Abstract
Purpose
B-cell leukemia/lymphoma-2 (BCL-2) overexpression is common in many non-Hodgkin lymphoma (NHL) subtypes. A phase I trial in patients with NHL was conducted to determine safety, pharmacokinetics, and efficacy of venetoclax, a selective, potent, orally bioavailable BCL-2 inhibitor.
Patients and Methods
A total of 106 patients with relapsed or refractory NHL received venetoclax once daily until progressive disease or unacceptable toxicity at target doses from 200 to 1,200 mg in dose-escalation and safety expansion cohorts. Treatment commenced with a 3-week dose ramp-up period for most patients in dose-escalation cohorts and for all patients in safety expansion.
Results
NHL subtypes included mantle cell lymphoma (MCL; n = 28), follicular lymphoma (FL; n = 29), diffuse large B-cell lymphoma (DLBCL; n = 34), DLBCL arising from chronic lymphocytic leukemia (Richter transformation; n = 7), Waldenström macroglobulinemia (n = 4), and marginal zone lymphoma (n = 3). Venetoclax was generally well tolerated. Clinical tumor lysis syndrome was not observed, whereas laboratory tumor lysis syndrome was documented in three patients. Treatment-emergent adverse events were reported in 103 patients (97%), a majority of which were grade 1 to 2 in severity. Grade 3 to 4 events were reported in 59 patients (56%), and the most common were hematologic, including anemia (15%), neutropenia (11%), and thrombocytopenia (9%). Overall response rate was 44% (MCL, 75%; FL, 38%; DLBCL, 18%). Estimated median progression-free survival was 6 months (MCL, 14 months; FL, 11 months; DLBCL, 1 month).
Conclusion
Selective targeting of BCL-2 with venetoclax was well tolerated, and single-agent activity varied among NHL subtypes. We determined 1,200 mg to be the recommended single-agent dose for future studies in FL and DLBCL, with 800 mg being sufficient to consistently achieve durable response in MCL. Additional investigations including combination therapy to augment response rates and durability are ongoing.
INTRODUCTION
Dysregulation of apoptosis via overexpression of the antiapoptotic protein B-cell leukemia/lymphoma-2 (BCL-2) is fundamental to the biology of several subtypes of non-Hodgkin lymphoma (NHL). The BCL-2 gene was first cloned in a lymphoid cell line1 and found to be a hallmark of the most common form of indolent NHL, follicular lymphoma (FL).2,3 BCL-2 is also overexpressed in approximately 30% of diffuse large B-cell lymphomas (DLBCLs),4 and amplification of chromosomal region 18q21 (which includes the BCL-2 locus) is frequently found in mantle cell lymphoma (MCL).5
Venetoclax is a highly selective BCL-2 inhibitor with potent activity against FL, DLBCL, and MCL cell lines, as well as in a t(14;18)-carrying xenograft model.6 The M12-175 study is a first-in-human dose-escalation trial of venetoclax in patients with relapsed or refractory chronic lymphocytic leukemia (CLL; arm A) or NHL (arm B). The results in the CLL arm showed a 79% response rate, including 20% of patients achieving complete remission.7 The potency of venetoclax in patients with CLL was illustrated by the development of clinical tumor lysis syndrome (TLS) in three of the initial 56 patients. Clinical TLS is defined as occurrence of two or more metabolic abnormalities during the same 24-hour period as well as an increased creatinine level, cardiac dysrhythmia, seizure, or death.8 With adjustments to the dose ramp-up schedule, there were no more clinical TLS events in 60 additional patients with CLL enrolled. Venetoclax is now approved by the US Food and Drug Administration for the treatment of patients with CLL with chromosome 17p deletion who have received at least one prior therapy.9
Here, we report the results for the NHL cohort of the M12-175 study. The objectives were to define the safety profile, maximum-tolerated dose (MTD), dose-limiting toxicities (DLTs), pharmacokinetics, and preliminary efficacy of venetoclax and determine a recommended phase II dose and schedule for future NHL studies.
PATIENTS AND METHODS
Study Design
Arm B of the M12-175 study enrolled patients with relapsed or refractory NHL between September 2011 and November 2014 and is ongoing. The data cutoff for this publication was April 5, 2016. The trial was conducted under the International Conference on Harmonisation Good Clinical Practice guidelines and according to the Declaration of Helsinki. A local institutional review or ethics board approved the study at each site. All patients provided written informed consent before participation.
Patient Eligibility
Detailed eligibility criteria are available in the protocol (Data Supplement). Briefly, patients were required to have adequate bone marrow function, an Eastern Cooperative Oncology Group performance status of 0 or 1, creatinine clearance ≥ 50 mL/min, liver transaminases ≤ 3.0× the upper limit of normal, and bilirubin ≤ 1.5× the upper limit of normal. One patient with multiple myeloma was enrolled early in the study, when the inclusion criteria permitted patients with other lymphoproliferative diseases. Patients were excluded if they had Burkitt or Burkitt-like lymphoma, lymphoblastic lymphoma or leukemia, post-transplantation lymphoproliferative disorder, active infection, previous allogeneic stem-cell transplantation (SCT) at any time, or autologous transplantation within 6 months of starting therapy. Antilymphoma therapy was not allowed within 14 days (8 weeks for monoclonal antibodies) before the first dose. Corticosteroids were allowed for a limited duration for medical indications other than antineoplastic intent.
Treatment
Venetoclax was administered orally once daily. Sequential dose-escalation cohorts of ≥ three patients were accrued to a 3 + 3 design.10 Patients in the initial dose-escalation cohorts reached target daily doses of 200 to 1,200 mg. Safety expansion cohorts included only patients with FL and DLBCL, who received a target dose of 1,200 mg daily. Patients continued receiving daily venetoclax until progression of disease, unacceptable toxicity, or elective withdrawal in remission to proceed to allogeneic SCT. Growth factor support, supportive care, and antimicrobial prophylaxis were provided according to institutional standards of care.
On the basis of the experience with CLL,7 an early amendment implemented strict measures for TLS prophylaxis, monitoring, and management (details provided in protocol; Data Supplement). Briefly, patients were generally treated as outpatients (except for those with MCL with maximum tumor diameter ≥ 10 cm, who received initial doses as inpatients), with oral or intravenous hydration, allopurinol, and in some cases rasburicase. Patients started at a low dose of venetoclax on day 1, with subsequent intrapatient dose ramp-up (Appendix Table A1, online only). If there was no evidence of TLS, patients received the next higher dose on day 2 or 8, with additional dose increases once per week until target dosing was reached. Laboratory monitoring for TLS was performed at a minimum of 8 and 24 hours postdose at each dose increase.
Study Assessments
Safety.
Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).11 The following events were reported as DLTs if they occurred during the observation period of the lead-in plus the first 3 weeks at target dosing and were thought to be at least possibly related to venetoclax: grade 4 neutropenia ≥ 7 days (despite growth factor support), grade 3 to 4 neutropenia with fever, grade 4 thrombocytopenia, grade ≥ 2 bleeding associated with thrombocytopenia, unexpected grade 2 toxicity that required dose modification or delay of ≥ 1 week, clinical TLS, or laboratory TLS that did not resolve within 72 hours despite intervention. Venetoclax could be reintroduced with dose reduction when a toxicity had improved to grade ≤ 1 or baseline if grade 2 at study entry.
Pharmacokinetics.
Pharmacokinetic assessments were performed predose and at 2, 3, 4, 6, 8, and 24 hours postdose, with additional sampling 8 hours postdose on day 1 of weeks 2 to 5 if the patient was increased to a higher dose.
Exploratory biomarkers.
Formalin-fixed, paraffin-embedded tumor samples were evaluated using immunohistochemistry (IHC) for BCL-2 (clone 124) and MYC (clone Y69) from Ventana Medical Systems (Tucson, AZ). BCL-2 immunostaining was scored on a 0 to 3 intensity scale; samples were coded BCL-2 high if ≥ 50% of lymphoma cells showed a cytoplasmic intensity score of 2+ or 3+ and BCL-2 low if positive in < 50% of lymphoma cells.12 Samples were coded MYC positive if ≥ 40% of lymphoma cells showed any level of MYC nuclear staining. DLBCL tumors IHC positive for BCL-2 and MYC were classified as double-expressor (DE) lymphoma.13 Classification of DLBCL tumor samples into activated B-cell (ABC) or germinal center B-cell (GCB) subtypes was determined using the Lymphoma Subtyping Test from NanoString Technologies (Seattle, WA).14
Efficacy.
Efficacy end points included overall response rate (ORR), progression-free survival (PFS), duration of response (DOR), time to progression, and overall survival. Responses were assessed according to the 2007 International Working Group or the Fourth International Workshop on Waldenström Macroglobulinemia criteria.15,16 Computed tomography or positron emission tomography with computed tomography was performed at screening, weeks 6, 12 or 16, and 24, and every 12 weeks thereafter. Patients were observed for survival after discontinuation of venetoclax.
Statistical Analysis
All patients who received at least one dose of venetoclax were included in the safety, pharmacokinetics, and survival analyses. Descriptive statistics including medians, standard deviations, and ranges were calculated. The Kaplan-Meier method was used for time-to-event analyses. PFS and DOR data were censored at the time of last tumor assessment in patients who did not experience an event or at time of data cutoff if an assessment was performed after the cutoff. Data were analyzed both by specific dose cohort and by pooling multiple cohorts. Pharmacokinetic parameters were assessed using a noncompartmental approach and biomarker studies by descriptive statistics.
RESULTS
Patient Demographic and Clinical Characteristics
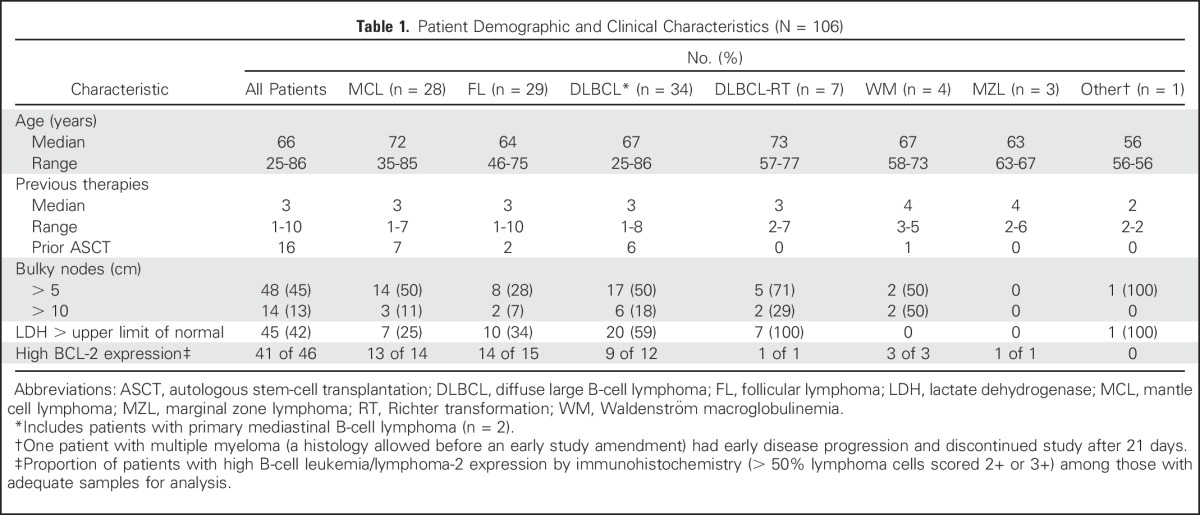
A total of 106 patients with a median age of 66 years (range, 25 to 86 years) were enrolled. Key demographic and clinical characteristics are summarized in Table 1. Seventy patients were treated in dose-escalation cohorts (target daily dose, 200 to 1,200 mg). Thirty-six patients (DLBCL, n = 21; FL, n = 15) were treated in the safety expansion cohort (target daily dose, 1,200 mg). The median number of prior therapies was three (range, one to 10). Among patients with MCL, none had received ibrutinib or lenalidomide, and five had received prior bortezomib-based therapy. Fifteen patients had received granulocyte colony-stimulating factor (G-CSF) within 6 months of study entry. Approximately half of the patients had bulky lymphadenopathy (> 5 cm, 45%; > 10 cm, 13%) at study entry.
Table 1.
Patient Demographic and Clinical Characteristics (N = 106)
Safety Profile and Disposition
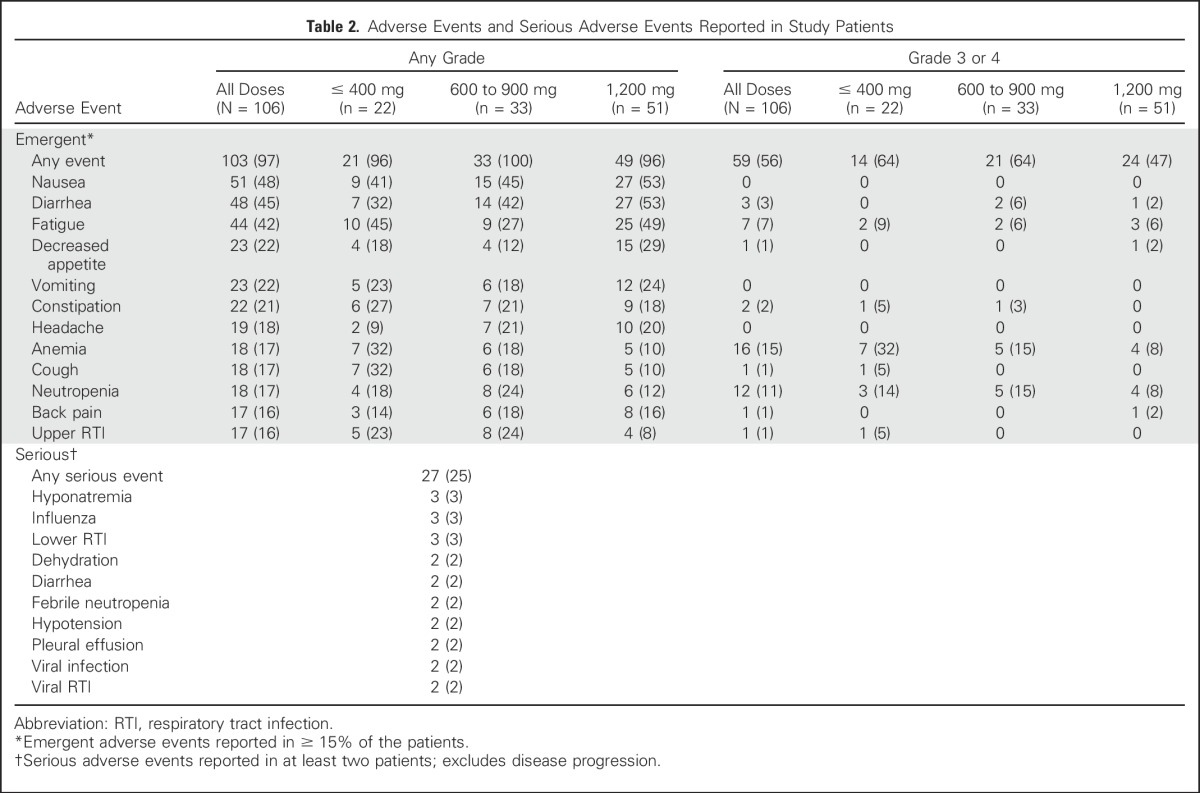
The mean duration of venetoclax treatment was 5.3 months (range, 0.2 to 46.0 months). The MTD of venetoclax was not reached. Two DLTs occurred, both in the 600-mg dose-escalation cohort: one grade 4 neutropenia in a patient with Richter transformation DLBCL (DLBCL-RT) that resolved after pegfilgrastim and a 9-day study drug interruption, and one grade 3 febrile neutropenia in a patient with DLBCL that resolved after a 2-day study drug interruption and G-CSF. A majority of AEs were grade 1 to 2 in severity; grade 3 to 4 events were reported in 59 patients (56%) and were independent of dose (Table 2). Grade 3 to 4 hematologic toxicities included anemia (15%), neutropenia (11%), and thrombocytopenia (9%). Serious AEs that occurred in > 2% of patients included hyponatremia, influenza, and lower respiratory tract infection (3% each; Table 2). There was no cumulative toxicity apparent with prolonged dosing and no clear association of toxicity with venetoclax dose. Fifteen patients required dose reductions, including nine of the 51 patients treated at 1,200 mg (five for nausea, four for diarrhea). Seventeen patients (16%) required G-CSF support (eight received filgrastim, seven pegfilgrastim, and two both at different times). Twenty-two total G-CSF doses were administered in 13 patients. Eight events of neutropenia prompted dose interruption, and five events prompted dose reduction. Only two patients received prolonged G-CSF support.
Table 2.
Adverse Events and Serious Adverse Events Reported in Study Patients
Eighty-seven patients have ended study participation: 77 had progressive disease, three had AEs (anemia, diarrhea and nausea, toxic myopathy), three underwent allogeneic SCT while in remission, two withdrew consent, one was noncompliant with the protocol, and one was removed at investigator discretion. Thirty-eight deaths have occurred: 10 within 30 days of venetoclax discontinuation (all related to disease progression) and 28 ≥ 30 days after discontinuation (of which 24 were the result of disease progression).
Given the TLS observed in CLL,7 there was particular vigilance for this potential toxicity. Clinical TLS was not observed in the NHL population. Three patients with bulky disease (maximal lymph node diameter, > 10 cm) had laboratory changes meeting Cairo-Bishop criteria for laboratory TLS17 within 24 hours of initial dosing. All three patients received TLS treatment and continued venetoclax as scheduled without dose interruption.
Pharmacokinetics
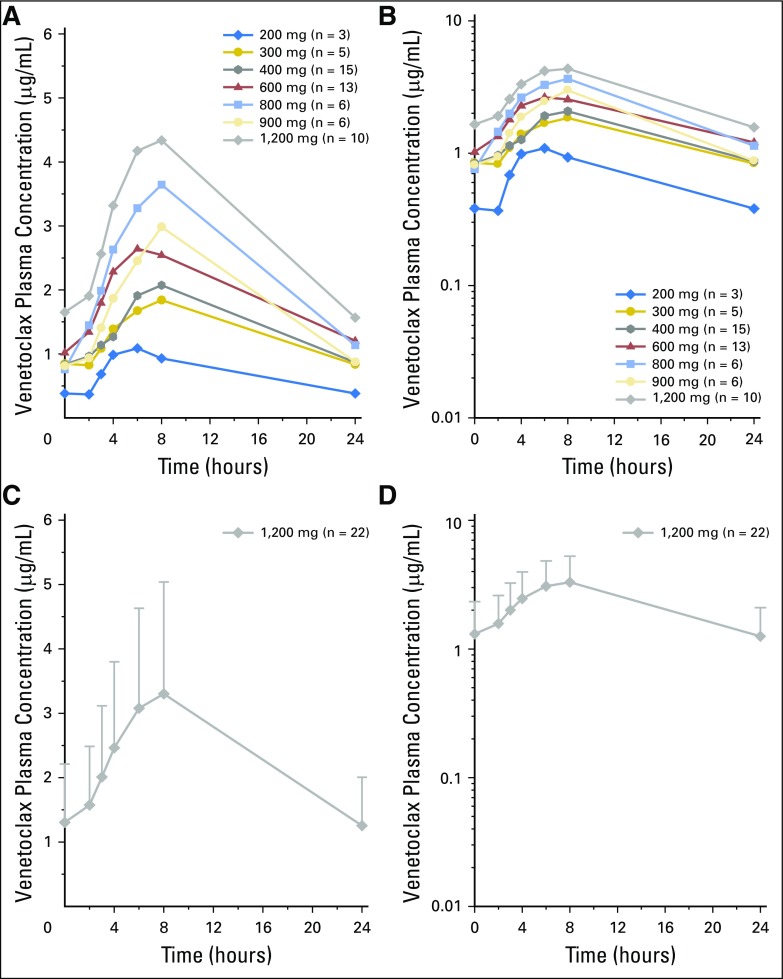
Venetoclax pharmacokinetics in patients with NHL (Appendix Fig A1, online only) were similar to those in patients with CLL.7 Plasma concentrations peaked approximately 6 to 8 hours after dosing, and the terminal half-life was approximately 16 hours. No trend was observed in dose-normalized steady-state trough levels over time.
Preliminary Efficacy
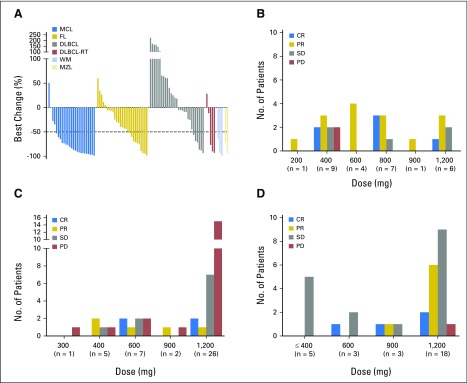
Of the total 106 patients enrolled, 44% achieved an objective response (complete response [CR] or partial response [PR]). Best response according to histology is summarized in Table 3. Responses were seen in all histologies, with the highest response rate in MCL (ORR, 75% [CR, 21%]). Significant antitumor activity was also observed in other histologies, including FL (ORR, 38% [CR, 14%]), DLBCL (ORR, 18% [CR, 12%]), DLBCL-RT (ORR, 43% [no CRs]), marginal zone lymphoma (MZL; ORR, 67% [no CRs]), and Waldenström macroglobulinemia (WM; ORR, 100% [no CRs]). The median time to first response for all responders was 42 days (range, 30 to 366 days; MZL, 36.5; MCL, 38; DLBCL, 39; FL, 40; WM, 74.5; DLBCL-RT, 100 days). The depth of nodal response across different histologies is shown in Figure 1A. For MCL, objective responses were seen even at doses ≤ 800 mg (ORR, 76%, including CR rate of 24%) and not at a higher rate at doses higher than this (Fig 1B). For DLBCL, responses were observed across dose cohorts, but a specific dose threshold for efficacy was not identified (Fig 1C). In FL, objective responses were more frequent at higher doses (> 600 mg), particularly at the 1,200-mg dose (nodal responses, 44%; Fig 1D).
Table 3.
Objective Responses by Histology (all doses, intention to treat)
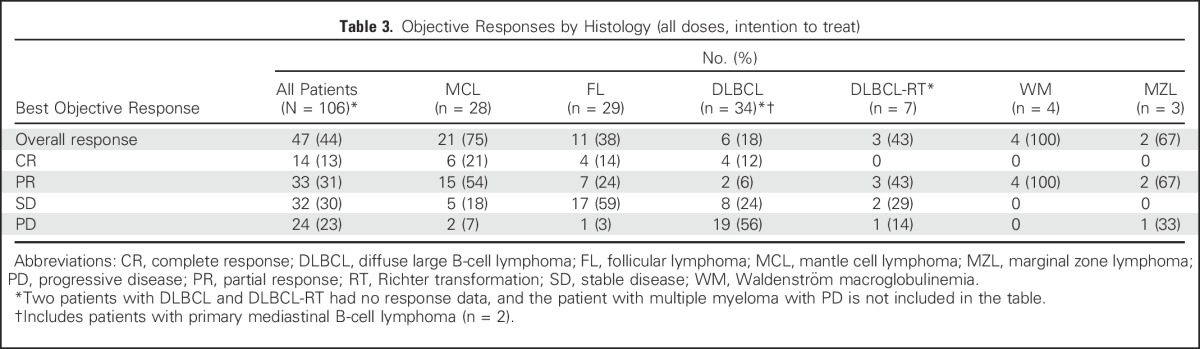
Fig 1.
Venetoclax induces response in several non-Hodgkin lymphoma histologies. (A) Waterfall plot depicting the best percentage change in the sum of the product of the diameters of target lymph nodes in patients with evaluable disease. Objective response by target dose level in patients with (B) mantle cell lymphoma (MCL), (C) diffuse large B-cell lymphoma (DLBCL), and (D) follicular lymphoma (FL). CR, complete response; MZL, marginal zone lymphoma; PD, progressive disease; PR, partial response; RT, Richter transformation; SD, stable disease; WM, Waldenström macroglobulinemia.
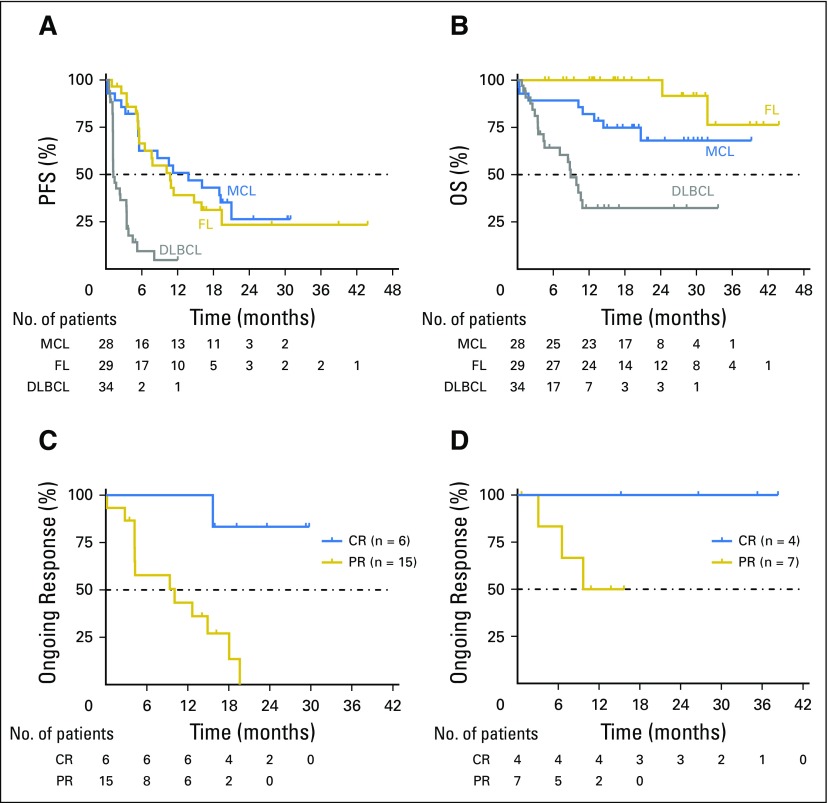
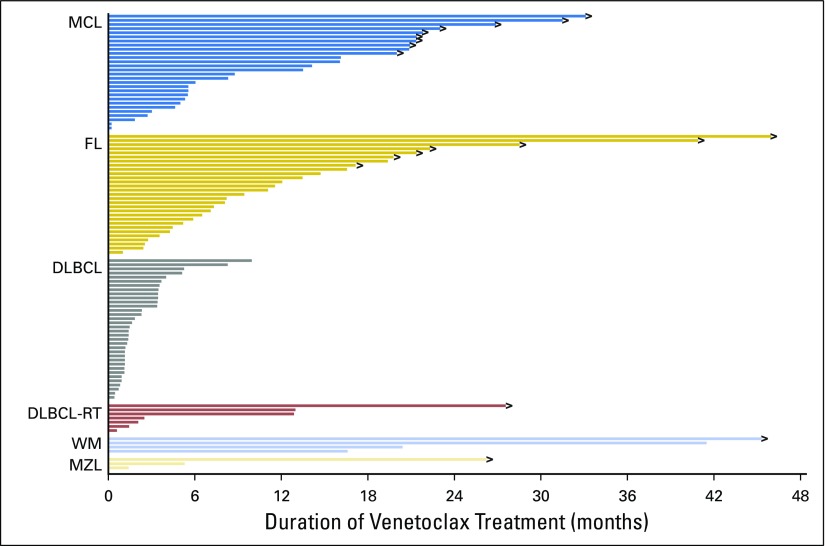
The estimated median PFS for all 106 patients was 6 months (95% CI, 4 to 10), but it varied by histology; for patients with MCL, FL, and DLBCL, it was 14, 11, and 1 months, respectively (Fig 2A). DOR for the four patients with WM was 11.1, 12.4, 38.2, and 41.5 months, and for the two patients with MZL, it was 2.3 and 23.6 months, respectively. The estimated 12-month overall survival for all patients was 70%, and it was 100%, 82%, and 32% for those with FL, MCL, and DLBCL, respectively (Fig 2B). Patients with MCL or FL who achieved CRs had more durable responses than those who achieved PRs as best response (Figs 2C and 2D). Three patients with chemotherapy-refractory disease, including DLBCL, primary mediastinal large B-cell lymphoma, and MCL, proceeded to allogeneic SCT after achieving remission with venetoclax (CR, PR, and PR, respectively); all three patients remained disease free without further treatment at 24.5, 24.5, and 22.7 months, respectively, after transplantation during the protocol-defined follow-up period of 2 years after venetoclax discontinuation. At the time of data cutoff, 19 patients continued to receive study treatment and derive clinical benefit (Fig 3).
Fig 2.
Durability of antitumor activity of venetoclax. Dashed lines indicate the 50% mark in each panel. (A) Progression-free survival (PFS) of patients with mantle cell lymphoma (MCL), follicular lymphoma (FL), and diffuse large B-cell lymphoma (DLBCL). Of note, three patients with previously chemotherapy-refractory disease, including DLBCL, primary mediastinal large B-cell lymphoma, and MCL, who proceeded to allogeneic stem-cell transplantation after achieving remission with venetoclax were censored when they left the study but remained disease free during the protocol-defined 2-year follow-up period after venetoclax therapy. (B) Overall survival (OS) of patients with MCL, FL, and DLBCL. Duration of response in (C) evaluable patients with MCL and (D) patients with FL who achieved a complete (CR) or partial response (PR).
Fig 3.
Patient status during study by histology, including mantle cell lymphoma (MCL), follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), DLBCL with Richter transformation (RT), Waldenström macroglobulinemia (WM), and marginal zone lymphoma (MZL). Right arrows indicate patients who continue to receive active treatment.
Exploratory Biomarkers
Baseline tissue was available from 46 patients; 41 samples (89%) were BCL-2 high by IHC. BCL-2 expression according to histology is summarized in Table 1. High expression was observed in all histologies, including MCL (13 [93%] of 14), FL (14 [93%] of 15), DLBCL (nine [75%] of 12), and WM (three [100%] of three), but did not correspond to observed responses. The ORR was similar in patients with BCL-2 low and BCL-2 high expression based on IHC staining (Appendix Table A2, online only). Cell-of-origin molecular subtype was determined for the five baseline DLBCL tumor samples that were available. Four patients had a GCB subtype, and one patient had an ABC subtype. CRs were observed in one patient each with ABC and GCB subtypes (Appendix Table A3, online only). BCL-2 and MYC DE lymphoma was observed in four of six evaluable DLBCL tumors. Two patients with DE lymphoma achieved an objective response (CR, n = 1; PR, n = 1; Appendix Table A4, online only). All five patients with DLBCL with sufficient tissue for fluorescent in situ hybridization analysis were negative for BCL-2 and MYC rearrangements. Two other patients had double-hit DLBCL noted in prior pathology reports, and neither patient achieved a response to venetoclax.
DISCUSSION
Venetoclax was well tolerated in patients with relapsed or refractory NHL. The high level of selectivity of venetoclax for BCL-26 led to enhanced antilymphoma activity without the dose-limiting thrombocytopenia observed with its predecessor navitoclax,18 a less selective BH3 mimetic. No clinical TLS was observed with starting doses of up to 400 mg daily (the dose now being used for studies of venetoclax in patients with CLL) or with ongoing dosing at 1,200 mg daily. Only three patients with high tumor burden had evidence of laboratory TLS, and all were managed successfully with intravenous fluids, corrective measures, and continuation of allopurinol. These results suggest that for NHL subtypes other than MCL, a dosing strategy starting at 400 mg daily for 1 week followed by weekly ramp-up from 800 mg daily to 1,200 mg daily thereafter with outpatient monitoring for TLS is well tolerated. Patients with high tumor burden, particularly those with MCL, are at increased risk for TLS and may be best managed with a more conservative dosing strategy, including a starting dose of 100 mg daily for 1 week, followed by weekly ramp-up to 200, 400, and 800 mg daily.
The other toxicities of venetoclax were mild and included mostly low-grade GI toxicity and neutropenia. The latter is likely an on-target effect of BCL-2 inhibition in neutrophil precursors19 and was well managed with supportive care including growth factor support administered concomitantly with treatment or dose interruption. Because a > 30% DLT rate was not seen at any dose level tested, no formal MTD was determined. The recommended phase II dose was therefore determined by the investigators to be the maximum cleared dose for FL and DLBCL (1,200 mg) and for DLBCL-RT (600 mg). For MCL, 800 mg was chosen given the significant activity observed at this dose and the risk of incremental toxicity at higher doses.
Venetoclax demonstrated significant single-agent activity across a broad range of NHL subtypes. The highest response rate was seen in MCL, with 75% of patients achieving responses, including 21% achieving CRs. Patients with MCL had a median PFS of 14 months, with only one progression to date among six complete responders and some patients receiving treatment > 33 months. These results are similar to those seen in MCL with the Bruton tyrosine kinase inhibitor ibrutinib (ORR, 68% [CR, 21%]; median PFS, 13.9 months).20
Patients with FL had a 38% response rate, with 14% achieving CRs, and a median PFS of 11 months, similar to the results seen with the delta isoform–specific phosphatidylinositol-3-kinase inhibitor idelalisib in FL (ORR, 47% to 57%; PFS, 7.6 to 11 months).21,22 Higher response rates were seen with higher doses of venetoclax in FL; the ORR was 44% for the 18 patients treated at 1,200 mg compared with an ORR of 27% for the 11 patients treated at ≤ 900 mg. These preliminary results need to be explored further in additional studies.
The response rate for patients with DLBCL was 18%, and unlike in FL, it did not seem to vary based on cohort dose. Nevertheless, four patients achieved CRs, including two patients who went on to undergo allogeneic SCT and remain in complete remission now > 2 years after transplantation. B-cell receptor pathway antagonists have shown limited efficacy in DLBCL, and therefore, novel agents such as venetoclax are urgently needed for combination regimens. Cell-of-origin data were only available in five patients with DLBCL, which is a limitation of this study. Significant responses to venetoclax were also noted in patients with MZL and WM, supporting the exploration of venetoclax in future studies in these diseases.
Understanding the heterogeneity of clinical responses observed will require further exploration. In the CLL arm, the depth of initial response to venetoclax was associated with the level of mitochondrial priming for apoptosis as determined by the BH3 profiling assay.23 Correlative samples were limited in the NHL arm and did not allow for systematic evaluation of parameters such as antiapoptotic family protein or mRNA expression, mutational analysis, or BH3 profiling. Exploratory analysis of the level of BCL-2 expression by IHC showed no significant association with clinical response or TLS risk in this patient group, where all patient samples tested had some BCL-2 expression. These results indicate that BCL-2 protein expression alone is not an adequate biomarker to predict clinical response or TLS risk, and functional assessments of the relative balance of the anti- and proapoptotic BCL-2 family proteins should be explored as biomarkers. Additional biomarker investigations will be important in future studies of venetoclax in NHL.
The optimal combination partners for venetoclax in NHL remain to be defined, and studies are under way to assess the safety and efficacy of combining venetoclax with chemotherapy, monoclonal antibodies, and B-cell receptor signaling inhibitors. Preclinical data suggest that abbreviated venetoclax use in combination with DNA-damaging cytotoxic therapies may be sufficient to achieve durable remissions.6,19,24 Combination with rituximab augmented the efficacy of the less selective BCL-2 inhibitor navitoclax in FL,25 and similar studies with venetoclax are ongoing.
We conclude that venetoclax is active as a single agent in NHL, with clinical benefit varying depending on histologic subtype. Targeted inhibition of BCL-2 will likely have a greater clinical impact when venetoclax is combined with other agents. The favorable toxicity profile and unique mechanism of action suggest that venetoclax will be a logical partner in a range of combination regimens. Ongoing trials will define the optimal use of venetoclax in NHL.
ACKNOWLEDGMENT
We thank the patients and their families, study coordinators, and support staff. Editorial support was provided by Sharanya Ford, of AbbVie, and by Evidence Scientific Solutions (Philadelphia, PA) and funded by AbbVie. Data programming and analysis support was provided by Joseph Beason.
Appendix
Table A1.
Intrapatient Ramp-Up Scheme in Arm B of the M12-175 Study
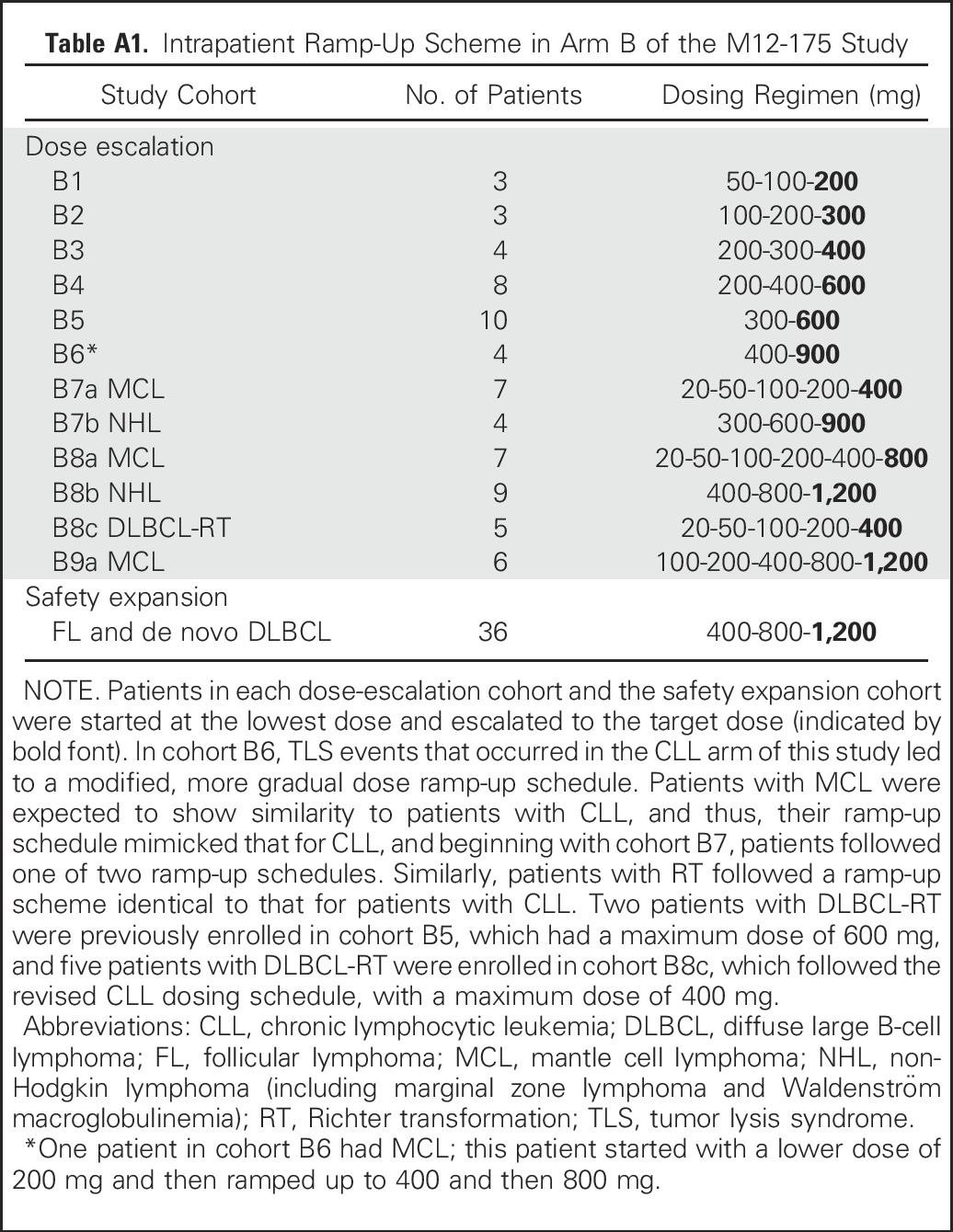
Table A2.
Summary of Objective Responses and PFS by BCL-2 Expression Data (n = 46)
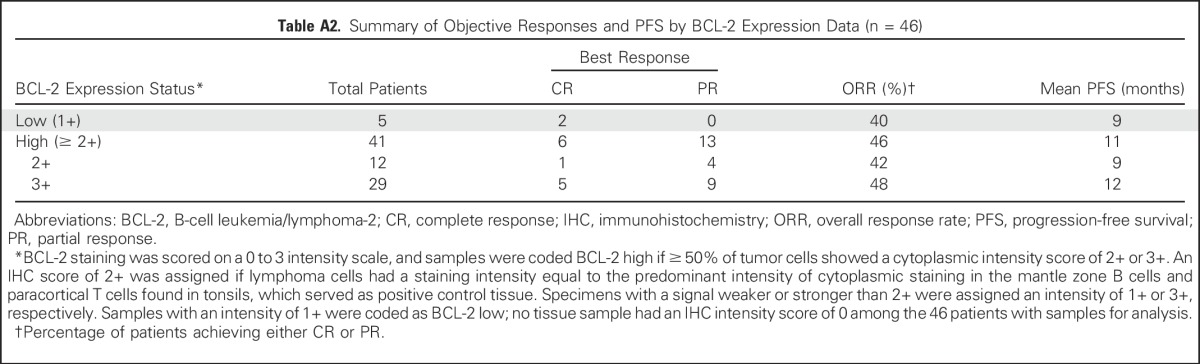
Table A3.
Objective Responses by DLBCL COO Subtype
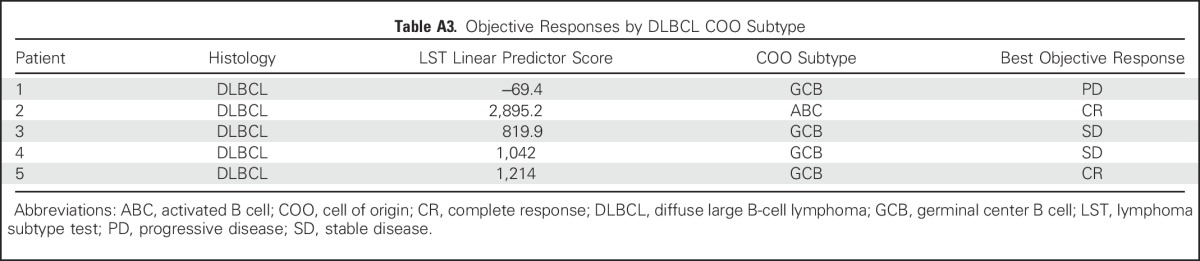
Table A4.
Objective Responses by BCL-2 and MYC DE Subtype
Fig A1.
Mean venetoclax plasma concentration. Time profiles in (A, B) dose-escalation and (C, D) safety expansion cohorts according to (A, C) linear and (B, D) log-linear scales. Error bars represent standard deviations.
Footnotes
Supported by AbbVie (which also funded editorial support) and Genentech; in part by the National Institutes of Health/National Cancer Institute Cancer Center Support Grant No. P30 CA008748 (J.F.G.); and in part by a Career Development Award from the American Society of Clinical Oncology (M.S.D.).
Presented in part at the 57th Annual Meeting of the American Society of Hematology (ASH), Orlando, FL, December 5-8, 2015; Pan Pacific Lymphoma Conference, Kohala Coast, HI, July 21-25, 2014; 19th Congress of the European Hematology Association (EHA), Milan, Italy, June 12-15, 2014; 50th Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, May 30-June 3, 2014; 55th ASH Annual Meeting, New Orleans, LA, December 7-10, 2013; 18th EHA Congress, Stockholm, Sweden, June 13-16, 2013; 49th ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2013; 12th International Conference on Malignant Lymphoma, Lugano, Switzerland, June 19-22, 2013; and 54th ASH Annual Meeting, Atlanta, GA, December 8-11, 2012.
Processed as a Rapid Communication manuscript.
Venetoclax is being developed in collaboration between AbbVie and Genentech, both of which provided financial support for the study and participated in the design, study conduct, analysis, and interpretation of data, as well as the writing, review, and approval of the manuscript.
Clinical trial information: NCT01328626.
AUTHOR CONTRIBUTIONS
Conception and design: Matthew S. Davids, Andrew W. Roberts, John F. Seymour, Rod A. Humerickhouse
Financial support: Ahmed Hamed Salem, Martin Dunbar, Ming Zhu, Franklin Peale, Jeremy A. Ross, Lori Gressick, Monali Desai, Su Young Kim, Maria Verdugo, Rod A. Humerickhouse, Gary B. Gordon
Provision of study materials or patients: Matthew S. Davids, Andrew W. Roberts, John F. Seymour, John M. Pagel, Brad S. Kahl, William G. Wierda, Soham Puvvada, Thomas J. Kipps, Mary Ann Anderson, John F. Gerecitano
Collection and assembly of data: Matthew S. Davids, Andrew W. Roberts, John F. Seymour, John M. Pagel, Brad S. Kahl, William G. Wierda, Soham Puvvada, Thomas J. Kipps, Mary Ann Anderson, John F. Gerecitano
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase I First-in-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Matthew S. Davids
Consulting or Advisory Role: Infinity Pharmaceuticals, Genentech, Gilead Sciences, Janssen Pharmaceuticals, Pharmacyclics, TG Therapeutics, Celgene, AbbVie
Research Funding: Genentech (Inst), TG Therapeutics (Inst), Infinity Pharmaceuticals (Inst), Pharmacyclics (Inst), AbbVie (Inst)
Andrew W. Roberts
Research Funding: AbbVie (Inst), Genentech (Inst), Amgen (Inst), BeiGene (Inst), Servier Laboratories (Inst)
Patents, Royalties, Other Intellectual Property: Employee of Walter and Eliza Hall Institute, which receives milestone and royalty payments related to venetoclax (Inst)
John F. Seymour
Consulting or Advisory Role: Roche, Genentech
Speakers’ Bureau: Roche, Genentech
Research Funding: AbbVie, Genentech
John M. Pagel
Consulting or Advisory Role: Pharmacyclics, Gilead Sciences
Brad S. Kahl
Consulting or Advisory Role: AbbVie, Celgene, Genentech, Millennium, Pharmacyclics, Seattle Genetics
Research Funding: AbbVie, Genentech
William G. Wierda
Consulting or Advisory Role: Sanofi, Genentech, Pharmacyclics, Celgene, Gilead Sciences, GlaxoSmithKline, Novartis, Genzyme, Merck, AbbVie, Emergent BioSolutions
Research Funding: GlaxoSmithKline, Novartis, AbbVie, Genentech, Karyopharm Therapeutics, Pharmacyclics, Acerta Pharma, Gilead Sciences, Janssen Pharmaceuticals, Emergent BioSolutions, Juno Therapeutics, Kite Pharma
Soham Puvvada
Consulting or Advisory Role: AbbVie, Genentech, Pharmacyclics, Seattle Genetics
Speakers’ Bureau: Gilead Sciences
Research Funding: Spectrum Pharmaceuticals (Inst), AbbVie (Inst), Genentech (Inst), Seattle Genetics (Inst), Takeda Pharmaceuticals (Inst), Janssen Pharmaceuticals (Inst)
Thomas J. Kipps
Consulting or Advisory Role: AbbVie
Research Funding: AbbVie
Mary Ann Anderson
Honoraria: AbbVie
Speakers’ Bureau: AbbVie
Research Funding: AbbVie (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: AbbVie
Ahmed Hamed Salem
Employment: AbbVie
Stock or Other Ownership: AbbVie
Martin Dunbar
Employment: AbbVie
Stock or Other Ownership: AbbVie
Ming Zhu
Employment: AbbVie
Stock or Other Ownership: AbbVie
Franklin Peale
Employment: Genentech
Stock or Other Ownership: Genentech
Jeremy A. Ross
Employment: AbbVie
Stock or Other Ownership: AbbVie
Lori Gressick
Employment: AbbVie
Stock or Other Ownership: AbbVie
Monali Desai
Employment: AbbVie
Stock or Other Ownership: AbbVie
Travel, Accommodations, Expenses: AbbVie
Su Young Kim
Employment: AbbVie
Stock or Other Ownership: AbbVie
Maria Verdugo
Employment: AbbVie
Stock or Other Ownership: AbbVie
Rod A. Humerickhouse
Employment: AbbVie
Stock or Other Ownership: AbbVie
Gary B. Gordon
Employment: AbbVie, Abbott Laboratories, Medtronic
Stock or Other Ownership: AbbVie
John F. Gerecitano
Honoraria: Genentech, AbbVie, Bayer HealthCare Pharmaceuticals, Gilead Sciences, Samus Therapeutics
Consulting or Advisory Role: Genentech, AbbVie, Bayer HealthCare Pharmaceuticals, Gilead Sciences, Samus Therapeutics
REFERENCES
- 1.Pegoraro L, Palumbo A, Erikson J, et al. A 14;18 and an 8;14 chromosome translocation in a cell line derived from an acute B-cell leukemia. Proc Natl Acad Sci USA. 1984;81:7166–7170. doi: 10.1073/pnas.81.22.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonnell TJ, Deane N, Platt FM, et al. Bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989;57:79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- 3.Tsujimoto Y, Finger LR, Yunis J, et al. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 4.Huang JZ, Sanger WG, Greiner TC, et al. The t(14;18) defines a unique subset of diffuse large B-cell lymphoma with a germinal center B-cell gene expression profile. Blood. 2002;99:2285–2290. doi: 10.1182/blood.v99.7.2285. [DOI] [PubMed] [Google Scholar]
- 5.Bentz M, Plesch A, Bullinger L, et al. t(11;14)-positive mantle cell lymphomas exhibit complex karyotypes and share similarities with B-cell chronic lymphocytic leukemia. Genes Chromosomes Cancer. 2000;27:285–294. [PubMed] [Google Scholar]
- 6.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 7.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AbbVie: Venclexta (venetoclax) tablets: Prescribing information. http://www.rxabbvie.com/pdf/venclexta.pdf
- 10.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101:708–720. doi: 10.1093/jnci/djp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Institutes of Health, National Cancer Institute: Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm [Google Scholar]
- 12.Punnoose EA, Leverson JD, Peale F, et al. Expression profile of BCL-2, BCL-XL, and MCL-1 predicts pharmacological response to the BCL-2 selective antagonist venetoclax in multiple myeloma models. Mol Cancer Ther. 2016;15:1132–1144. doi: 10.1158/1535-7163.MCT-15-0730. [DOI] [PubMed] [Google Scholar]
- 13.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott DW, Wright GW, Williams PM, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood. 2014;123:1214–1217. doi: 10.1182/blood-2013-11-536433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 16.Dimopoulos MA, Gertz MA, Kastritis E, et al. Update on treatment recommendations from the Fourth International Workshop on Waldenstrom’s Macroglobulinemia. J Clin Oncol. 2009;27:120–126. doi: 10.1200/JCO.2008.17.7865. [DOI] [PubMed] [Google Scholar]
- 17.Cairo MS, Bishop M. Tumour lysis syndrome: New therapeutic strategies and classification. Br J Haematol. 2004;127:3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- 18.Wilson WH, O’Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: A phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leverson JD, Phillips DC, Mitten MJ, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. 2015;7:279ra40. doi: 10.1126/scitranslmed.aaa4642. [DOI] [PubMed] [Google Scholar]
- 20.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flinn IW, Kahl BS, Leonard JP, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-δ, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014;123:3406–3413. doi: 10.1182/blood-2013-11-538546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson MA, Deng J, Seymour JF, et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood. 2016;127:3215–3224. doi: 10.1182/blood-2016-01-688796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason KD, Vandenberg CJ, Scott CL, et al. In vivo efficacy of the Bcl-2 antagonist ABT-737 against aggressive Myc-driven lymphomas. Proc Natl Acad Sci USA. 2008;105:17961–17966. doi: 10.1073/pnas.0809957105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts AW, Advani RH, Kahl BS, et al. Phase 1 study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20+ lymphoid malignancies. Br J Haematol. 2015;170:669–678. doi: 10.1111/bjh.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]