fibrosis (n = 5) | Control Subjects without Lung Disease (n = 5) | |
|---|---|---|
| Age, yr (SD) | 27.6 (3.4) | 26.7 (2.2) |
| Race | 5/5 white (100%) | 5/5 white (100%) |
| Sex | 3/5 male (60%) | 4/5 male (80%) |
| 2/5 female (40%) | 1/5 female (20%) | |
| Mutation | 3/5 ΔF508 homozygous (60%) | ND |
| 2/5 ΔF508 heterozygous (40%) | ||
| Bacteriology | 4/5 mucoid Pseudomonas aeruginosa (80%) | ND |
| 1/5 nonmucoid P. aeruginosa (20%) | ||
| FEV1, liters (mean ± SD) | 2.3 ± 1.0 | ND |
| FEV1, % (mean ± SD) | 55.8 ± 22.2 | ND |
Definition of abbreviation: ND, not determined.
Murine LPS Model
Female C3He/J or C3He/B mice (6–8 wk old) were intratracheally administered either PBS or LPS (75 μg/50 μl) once a day over a 3-day period and then underwent bronchoalveolar lavage as previously described (21, 23). All studies were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Antibodies and Reagents
Rabbit polyclonal anti-PE antibodies were obtained from Sigma (St. Louis, MO) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse monoclonal anti–α-tubulin and antiactin antibodies, LPS from Pseudomonas aeruginosa, PE-specific inhibitor S17092, was purchased from Sigma. Mouse monoclonal anti-CD63 antibody was from Millipore (Temecula, CA). Mouse monoclonal anti–Flotillin-1, anti-EEA1, and anti-GM130 antibodies were from BD Transduction Laboratories (San Jose, CA). Mouse monoclonal anti-Alix, anti-CD81 (5A6), and anti-CD9 (C-4) antibodies were from Santa Cruz Biotechnology, Inc. Rabbit polyclonal anti-TSG101 and mouse monoclonal anti-mannose 6 phosphate receptor antibodies were from Abcam (Cambridge, MA). Mouse monoclonal anti-ERGIC53 antibody G1/93 were a generous gift from Dr. Hauri (University of Basel, Basel, Switzerland). The LDH cytotoxicity kit was from Cayman Chemical (Ann Arbor, MI). The recombinant PE was created in our laboratory as previously described (24).
Small Interfering RNA Treatment
Small interfering RNA (siRNA) against human TLR4 (cat. #4390824) and nontargeting (scramble) siRNA (cat. #AM4611) were designed and synthesized as annealed primers by Ambion (Austin, TX). Cells were transfected with siRNA using siLentFect lipid from BioRad Laboratories (Hercules, CA) according to the manufacturer’s instructions. Cells were processed for Western blotting after 72 hours of incubation with siRNAs (25).
Cell Culture and Treatment
The human bronchial epithelial cell line (16HBE14o-), the human submucosal bronchial gland cell line (Calu-3), the CF bronchial epithelial cell line (CFBE41o- par), and CFBE cells homozygous for either the ΔF508 mutation (CFBE F508) or its isogenic wild-type CFTR complement counterpart (CFBE WT) were used. Primary human bronchial epithelial cells were maintained in the UAB Cystic Fibrosis Research Center. The cell lines were grown in modified Eagle’s medium. Primary human bronchial epithelial cells were grown on 6-well Transwell inserts (Corning Inc. Life Sciences, Lowell, MA) under air–liquid interface conditions for a minimum of 14 days to achieve polarization and differentiation using bronchial epithelial growth medium with supplements.
Stimulation of epithelial cultures was performed with 1.0 μg/ml LPS (P. aeruginosa) (Sigma). PE activity neutralization in cell lysates was performed in the presence or absence of S17092. After stimulation, lysates were collected for PE activity or enzyme concentration.
Immunocytochemistry
For immunofluorescence, cells on cover slips were as previously described (25). Briefly, for immunofluorescence, cells on cover slips were washed in PBS, fixed in 3% paraformaldehyde, and quenched with 10 mM ammonium chloride. Cells were permeabilized with 0.1% Triton-X-100 in PBS. After washing with PBS, cells were blocked in PBS with 2.5% goat serum and 0.2% Tween-20, followed by blocking in PBS with 0.4% fish skin gelatin and 0.2% Tween-20. Cells were then processed for double-labeling with the indicated primary antibody diluted in PBS containing 0.4% fish skin gelatin and 0.2% Tween-20. After 1 hour of incubation at room temperature, cells were washed in PBS with 0.2% Tween and blocked as described above. Next, cells were incubated for 1 hour at room temperature with a secondary antibody labeled with either Alexa Fluor 488 or Alexa Fluor 594 (Invitrogen, Eugene, OR) diluted in PBS containing 2.5% goat serum and 0.2% Tween-20. Nuclei were stained with Hoechst (Sigma). Cells were washed as described above. Cover slips were washed in PBS with 0.2% Tween-20 and mounted on slides in PermaFluor (Thermo Scientific, Waltham, MA).
Microscopy
Fluorescence imaging was performed using an inverted microscope (model 2000U; Nikon, Melville, KY) outfitted with a UltraVIEW ERS 6FE-US spinning disk laser apparatus (PerkinElmer, Shelton, CT). Optical sections through the Z axis were generated using a computer-controlled focus step motor. Flattened projections of image stacks were prepared using proprietary confocal imaging Volocity 6.1 software (Improvision Inc., Waltham, MA). The 100×, 60×, or 40× oil (NA 1.4) objectives were used depending on the experimental protocol (26).
For negative stain electron microscopy, isolated exosomes were resuspended in 50 μl double-distilled water, and an aliquot of 3 μl of resuspended exosomes was applied to glo-discharged Quantifoil holey film (Quantifoil MicroTools, Jena, Germany). Samples were washed with water and treated with 1% uranyl acetate for 15 seconds. The samples were then observed in an FEI Tecnai F20 200kV field-emission gun microscope equipped with a high-sensitivity Gatan Ultrascan 4000 CCD camera (27).
PCR Reactions
Total RNA was purified from different human airway epithelial cells samples using an Aurum Total RNA kit (Bio-Rad Laboratories). The PE and TLR4 gene expression analyses were performed first by RT-PCR using One Step RT-PCR Kit (Qiagen, Germantown, MD) and a second using quantitative real-time PCR (qPCR) in TaqMan One Step RT-PCR Master Mix Reagent Assay (Life Technologies, Carlsbad, CA). In qPCR, a gene expression was normalized to GAPDH expression. The relative expression was calculated using the comparative relative standard curve method.
PE Activity Assay
Cell were lysed in lysis buffer (50 mM Tris-HCl [pH 8.0], 5 mM EDTA, 150 mM NaCl, 1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail), and lysates were incubated with assay buffer (25 mM Tris, 0.25 M NaCl [pH 7.5], 2.0 mM DTT) containing a specific flurogenic substrate Suc-Gly-Pro-7-amido-4-methylcoumarin (Suc-Gly-Pro-AMC) (Bachem, Bubendorf, Switzerland) at 37°C and 5% CO2. The cleavage of AMC from substrate by PE was detected using a Fluometer Reader at an excitation wavelength of 360 nm and an emission wavelength of 460 nm and compared with a generated standard curve for PE activity (24).
Exosome Isolation
Exosomes from media collected from CFBE WT cells (+/−LPS treatment) were isolated through differential centrifugation (at 300 × g for 10 min to eliminate cells and large cellular debris, then at 2,000 × g for 20 min followed by 10,000 × g for 30 min to eliminate any remaining membranous debris). Exosomes were pelleted by centrifuging the supernatant at approximately 150,000 × g for 2 hours, and the supernatant was removed. Pellets were resuspended in PBS and centrifuged at approximately 500,000 × g for 15 minutes to eliminate any contaminants. The supernatant was removed, and exosomes were resuspended in the appropriate buffer (27).
Semiquantitation of Exosomes in Conditioned Media
Exosomes in cell culture supernatants were concentrated by differential centrifugation and, after resuspension, were incubated for 24 hours at room temperature with anti-CD63 antibody–coated superparagmagnetic polystyrene beads (Life Technologies). Various bead and culture supernatant concentrations were used to obtain unsaturated beads for semiquantitation as previously described (28). Exosome-coated beads were magnetically separated, washed, and labeled with an anti-CD63 antibody (clone H5C6) conjugated with phycoerythrin (BioLegend, San Diego, CA) for 45 minutes. After washing, beads were examined using a Becton-Dickinson Custom LSRII (Franklin Lakes, NJ), and data were analyzed using FlowJo V7.6.5 (Treestar, Ashland, OR). Single beads were gated based on forward scatter, side scatter, and autofluorescence measured in the detector for PerCP-Cy5.5.
Quantitation of Exosomes in Mouse Bronchoalveolar Lavage Fluid
For measurement of murine exosome content, the Nanosight NS300 (Malvern Instruments, Worcestershire, UK) was used. Briefly, cell-derived vesicles from bronchoalveolar lavage fluid from C3He/B or C3He/J mice treated with LPS or vehicle alone were stained using QTracker 565 (Life Technologies) and examined by nanoparticle tracking analysis using an NS300 equipped with a 488-nm laser module and a 488-nm long pass filter. After staining with QTracker 565, samples were diluted, and only QTracker 565–stained vesicles were visualized using the 488-nm long pass filter. Data were recorded and analyzed using NTA 2.3 software (Malvern Instruments).
Statistical Analysis
Descriptive statistics, including mean and SD, were conducted for all quantitative measures. The two-tailed Student t test was used for comparisons between two groups, and one-sided ANOVA was used for comparisons between three or more groups. The results were considered significant at the 95% confidence level or at P values ≤0.05.
Results
PE Is Present in Human Airway Epithelial Cells
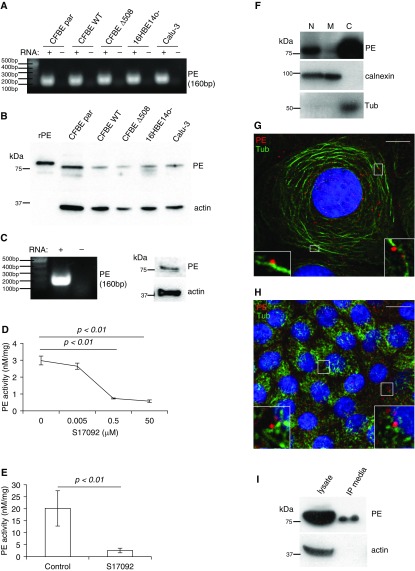
To explore the potential of airway epithelial cells as a source for PE release, we first examined expression of this protease in various airway epithelial cell types. After isolation of total RNA, we performed one-step RT-PCR, confirming the expression of PE mRNA in numerous epithelial cell models (Figure 1A). Cell lysates also demonstrated PE protein expression with a band observed at approximately 80 kD, consistent with the expected molecular weight of PE (29) (Figure 1B). These findings were complemented by the use of fully differentiated primary human bronchial epithelial cells (30), which also demonstrated both mRNA and protein expression for PE (Figure 1C). To further establish that both the mRNA and protein relate to active PE, CFBE WT cells (Figure 1D) and primary airway cells (Figure 1E) were measured for PE activity using a cleavage assay for the PE-specific substrate Suc-Gly-Pro-AMC. The lysates from these cells exhibited elevated PE activity, which was inhibited by the PE-specific inhibitor S17092 (31). These results clearly demonstrate the presence of active PE in airway epithelial cells.
Figure 1.
Human airway epithelial cells express active prolyl endopeptidase (PE) that is secreted from cells. (A) Various airway epithelial cell lines were lysed, and total RNA was purified and probed for PE (160 base pair [bp]) via RT-PCR. (B) Airway epithelial cell lines were lysed, and proteins were resolved in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and probed with anti-PE antibody. The recombinant PE (rPE) serves as a positive control and has slightly higher molecular weight owing to His and Xpress tags. Actin serves as a loading control. (C) Primary human airway epithelial cells were lysed, and total RNA was purified and subjected to RT-PCR for PE or the lysate was resolved in SDS-PAGE and probed with PE antibody. (D) Cystic fibrosis (CF) bronchial epithelial (CFBE) wild-type (WT) cell lysate was subjected to PE activity assay in different concentrations of the PE-specific inhibitor S17092. Data were obtained from three independent experiments. P < 0.01 for 0.5 and 50 μM when compared with control. (E) Primary human airway epithelial cell lysate was subjected to PE activity assay in the presence of S17092. Data were obtained from five independent experiments (P < 0.01). (F) CFBE WT cells were homogenized and subjected to differential centrifugation to separate cell cytosol from the nuclei and cell membranes. After fractionation, equivalent amount of the nuclear (N), cytosolic (C), and membrane (M) fractions were resolved by SDS-PAGE and Western blotted with anti-PE antibody. The nitrocellulose was probed with anticalnexin and antitubulin antibodies to provide recovery standards for membranes (M) and cytosol (C), respectively. CFBE WT (G) and primary human epithelial (H) cells underwent immunofluorescence with anti-PE (red) and antitubulin (Tub; green) antibodies. In both cell types, PE localizes to cell nuclei and cell periphery in a disperse pattern. Most of the PE in the cell periphery aligns with microtubules (insets). Scale bar, 10 μm. (I) Media from CFBE WT cells was collected and immunoprecipitated with anti-PE antibody and compared with PE isolated from CFBE WT cell lysate. Actin serves as a purity control. CFBE par, parental CF bronchial.
Cellular Localization of PE
The presence of PE in airway epithelia raises a question about the cellular compartment wherein PE resides. To identify the cellular localization of PE, we performed fractionation experiments. Cell fractionation showed that most of the PE was recovered in the cytosolic fraction of cells, with some PE in the nuclear fraction. We also detected small amount of PE in the membrane fraction after prolonged film exposure (Figure 1F). Subsequent immunofluorescence staining of CFBE WT cells and polarized human primary bronchial epithelial cells showed a dispersed PE pattern (Figures 1G and 1H). Most of the PE aligned on microtubules (Figures 1G and 1H, insets), suggesting that PE might be actively secreted from cells. We addressed the question by immunoprecipitating PE from CFBE WT growth media. Although PE is lacking a signal sequence for the classical secretory pathway, it is clear that PE is present in the growth media (Figure 1I). However, this result does not account for the particular mechanism by which PE is released from airway epithelial cells.
LPS Stimulates Secretion of PE
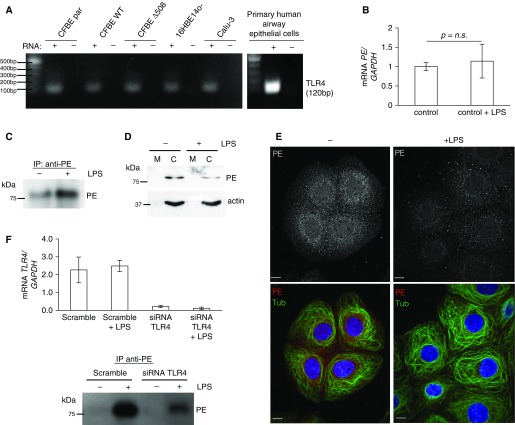
We next investigated whether a specific molecular mechanism could induce the release of PE from these airway cells. Previous data had suggested that cigarette smoke (a potent TLR4 activator) (32) could serve as a mediator of increased PE release in a murine model of lung disease (33). All epithelial cell lines tested demonstrated the expression of mRNA for TLR4 (Figure 2A), although expression was variable (qPCR data) with increased expression in CFBE WT cells relative to other cell lines (see Figure E1A in the online supplement). In addition, previous data showed that TLR4 surface expression in CFBE WT cells increases significantly after prolonged exposure to LPS without an increase in associated TLR4 mRNA (34). These findings highlighted CFBE WT cells as a relevant model for further TLR4-related studies.
Figure 2.
Secretion of PE is regulated by Toll-like receptor 4 (TLR4) after LPS engagement. (A) Various airway epithelial cell lines and human primary epithelial cells were lysed, and total RNA was purified and probed for TLR4 (120 bp) via RT-PCR. (B) CFBE WT cells were treated for 24 hours with LPS. After total RNA was extracted from cells, RNA was subjected to quantitative PCR (qPCR) for PE expression and displayed as mean of three independent RNA isolations (± SD) (relative to GAPDH mRNA). The result is expressed as a fold change compared with no treatment condition. (C) CFBE WT cells were incubated with LPS (1 μg/ml) for 24 hours, and media was subjected to immunoprecipitation with anti-PE antibody. Precipitates were resolved on SDS-PAGE and probed with anti-PE antibody. (D) CFBE WT cells were incubated for 24 hours with LPS (1 μg/ml), and cell lysates were subjected to cell fractionation to separate cell membranes (M) from cell cytosol (C). Cell fractions were resolved on SDS-PAGE and probed with anti-PE antibody. Actin serves as a fractionation purity control. (E) CFBE WT cells were incubated for 24 hours with LPS (1 μg/ml) and subjected to immunofluorescence with anti-PE (red) and antitubulin (green) antibodies. Confocal images were taken with same exposure time for each image. Scale bar, 10 μm. (F) CFBE WT cells were depleted of TLR4 via siRNA for 72 hours with >90% TLR4 mRNA depletion. After 48 hours of depletion of TLR4, these cells were treated for 24 hours with LPS (1 μg/ml), and the media was immunoprecipitated with anti-PE antibody. Precipitates were immunoblotted for PE on SDS-PAGE. qPCR data are displayed as the mean of three independent RNA isolations (± SD) and as a relative change of expression after normalization to mRNA GAPDH. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; n.s., not significant.
Although 24-hour LPS stimulation of CFBE WT cells did not significantly affect expression of the mRNA encoding PE (Figure 2B), such stimulation induced PE secretion (61% increase) from epithelial cells into the surrounding media (Figure 2C) without increasing cytotoxicity (Figure E1B). Fractionation of cellular lysates after LPS stimulation demonstrated 45% decline in cytosolic PE (Figure 2D). It is important to stress that the less intense cytosolic band in this blot did not correlate with a more intense band in the membrane or nuclear fractions, indicating that PE was released from the cytosol (data not shown). Immunostaining of LPS-treated CFBE WT cells correlated with the fractionation data. The intensity of PE staining was weaker in LPS-treated cells, and such treatment did not affect the cell morphology or microtubule pattern. Cells without LPS demonstrated a more centralized pattern of PE compared with a more dispersed pattern with larger peripheral clusters after LPS treatment (Figure 2E). Because TLR4 serves as a primary receptor for LPS, we performed siRNA-mediated depletion of TLR4 and analyzed PE release. Depletion of TLR4 resulted in over 90% loss of TLR4 mRNA by qPCR and attenuated PE release from CFBE WT cells after LPS stimulation, as measured by PE immunoprecipitation from the media (Figure 2F).
PE Is Released by Exosomes
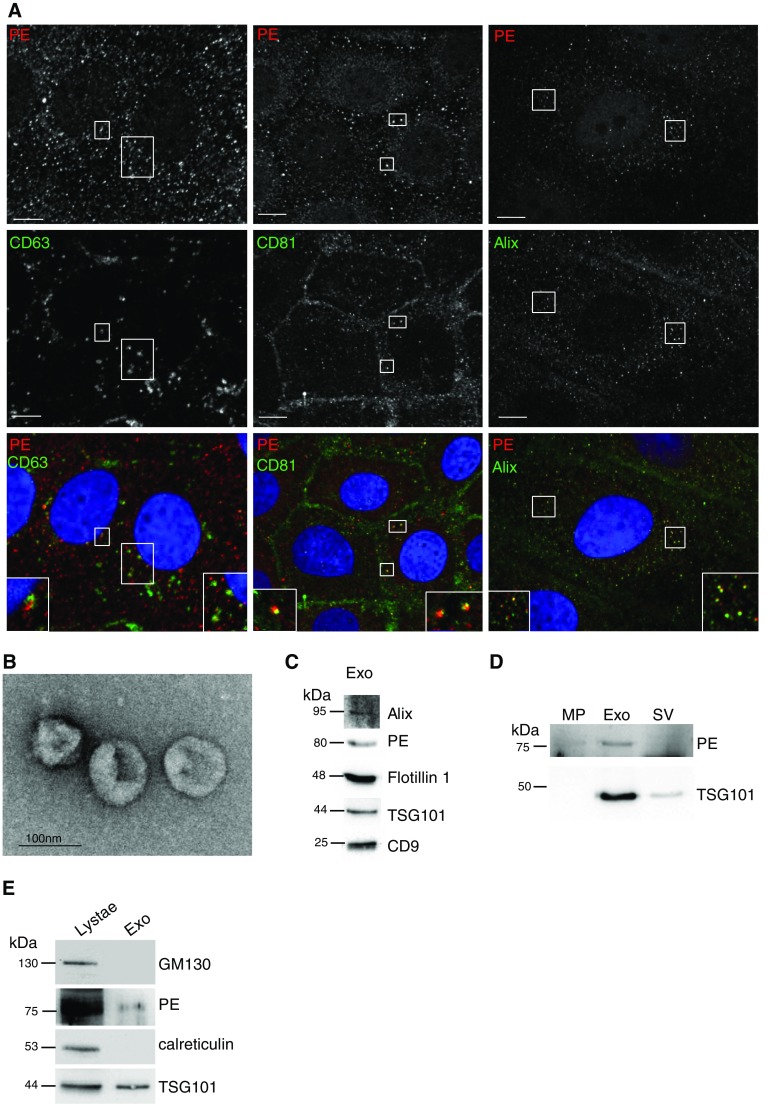
To determine the specific mechanism regulating PE release, we used a variety of intracellular trafficking compartment markers to determine the specific subcellular localization of PE. PE did not colocalize with the ER-to-Golgi intermediate compartment ERGIC (ERGIC53), the cis-Golgi (GM130), or early endosomal (EEA1, AP1) markers (Figure E2A). PE, however, did colocalize with late endosomal/lysosomal markers such as syntaxin 6 and LAMP-1 (Figure E2B), which are associated with the biogenesis of the multivesicular body, the organelle from which exosomes originate (4). Taken together, these findings suggest that PE could be released from airway epithelial cells through an unconventional secretory mechanism.
Among the most common exosomal markers are ALG-2–interacting protein (Alix) and the tetraspanins CD63 and CD81 (35, 36). Immunofluorescence analysis of these markers together with PE showed strong colocalization of PE, suggesting the possibility that PE may be released from cells through exosomes (Figure 3A, and insets). Using LPS-treated CFBE WT cells, we isolated exosomes from the associated media via differential centrifugation, as verified by negative-stain electron microscopy (Figure 3B). The use of additional markers validated a population of vesicles as exosomes (Figure 3C). PE was enriched (85%) in the exosomal fraction, and the exosomal marker TSG101 (88% enrichment) best distinguished exosomes from microparticles (10,000 × g spin for 45 min) or small vesicle after prolonged centrifugation (150,000 × g spin for 8 h) (Figure 3D). The purified exosomal fraction was free of cellular organelles, such as the Golgi (GM130) or ER membranes (calreticulin) (Figure 3E).
Figure 3.
PE localizes to exosomes in airway epithelial cells and generates the neutrophil chemoattractant tripeptide Pro-Gly-Pro. (A) CFBE WT cells underwent immunofluorescence for PE (top panels) and classic exosomal markers of CD63, CD81, and Alix (middle panels and associated insets). Scale bar, 10 μm. (B) Media from CFBE WT cells underwent differential centrifugation to purify exosomes. Exosomal fraction was negatively stained for electron microscopy analyses. Representative image shows purity of exosomal fraction. Scale bar, 100 nm. (C) Media from CFBE WT cells were subjected to differential centrifugation, and exosomal fraction was resolved in SDS-PAGE with antibody for indicated markers. (D) To further confirm purity of exosomes, microparticle (MP, after 10,000 × g for 30 min), exosomal (Exo, 150,000 × g for 2 h), and small vesicle (SV, after 150,000 × g for 8 h) fractions were resolved in SDS-PAGE and blotted with anti-PE and anti-TSG101 antibodies. (E) Exosome fraction was stained with marker for the Golgi (GM130) or endoplasmic reticulum (calreticulin) membranes enriched in the cell lysate.
TLR4 Activation Induces Secretion of Airway Exosomes
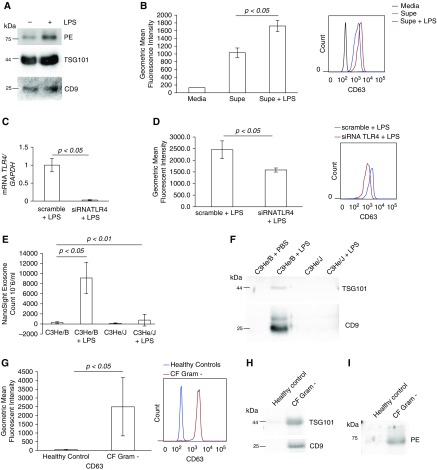
Our data demonstrate an increase in LPS-mediated secretion of PE (Figures 2C–2E), which was attenuated by siRNA depletion of TLR4 (Figure 2F). To explore if LPS treatment induces the packaging of PE into exosomes or increases a total number of released exosomes, we first purified exosomes using differential centrifugation and then performed Western blotting with anti-PE and exosome-specific markers. Upon stimulation of CFBE WT cells with LPS, the expression of two exosomal markers TSG101 and CD9 was increased (43 and 28% increase, respectively) in the media along with expression of PE (43% increase) (Figure 4A). Flow cytometry analysis of purified exosomes further demonstrated significant increase of exosomes in the media after LPS stimulation (Figure 4B). TLR4 was successfully siRNA depleted from cells (Figure 4C), which, upon stimulation by LPS, demonstrated reduced exosome release into the extracellular media compared with TLR4-replete cells (Figure 4D).
Figure 4.
Exosomes are released by TLR4 engagement. (A) Media from CFBE WT cells after incubation for 24 hours with LPS (1 μg/ml) were subjected to differential centrifugation to purify exosomes. The purified exosomal fraction was resolved in SDS-PAGE and blotted for PE and two exosomal markers, TSG101 and CD9. (B) CFBE WT cells were incubated for 24 hours with LPS (1 μg/ml), and the media was subjected to differential centrifugation to purify exosomes. Exosomal fractions were incubated with magnetic beads with bound anti-CD63 antibody. After 24 hours of incubation on shaker, beads were washed, incubated with anti–CD63-phycoerythrin antibody, and analyzed by flow cytometry. Data represent the mean ± SD of three independent exosome isolations (P < 0.05). (C) CFBE WT cells were incubated for 48 hours with TLR4 siRNA. LPS (1 μg/ml) was subsequently added, and cells were incubated for an additional 24 hours. The cell media was collected and underwent exosome purification and analysis (as described in B). CFBE WT cells demonstrated over 95% reduction for TLR4 mRNA. Data represent mean ± SD of three independent exosome isolations and corresponding RNA purifications (P < 0.05). (D) CFBE WT cells underwent siRNA depletion of TLR4 or scramble siRNA and subsequent stimulation with LPS (1 μg/ml) over 24 hours and were quantified by flow cytometry as described in B. Data represent the mean ± SD of three independent exosome isolations (P < 0.05). (E) C3He/J (TLR4 mutant) or C3He/B (TLR4 intact) mice (n = 5 per group) were treated intratracheally for 3 days with 75 μg of LPS (or PBS) daily and then underwent bronchoalveolar lavage (BAL). BAL was quantified for exosome content via Nanosight (P < 0.01 via ANOVA for all groups and P < 0.05 via post-hoc analysis). (F) BAL samples from mice were pooled into groups and resolved in SDS-PAGE and blotted for two exosomal markers, TSG101 and CD9. (G) Sputum from gram-negative colonized subjects with CF (n = 5) and control subjects without lung disease (n = 5) (normalized by total protein) were spun at 10,000 × g for 45 minutes, and the supernatant was quantified using flow cytometry as described in B (P < 0.05). Sputum was pooled into groups and resolved in SDS-PAGE and blotted for two exosomal markers, TSG101 and CD9 (H) and also for PE (I). Supe, supernatant.
Previous data from our group have demonstrated that LPS exposure in the lungs of mice leads to the generation of PGP in vivo (23). Therefore, we next tested the in vivo release of exosomes via intratracheal LPS in mice with intact TLR4 signaling (C3He/B) and a variant strain with impaired TLR4 signaling (C3He/J) (37). In TLR4-replete C3He/B mice, intratracheal LPS administration led to an increase in BAL exosome number compared with saline administration. By contrast, in TLR4-signaling–deficient C3He/J mice, there was no significant increase in BAL exosome count upon LPS treatment (Figure 4E). This difference was further validated by an increased expression of exosome markers upon LPS treatment in C3He/B, but not C3He/J, mice (Figure 4F). In total, these results highlight the importance of this specific pattern recognition receptor in inducing the release of exosomes in the lung.
Increased Exosome Burden Is Observed in the Airways of Subjects with CF
We next inquired if increased exosomes were present in the airway secretions of individuals with CF, a genetic disorder characterized by progressive airway remodeling, unrelenting neutrophilic inflammation, chronic airway colonization by gram-negative bacteria, and elevated PGP peptide levels (9, 21). When compared with control subjects without lung disease, individuals with CF demonstrated a >700-fold increase in CD63+ exosomes via flow cytometry (Figure 4G). These results paralleled an increase in both exosome markers (Figure 4H) and PE (Figure 4I) in the specimens of individuals with CF compared with control subjects without lung disease, highlighting that this novel nonstochastic mechanism of proteolytic exosome release is likely operative in human lung disease.
Discussion
The findings from the current study provide a novel mechanism coupling the pattern recognition receptor TLR4 to the increased release of exosomes, leading to a new potential pathway for the nonclassical release of proteases into the extracellular environment. Although exosomes are a well-described mechanism for the release of proteins lacking a signaling sequence into the extracellular environment, determination of specific extracellular cues regulating the release of these vesicles has been greatly limited. Our study also demonstrates, for the first time, the presence of protease-rich exosomes in the airway secretions from subjects with CF lung disease colonized by gram-negative bacteria. As exosome isolation technology and characterization improves, we hope to isolate disease-related exosomes from small volumes of clinical specimens (e.g., sputum) to conduct additional functional assays on the isolated exosomes. Although these results are statistically significant, further studies with larger cohorts of subjects with CF should be conducted to better assess the association between exosome content and CF lung disease phenotype.
Our work describes the interaction of a specific inflammatory ligand (LPS) with a particular innate immune receptor (TLR4) on airway epithelial cells and a murine model of pulmonary inflammation that clearly demonstrate that TLR4 induces the release of exosomes. TLRs are evolutionarily conserved receptors that predominantly recognize LPS, although TLRs have been shown to also signal via danger-associated molecular pattern (DAMP) ligands (38).
Although our in vitro work focuses directly on the impact of TLR4 engagement in airway epithelia, the ability to specifically identify epithelial-derived exosomes from total exosome burden in complex biologic specimens such as murine BAL and human sputum is limited owing to the current state of exosome-related technologies. However, the TLR4 dependency of exosome release in these specimens clearly highlights the global impact of this signaling pathway in pulmonary exosome regulation, potentially involving both structural and immune cells in the lung microenvironment.
With recent advances in proteomics, a greater understanding of the potential roles of exosomes in the airways of patients has become possible. A previous study identified exosome-like vesicles from airway epithelial cells and suggests the potential for these vesicles to harbor proteins critical in immune defense (39) but did not delineate the presence of proteases in these exosome-like vesicles. Previous studies have identified PE in the cytosol of a variety of cells, although the specific mechanism regarding its release has been poorly understood (40, 41). Our results demonstrate localization of PE to exosomes in human airway epithelial cells, a mechanism bypassing the classical secretory pathway. Overall, the results of these exosomes harboring active proteolytic activity reflect recent evidence suggesting that tumor-associated exosomes are proteolytically active and may have a role in local cancer invasion (42). It is likely that, in addition to exosome release, PE release may also be mediated by additional nonclassic secretory mechanisms, as observed with other ligands such as IL-1β (43). Regardless, the identification of PE as exosomal cargo underscores a novel mechanism of how this important class of biologically active proteases is released extracellularly.
In conclusion, the nonclassical pathway of PE release establishes a new paradigm coupling pattern recognition receptors of the innate immune system to an exosome-mediated secretory system. The increased presence of these exosomes in chronic lung disease suggests that this pathway is operative and may affect disease-related outcomes. The potential implications of this new exosome release pathway are quite broad because the presence of TLR4 and its respective PAMPs/DAMPs are observed at increased levels in numerous inflammatory disease states (44). These results provide novel avenues for the potential of therapeutic targeting in inflammatory disorders characterized by excessive protease secretion.
Acknowledgments
Acknowledgments
The authors thank Drs. Anuj Gaggar and Eric J. Sorscher for careful reading and critical evaluation of this manuscript; the Gregory Fleming James Cystic Fibrosis Research Center (CF Clinical and Translational Core, principal investigator: Steven M. Rowe) and especially Marina Mazur for access to primary human airway cells used in our experiments; Vin Tangpricha, M.D., for recruitment of subjects with cystic fibrosis; and Camilla Thompson (Department of Pathology, University of Alabama at Birmingham) for technical support with electron microscopy imaging.
Footnotes
This work was supported by an American Heart Association postdoctoral fellowship (T.S.); by National Institutes of Health training grant 5T32HL007457; by National Heart, Lung, and Blood Institute grants HL07783, HL090999, and HL087824 (J.E.B.) and HL102371 (A.G.); by Veterans Administration grant 1 I01BX001756 (A.G.); and by the Ismail Moustapha Scholar Fund (A.G.). Research reported in this publication was supported by the National Institutes of Health and the Family Smoking Prevention and Tobacco Control Act. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Author Contributions: Conception and design: T.S., P.E.B., K.B.F., M.K., E.S., S.R., J.E.B., X.X., and A.G. Analysis and interpretation: T.S., P.E.B., E.S., J.E.B., X.X., and A.G. Drafting the manuscript for important intellectual content: T.S., P.E.B., M.K., R.T., S.I., J.E.B., X.X., and A.G.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0108OC on July 29, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Szul T, Sztul E. COPII and COPI traffic at the ER-Golgi interface. Physiology (Bethesda) 2011;26:348–364. doi: 10.1152/physiol.00017.2011. [DOI] [PubMed] [Google Scholar]
- 2.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 3.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 5.Segura E, Guérin C, Hogg N, Amigorena S, Théry C. CD8+ dendritic cells use LFA-1 to capture MHC-peptide complexes from exosomes in vivo. J Immunol. 2007;179:1489–1496. doi: 10.4049/jimmunol.179.3.1489. [DOI] [PubMed] [Google Scholar]
- 6.Clayton A, Turkes A, Dewitt S, Steadman R, Mason MD, Hallett MB. Adhesion and signaling by B cell-derived exosomes: the role of integrins. FASEB J. 2004;18:977–979. doi: 10.1096/fj.03-1094fje. [DOI] [PubMed] [Google Scholar]
- 7.Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaggar A, Li Y, Weathington N, Winkler M, Kong M, Jackson P, Blalock JE, Clancy JP. Matrix metalloprotease-9 dysregulation in lower airway secretions of cystic fibrosis patients. Am J Physiol Lung Cell Mol Physiol. 2007;293:L96–L104. doi: 10.1152/ajplung.00492.2006. [DOI] [PubMed] [Google Scholar]
- 10.Bond JS, Beynon RJ. Proteolysis and physiological regulation. Mol Aspects Med. 1987;9:173–287. doi: 10.1016/0098-2997(87)90021-5. [DOI] [PubMed] [Google Scholar]
- 11.O’Reilly PJ, Gaggar A, Blalock JE. Interfering with extracellular matrix degradation to blunt inflammation. Curr Opin Pharmacol. 2008;8:242–248. doi: 10.1016/j.coph.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Den Steen PE, Wuyts A, Husson SJ, Proost P, Van Damme J, Opdenakker G. Gelatinase B/MMP-9 and neutrophil collagenase/MMP-8 process the chemokines human GCP-2/CXCL6, ENA-78/CXCL5 and mouse GCP-2/LIX and modulate their physiological activities. Eur J Biochem. 2003;270:3739–3749. doi: 10.1046/j.1432-1033.2003.03760.x. [DOI] [PubMed] [Google Scholar]
- 13.Hartl D, Latzin P, Hordijk P, Marcos V, Rudolph C, Woischnik M, Krauss-Etschmann S, Koller B, Reinhardt D, Roscher AA, et al. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat Med. 2007;13:1423–1430. doi: 10.1038/nm1690. [DOI] [PubMed] [Google Scholar]
- 14.Buzza MS, Netzel-Arnett S, Shea-Donohue T, Zhao A, Lin CY, List K, Szabo R, Fasano A, Bugge TH, Antalis TM. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proc Natl Acad Sci USA. 2010;107:4200–4205. doi: 10.1073/pnas.0903923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson TJ, Singh RK. Proteases as modulators of tumor-stromal interaction: primary tumors to bone metastases. Biochim Biophys Acta. 2008;1785:85–95. doi: 10.1016/j.bbcan.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overbeek SA, Braber S, Koelink PJ, Henricks PA, Mortaz E, LoTam Loi AT, Jackson PL, Garssen J, Wagenaar GT, Timens W, et al. Cigarette smoke-induced collagen destruction; key to chronic neutrophilic airway inflammation? PLoS One. 2013;8:e55612. doi: 10.1371/journal.pone.0055612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goswami S, Angkasekwinai P, Shan M, Greenlee KJ, Barranco WT, Polikepahad S, Seryshev A, Song LZ, Redding D, Singh B, et al. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat Immunol. 2009;10:496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol. 2008;180:5662–5669. doi: 10.4049/jimmunol.180.8.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, Gaggar A, Shastry S, Rowe SM, Shim YM, Hussell T, et al. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science. 2010;330:90–94. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 21.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 22.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1β by microvesicle shedding. Immunity. 2001;15:825–835. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 23.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Reilly PJ, Hardison MT, Jackson PL, Xu X, Snelgrove RJ, Gaggar A, Galin FS, Blalock JE. Neutrophils contain prolyl endopeptidase and generate the chemotactic peptide, PGP, from collagen. J Neuroimmunol. 2009;217:51–54. doi: 10.1016/j.jneuroim.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szul T, Grabski R, Lyons S, Morohashi Y, Shestopal S, Lowe M, Sztul E. Dissecting the role of the ARF guanine nucleotide exchange factor GBF1 in Golgi biogenesis and protein trafficking. J Cell Sci. 2007;120:3929–3940. doi: 10.1242/jcs.010769. [DOI] [PubMed] [Google Scholar]
- 26.Tower-Gilchrist C, Styers ML, Yoder BK, Berbari NF, Sztul E. Monitoring endosomal trafficking of the G protein-coupled receptor somatostatin receptor 3. Methods Enzymol. 2014;534:261–280. doi: 10.1016/B978-0-12-397926-1.00015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser KB, Moehle MS, Daher JP, Webber PJ, Williams JY, Stewart CA, Yacoubian TA, Cowell RM, Dokland T, Ye T, et al. LRRK2 secretion in exosomes is regulated by 14-3-3. Hum Mol Genet. 2013;22:4988–5000. doi: 10.1093/hmg/ddt346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA, Newman GR, Jasani B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods. 2001;247:163–174. doi: 10.1016/s0022-1759(00)00321-5. [DOI] [PubMed] [Google Scholar]
- 29.Shirasawa Y, Osawa T, Hirashima A. Molecular cloning and characterization of prolyl endopeptidase from human T cells. J Biochem. 1994;115:724–729. doi: 10.1093/oxfordjournals.jbchem.a124402. [DOI] [PubMed] [Google Scholar]
- 30.Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, Levin E, Raju SV, Li Y, Mazur M, et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One. 2012;7:e39809. doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barelli H, Petit A, Hirsch E, Wilk S, De Nanteuil G, Morain P, Checler F. S 17092-1, a highly potent, specific and cell permeant inhibitor of human proline endopeptidase. Biochem Biophys Res Commun. 1999;257:657–661. doi: 10.1006/bbrc.1999.0366. [DOI] [PubMed] [Google Scholar]
- 32.Mortaz E, Henricks PA, Kraneveld AD, Givi ME, Garssen J, Folkerts G. Cigarette smoke induces the release of CXCL-8 from human bronchial epithelial cells via TLRs and induction of the inflammasome. Biochim Biophys Acta. 2011;1812:1104–1110. doi: 10.1016/j.bbadis.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Braber S, Koelink PJ, Henricks PA, Jackson PL, Nijkamp FP, Garssen J, Kraneveld AD, Blalock JE, Folkerts G. Cigarette smoke-induced lung emphysema in mice is associated with prolyl endopeptidase, an enzyme involved in collagen breakdown. Am J Physiol Lung Cell Mol Physiol. 2011;300:L255–L265. doi: 10.1152/ajplung.00304.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.John G, Yildirim AO, Rubin BK, Gruenert DC, Henke MO. TLR-4-mediated innate immunity is reduced in cystic fibrosis airway cells. Am J Respir Cell Mol Biol. 2010;42:424–431. doi: 10.1165/rcmb.2008-0408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 36.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Qureshi ST, Larivière L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D. Endotoxin-tolerant mice have mutations in Toll-like receptor 4 (Tlr4) J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erridge C. Endogenous ligands of TLR2 and TLR4: agonists or assistants? J Leukoc Biol. 2010;87:989–999. doi: 10.1189/jlb.1209775. [DOI] [PubMed] [Google Scholar]
- 39.Kesimer M, Scull M, Brighton B, DeMaria G, Burns K, O’Neal W, Pickles RJ, Sheehan JK. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J. 2009;23:1858–1868. doi: 10.1096/fj.08-119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulz I, Zeitschel U, Rudolph T, Ruiz-Carrillo D, Rahfeld JU, Gerhartz B, Bigl V, Demuth HU, Rossner S. Subcellular localization suggests novel functions for prolyl endopeptidase in protein secretion. J Neurochem. 2005;94:970–979. doi: 10.1111/j.1471-4159.2005.03237.x. [DOI] [PubMed] [Google Scholar]
- 41.Dresdner K, Barker LA, Orlowski M, Wilk S. Subcellular distribution of prolyl endopeptidase and cation-sensitive neutral endopeptidase in rabbit brain. J Neurochem. 1982;38:1151–1154. doi: 10.1111/j.1471-4159.1982.tb05362.x. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi M, Salomon C, Tapia J, Illanes SE, Mitchell MD, Rice GE. Ovarian cancer cell invasiveness is associated with discordant exosomal sequestration of Let-7 miRNA and miR-200. J Transl Med. 2014;12:4. doi: 10.1186/1479-5876-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eder C. Mechanisms of interleukin-1β release. Immunobiology. 2009;214:543–553. doi: 10.1016/j.imbio.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Casanova JL, Abel L, Quintana-Murci L. Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev Immunol. 2011;29:447–491. doi: 10.1146/annurev-immunol-030409-101335. [DOI] [PubMed] [Google Scholar]