Abstract
Angiogenesis, the growth of new blood vessels, plays a key role in organ development, homeostasis, and regeneration. The cooperation of multiple angiogenic factors, rather than a single factor, is required for physiological angiogenesis. Recently, we have reported that soluble platelet-rich plasma (PRP) extract, which contains abundant angiopoietin-1 and multiple other angiogenic factors, stimulates angiogenesis and maintains vascular integrity in vitro and in vivo. In this report, we have demonstrated that mouse PRP extract increases phosphorylation levels of the Wnt coreceptor low-density lipoprotein receptor–related protein 5 (LRP5) and thereby activates angiogenic factor receptor Tie2 in endothelial cells (ECs) and accelerates EC sprouting and lung epithelial cell budding in vitro. PRP extract also increases phosphorylation levels of Tie2 in the mouse lungs and accelerates compensatory lung growth and recovery of exercise capacity after unilateral pneumonectomy in mice, whereas soluble Tie2 receptor or Lrp5 knockdown attenuates the effects of PRP extract. Because human PRP extract is generated from autologous peripheral blood and can be stored at −80°C, our findings may lead to the development of novel therapeutic interventions for various angiogenesis-related lung diseases and to the improvement of strategies for lung regeneration.
Keywords: angiogenesis, lung regeneration, platelet-rich plasma extract, LRP5, Tie2
Clinical Relevance
Our findings may improve the efficiency of lung regeneration and lung organ engineering. Because deregulated angiogenesis contributes to the pathogenesis of various chronic lung diseases, such as bronchopulmonary dysplasia and emphysema, the findings regarding the mechanism by which platelet-rich plasma (PRP) extract stimulates angiogenesis and lung regeneration after pneumonectomy may improve understanding of how these processes are deregulated in diseased lungs and may potentially lead to the development of new therapeutic strategies for chronic lung diseases. Given that PRP extract is generated from autologous peripheral blood by a simple method and can be preserved stably in the freezer for a long period, PRP extract could be a potentially good therapeutic tool for regenerating damaged or lost adult lung tissue.
More than 35 million Americans suffer from chronic lung diseases, including chronic obstructive pulmonary disease, pulmonary fibrosis, and asthma. Chronic lung diseases are the third leading cause of death in the United States, causing approximately 400,000 deaths every year in the United States. These pulmonary diseases are primarily caused by the loss or deregulated remodeling of alveolar septa, which results in compromised gas exchange at the alveolar–capillary interface and in long-term impairment of patients’ quality of life. To date, lung transplantation is the only way to save patients with end-stage chronic lung diseases. However, because of the shortage of transplant donors, high cost, serious complications, and low survival rate (1), lung transplantation is not an optimal approach. It has been recognized that human lungs continue to grow until adolescence by neoalveolarization (2) and that adult human lungs may have the potential to regenerate after pneumonectomy (PNX) (3). Thus, stimulating the regeneration of adult lungs could be a good therapeutic strategy for chronic lung diseases.
Capillary blood vessels not only transport oxygen, nutrients, and specific cells to the lung; they also provide instructive signals to the surrounding local microenvironment in the lung. Therefore, angiogenesis plays key roles in physiological lung development and regeneration (4–9), and angiogenesis-targeted therapy may be a good strategy to regenerate damaged or lost adult lung tissues. Most current angiogenic therapies for various pathological conditions (e.g., ischemic diseases) rely on the use of a single angiogenic factor, such as vascular endothelial growth factor (VEGF) (10, 11). However, angiogenesis is controlled by multiple angiogenic signaling pathways (12–14), and an appropriate combination of angiogenic factors rather than a single factor is preferred to generate stable and functional blood vessels in tissues (15–17).
Aside from coagulation factors, platelets store and release many bioactive angiogenic factors, including platelet-derived epidermal growth factor, platelet-derived growth factor (PDGF), VEGF, basic fibroblast growth factor, angiopoietin (Ang), and transforming growth factor β (18), and attract endothelial cells (ECs), macrophages, mesenchymal stem cells, and osteoblasts to local sites, enhancing angiogenesis, tissue regeneration, and wound healing (19, 20). Therefore, platelet-rich plasma (PRP), which includes concentrated platelets, has been extensively used to accelerate tissue regeneration in the orthopedic and periodontal fields (21). However, the signaling mechanism by which PRP controls angiogenesis and tissue regeneration remains unknown. Recently, we have reported that soluble extract from mouse PRP (PRP extract), which contains abundant Ang1 and a lower amount of multiple other angiogenic factors, stimulates angiogenesis and maintains lung vascular integrity through endothelial Ang1–Tie2 signaling in mice (17, 22). We have also shown that low-density lipoprotein receptor–related protein 5 (LRP5), a component of the Wnt ligand–receptor complex, stimulates angiogenesis and alveolar formation in neonatal mouse lungs by activating Tie2 in ECs (5, 23). Thus, PRP extract may stimulate regenerative alveolar formation by accelerating angiogenesis through the Ang1–LRP5–Tie2 pathway in adult mice.
In this report, we have demonstrated that mouse PRP extract promotes vascular and alveolar regeneration after unilateral PNX through the Ang1–LRP5–Tie2 pathway in adult mice. Because human autologous PRP extract is generated from whole blood with a simple method, can be stably stored at −80°C (22), and has little immunological reaction and no risk of unnecessary pathogen transfer (21, 24), PRP extract could be an efficient therapeutic tool for lung regeneration and chronic lung diseases.
Materials and Methods
Materials
Soluble Tie2 receptor and Ang1 were from R&D (Minneapolis, MN). Anti-CD31 antibody was from Transduction Laboratories (Lexington, KY). Anti–aquaporin 5 (AQP5), anti–E-cadherin, anti–surfactant protein B, and anti-LRP5 antibodies were from Abcam (Cambridge, MA). Anti–β-actin monoclonal antibody was from Sigma (St. Louis, MO). Anti-Tie2 monoclonal antibody was from Upstate (Lake Placid, NY). Anti–phospho-Tie2 (Tyr 992) antibody was from R&D. Anti–phospho-LRP5 antibody (Thr 1492) was from AbboMax (San Jose, CA). Human umbilical vein endothelial (HUVE) cells from Lonza (Hopkinton, MA) were cultured in EBM2 medium containing 5% FBS and growth factors (VEGF, basic fibroblast growth factor, and PDGF). Human adult lung fibroblasts (ATCC, Manassas, VA) and human immortalized bronchial epithelial cells (HBEC3-KT; ATCC), which have been reported to differentiate into alveolar epithelial cells and to exhibit a bronchoalveolar phenotype (25, 26), were cultured in Eagle’s minimum essential medium (ATCC) and airway epithelial cell basal medium (ATCC), respectively.
Preparation of the PRP Extract
All animal studies were reviewed and approved by the Animal Care and Use Committee of Boston Children’s Hospital. Unless otherwise indicated, CD1 (Charles River Laboratories, Wilmington, MA) and C57BL/6 mice (stock no. 664; Jackson Laboratory, Bar Harbor, ME) were used for the study. The Lrp5−/− mice (stock no. 005823; Jackson Laboratory) were developed by Deltagen, Inc. (San Mateo, CA) (5, 23). We prepared PRP extract as previously described (22). Details are provided in the online supplement.
In Vitro Fibrin Gel Angiogenesis Assay
Fibrin gel angiogenesis assays were performed as previously described (27). Details are provided in the online supplement.
Coculture of HBEC3-KT and HUVE Cells
Coculture of HBEC3-KT and HUVE cells was performed as previously described with slight modifications (25). Details are provided in the online supplement.
Unilateral PNX
Unilateral PNX was performed as described (28). Details are provided in the online supplement.
Static Lung Compliance
Static lung compliance was evaluated 7 days after PNX as described (17, 29). Details are provided in the online supplement.
Measurement of Alveolar–Arterial Oxygen Difference
Mice were anesthetized with ketamine/xylazine (intraperitoneally). The chest was surgically opened, and arterial blood was obtained by puncturing the left ventricle using a heparinized needle and syringe. Arterial blood gas was analyzed as described in the online supplement (30).
In Vivo Pulmonary Vascular Permeability Assay
Lung vascular permeability was assessed 7 days after PNX using Evans blue dye leakage (17, 31, 32). Details are provided in the online supplement.
Exercise Capacity
Mice were run according to a predetermined protocol, and we assessed the ability of mice to run for distance (17, 32). Details are provided in the online supplement.
Statistical Analysis
All phenotypic analysis was performed by masked observers unaware of the identity of the experimental groups. Error bars (SEM) and P values were determined from the results of three or more independent experiments. The F test (for two samples) or the Levene test (for more than two samples) was performed to confirm that the variances are homogeneous. Student’s t test was used for statistical significance between two groups. For more than two groups, one-way ANOVA with a post hoc analysis using the Bonferroni test was conducted.
Results
PRP Extract Stimulates Angiogenesis through LRP5–Tie2 Signaling In Vitro
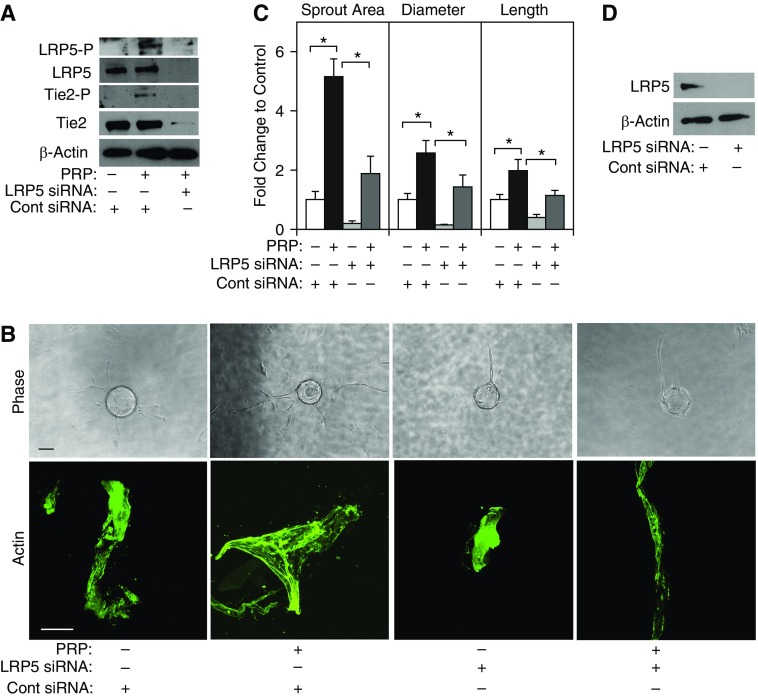
We have reported that PRP extract, which contains abundant Ang1, stimulates angiogenesis through endothelial Ang1–Tie2 signaling in mice (17, 22). Because the Wnt coreceptor LRP5 enhances angiogenesis in neonatal mouse lungs by increasing Tie2 activity (5), we examined whether PRP extract stimulates Tie2 activity in HUVE cells through LRP5 signaling. The threonine phosphorylation levels of LRP5 were 3.8-fold higher (Figure 1A) and the tyrosine phosphorylation levels of Tie2 were 9.1-fold higher in HUVE cells treated with PRP extract compared with those in cells treated with protein concentration–matched control serum (Figure 1A). Importantly, when we knocked down LRP5 expression using small interfering RNA (siRNA) transfection, LRP5 siRNA decreased the protein levels of LRP5 and Tie2, the threonine phosphorylation levels of LRP5, and the tyrosine phosphorylation levels of Tie2 induced by PRP extract in HUVE cells (Figure 1A). siRNA-based knockdown of LRP5 also decreases the protein levels of β-catenin in ECs (5) (not shown), suggesting that LRP5 mediates PRP extract–induced increases in Tie2 tyrosine phosphorylation in ECs via the canonical Wnt pathway.
Figure 1.
Platelet-rich plasma (PRP) extract induces endothelial sprouting through lipoprotein receptor–related protein 5 (LRP5) signaling in vitro. (A) Immunoblots showing threonine-phosphorylated LRP5, tyrosine-phosphorylated Tie2, LRP5, Tie2, and β-actin protein levels in human umbilical vein endothelial (HUVE) cells treated with PRP extract or in combination with LRP5 small interfering RNA (siRNA) or control (Cont) siRNA with irrelevant sequences. Protein concentration–matched mouse serum is used as a control vehicle. (B) Phase contrast images showing endothelial cells sprouting from each bead (top; scale bar, 100 μm) and immunofluorescent images of actin staining (bottom; scale bar, 25 μm) in HUVE cells treated with PRP extract or in combination with LRP5 siRNA or control siRNA with irrelevant sequences. Protein concentration–matched mouse serum is used as a control vehicle. (C) Graph showing changes in sprout area, diameter, and length of the longest sprout in HUVE cells treated with PRP extract or in combination with LRP5 siRNA or control siRNA with irrelevant sequences. *P < 0.05. Error bars represent SEM of three independent experiments. (D) Immunoblots showing LRP5 and β-actin protein levels in HUVE cells treated with LRP5 siRNA or control siRNA with irrelevant sequences.
To examine whether PRP extract modulates blood vessel formation through the LRP5–Tie2 pathway in vitro, we performed a three-dimensional EC sprouting assay inside the fibrin gel (27). After culturing beads coated with HUVE cells in the fibrin gel for 5 days, sprouting from the beads was quantified (Figures 1B and 1C). PRP extract increased the area, lumen diameter, and length of the EC sprouts by 5.1-, 2.5-, and 2.1-fold, respectively, whereas LRP5 knockdown using siRNA transfection (Figure 1D) inhibited sprout formation induced by PRP extract (Figures 1B and 1C). Actin staining of the sprouted area also revealed that PRP extract significantly increased the diameter of the sprouts, whereas LRP5 knockdown inhibited this effect (Figures 1B and 1C). Tie2 knockdown using siRNA transfection (see Figure E1A in the online supplement) also decreased sprout area and length, but it did not change the diameter of sprouts induced by PRP extract (Figure E1A). Inhibition of Ang1–Tie2 signaling using soluble Tie2 receptor, which blocks Ang1, also inhibited the formation of EC sprouts induced by PRP extract (Figure E1B), suggesting that PRP extract stimulates EC sprouting through Ang1–LRP5–Tie2 signaling. Although Ang1 is the major angiogenic factor in PRP extract, PRP extract also contains lower amounts of other factors and effectively stimulates angiogenesis and maintains lung vascular integrity (17, 22). To prove that these multiple other factors in PRP extract are required for stimulation of angiogenesis, we treated HUVE cells with recombinant Ang1 (100 ng/ml) and performed the assay. Although recombinant Ang1 increased sprout area, diameter, and length in HUVE cells, the magnitude of the changes was smaller compared with those treated with PRP extract (Figure E1C), suggesting that small amounts of other factors in PRP extract may be required for the action of PRP extract or that PRP extract contains more biologically active Ang1.
PRP Extract Stimulates Epithelial Budding through LRP5–Tie2 Signaling In Vitro
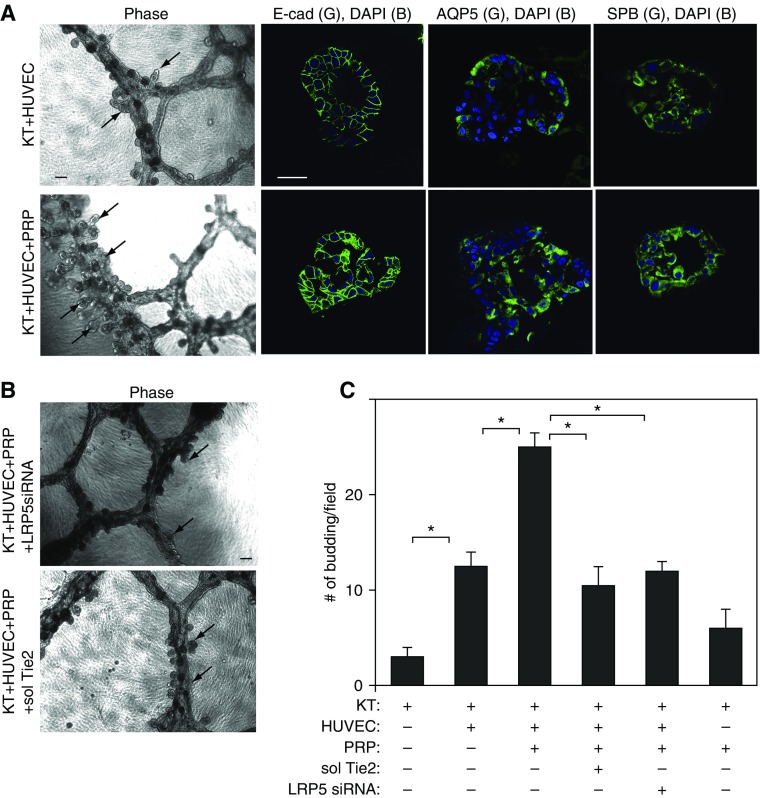
It has been recognized that ECs stimulate alveolar morphogenesis by instructing lung epithelial cells (7, 8). Because PRP extract stimulates EC sprouting in vitro (Figures 1 and E1) and in vivo (22), we next examined whether PRP extract activates alveolar morphogenesis by stimulating angiogenesis. It has been reported that immortalized bronchial epithelial cells (HBEC3-KT) differentiate into alveolar epithelial cells and exhibit a bronchoalveolar phenotype when cultured on growth factor–reduced Matrigel that is overlaid on top of a monolayer of human lung fibroblasts (25). Therefore, we used this system to examine whether ECs stimulate alveolar morphogenesis in HBEC3-KT cells when these cells are cocultured. When HBEC3-KT cells were cocultured with HUVE cells on the growth factor–reduced Matrigel, the cells self-assembled into tubule-like structures (Figure 2A) similar to those observed in organotypic cultures with ECs (22) or HBEC3-KT cells alone (Figure E2A) (25). Hematoxylin and eosin (H&E) staining of the cells in the small bud-like structures emerging from the tubules demonstrated that cells within the buds display a cuboidal morphology, and there seems to be a lumen within the budding structure (Figure E2B). These budding structures expressed alveolar epithelial markers (E-cadherin, aquaporin 5, and surfactant protein B) (Figure 2A), suggesting that these cells differentiated into alveolar epithelial cells. In the coculture, the number of alveolar buds that had lumens and expressed alveolar epithelial markers increased by 6 times compared with cultures of HBEC3-KT cells alone (Figures 2A, 2C, and E2A). Importantly, PRP extract (1/1,000 vol/vol) further stimulated alveolar bud formation in cocultures of HBEC3-KT and HUVE cells (Figures 2A and 2C). Although PRP extract stimulated alveolar bud formation in HBEC3-KT cells cultured without HUVE cells, the number of alveolar buds was significantly lower compared with that in coculture with HUVE cells and treated with PRP extract (Figures E2A and 2C). These results suggest that coculture with HUVE cells stimulates alveolar bud formation, which is further enhanced by PRP extract. We then examined whether LRP5–Tie2 signaling mediates the effects of PRP extract on alveolar bud formation in vitro. When we knocked down LRP5 expression in HUVE cells using siRNA transfection or depleted Ang1 using soluble Tie2 receptor and cocultured with HBEC3-KT cells, epithelial budding induced by PRP extract was inhibited (Figures 2B and 2C), suggesting that PRP extract stimulates alveolar bud formation through Ang1–LRP5–Tie2 signaling in ECs and that communication between ECs and epithelial cells is necessary for alveolar bud formation. Because most of the increases in endothelial sprouting were smaller in HUVE cells treated with recombinant Ang1 alone compared with those treated with PRP extract (Figure E1C), we also examined the effects of recombinant Ang1 on epithelial budding. Consistent with the results of the EC sprouting assay, treatment with recombinant Ang1 (100 ng/ml) enhanced epithelial budding, but the number of buds was significantly smaller than those treated with PRP extract (Figure E2C), suggesting that small amounts of other angiogenic factors in PRP extract may also be required for epithelial budding in vitro.
Figure 2.
PRP extract induces lung epithelial budding through LRP5-Tie2 signaling in vitro. (A) Phase contrast images showing epithelial cell budding (left; scale bar, 100 μm) and immunofluorescent images (scale bar, 50 μm) showing epithelial cell buds detected by staining with anti–E-cadherin (E-cad) (second), anti–aquaporin 5 (AQP5) (third), or anti–surfactant protein B (SPB) (right) antibodies in coculture of HBEC3-KT (KT) and HUVE cells (top) and coculture of KT and HUVE cells treated with PRP extract (bottom) for 5 days. Arrows indicate the bud formation. Protein concentration–matched mouse serum is used as a control vehicle for PRP extract. (B) Phase contrast images showing epithelial cell budding (scale bar, 100 μm) in coculture of KT and HUVE cells treated with PRP extract in combination with LRP5 siRNA (top) or soluble Tie2 (sol Tie2, bottom) for 5 days. Arrows indicate the bud formation. (C) Graph showing the number of buds in coculture of KT and HUVE cells treated with PRP extract or in combination with soluble Tie2 or LRP5 siRNA for 5 days. *P < 0.05. Error bars represent SEM of three independent experiments. DAPI, 4′,6-diamidino-2-phenylindole.
PRP Extract Stimulates Compensatory Lung Growth after PNX
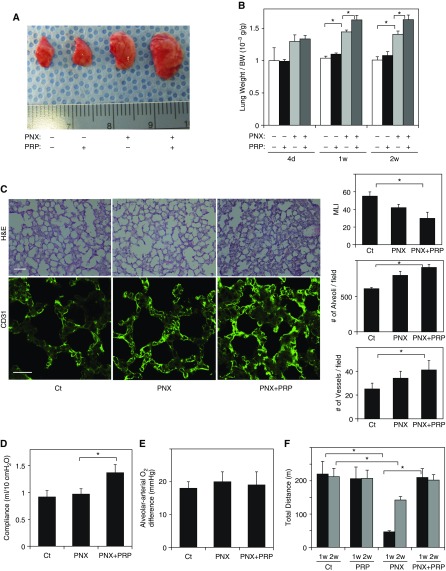
Because PRP extract stimulates morphogenesis of ECs (Figure 1) and epithelial cells (Figure 2) in vitro, we next examined whether PRP extract stimulates alveolar regeneration in mouse lung in vivo. PNX is a good system to study the mechanisms by which an angiogenic signaling pathway controls lung vascular and alveolar regeneration in the adult mouse because (1) compensatory lung growth after PNX in adult mice is partly due to neoalveolarization (33) and (2) PNX induces angiogenesis, and PNX-induced angiogenesis produces growth factors and stimulates alveolar formation (7, 34). Consistent with previous reports (28), when we performed left unilateral PNX, there was a significant increase in the size of right lung lobe (Figure 3A) and the weight of the remaining right lung cardiac lobe (measured as a ratio of the weight of cardiac lobe to mouse weight) within 7 to 14 days after left unilateral PNX (Figure 3B). The original lung weight to body weight ratio was 1.01 × 10−3 (g/g), but it increased by 1.4-fold in the lungs 7 to 14 days after PNX (Figures 3A and 3B). When we treated mice with PRP extract (20 μl, every day, intraperitoneally) after PNX, compensatory lung growth was further stimulated; the ratio of lung weight to body weight was 14% higher in PRP extract–treated lungs 7 and 14 days after PNX compared with untreated lungs, whereas lung weight did not change when we treated sham-operated mice with PRP extract (Figures 3A and 3B). Morphometric analysis of H&E-stained mouse lungs also revealed that lungs treated with PRP extract after PNX exhibited thickened alveolar septa, a decrease in the size of the alveolar space measured by mean linear intercept, and an increase in the number of alveoli compared with control untreated lung (Figure 3C). In addition, when we analyzed blood vessel density in the lung using immunohistochemical staining for the blood vessel marker CD31, PRP extract increased the blood vessel density in the mouse lung 7 days after PNX (Figure 3C). These findings suggest that PRP extract enhances lung vascular and alveolar regeneration after PNX.
Figure 3.
PRP extract induces post-pneumonectomy (PNX) compensatory lung growth. (A) Representative micrograph showing the right lung lobe of control mouse, mouse treated with PRP extract for 7 days, mouse 7 days after PNX, or in combination with treatment with PRP extract after PNX for 7 days. Protein concentration–matched mouse serum is used as a control vehicle. (B) Graph showing the ratio of the weight of right lung cardiac lobe to mouse body weight (BW) after PNX, treatment with PRP extract, or PNX in combination with treatment with PRP extract (n = 7; mean ± SEM). *P < 0.05. (C) Hematoxylin and eosin (H&E)–stained mouse lungs (top; scale bar, 50 μm) and CD31-positive blood vessels (bottom; scale bar, 20 μm) in the cardiac lobe of control (Ct) mouse, mouse 7 days after PNX (PNX), or in combination with treatment with PRP extract after PNX for 7 days (PNX+PRP). Graphs showing quantification of alveolar size (mean linear intercept [MLI]; right top), alveolar number (right middle), and vessel number (right bottom) in the lungs after PNX with or without treatment with PRP extract (n = 7; mean ± SEM). *P < 0.05. (D) Static lung compliance measured 7 days after PNX or in combination with treatment with PRP extract for 7 days (n = 6; mean ± SEM). *P < 0.05. (E) Alveolar-arterial oxygen differences in the control sham-operated mice or mice with or without PRP extract treatment after PNX for 7 days (n = 6; mean ± SEM). *P < 0.05. (F) Exercise capacity of sham-operated mice or mice undergoing PNX with or without treatment with PRP extract for 7 or 14 days assessed by a total running distance using a rodent treadmill exercise protocol (n = 7; mean ± SEM). *P < 0.05.
To examine the effects of PRP extract on lung function after PNX, we analyzed lung compliance, alveolar–arterial oxygen difference, and lung vascular permeability and compared these parameters between mouse lungs with or without PRP extract treatment after PNX. Lung compliance was approximately 30% higher in the lungs treated with PRP extract, whereas alveolar–arterial oxygen difference was not changed in the mice after PNX under resting conditions on room air (Figures 3D and 3E). Lung vascular permeability measured by the leakage of intravenously injected Evans blue dye into the alveolar space was also not changed by treatment with PRP extract after PNX (Figure E3A). These results suggest that PNX and PRP extract did not have inflammatory effects and that PRP extract stimulates expansion of functional alveolar units after PNX. We also analyzed exercise capacity by measuring the total distance mice were able to run using a rodent treadmill exercise system. The total running distance decreased by 84 and 29% in mice 7 and 14 days after PNX, respectively, compared with control sham-operated mice (Figure 3F), suggesting that physiological lung function is impaired after PNX even after the remaining lung has undergone some compensatory growth. Treatment with PRP extract after PNX restored running distance to the levels of control mice without PNX (Figure 3F), indicating that PRP extract accelerates the functional recovery from PNX. Importantly, the effects of PRP extract on compensatory lung growth and lung function after PNX seem to be lung specific rather than systemic effects of PRP extract because intratracheal administration of PRP extract had similar effects on compensatory lung growth and lung function after PNX as systemic intraperitoneal administration (Figures E3B and E3C).
PRP Extract Stimulates Post-PNX Compensatory Lung Growth through Ang1–Tie2 Signaling
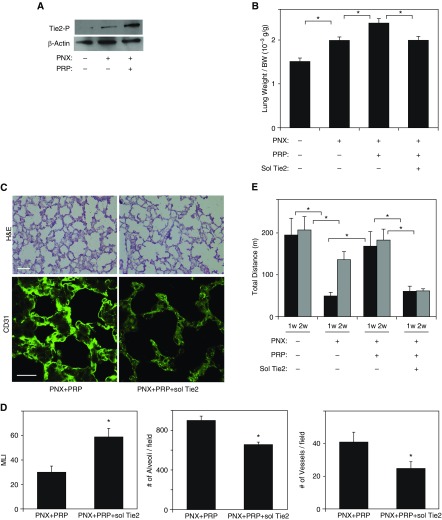
We have reported that postnatal alveolarization is regulated by the Ang1–Tie2 pathway (5, 23); Ang1 activates Tie2 receptor and stimulates lung vascular and alveolar formation during postnatal lung development in mice (5). Because PRP extract contains abundant Ang1 (22) and stimulates endothelial and epithelial morphogenesis in vitro (Figures 1, 2, E1, and E2), we next explored whether PRP extract enhances angiogenesis after PNX through Ang1–Tie2 signaling. Tie2 tyrosine phosphorylation levels were higher in mouse lungs 7 days after PNX, and, consistent with in vitro results using HUVE cells, treatment with PRP extract further increased the levels of Tie2 phosphorylation compared with those in untreated lungs after PNX (Figure 4A), suggesting that PRP extract may stimulate lung vascular and alveolar regeneration through Ang1–Tie2 signaling in vivo.
Figure 4.
PRP extract induces angiogenesis and compensatory lung growth in mouse lung after PNX through Ang1–Tie2 signaling. (A) Immunoblots showing tyrosine-phosphorylated Tie2 and β-actin protein levels in the right lung cardiac lobe of control mouse, mouse 7 days after PNX, or in combination with treatment with PRP extract for 7 days after PNX. (B) Graph showing the ratio of weight of the right lung cardiac lobe to mouse body weight after PNX treated with PRP extract or in combination with soluble (sol) Tie2 receptor for 7 days (n = 7; mean ± SEM). *P < 0.05. Protein concentration–matched mouse serum and control vehicle are used as a control. (C) H&E–stained mouse right lung cardiac lobe treated with PRP extract or in combination with sol Tie2 for 7 days after PNX (top; scale bar, 50 μm). Immunofluorescence micrographs showing CD31-positive blood vessels in right lung cardiac lobe of mouse treated with PRP extract or in combination with sol Tie2 for 7 days after PNX (bottom; scale bar, 20 μm). (D) Graphs showing the quantification of alveolar size (MLI, left), the alveolar number (middle), and the vessel number of CD31-positive endothelial cells (right) in the right lung cardiac lobe after each treatment (n = 7; mean ± SEM). *P < 0.05. (E) Exercise capacity of mice 7 and 14 days after PNX or in combination with treatment with PRP extract and/or sol Tie2 receptor after PNX for 7 and 14 days assessed by a total running distance using a rodent treadmill exercise protocol (n = 7; mean ± SEM). *P < 0.05.
To directly examine whether PRP extract enhances angiogenesis and alveolar formation after PNX through Ang1–Tie2 signaling, we depleted Ang1 by treating mice with soluble Tie2 receptor. Treatment of mice with soluble Tie2 receptor (1 μg, every day, intraperitoneally) for 7 days suppressed the effects of PRP extract on lung weight after PNX, reducing it to the levels of that in mouse lung with PNX alone (Figure 4B). Morphometric analysis of H&E-stained mouse lungs also revealed that soluble Tie2 attenuated alveolar formation stimulated by PRP extract in the lung after PNX; septation was incomplete, alveolar spaces measured by mean linear intercept were twice as large, and the number of alveoli was 27% smaller in the lungs treated with soluble Tie2 in combination with PRP extract after PNX compared with those treated with PRP extract alone (Figures 4C and 4D). Furthermore, soluble Tie2 decreased blood vessel density detected by CD31 staining in mouse lung treated with PRP extract after PNX by 41% (Figures 4C and 4D). Exercise capacity as measured by a rodent treadmill exercise protocol also revealed that total running distance restored by PRP extract after PNX was attenuated by soluble Tie2 treatment (Figure 4E). These results suggest that PRP extract accelerates compensatory lung growth and recovery of lung function after PNX through Ang1–Tie2 signaling.
LRP5–Tie2 Signaling Mediates Compensatory Lung Growth after PNX
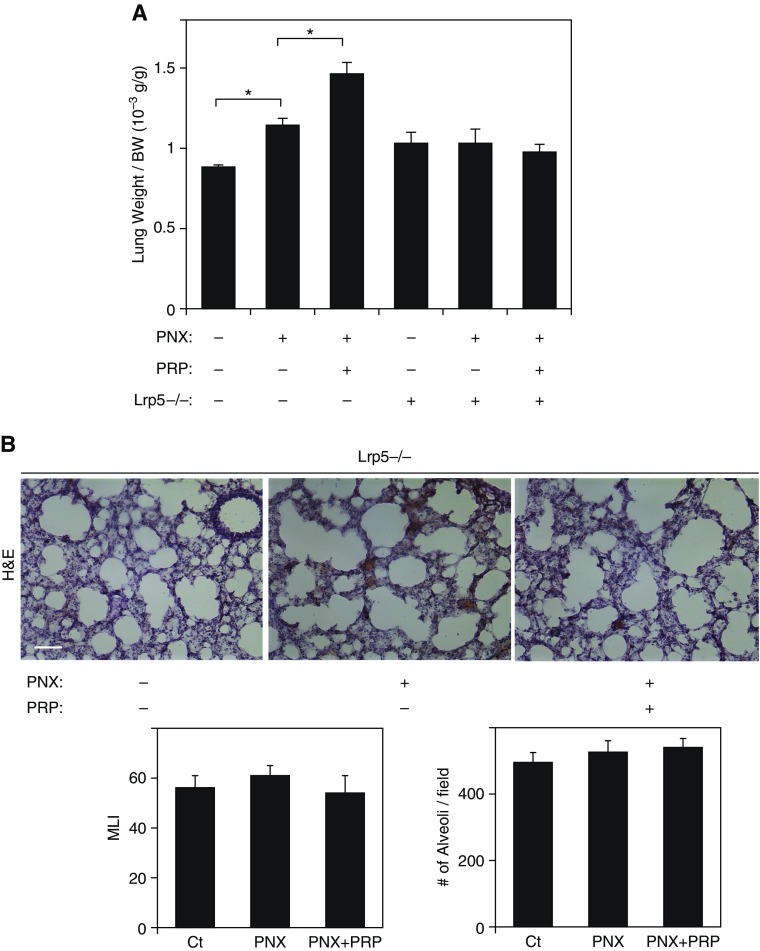
We have found that LRP5 knockdown decreases Tie2 phosphorylation in HUVE cells (Figure 1) and inhibits endothelial sprouting and lung epithelial budding induced by PRP extract in vitro (Figures 1 and 2). Tie2 phosphorylation levels were also higher after PNX, and were further increased by PRP extract in mouse lungs (Figure 4A). Thus, we next examined whether LRP5–Tie2 signaling mediates compensatory alveolar growth after PNX in mice. When we performed PNX on Lrp5−/− mice, in which LRP5 and Tie2 expression are decreased in the lungs (Figure E4A), and treated the mice with PRP extract for 7 days, PNX alone and PNX in combination with treatment with PRP extract failed to increase lung weight as observed in the wild-type (WT) mouse lungs (Figure 5A). Morphometric analysis of H&E-stained mouse lungs also revealed that PRP extract did not stimulate alveolar formation in Lrp5−/− mice after PNX; septation was incomplete, alveolar spaces were large, and the alveolar number was small in the Lrp5−/− mouse lungs treated with PRP extract after PNX, similar to the untreated Lrp5−/− mouse lungs (Figure 5B) but unlike the effects observed in control mice after PNX (Figures 3C and E4B), suggesting that LRP5–Tie2 signaling mediates compensatory lung growth after PNX in mice.
Figure 5.
LRP5–Tie2 signaling mediates compensatory lung growth after PNX. (A) Graph showing the ratio of weight of the lung cardiac lobe to mouse body weight 7 days after PNX with or without treatment with PRP extract in wild-type C57BL/6 mice or Lrp5−/− mice (n = 7; mean ± SEM). *P < 0.05. (B) H&E–stained mouse right lung cardiac lobe 7 days after PNX with or without treatment with PRP extract in Lrp5−/− mice. Scale bar, 50 μm. Graph showing the quantification of alveolar size (MLI, left) and the alveolar number (right) (n = 7; mean ± SEM). *P < 0.05.
Discussion
It has been recognized that rebooting the process of embryonic development by stimulating the morphogenesis signaling pathways or by recapitulating embryonic microenvironments may be an ideal strategy for the regeneration of adult organs (35). Angiogenesis plays a key role in alveolar formation in developing and regenerating lungs (4–9, 33), whereas deregulated angiogenesis contributes to various lung developmental disorders such as bronchopulmonary dysplasia (BPD) (5, 9, 23) and impairment of adult lung regeneration in mice (7, 8, 28, 36). ECs interact with alveolar epithelial cells, and endothelial signaling plays an important role in the process of lung regeneration (7, 8). Therefore, modulation of angiogenesis could be a potential strategy for regeneration of lost or damaged adult lung tissues. Recently, we have reported that PRP extract, which contains abundant Ang1 and lower amounts of other angiogenic growth factors, stimulates angiogenesis and maintains vascular integrity through the Ang1–Tie2 pathway in vitro and in vivo (17, 22). However, the mechanism by which PRP controls vascular and alveolar morphogenesis during lung regeneration has not been well characterized. In this report, we have demonstrated that PRP extract stimulates EC sprouting and lung epithelial bud formation through Ang1–LRP5–Tie2 signaling in vitro. We have also found that PRP extract stimulates angiogenesis and accelerates compensatory lung growth after unilateral PNX through Ang1–LRP5–Tie2 signaling in adult mice in vivo.
The establishment of stable and functional blood vessel networks requires cooperation of multiple angiogenic growth factors rather than relying on a single factor (16). For example, VEGF and Ang work together during vascular development; VEGF acts at early stages of vessel formation, whereas Ang1 acts later during vessel remodeling, maturation, and stabilization (15, 37). PRP extract contains abundant Ang1 and a number of other angiogenic growth factors including VEGF (22), which is also required for compensatory lung growth after PNX (28). Importantly, the magnitude of the endothelial sprouting and epithelial budding induced by PRP extract was larger than that induced by recombinant Ang1 alone (Figures E1C and E2C), suggesting that small amounts of these other factors in PRP extract may be required for angiogenesis and alveolar formation. In addition to Ang1, PRP extract contains Ang2, which antagonizes the role of Ang1. Although the concentration of Ang2 is far below Ang1 (Ang1:Ang2 = 100:3) (22) and thus Ang1 is likely dominant in the PRP extract–treated cells, recently it has been reported that Ang2 not only antagonizes the role of Ang1 but also coordinates angiogenesis and stimulates liver regeneration (38, 39). Thus, Ang2 may cooperate with Ang1 to accelerate lung regeneration after PNX in mice treated with PRP extract. PRP extract also contains various epithelial stimulating factors, such as keratinocyte growth factor (40) and hepatocyte growth factor (41), which stimulate proliferation and differentiation of epithelial cells, and therefore PRP extract may stimulate lung regeneration after PNX by directly stimulating epithelial cells as well (41, 42). In fact, PRP extract stimulates lung epithelial bud formation to some degree in the absence of ECs (Figure E2A), suggesting that these epithelial-stimulating components in PRP extract (41, 42) may stimulate alveolar morphogenesis in vitro. PRP extract also contains many chemical stimulators (e.g., PDGF and FGF2) that modulate Wnt signaling (43, 44) and may contain Wnt ligands, which bind to LRP5. It has been reported that Wnt3a promotes definitive endoderm differentiation (45) and that Wnt7b signals through LRP5 and regulates lung airway and vascular development (46). Wnt2–Wnt7b signaling also promotes Wnt activity in mesenchymal progenitors, which is required for lung development (43), and these Wnt ligands may potentially enhance lung regeneration after PNX. Thus, although Ang1–LRP5–Tie2 signaling seems to be one of the major pathways by which PRP extract stimulates lung regeneration after PNX, other angiogenic or epithelial factors in PRP extract may contribute to lung angiogenesis and alveolar formation. In fact, although LRP5 knockdown or soluble Tie2 receptor, which blocks Ang1, decreased EC sprout diameter induced by PRP extract in vitro, Tie2 knockdown did not change it, suggesting that LRP5 and Ang1 control EC sprouting through mechanisms other than Tie2.
Our results indicate that compensatory lung growth appears to occur mainly within the first week of PNX and that PRP extract restores the exercise capacity within the first week in mice. However, the effects of PRP extract on the recovery of exercise capacity were different between Weeks 1 and 2. Given that PRP extract did not change lung weight and exercise capacity in sham-operated mice and that intratracheal administration of PRP extract had similar effects on compensatory lung growth and recovery from exercise intolerance after PNX as systemic treatment (intraperitoneal injection), the distinct response of exercise capacity to PRP extract may be potentially due to the recovery from PNX and not a result of the systemic effects of PRP extract.
In pathological lungs, regenerative alveolarization may be impaired because specific chemical factors, their receptors, and/or lineages of progenitor cells are depleted (47). Supplementation of these factors or progenitor cells in the lung in combination with PRP extract may be able to stimulate regeneration of functional blood vessels and alveolar structures in diseased lungs more efficiently, which will greatly expand the range of potential applications of PRP extract. Although our coculture system suggests that LRP5–Tie2 signaling in ECs controls alveolar bud formation, it is still not clear how PRP extract stimulates LRP5 in the ECs and how ECs use Ang1–LRP5–Tie2 signaling to modulate alveolar morphogenesis. Also, the molecular mechanism by which LRP5 controls Tie2 expression remains unknown. It has been reported that LRP5 controls the expression of Twist1, a basic helix-loop-helix transcription factor, which binds to E-box promoter regions (48). Because the Tie2 promoter region contains E-box sequences and Twist1 knockdown decreases Tie2 expression (32), one possible mechanism is that LRP5 knockdown down-regulates Twist1 expression and thereby decreases Tie2 transcriptional activity and gene expression. PRP extract may stimulate lung regeneration after PNX through the LRP5–Twist1–Tie2 pathway. Further characterization of these mechanisms could lead to the development of angiogenesis-targeting strategies for regeneration of damaged or lost lung tissues. In addition to signals from the chemical microenvironment, properties of the micromechanical environment, such as tissue stiffness and extracellular matrix structures, contribute to lung regeneration and various lung diseases, including fibrosis, acute respiratory distress syndrome, BPD, and inflammation (23, 31, 49). Thus, in addition to optimization of growth factor concentrations, modification of the micromechanical environment using extracellular matrix modifiers may also improve the ability of PRP extract to promote lung regeneration.
We have reported that LRP5 controls Tie2 expression in lung human microvascular endothelial cells (5). However, although PRP extract increases phosphorylation levels of LRP5 and Tie2 in HUVE cells, it did not change Tie2 expression in HUVE cells. This may be because of underlying differences between the cell lines. Alternatively, other factors in PRP extract may inhibit Tie2 expression in HUVE cells in vitro.
In summary, we have demonstrated that PRP extract stimulates adult mouse lung vascular and alveolar regeneration through Ang1–LRP5–Tie2 signaling. These findings may improve the efficiency of lung regeneration and lung organ engineering. Because deregulated angiogenesis contributes to pathogenesis of various chronic lung diseases such as BPD and emphysema, the findings regarding the mechanism by which PRP extract stimulates angiogenesis and lung regeneration after PNX may improve understanding of how these processes are deregulated in diseased lungs and potentially lead to the development of new therapeutic strategies for chronic lung diseases. Given that PRP extract is generated from autologous peripheral blood by a simple method and can be preserved stably in the freezer for a long period, PRP extract could be a potentially good therapeutic tool for regenerating damaged or lost adult lung tissue.
Footnotes
This work was supported by funds from the American Heart Association (A.M.), by a Boston Children’s Hospital Faculty Career Development Fellowship (T.M. and A.M.), by Department of Defense grant W81XWH-10-1-0565 (D.E.I.), and by Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center grant P30 HD18655.
Author Contributions: Conceived and designed the experiments: T.M. and A.M. Performed the experiments: T.M., Z.C., A.J., E.J., and A.M. Analyzed the data: T.M. and A.M. Contributed reagents/materials/analysis tools: T.M. and A.M. Wrote the paper: T.M., D.E.I., and A.M.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0045OC on June 19, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Trulock EP, Christie JD, Edwards LB, Boucek MM, Aurora P, Taylor DO, Dobbels F, Rahmel AO, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26:782–795. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Narayanan M, Owers-Bradley J, Beardsmore CS, Mada M, Ball I, Garipov R, Panesar KS, Kuehni CE, Spycher BD, Williams SE, et al. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am J Respir Crit Care Med. 2012;185:186–191. doi: 10.1164/rccm.201107-1348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler JP, Loring SH, Patz S, Tsuda A, Yablonskiy DA, Mentzer SJ. Evidence for adult lung growth in humans. N Engl J Med. 2012;367:244–247. doi: 10.1056/NEJMoa1203983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- 5.Mammoto T, Chen J, Jiang E, Jiang A, Smith LE, Ingber DE, Mammoto A. LRP5 regulates development of lung microvessels and alveoli through the angiopoietin-Tie2 pathway. PLoS One. 2012;7:e41596. doi: 10.1371/journal.pone.0041596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeLisser HM, Helmke BP, Cao G, Egan PM, Taichman D, Fehrenbach M, Zaman A, Cui Z, Mohan GS, Baldwin HS, et al. Loss of PECAM-1 function impairs alveolarization. J Biol Chem. 2006;281:8724–8731. doi: 10.1074/jbc.M511798200. [DOI] [PubMed] [Google Scholar]
- 7.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, Crystal RG, Simons M, Sato TN, Worgall S, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, Wagers AJ, Tseng YH, Ryeom S, Kim CF. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell. 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thebaud B. Angiogenesis in lung development, injury, and repair. PVRI Review. 2010;2:62–68. doi: 10.1159/000101344. [DOI] [PubMed] [Google Scholar]
- 10.Wang B, Cheheltani R, Rosano J, Crabbe DL, Kiani MF. Targeted delivery of VEGF to treat myocardial infarction. Adv Exp Med Biol. 2013;765:307–314. doi: 10.1007/978-1-4614-4989-8_43. [DOI] [PubMed] [Google Scholar]
- 11.Navaratna D, Guo S, Arai K, Lo EH. Mechanisms and targets for angiogenic therapy after stroke. Cell Adhes Migr. 2009;3:216–223. doi: 10.4161/cam.3.2.8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benest AV, Salmon AH, Wang W, Glover CP, Uney J, Harper SJ, Bates DO. VEGF and angiopoietin-1 stimulate different angiogenic phenotypes that combine to enhance functional neovascularization in adult tissue. Microcirculation. 2006;13:423–437. doi: 10.1080/10739680600775940. [DOI] [PubMed] [Google Scholar]
- 16.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 17.Mammoto T, Jiang A, Jiang E, Mammoto A. Platelet-rich-plasma extract prevents pulmonary edema through angiopoietin-Tie2 signaling. Am J Respir Cell Mol Biol. 2015;52:56–64. doi: 10.1165/rcmb.2014-0076OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klement GL, Yip TT, Cassiola F, Kikuchi L, Cervi D, Podust V, Italiano JE, Wheatley E, Abou-Slaybi A, Bender E, et al. Platelets actively sequester angiogenesis regulators. Blood. 2009;113:2835–2842. doi: 10.1182/blood-2008-06-159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borzini P, Mazzucco L. Platelet gels and releasates. Curr Opin Hematol. 2005;12:473–479. doi: 10.1097/01.moh.0000177831.70657.e8. [DOI] [PubMed] [Google Scholar]
- 20.Demidova-Rice TN, Wolf L, Deckenback J, Hamblin MR, Herman IM. Human platelet-rich plasma- and extracellular matrix-derived peptides promote impaired cutaneous wound healing in vivo. PLoS One. 2012;7:e32146. doi: 10.1371/journal.pone.0032146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Gonzalez DJ, Mendez-Bolaina E, Trejo-Bahena NI. Platelet-rich plasma peptides: key for regeneration. Int J Pept. 2012;2012:532519. doi: 10.1155/2012/532519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mammoto T, Jiang A, Jiang E, Mammoto A. Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvasc Res. 2013;89:15–24. doi: 10.1016/j.mvr.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Mammoto T, Jiang E, Jiang A, Mammoto A. ECM structure and tissue stiffness control postnatal lung development through the LRP5-Tie2 signaling system. Am J Respir Cell Mol Biol. 2013;49:1009–1018. doi: 10.1165/rcmb.2013-0147OC. [DOI] [PubMed] [Google Scholar]
- 24.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 25.Kaisani A, Delgado O, Fasciani G, Kim SB, Wright WE, Minna JD, Shay JW. Branching morphogenesis of immortalized human bronchial epithelial cells in three-dimensional culture. Differentiation. 2014;87:119–126. doi: 10.1016/j.diff.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado O, Kaisani AA, Spinola M, Xie XJ, Batten KG, Minna JD, Wright WE, Shay JW. Multipotent capacity of immortalized human bronchial epithelial cells. PLoS One. 2011;6:e22023. doi: 10.1371/journal.pone.0022023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakatsu MN, Davis J, Hughes CC. Optimized fibrin gel bead assay for the study of angiogenesis. J Vis Exp. 2007;(3):186. doi: 10.3791/186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakurai MK, Lee S, Arsenault DA, Nose V, Wilson JM, Heymach JV, Puder M. Vascular endothelial growth factor accelerates compensatory lung growth after unilateral pneumonectomy. Am J Physiol Lung Cell Mol Physiol. 2007;292:L742–L747. doi: 10.1152/ajplung.00064.2006. [DOI] [PubMed] [Google Scholar]
- 29.Akinbi HT, Breslin JS, Ikegami M, Iwamoto HS, Clark JC, Whitsett JA, Jobe AH, Weaver TE. Rescue of SP-B knockout mice with a truncated SP-B proprotein: function of the C-terminal propeptide. J Biol Chem. 1997;272:9640–9647. doi: 10.1074/jbc.272.15.9640. [DOI] [PubMed] [Google Scholar]
- 30.Allen GB, Pavone LA, DiRocco JD, Bates JH, Nieman GF. Pulmonary impedance and alveolar instability during injurious ventilation in rats. J Appl Physiol (1985) 2005;99:723–730. doi: 10.1152/japplphysiol.01339.2004. [DOI] [PubMed] [Google Scholar]
- 31.Mammoto A, Mammoto T, Kanapathipillai M, Yung CW, Jiang E, Jiang A, Lofgren K, Gee EPS, Ingber DE. Control of lung vascular permeability and endotoxin-induced pulmonary edema by changes in extracellular matrix mechanics. Nat Commun. 2013;4:1759. doi: 10.1038/ncomms2774. [DOI] [PubMed] [Google Scholar]
- 32.Mammoto T, Jiang E, Jiang A, Lu Y, Juan AM, Chen J, Mammoto A. Twist1 controls lung vascular permeability and endotoxin-induced pulmonary edema by altering Tie2 expression. PLoS One. 2013;8:e73407. doi: 10.1371/journal.pone.0073407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fehrenbach H, Voswinckel R, Michl V, Mehling T, Fehrenbach A, Seeger W, Nyengaard JR. Neoalveolarisation contributes to compensatory lung growth following pneumonectomy in mice. Eur Respir J. 2008;31:515–522. doi: 10.1183/09031936.00109407. [DOI] [PubMed] [Google Scholar]
- 34.Yan X, Bellotto DJ, Foster DJ, Johnson RL, Jr, Hagler HK, Estrera AS, Hsia CC. Retinoic acid induces nonuniform alveolar septal growth after right pneumonectomy. J Appl Physiol. 2004;96:1080–1089. doi: 10.1152/japplphysiol.00771.2003. [DOI] [PubMed] [Google Scholar]
- 35.Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med. 2014;20:822–832. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konerding MA, Gibney BC, Houdek JP, Chamoto K, Ackermann M, Lee GS, Lin M, Tsuda A, Mentzer SJ. Spatial dependence of alveolar angiogenesis in post-pneumonectomy lung growth. Angiogenesis. 2012;15:23–32. doi: 10.1007/s10456-011-9236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 38.Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, Bartol A, Kienast Y, Vettel C, Loos EK, et al. Angiopoietin-2 differentially regulates angiogenesis through Tie2 and integrin signaling. J Clin Invest. 2012;122:1991–2005. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu J, Srivastava K, Wieland M, Runge A, Mogler C, Besemfelder E, Terhardt D, Vogel MJ, Cao L, Korn C, et al. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014;343:416–419. doi: 10.1126/science.1244880. [DOI] [PubMed] [Google Scholar]
- 40.Rozman P, Bolta Z. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol Alp Panonica Adriat. 2007;16:156–165. [PubMed] [Google Scholar]
- 41.Zhang J, Middleton KK, Fu FH, Im HJ, Wang JH. HGF mediates the anti-inflammatory effects of PRP on injured tendons. PLoS One. 2013;8:e67303. doi: 10.1371/journal.pone.0067303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Sharkawy H, Kantarci A, Deady J, Hasturk H, Liu H, Alshahat M, Van Dyke TE. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol. 2007;78:661–669. doi: 10.1902/jop.2007.060302. [DOI] [PubMed] [Google Scholar]
- 43.Miller MF, Cohen ED, Baggs JE, Lu MM, Hogenesch JB, Morrisey EE. Wnt ligands signal in a cooperative manner to promote foregut organogenesis. Proc Natl Acad Sci USA. 2012;109:15348–15353. doi: 10.1073/pnas.1201583109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fei Y, Xiao L, Doetschman T, Coffin DJ, Hurley MM. Fibroblast growth factor 2 stimulation of osteoblast differentiation and bone formation is mediated by modulation of the Wnt signaling pathway. J Biol Chem. 2011;286:40575–40583. doi: 10.1074/jbc.M111.274910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roszell B, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH, Lelkes PI, Finck CM. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tissue Eng Part A. 2009;15:3351–3365. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Shu W, Lu MM, Morrisey EE. Wnt7b activates canonical signaling in epithelial and vascular smooth muscle cells through interactions with Fzd1, Fzd10, and LRP5. Mol Cell Biol. 2005;25:5022–5030. doi: 10.1128/MCB.25.12.5022-5030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomperts BN, Strieter RM. Stem cells and chronic lung disease. Annu Rev Med. 2007;58:285–298. doi: 10.1146/annurev.med.58.081905.134954. [DOI] [PubMed] [Google Scholar]
- 48.Guo Y, Zi X, Koontz Z, Kim A, Xie J, Gorlick R, Holcombe RF, Hoang BH. Blocking Wnt/LRP5 signaling by a soluble receptor modulates the epithelial to mesenchymal transition and suppresses met and metalloproteinases in osteosarcoma Saos-2 cells. J Orthop Res. 2007;25:964–971. doi: 10.1002/jor.20356. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman AM, Shifren A, Mazan MR, Gruntman AM, Lascola KM, Nolen-Walston RD, Kim CF, Tsai L, Pierce RA, Mecham RP, et al. Matrix modulation of compensatory lung regrowth and progenitor cell proliferation in mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L158–L168. doi: 10.1152/ajplung.90594.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]