Abstract
Purpose
To test the efficacy of 4 weeks of intravenous (IV) induction with high-dose interferon (IFN) as part of the Eastern Cooperative Oncology Group regimen compared with observation (OBS) in patients with surgically resected intermediate-risk melanoma.
Patients and Methods
In this intergroup international trial, eligible patients had surgically resected cutaneous melanoma in the following categories: (1) T2bN0, (2) T3a-bN0, (3) T4a-bN0, and (4) T1-4N1a-2a (microscopic). Patients were randomly assigned to receive IFN α-2b at 20 MU/m2/d IV for 5 days (Monday to Friday) every week for 4 weeks (IFN) or OBS. Stratification factors were pathologic lymph node status, lymph node staging procedure, Breslow depth, ulceration of the primary lesion, and disease stage. The primary end point was relapse-free survival. Secondary end points included overall survival, toxicity, and quality of life.
Results
A total of 1,150 patients were randomly assigned. At a median follow-up of 7 years, the 5-year relapse-free survival rate was 0.70 (95% CI, 0.66 to 0.74) for OBS and 0.70, (95% CI, 0.66 to 0.74) for IFN (P = .964). The 5-year overall survival rate was 0.83 (95% CI, 0.79 to 0.86) for OBS and 0.83 (95% CI, 0.80 to 0.86) for IFN (P = .558). Treatment-related grade 3 and higher toxicity was 4.6% versus 57.9% for OBS and IFN, respectively (P < .001). Quality of life was worse for the treated group.
Conclusion
Four weeks of IV induction as part of the Eastern Cooperative Oncology Group high-dose IFN regimen is not better than OBS alone for patients with intermediate-risk melanoma as defined in this trial.
INTRODUCTION
Of the 76,380 new patients with melanoma projected for 2016 in the United States,1 those with deeper primary lesions or regional lymph node involvement, classified in the 6th edition of the American Joint Committee on Cancer (AJCC) staging system as T3N0M0 (1.5 to 4 mm deep [IIA]) or T4N0M0 (> 4 mm deep [IIB]), will have 5-year survival rates diminishing from 75% to 66%, and those with regional nodal involvement (TxN1-2a-bM0 [IIIA-B-C]) will have 5-year survival rates diminishing from 59% to 40%.2,3
Interferon-α-2b (IFN-α-2b; Intron A, Merck, NJ) was the first agent approved for adjuvant therapy for patients with high-risk surgically resected melanoma (stages IIB and III) on the basis of the results of the Eastern Cooperative Oncology Group (ECOG) E1684 trial, which tested high-dose interferon (HDI; IFN-α-2b, 20 MU/m2 intravenously [IV] for 5 of 7 days per week [Monday to Friday] for 4 weeks [induction], followed by IFN-α-2b, 10 MU/m2 three times per week [Monday, Wednesday, Friday, maintenance] for 11 months) versus observation (OBS). This trial showed statistically significant benefits of treatment for relapse-free survival (RFS; P = .002) and for overall survival (OS, P = .024).4
The Kaplan-Meier RFS and OS curves from E1684 showed early separation and sustained benefit throughout treatment and follow-up. It was hypothesized that the major source of benefit from the full HDI regimen may have been the initial IV induction treatment administered over 4 weeks. The current study (E1697) was proposed to test the impact of administering only 4 weeks of high-dose IFN-α-2b IV 5 of 7 days per week, as in the E1684 trial induction phase, compared with OBS for patients with resected melanoma in the following categories: (1) T2bN0, (2) T3a-bN0, (3) T4a-bN0 (> 4 mm), and (4) T1-4N1a-2a (microscopic). These patients were selected for study because they were considered to be at intermediate risk for recurrence and particularly likely to be motivated to pursue a shorter and potentially less toxic adjuvant therapy regimen with less disruption of quality of life (QOL).
PATIENTS AND METHODS
Eligibility
Eligibility criteria included completely resected, histologically confirmed cutaneous melanoma in the following categories: (1) T2bN0, (2) T3a-bN0, (3) T4a-bN0, and (4) T1-4N1a2a (microscopic). Participants also had to be > 10 years of age, have completed all primary surgical therapy (wide excision with or without lymphadenectomy) and be randomly assigned within 84 days of surgery, have an ECOG performance status of 0 to 1, have adequate hematologic and biochemical parameters, and have no major comorbidity or concurrent malignancy. The absence of regional or distant metastatic disease was required to be documented by physical examination and appropriate radiologic studies. Surgical staging of regional lymph nodes by sentinel node mapping was encouraged but not mandatory.
Treatment
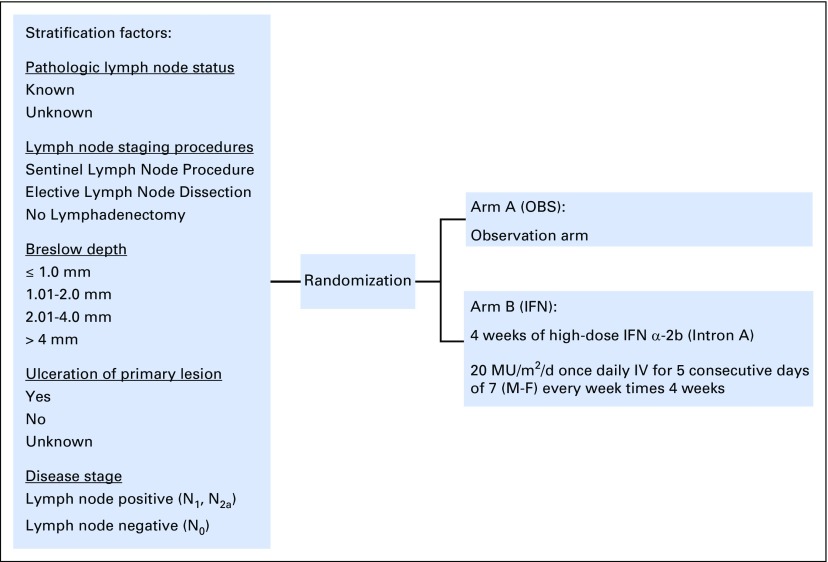
The protocol (Data Supplement) was approved by the ethics committees at participating sites. All patients provided written informed consent. Patients were randomly assigned to OBS or IFN, stratified by pathologic lymph node status (known, unknown), lymph node staging procedures (sentinel lymph node procedure, elective lymph node dissection, no lymphadenectomy), Breslow depth (≤ 1.00 mm, 1.01 to 2.00 mm, 2.01 to 4.00 mm, > 4.00 mm), ulceration of the primary lesion (yes, no, unknown), and disease stage (lymph node positive [N1, N2a], lymph node negative [N0]; Fig 1).
Fig 1.
Study schema for the phase III randomized trial of 4 weeks of high-dose interferon-α-2b in patients with intermediate-risk melanoma. IFN, interferon group; IV, intravenously; M-F, Monday to Friday; OBS, observation group.
Patients randomly assigned to the IFN group received IFN-α-2b at 20 MU/m2/d IV for 5 of 7 days per week (Monday to Friday) for 4 weeks. Patients who experienced grade 3 or higher toxicity per National Cancer Institute Criteria for Adverse Events according to Common Terminology Criteria for Adverse Events (version 2.0) and protocol-specific criteria were specified to have treatment withheld or discontinued.
End Points
The primary objective was to compare RFS between IFN and OBS. Secondary objectives were to compare OS and assess toxicity and QOL. RFS was defined as time from randomization to first recurrence or death without recurrence. For censored patients, time from randomization to the last date of assessment was used. OS was defined as time from randomization to death from any cause. For censored patients, time from randomization to the last known date alive was used. Adverse events (AEs) were coded and graded according to the National Cancer Institute’s Common Terminology for Criteria for Adverse Events (version 2.0). QOL data were collected longitudinally. Collection schedules, statistical analysis, and results are available in the Appendix and Table A1 (online only).
Statistical Design and Analysis
A cure rate model5,6 was assumed when assessing power and sample size during the design stage of the trial. The model assumes that the target population is a mixture, with proportion p being those who would be cured and 1 − p being those who would fail according to an exponential distribution with rate λ. Thus, the survival function used to assess power and sample size is expressed as S(t) = p + (1 − p) exp(−λt). It was assumed that IFN would provide a 15% relative increase in median time to event (from OBS RFS = 1.5 years, OS = 2.5 years) for those not cured and a 7.5% absolute increase in the cure rate (from OBS RFS = 65%, OS = 75%). This design required 1,420 total patients (with 1,278 eligible), 339 RFS events, and 186 deaths to achieve 88% power, and a two-sided type I error rate of 5% for each end point. Interim analyses were planned, starting at 25% information time. O'Brien-Fleming boundaries were used for efficacy and conditional power for futility monitoring.
All RFS and OS analyses were based on the intent-to-treat population. The distributions of RFS and OS were estimated using the Kaplan-Meier method,7 with 95% CIs calculated using Greenwood’s equation.8 Differences in treatment effect were tested using stratified log rank tests. Stratified Cox proportional hazards models9 were used to estimate hazard ratios (HRs) for the treatment effect for RFS and OS. Repeated CIs (RCIs)10 were provided to adjust for interim analyses. Toxicity data were compared using Fisher's exact tests.11 All reported P values were for two-sided tests, and the significance level was set at .05. Statistical analysis was conducted using SAS/STAT software (Version 9.2, SAS Institute, Cary, NC).
RESULTS
This intergroup study was initiated by ECOG in 1998, with participation from the Southwest Oncology Group (SWOG), the National Cancer Institute of Canada, the CALGB (Cancer and Leukemia Group B), and the Sydney Melanoma Unit. At the third interim analysis, the data safety monitoring committee recommended that the trial be stopped early on the basis of futility analysis. The study was terminated in October 2010.
Total accrual was 1,150 patients. Baseline demographic and clinical characteristics are listed in Table 1. Randomization was well balanced between treatment groups for sex, race, age, lymph node status, and staging procedure, Breslow depth, Clark level, ulceration, disease stage, primary site, and ECOG performance status.
Table 1.
Patient Characteristics (n = 1,150)
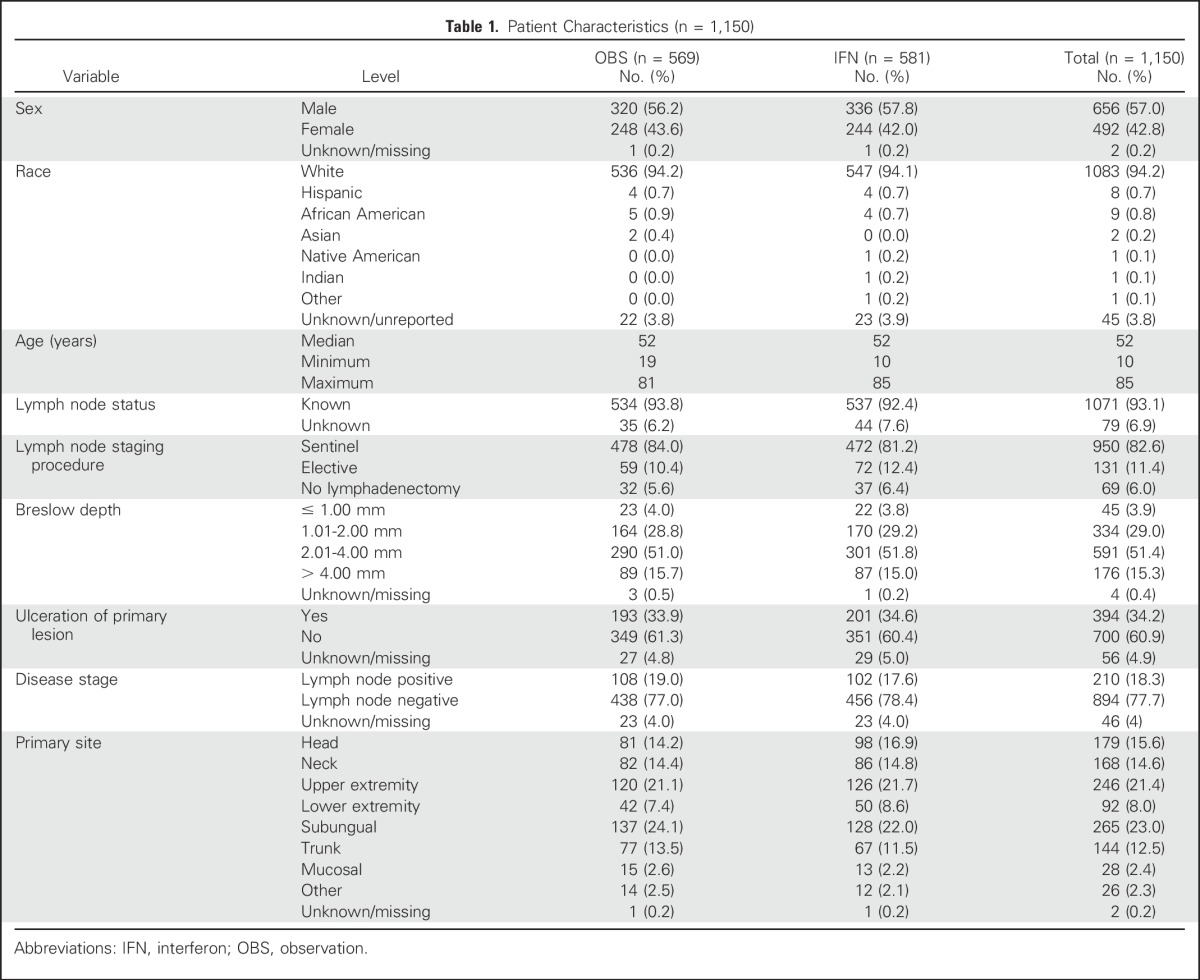
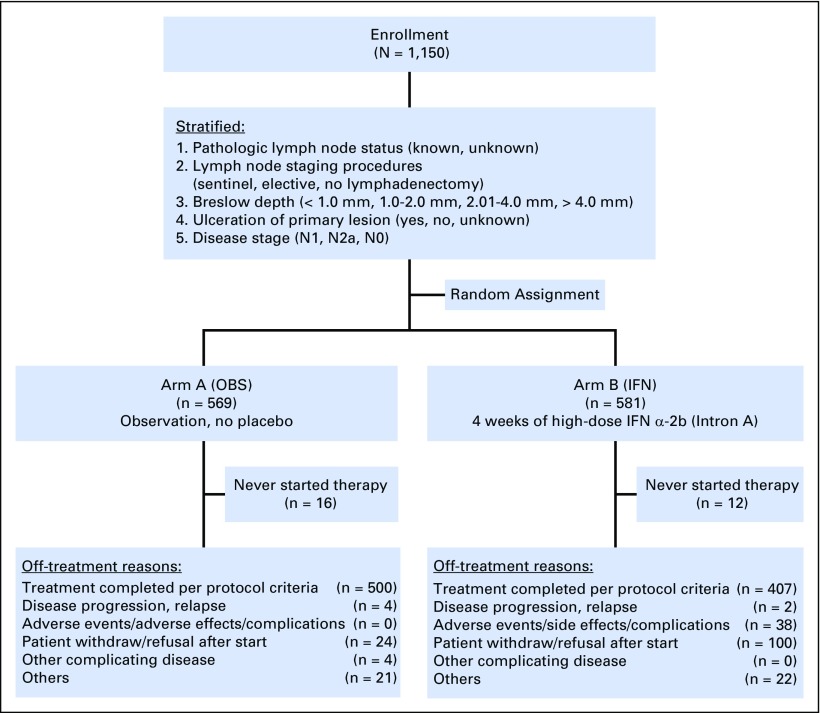
The data as of a cutoff date of January 2015 were used, with a median follow-up time of 7 years. There were 50 and 52 ineligible patients in the OBS and IFN groups, respectively. As shown in the CONSORT diagram (Fig 2), 17% of patients refused to start or withdrew consent after being randomly assigned, and 6.5% discontinued because of adverse events. Among the 581 patients randomly assigned to receive IFN, 12 never started treatment. The median duration of treatment was 26 days (range, 1 to 47 days).
Fig 2.
CONSORT diagram. Note that the patients in the observation (OBS) arm who refused participation were counted as never started assigned therapy. IFN, interferon arm.
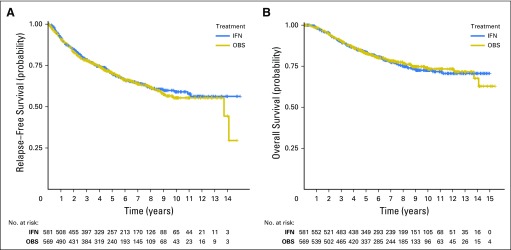
Regardless of eligibility, an intent-to-treat analysis was conducted. Neither RFS nor OS was significantly different between the two arms of the study. There were a total of 367 RFS events (319 recurrences and 48 deaths without recurrences). The 5-year RFS rate was 0.70 (95% CI, 0.66 to 0.74) for OBS and 0.70 (95% CI, 0.66 to 0.74) for IFN (P = .964). Figure 3A displays the Kaplan-Meier plot of RFS. The HR for IFN versus OBS was 0.98, with a 95% RCI of 0.79 to 1.22.
Fig 3.
Kaplan-Meier plot of (A) relapse-free survival and (B) overall survival (OS) by treatment arm for patients with intermediate risk melanoma.
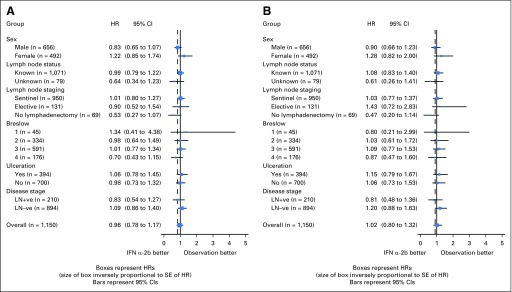
There were a total of 241 deaths. The 5-year OS rate was 0.83 (95% CI, 0.79 to 0.86) for OBS and 0.83 (95% CI, 0.80 to 0.86) for IFN (P = .558). Figure 3B displays the Kaplan-Meier plots for OS. The HR for IFN versus OBS was 1.08, with a 95% RCI of 0.82 to 1.41. Figures 4A and 4B display the forest plot for the effect of IFN for RFS and OS in the subgroups. None of the subgroups indicated a significant treatment difference.
Fig 4.
Forest plot for (A) relapse-free survival and (B) overall survival (with hazard ratios [HRs] for interferon [IFN] v observation [OBS]). Note that the CIs presented here are not recurrent CIs. SE, standard error; LN, lymph node; −ve, negative; +ve, positive.
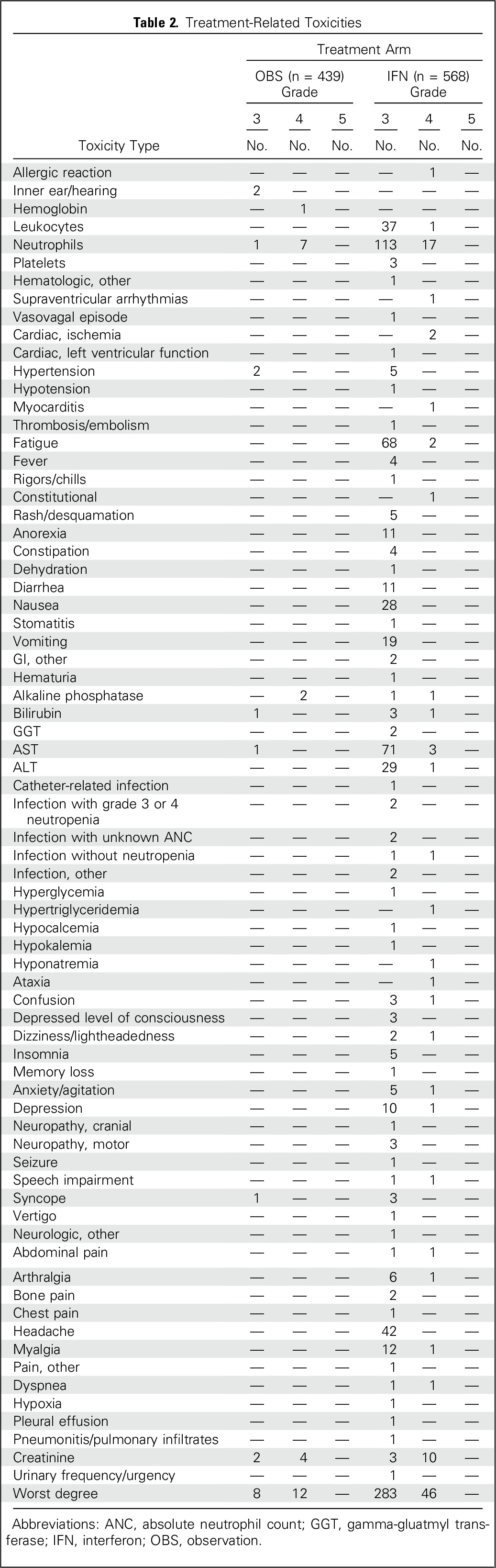
Toxicity is listed in Table 2. A total of 439 patients in the OBS group and 568 in the IFN group were included in the toxicity assessment. Treatment-related grade 3 and higher toxicities were seen in 4.6% of patients in the OBS group versus 57.9% of patients in the IFN group. This difference was significant (P < .001). The most common grade 3 or higher toxicities for patients receiving IFN were neutropenia (21%), fatigue (13%), elevated liver enzymes (13%), and headache (8%). The QOL analysis is provided in the Appendix.
Table 2.
Treatment-Related Toxicities
DISCUSSION
Two decades after the first US Food and Drug Administration approval of an adjuvant therapy for high-risk melanoma (defined as AJCC stage IIB and III),12 debate and controversy around the optimal clinical use of adjuvant IFN continues. The 1-year HDI regimen comprises a high-dose IV (induction) phase followed by a subcutaneous (maintenance) phase. On the basis of the results of trial E1684,4 this regimen has been accepted as a standard in the United States and many other countries and has served as the control arm of most ECOG- and SWOG-led US intergroup randomized trials of adjuvant therapy, including E1609 and S0008. However, the toxicity associated with this regimen and the 12-month duration of treatment has been an impediment to its universal acceptance. The lack of an improvement in OS in one subsequent trial (E1690) has further fueled controversy.13 In the absence of defined alternative treatment options, modifications of this regimen attempting to lower the dose or shorten the duration of therapy have been pursued.
Adjuvant trials have traditionally targeted patients at high risk. However, patients with melanoma of Breslow thickness between 1.5 and 4.0 mm without nodal metastases (stage IIA, 6th edition AJCC) account for 31% of new diagnoses, comprising approximately 25,000 patients per year.14 These patients have a significant relapse rate of 30% at 5 years,15,16 but have uniformly been excluded from prior cooperative group trials of adjuvant therapy.
At the time we designed E1697, information from two European trials for stage II patients were available. The Austrian Melanoma Cooperative Group17 and the French Cooperative Group on Melanoma18 both tested lower doses of IFN-α (3 MU three times per week, termed low-dose IFN [LDI]) for varying durations compared with OBS. Both trials showed improvement in RFS that diminished over time without significant impact on OS, potentially implying transient benefits without a fraction of patients who are cured. The WHO trial No. 16 for patients with stage III disease tested the benefit of 3 years of LDI and showed no benefit in either RFS or OS.19 Intergroup trial E 1690 tested HDI or LDI versus OBS for patients with stage IIB and III melanoma, showing no significant benefit for LDI in either RFS or OS.20
Analysis of the hazard functions observed in the HDI arm in E1684 showed maximal benefit early, during the IV induction phase, that was subsequently sustained.4 This IV induction phase distinguished these trials from European trials of low- or intermediate-dosage IFN. We hypothesized that this induction phase might be necessary and sufficient for the benefit of the 1-year HDI regimen. In addition, toxicity in the E1684 trial showed two major adverse effects that influenced QOL for patients—namely, fatigue and depression—that were cumulative over the year of treatment. E1697 was designed to test the potential benefits of 1 month of high-dose IV IFN-α-2b alone in patients with intermediate-risk melanoma and defined this initially as T3N0 disease. Subsequently, eligibility was expanded to include patients with T4N0 and TanyN1a disease, as well.
The results of E1697 clearly show no impact of induction therapy alone in terms of either RFS or OS (P values of .964 and .558, and HRs for arm B versus arm A of 0.98 and 1.08, respectively). Subgroup and subset analyses on the basis of the various stratification factors, including T3, T4, and N1a disease as well as those with ulcerated primary lesions, also showed no differences between the treatment arms. QOL, not surprisingly, was worse for the patients who received treatment compared with OBS.
Other trials have attempted to address the issue of the value of IV induction therapy with IFN compared with the full year of treatment. The Hellenic Cooperative Oncology Group conducted trial 13A/98 to test the noninferiority of a modified IFN IV induction (15 MU/m2 per day for 5 days per week for 4 weeks) compared with the same induction regimen followed by a modified maintenance phase (10 MU flat dose, three times per week for 48 weeks). This relatively small trial (364 patients) failed to show differences in RFS or OS between the two arms.21 The modification of both the induction and the maintenance phases of this trial compared with the E1684 trial, and lack of a control arm, make interpretation difficult.22 Payne et al23 conducted a pilot trial with 194 patients with stage IIB-IIIC melanoma, randomly assigning a higher-risk population, among which 77% had gross nodal disease, to standard 1-year HDI or the induction phase alone using doses and schedules identical to E1684. The OS outcome for the induction-only arm was statistically inferior to the full year of HDI.
Other trials have tested the role of multiple courses of IV induction in patients with high-risk melanoma.24,25 A trial from the Dermatologic Cooperative Oncology Group (DeCOG) randomly allocated 649 patients to either standard HDI or IV induction given three times every fourth month. No significant difference in survival was observed between the two arms of the study.26 An Italian trial randomly assigned 336 patients in a similar design but with four courses of IV induction, once every third month. No significant differences were observed between the two arms of the trial.27 Of note, both of these trials included only patients with stage III disease.
E1697 is the only trial to date to test the role of 1 month of IV induction versus OBS. The lack of any impact of this treatment on either RFS or OS in any subset of patients suggests that IV induction therapy does not, by itself, have a meaningful therapeutic role and that the overall benefit of the E1684 regimen requires additional IFN, either maintenance treatment of 11 months per the HDI regimen or possibly repeated courses of IV induction per the two European trials cited previously.
An alternate hypothesis regarding adjuvant IFN therapy is that treatment duration may be as important as dose intensity. A trial from the European Organization for the Research and Treatment of Cancer (EORTC 18991) tested the role of 5 years of pegylated interferon in 1,256 patients with stage III melanoma compared with OBS.28 The difference in RFS between the treatment and the OBS groups (HR, 0.87; 95% CI, 0.76 to 1.00, P = .05) was statistically significant, but was not accompanied by any impact on OS. This regimen received regulatory approval in the United States for patients with resected stage III disease.
Taken in aggregate, a single IV induction course of IFN does not seem to benefit patients with intermediate- or high-risk melanoma when administered without the maintenance phase. For patients seeking additional options for adjuvant therapy with IFN, those with stage IIB and higher disease are eligible to receive standard HDI (IV induction followed by maintenance) and those with stage III disease unable or unwilling to pursue HDI may choose pegylated IFN for up to 5 years.
Recent advances using checkpoint inhibitors in advanced melanoma have prompted investigation in the adjuvant arena. EORTC 18071 showed a statistically significant RFS and OS benefit in 951 patients randomly allocated to receive ipilimumab 10 mg/kg versus placebo once every 3 weeks for four doses and then every 3 months for up to 3 years. Adverse events led to discontinuation of ipilimumab in 52% of patients who started ipilimumab, including 39% during the initial induction phase of four doses.29,30 Five patients (1%) died as a result of drug-related toxicity. Importantly, data related to distant metastasis–free survival and OS end points are still pending. This regimen has recently received regulatory approval in the United States for patients with stage III disease. The ECOG–American College of Radiology Imaging Network–led intergroup E1609 trial compared low-dose (3 mg/kg) and high-dose (10 mg/kg) ipilimumab with HDI and completed accrual in 2014, whereas the current SWOG-led intergroup S1404 tests the anti–programmed death-1 antibody pembrolizumab versus HDI or high-dose ipilimumab (10 mg/kg) in the adjuvant setting. Furthermore, CheckMate-238, testing nivolumab versus ipilimumab 10 mg/kg, completed accrual in 2015, and KEYNOTE-054, testing pembrolizumab versus placebo, are ongoing. Adjuvant trials testing molecularly targeted therapy with dabrafenib-trametinib versus placebo and vemurafenib versus placebo have also been completed and are pending analysis.
These trials will provide further guidance for physicians and patients to help negotiate the complexity of choices that are available for the adjuvant therapy of high-risk melanoma. Meanwhile, standard HDI therapy is still a viable option. The results of E1697 presented here clarify that 1 month of IV induction is insufficient treatment and that the traditional 1-year HDI regimen should not be abbreviated or modified at this time.
ACKNOWLEDGMENT
This study was coordinated by the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group (Robert L. Comis and Mitchell D. Schnall, Group Co-Chairs).
Appendix
Assessment of patients’ quality of life (QOL) was one of the secondary end points for this study. This section summarizes the QOL analysis.
Statistical Analysis
The QOL was collected at the baseline, day 22, every 3 months after registration until 2 years, and then every 6 months until 5 years. The questions included: Main Activity (1 = work normally, 2 = need help, 3 = unable to work); Daily Living (1 = normal, 2 = need assistance, 3 = cannot manage personal care); Health (1 = feel well most of the time, 2 = feel well some of the time, 3 = feel lousy most of the time); Support (1 = strong support from family members, 2 = limited support, 3 = no support); and Outlook (1 = good, 2 = anxious and depressed at times, 3 = frightened and confused). The last question was a Patient Score of overall well-being over the past 2 weeks (0-100, with 0 being the worst possible health state and 100 being an excellent health state). To compare the QOL measurements between the two treatment arms, the χ2 test was used for the first five questions (categorical measure) and the two-sample t test for the Patient Score (continuous measure) at each time point.
Because the patients in the treatment arm received only a single 4-week treatment of interferon-α-2b, it was hypothesized that the most pronounced changes of QOL would be between baseline and day 22.
For the patients with QOL measurements at baseline and day 22, a change in QOL score was estimated (by subtracting the baseline value from day 22 value). The difference in this change between the two treatment arms was compared. For the QOL measurements with the categorical variables (Main Activity, Daily Living, Health, Support, and Outlook) with values 1, 2, and 3 at each time point, this change could be −2, −1, 0, 1, and 2: negative measures indicating a change toward the better QOL at day 22, 0 indicating no change and positive measures indicating a change toward the worse QOL at day 22. This change was compared using the χ2 test. The change in the Patient Score was compared using the two-sample t test. As exploratory analyses, the change in QOL measures between a later time point and baseline was evaluated in a similar manner.
All reported P values are for two-sided tests. Given the exploratory nature of this study, no adjustments were made for multiple testing.
Results
Of the 1,150 patients randomly assigned, 1,044 participated in the QOL study. Compliance rate varied over time, ranging from 93% (baseline) to 42% (5-year), using the number of patients reaching the specific time point as a denominator.
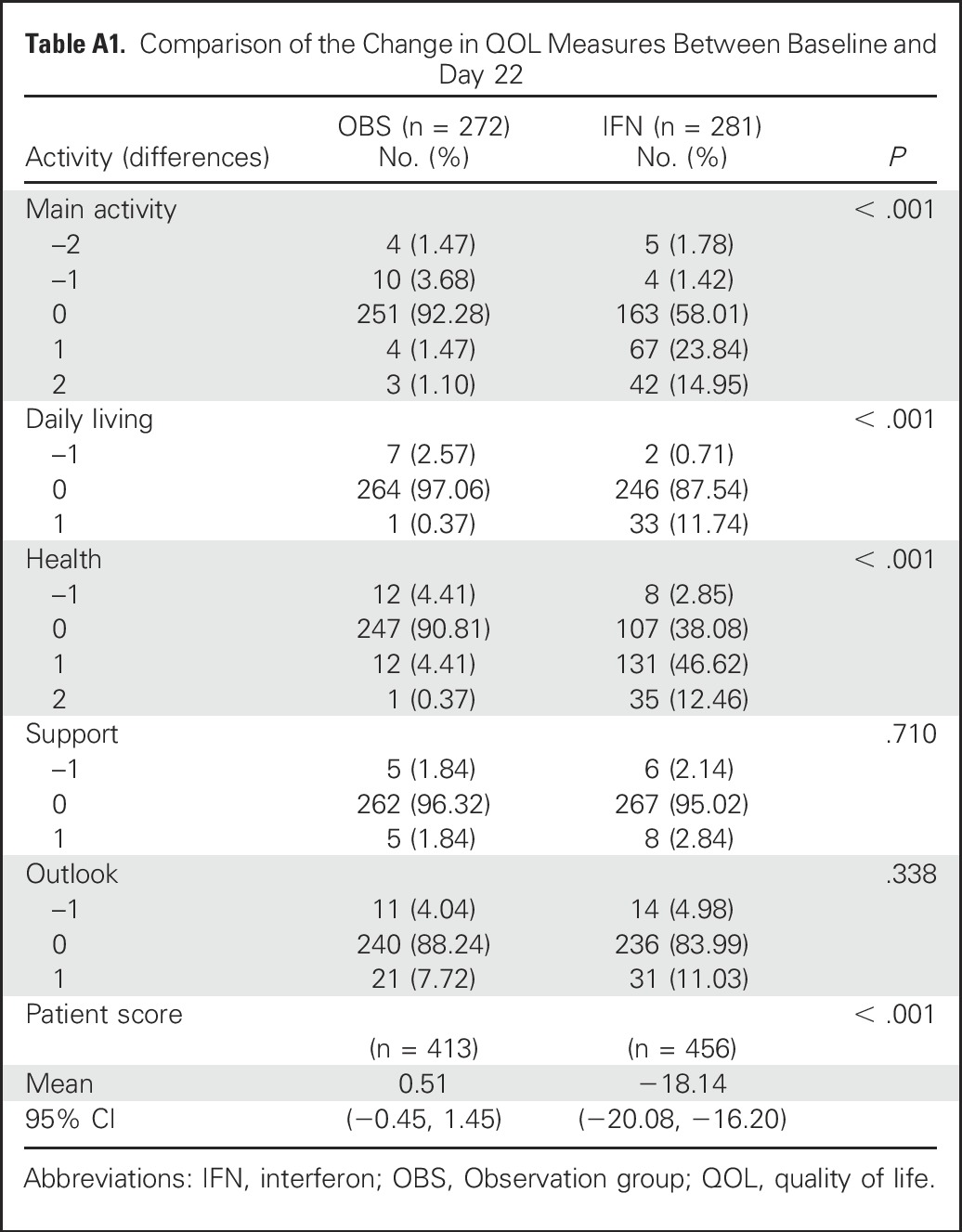
The primary analysis for QOL data were descriptive. First, QOL scores of Main Activity, Daily Living, Health, Support, and Outlook were compared at each time point between the two treatment arms. The QOL measures of Main Activity, Daily Living, Health, and Patient Score were significantly different between the two treatment arms at day 22 (each comparison with P < .001). No other comparisons were significantly different. For the Patient Score, only the measurement from day 22 comparison was significantly different between the two treatment arms (P < .001).
Comparing the change in QOL scores from baseline to day 22, only Main Activity, Daily Living, Health, and Patient Score were statistically significant, with P values < .001. The detailed results are presented in Table A1. No other comparisons from baseline to any other time points were significantly different.
Conclusion
QOL data suggest that patients experience lower QOL during the interferon-α-2b treatment.
Table A1.
Comparison of the Change in QOL Measures Between Baseline and Day 22
Footnotes
Supported by the National Cancer Institute of the National Institutes of Health under award Nos. CA180820, CA21115, CA180794, CA23318, CA66636, CA180844, CA39229, CA180802, CA16116, CA17145, CA180853, CA14548, CA180799, CA21076, CA80775, CA189808, CA35431, CA180855, CA180847, CA49957, CA180888, CA32102, CA180835, CA14028, CA73950, CA077658, CA077202, CA180886, CA180899, CA098543, and CA098413, and Canadian Cancer Society Research Institute Grant No.021039. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Clinical trial information: NCT00003641.
AUTHOR CONTRIBUTIONS
Conception and design: Sanjiv S. Agarwala, Sandra J. Lee, Gary I. Cohen, Michael B. Atkins, John M. Kirkwood
Provision of study materials or patients: Ahmad A. Tarhini, Joanna M. Brell, Michael B. Atkins, Richard F. Kefford
Collection and assembly of data: Sanjiv S. Agarwala, Sandra J. Lee, Waiki Yip, Uma N. Rao, Ahmad A. Tarhini, Douglas S. Reintgen, Michael B. Atkins, Shaker R. Dakhil, Robert M. Conry, Vernon K. Sondak, William E. Carson, Michael G. Smylie, Alberto S. Pappo, Richard F. Kefford
Data analysis and interpretation: Sanjiv S. Agarwala, Sandra J. Lee, Waiki Yip, Ahmad A. Tarhini, Terry L. Evans, Joanna M. Brell, Mark R. Albertini, Jeffrey A. Sosman, Lawrence E. Flaherty, Vernon K. Sondak
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase III Randomized Study of 4 Weeks of High-Dose Interferon-α-2b in Stage T2bNO, T3a-bNO, T4a-bNO, and T1-4N1a-2a (microscopic) Melanoma: A Trial of the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network Cancer Research Group (E1697)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Sanjiv S. Agarwala
No relationship to disclose
Sandra J. Lee
Employment: NantKwest (I)
Leadership: Osiris (I)
Stock or Other Ownership: NantKwest (I)
Consulting or Advisory Role: Genentech
Waiki Yip
Employment: Foundation Medicine
Stock or Other Ownership: Abbvie, Eli Lilly
Travel, Accommodations, Expenses: Foundation Medicine
Uma N. Rao
Research Funding: Eastern Cooperative Oncology Group
Ahmad A. Tarhini
Consulting or Advisory Role: Bristol-Myers Squibb
Research Funding: Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Amgen (Inst), Novartis (Inst), Prometheus (Inst), Incyte (Inst), GreenPeptide (Inst)
Gary I. Cohen
Stock or Other Ownership: Nymox, Celgene, Foundation Medicine, Abbvie
Consulting or Advisory Role: NantHealth
Speakers' Bureau: NantHealth, Genzyme
Douglas S. Reintgen
No relationship to disclose
Terry L. Evans
No relationship to disclose
Joanna M. Brell
No relationship to disclose
Mark R. Albertini
No relationship to disclose
Michael B. Atkins
Honoraria: Bristol-Myers Squibb
Consulting or Advisory Role: Genentech, Pfizer, Novartis, Amgen, Genoptix, Bristol-Myers Squibb, Nektar, Merck, Agenus, Infinity Pharmaceuticals, Alkermes, Celldex
Shaker R. Dakhil
No relationship to disclose
Robert M. Conry
Speakers' Bureau: Novartis, Bristol-Myers Squibb, Merck
Jeffrey A. Sosman
No relationship to disclose
Lawrence E. Flaherty
Consulting or Advisory Role: Merck/Schering Plough, Caris Life Sciences
Travel, Accommodations, Expenses: Merck/Schering Plough, Caris Life Sciences
Vernon K. Sondak
Consulting or Advisory Role: Merck/Schering-Plough, Provectus Pharmaceuticals, Novartis, GlaxoSmithKline, Bristol-Myers Squibb
William E. Carson
No relationship to disclose
Michael G. Smylie
Honoraria: Bristol-Myers Squibb, Merck, Novartis
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Novartis
Speakers' Bureau: Merck, Bristol-Myers Squibb
Alberto S. Pappo
Consulting or Advisory Role: Eli Lilly
Richard F. Kefford
Honoraria: Merck, Bristol-Myers Squibb, Novartis
Consulting or Advisory Role: Roche (Inst), Amgen (Inst), Bristol-Myers Squibb (Inst), Merck (Inst), Novartis (Inst), TEVA
Speakers' Bureau: GlaxoSmithKline, Merck
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck
John M. Kirkwood
Consulting or Advisory Role: Bristol-Myers Squibb, Amgen, GreenPeptide, Roche, Genentech
Research Funding: Prometheus (Inst), Merck (Inst)
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Koh HK. Cutaneous melanoma. N Engl J Med. 1991;325:171–182. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: The Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol. 1996;14:7–17. doi: 10.1200/JCO.1996.14.1.7. [DOI] [PubMed] [Google Scholar]
- 5.Berkson J, Gage RP. Calculation of survival rates for cancer. Proc Staff Meet Mayo Clin. 1950;25:270–286. [PubMed] [Google Scholar]
- 6.Goldman AI. Survivorship analysis when cure is a possibility: A Monte Carlo study. Stat Med. 1984;3:153–163. doi: 10.1002/sim.4780030208. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan EL , Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 8.Greenwood, M, Topley WW: Experimental epidemiology: Some general considerations. Proc R Soc Med, 19:31-44, 1926 [DOI] [PMC free article] [PubMed]
- 9.Cox DR. Regression models and life-tables. J R Stat Soc (Ser A) 1972;34:187–220. [Google Scholar]
- 10.Jennison C, Turnbull BW. Repeated confidence intervals for group sequential clinical trials. Control Clin Trials. 1984;5:33–45. doi: 10.1016/0197-2456(84)90148-x. [DOI] [PubMed] [Google Scholar]
- 11.Agresti A, Min Y. Unconditional small-sample confidence intervals for the odds ratio. Biostatistics. 2002;3:379–386. doi: 10.1093/biostatistics/3.3.379. [DOI] [PubMed] [Google Scholar]
- 12.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 13.Kirkwood JM, Ibrahim JG, Sondak VK, et al. High- and low-dose interferon alfa-2b in high-risk melanoma: First analysis of intergroup trial E1690/S9111/C9190. J Clin Oncol. 2000;18:2444–2458. doi: 10.1200/JCO.2000.18.12.2444. [DOI] [PubMed] [Google Scholar]
- 14.Balch CM, Soong S, Shaw HM, et al: Changing trends in the clinical and pathologic features of melanoma (ed 2). Cutaneous melanoma Balch CM (ed): Philadelphia, PA, J.B. Lippincott, 1992 xxiv, 583 p. 5 [Google Scholar]
- 15.Soong SJ, Shaw HM, Balch CM, et al. Predicting survival and recurrence in localized melanoma: A multivariate approach. World J Surg. 1992;16:191–195. doi: 10.1007/BF02071520. [DOI] [PubMed] [Google Scholar]
- 16.Slingluff CL, Jr, Dodge RK, Stanley WE, et al. The annual risk of melanoma progression. Implications for the concept of cure. Cancer. 1992;70:1917–1927. doi: 10.1002/1097-0142(19921001)70:7<1917::aid-cncr2820700719>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Pehamberger H, Soyer HP, Steiner A, et al. Adjuvant interferon alfa-2a treatment in resected primary stage II cutaneous melanoma. J Clin Oncol. 1998;16:1425–1429. doi: 10.1200/JCO.1998.16.4.1425. [DOI] [PubMed] [Google Scholar]
- 18.Grob JJ, Dreno B, de la Salmonière P, et al. Randomised trial of interferon alpha-2a as adjuvant therapy in resected primary melanoma thicker than 1.5 mm without clinically detectable node metastases. Lancet. 1998;351:1905–1910. doi: 10.1016/s0140-6736(97)12445-x. [DOI] [PubMed] [Google Scholar]
- 19.Cascinelli N, Belli F, MacKie RM, et al. Effect of long-term adjuvant therapy with interferon alpha-2a in patients with regional node metastases from cutaneous melanoma: A randomised trial. Lancet. 2001;358:866–869. doi: 10.1016/S0140-6736(01)06068-8. [DOI] [PubMed] [Google Scholar]
- 20.Agarwala SS, Kirkwood JM, Bryant J. Phase 1, randomized, double-blind trial of 7-allyl-8-oxoguanosine (loxoribine) in advanced cancer. Cytokines Cell Mol Ther. 2000;6:171–176. doi: 10.1080/mccm.6.4.171.176. [DOI] [PubMed] [Google Scholar]
- 21.Pectasides D, Dafni U, Bafaloukos D, et al. Randomized phase III study of 1 month versus 1 year of adjuvant high-dose interferon alfa-2b in patients with resected high-risk melanoma. J Clin Oncol. 2009;27:939–944. doi: 10.1200/JCO.2008.16.3121. [DOI] [PubMed] [Google Scholar]
- 22.Agarwala SS, Gray RJ, Wong MK: Duration of high-dose interferon alpha-2b regimen for resected high-risk melanoma. J Clin Oncol 27:e82-e83 [DOI] [PubMed]
- 23.Payne MJ, Argyropoulou K, Lorigan P, et al. Phase II pilot study of intravenous high-dose interferon with or without maintenance treatment in melanoma at high risk of recurrence. J Clin Oncol. 2014;32:185–190. doi: 10.1200/JCO.2013.49.8717. [DOI] [PubMed] [Google Scholar]
- 24.Mao L, Si L, Chi Z, et al. A randomised phase II trial of 1 month versus 1 year of adjuvant high-dose interferon α-2b in high-risk acral melanoma patients. Eur J Cancer. 2011;47:1498–1503. doi: 10.1016/j.ejca.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Malczewski A, Marshall A, Payne MJ, et al. Intravenous high-dose interferon with or without maintenance treatment in melanoma at high risk of recurrence: Meta-analysis of three trials. Cancer Med. 2016;5:17–23. doi: 10.1002/cam4.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohr P, Hauschild A, Trefzer U, et al. Intermittent high-dose intravenous interferon alfa-2b for adjuvant treatment of stage III melanoma: Final analysis of a randomized phase III Dermatologic Cooperative Oncology Group trial. J Clin Oncol. 2015;33:4077–4084. doi: 10.1200/JCO.2014.59.6932. [DOI] [PubMed] [Google Scholar]
- 27.Chiarion-Sileni V, Guida M, Romanini A: Intensified high-dose intravenous interferon alpha 2b (IFNa2b) for adjuvant treatment of stage III melanoma: A randomized phase III Italian Melanoma Intergroup (IMI) trial [ISRCTN75125874]. J Clin Oncol 29:527s, 2011 (suppl; abstr 8506) [Google Scholar]
- 28.Eggermont AM, Suciu S, Santinami M, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: Final results of EORTC 18991, a randomised phase III trial. Lancet. 2008;372:117–126. doi: 10.1016/S0140-6736(08)61033-8. [DOI] [PubMed] [Google Scholar]
- 29.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 30.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]